Abstract
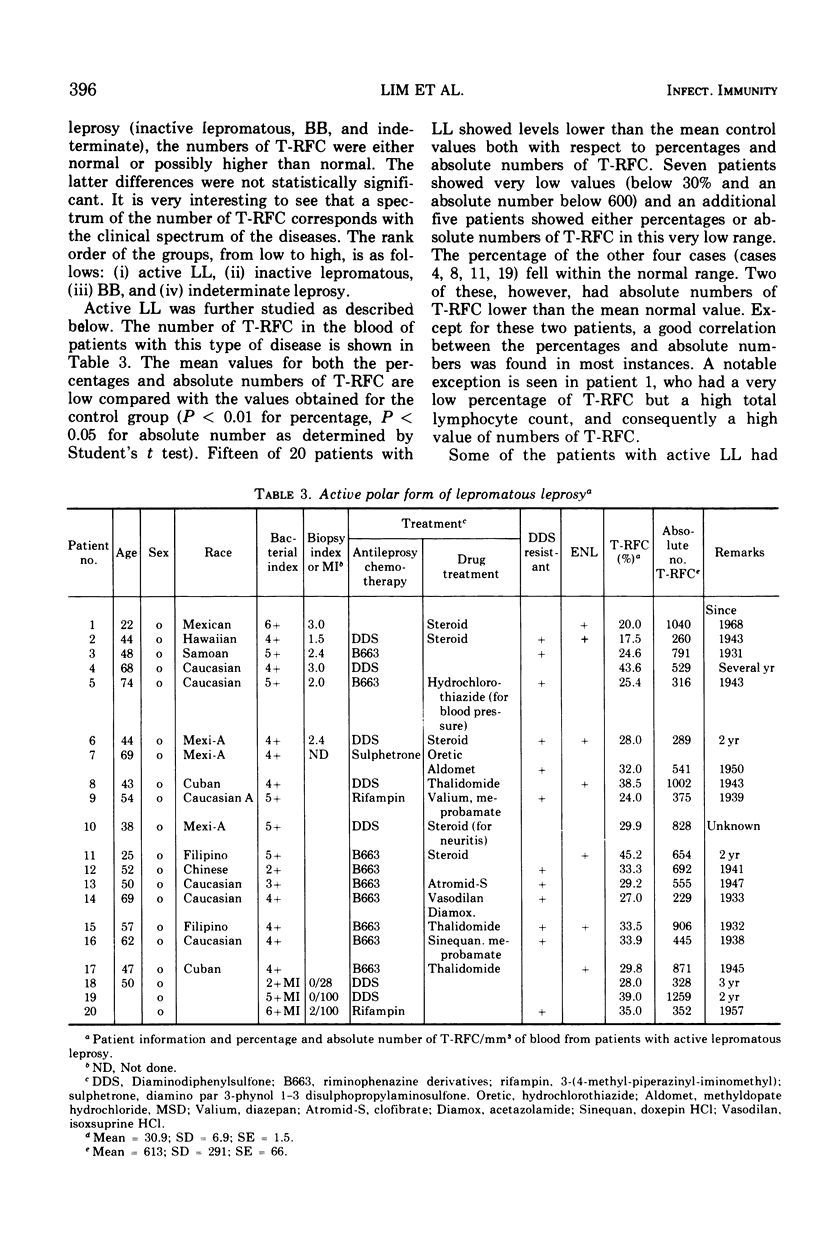
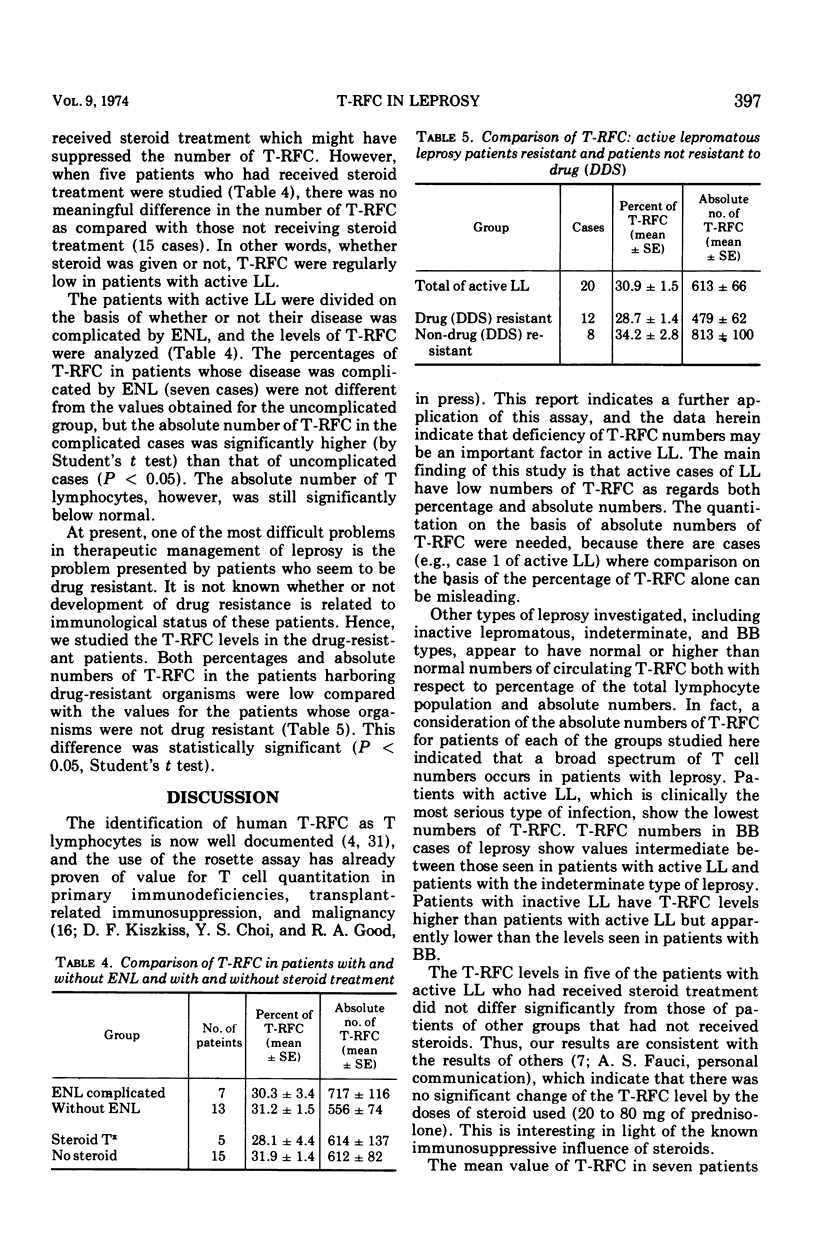
Study of the numbers of thymus-derived lymphocytes by the rosette assay (T-RFC) in patients with leprosy reveals that lower than normal numbers of T-RFC are regularly seen in those patients with the active lepromatous form of this disease. Essentially normal numbers of T-RFC were found in inactive lepromatous, borderline, and indeterminate types of leprosy. The lowest percentages and lowest absolute numbers of T-RFC were encountered in patients with lepromatous leprosy resistant to chemotherapy. Patients with lepromatous leprosy complicated by erythema nodosum leprosum show numbers of T-RFC that are more nearly normal than the numbers of T-RFC in patients with uncomplicated lepromatous leprosy. These findings are discussed with respect to the pathogenesis of lepromatous leprosy and the T-RFC deficiency demonstrated in this disease. The possibility that transient defects in T-RFC numbers or function may predispose to lepromatous leprosy is proposed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain P., Gordon J., Willetts W. A. Rosette formation by peripheral lymphocytes. Clin Exp Immunol. 1970 May;6(5):681–688. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Jr, Fasal P. Studies of immune mechanisms in leprosy. 3. The role of cellular and humoral factors in impairment of the in vitro immune response. J Immunol. 1971 Apr;106(4):888–899. [PubMed] [Google Scholar]
- Coombs R. R., Gurner B. W., Wilson A. B., Holm G., Lindgren B. Rosette-formation between human lymphocytes and sheep red cells not involving immunoglobulin receptors. Int Arch Allergy Appl Immunol. 1970;39(5-6):658–663. doi: 10.1159/000230390. [DOI] [PubMed] [Google Scholar]
- Dierks R. E., Shepard C. C. Effect of phytohemagglutinin and various mycobacterial antigens on lymphocyte cultures from leprosy patients. Proc Soc Exp Biol Med. 1968 Feb;127(2):391–395. doi: 10.3181/00379727-127-32698. [DOI] [PubMed] [Google Scholar]
- Dwyer J. M., Bullock W. E., Fields J. P. Disturbance of the blood T:B lymphocyte ratio in lepromatous leprosy. Clinical and immunologic correlations. N Engl J Med. 1973 May 17;288(20):1036–1039. doi: 10.1056/NEJM197305172882002. [DOI] [PubMed] [Google Scholar]
- Gaafar S. M., Turk J. L. Granuloma formation in lymph-nodes. J Pathol. 1970 Jan;100(1):9–20. doi: 10.1002/path.1711000103. [DOI] [PubMed] [Google Scholar]
- Gajl-Peczalska K. J., Lim S. D., Jacobson R. R., Good R. A. B lymphocytes in lepromatous leprosy. N Engl J Med. 1973 May 17;288(20):1033–1035. doi: 10.1056/NEJM197305172882001. [DOI] [PubMed] [Google Scholar]
- Gaugas J. M. Enhancing effect of antilymphocytic globulin on human leprosy infection in thymectomized mice. Nature. 1968 Dec 21;220(5173):1246–1248. doi: 10.1038/2201246a0. [DOI] [PubMed] [Google Scholar]
- Gaugas J., Rees R. J. Enhancing effect of antilymphocytic serum on mycobacterial infections in mice. Nature. 1968 Jul 27;219(5152):408–409. doi: 10.1038/219408a0. [DOI] [PubMed] [Google Scholar]
- Han S. H., Weiser R. S., Kau S. T. Prolonged survival of skin allografts in leprosy patients. Int J Lepr Other Mycobact Dis. 1971 Jan-Mar;39(1):1–6. [PubMed] [Google Scholar]
- Han S. H., Weiser R. S., Lin Y. C. Transformation of leprous lymphocytes by leprolin, tuberculin and phytohemagglutinin. Int J Lepr Other Mycobact Dis. 1971 Oct-Dec;39(4):789–795. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S. I., DeBetz B. H., Zaias N. Production of macrophage inhibitory factor by patients with leprosy. Arch Dermatol. 1971 Apr;103(4):358–361. [PubMed] [Google Scholar]
- Lay W. H., Mendes N. F., Bianco C., Nussenzweig V. Binding of sheep red blood cells to a large population of human lymphocytes. Nature. 1971 Apr 23;230(5295):531–532. doi: 10.1038/230531a0. [DOI] [PubMed] [Google Scholar]
- Nelson D. S., Nelson M., Thurston J. M., Waters M. F., Pearson J. M. Phytohaemagglutinin-induced lymphocyte transformation in leprosy. Clin Exp Immunol. 1971 Jul;9(1):33–43. [PMC free article] [PubMed] [Google Scholar]
- Pettit J. H., Rees R. J., Ridley D. S. Studies on sulfone resistance in leprosy. I. Detection of cases. Int J Lepr Other Mycobact Dis. 1966 Oct-Dec;34(4):375–390. [PubMed] [Google Scholar]
- Pettit J. H., Waters M. F. The etiology of erythema nodosum leprosum. Int J Lepr Other Mycobact Dis. 1967 Jan-Mar;35(1):1–10. [PubMed] [Google Scholar]
- Rees R. J., Waters M. F., Weddell A. G., Palmer E. Experimental lepromatous leprosy. Nature. 1967 Aug 5;215(5101):599–602. doi: 10.1038/215599a0. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Turk J. L., Waters M. F. Cell-mediated immunity in patients with leprosy. Lancet. 1969 Aug 2;2(7614):243–246. doi: 10.1016/s0140-6736(69)90009-9. [DOI] [PubMed] [Google Scholar]
- Turk J. L., Waters M. F. Immunological basis for depression of cellular immunity and the delayed allergic response in patients with lepromatous leprosy. Lancet. 1968 Aug 24;2(7565):436–438. doi: 10.1016/s0140-6736(68)90472-8. [DOI] [PubMed] [Google Scholar]
- Waldorf D. S., Sheagren J. N., Trautman J. R., Block J. B. Impaired delayed hypersensitivity in patients with lepromatous leprosy. Lancet. 1966 Oct 8;2(7467):773–776. doi: 10.1016/s0140-6736(66)90366-7. [DOI] [PubMed] [Google Scholar]
- Wong P. C., Chan-Teoh C. H., Kendall F. H. Transformation of lymphocytes by phytohemagglutinin in leprosy sera. Int J Lepr Other Mycobact Dis. 1971 Jan-Mar;39(1):7–13. [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]


