Abstract
In this study the role of P2Y12 receptors (P2Y12R) was explored in rodent models of inflammatory and neuropathic pain and in acute thermal nociception. In correlation with their activity to block the recombinant human P2Y12R, the majority of P2Y12R antagonists alleviated mechanical hyperalgesia dose-dependently, following intraplantar CFA injection, and after partial ligation of the sciatic nerve in rats. They also caused an increase in thermal nociceptive threshold in the hot plate test. Among the six P2Y12R antagonists evaluated in the pain studies, the selective P2Y12 receptor antagonist PSB-0739 was most potent upon intrathecal application.
P2Y12R mRNA and IL-1β protein were time-dependently overexpressed in the rat hind paw and lumbar spinal cord following intraplantar CFA injection. This was accompanied by the upregulation of TNF-α, IL-6 and IL-10 in the hind paw. PSB-0739 (0.3 mg/kg i.t.) attenuated CFA-induced expression of cytokines in the hind paw and of IL-1β in the spinal cord. Subdiaphragmatic vagotomy and the α7 nicotinic acetylcholine receptor antagonist MLA occluded the effect of PSB-0739 (i.t.) on pain behavior and peripheral cytokine induction. Denervation of sympathetic nerves by 6-OHDA pretreatment did not affect the action of PSB-0739. PSB-0739, in an analgesic dose, did not influence motor coordination and platelet aggregation. Genetic deletion of the P2Y12R in mice reproduced the effect of P2Y12R antagonists on mechanical hyperalgesia in inflammatory and neuropathic pain models, on acute thermal nociception and on the induction of spinal IL-1β.
Here we report the robust involvement of the P2Y12R in inflammatory pain. The anti-hyperalgesic effect of P2Y12R antagonism could be mediated by the inhibition of both central and peripheral cytokine production and involves α7-receptor mediated efferent pathways.
Abbreviations: reactive blue 2, 1-amino-4-[[4-[[4-chloro-6-[[3- (or 4-)sulfophenyl]amino]-1,3,5-triazin-2-yl]amino]-3-sulfophenyl]amino]-9,10-dihydro-9,10-dioxo-2-anthracenesulfonic acid; PSB-0739, 1-amino-4-[4-phenylamino-3-sulfophenylamino]-9,10-dioxo-9,10-dihydroanthracene-2-sulfonate; α7 nAChR, α7-nicotinic acetylcholine receptors; CFA, complete Freund's adjuvant; cangrelor, [dichloro-[[[(2R,3S,4R,5R)-3,4-dihydroxy-5-[6-(2-methylsulfanylethylamino)-2-(3,3,3-trifluoropropylsulfanyl)purin-9-yl]oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyhydroxyphosphoryl]methyl]phosphonic acid; MRS2395, 2,2-dimethylpropionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethylpropionyloxymethyl)propyl ester; DMSO, dimethylsulfoxide; HPLC, high performance liquid chromatography; 6-OHDA, 6-hydroxydopamine; MLA, interleukin-1β, IL-1β, methyllycaconitine; mED, minimal effective dose; PWT, paw withdrawal threshold; PEG, polyethyleneglycol 300; P2Y12R, P2Y12 receptor; P2ry12−/− mice, P2Y12R deficient mice
Keywords: P2Y12 receptor, Purine receptor, Inflammatory pain, Neuropathic pain, Spinal cord, Interleukin-1β
Highlights
-
•
Pharmacological blockade of P2Y12 receptors alleviates inflammatory and neuropathic pain.
-
•
Central inhibition of P2Y12 receptors attenuates cytokine production in the spinal cord.
-
•
Central P2Y12 receptor inhibition attenuates cytokine production in the inflamed hind paw.
-
•
α7-Receptors mediate the effect of P2Y12 receptor blockade on hyperalgesia and cytokine level.
-
•
Genetic deletion of P2Y12 receptors alleviates inflammatory, neuropathic and acute pain.
Introduction
Purine nucleotides activating P2X and P2Y receptors are key extracellular modulators of different forms of pain (Burnstock et al., 2011, Koles et al., 2011). P2X receptors are ligand-gated non-selective cation channels, forming trimeric co-assemblies of seven individual subunits (P2X1, P2X2, P2X3, P2X4, P2X5, P2X6 and P2X7), whereas P2Y receptors belong to the G protein-coupled metabotropic receptor family consisting of eight different subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) (Coddou et al., 2011, von Kügelgen and Harden, 2011). Both P2X and P2Y receptors are expressed along nociceptive pathways and rapidly accumulating data support their involvement in the processing of pathological pain (Trang and Salter, 2012, Tsuda et al., 2012). Whereas P2X3, P2X4, and P2X7 antagonists are currently under development for treating inflammatory and neuropathic pain (Gum et al., 2012, Jarvis, 2010), among P2Y receptors, the P2Y12 receptor (Hollopeter et al., 2001) has only recently been implicated in the generation of pathological pain (Trang et al., 2012). P2Y12R antagonists, such as clopidogrel, are widely used as safe drugs for the prevention of myocardial infarction and stroke and have been among the world's best selling drugs in the recent years (Debnath et al., 2010, Raju et al., 2008). Accordingly, P2Y12Rs are highly expressed on platelets and their activation by ADP results in rapid thrombocyte aggregation. However, P2Y12Rs are also expressed in the CNS, in particular in microglia (Haynes et al., 2006). P2Y12R mRNA is upregulated in microglia and the putative P2Y12R antagonist MRS2395 and a P2Y12R specific antisense oligonucleotide inhibited hyperalgesia after partial sciatic nerve ligation (PSNL, Seltzer model) in rats (Kobayashi et al., 2008). These results were then confirmed using another P2Y12R antagonist, cangrelor (AR-C69931MX), and by genetic invalidation of P2Y12R (Tozaki-Saitoh et al., 2008). We have recently found that MRS2395 attenuated not only neuropathic, but also inflammatory pain and acute thermal nociception (Ando et al., 2010). The above studies strongly indicated the participation of P2Y12Rs in different pain modalities. However, besides P2Y12R, other metabotropic P2 receptors, such as P2Y6 and P2Y14 may also play a role in the modulation of neuropathic pain (Kobayashi et al., 2012). Antagonists of P2Y receptors used in previous studies, however, were not selective enough to obtain conclusive evidence for the inhibition of P2Y12R as a new approach for inflammatory analgesia. Moreover, the mechanism of such an action has also remained enigmatic until now.
In this study we have systematically examined the effect of genetic deletion and different P2Y12R antagonists, including the selective P2Y12R antagonist PSB-0739 (Baqi et al., 2009, Hoffmann et al., 2009) on inflammatory and neuropathic pain and acute thermal nociception in parallel with their in vitro efficacy to inhibit the human recombinant P2Y12R. Our data show that both genetic ablation and pharmacological blockade of P2Y12R reduce pain in all three models. As a potential underlying mechanism of these effects, we demonstrate the alleviation of cytokine production in the spinal cord and in the inflamed paw after central PSB-0739 treatment and the participation of α7 nAChRs in mediating this effect. Our findings indicate that central P2Y12R inhibition is a plausible strategy to combat inflammatory and neuropathic pain.
Materials and methods
Animals
All studies were conducted in strict accordance with the principles and procedures outlined in Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the ARRIVE guidelines. The local Animal Care Committee of the IEM HAS approved all experimental procedures (Permission No: 22.1/3671/003/2008). Animals were kept under standard laboratory conditions with food and water ad libitum. All efforts were made to minimize animal suffering and reduce the number of animals used. All the experiments were carried out between 9:00 and 14:00 in the housing room of the animals. Experiments were performed on male Wistar rats obtained from the local animal house and on male wild-type (C57/Bl6) and P2Y12R deficient mice (P2ry12−/−) weighting 25–30 g. B6;129-P2ry12tm1Dgen/H knockout mice (P2ry12−/−) were established by Deltagen Inc. (San Matteo, CA, USA). Mutation was generated with the insertion of lacZ and neo genes into the p2ry12 gene in an R1 ES cell. The chimeric mouse was crossed into C57Bl6 background and reposited and archived in EMMA (EM:02301). After rederivation of mice from frozen stocks, mutants were additionally crossed with C57Bl6/J mice in the Medical Gene Technology Unit of the IEM HAS. Heterozygous breeding pairs were then crossed to generate homozygous knock-outs and littermate controls. Mice that were homozygous for the targeted allele were viable, fertile, normal in size and did not display any gross physical or behavioral abnormalities. Mice were phenotyped by physical examination, necropsy, including body weight, body length and organ weight measurements, histopathology, clinical chemistry, aging and behavior. Individual homozygous or heterozygous mutant mice had only occasional minor differences in observed physical features compared to wild-type control mice. These findings are considered to represent individual variability, background features occasionally seen in this strain of mice, findings due to spontaneous disease, age-related findings, procedural artifacts, and/or findings of nonspecific document etiology. However, none of these differences was regarded as biologically significant or genotype related. For the complete phenotype data of the original mouse see Deltagen Inc. (https://deltaone.deltagen.com). Genomic DNA was isolated from mouse-tail biopsies and genotypes were identified by a multiplex quantitative PCR. We have used a Neo gene specific Taqman assay (Lifetech Mr00299300_cn) simultaneously with a general Taqman assay for the endogenous Tert gene (Lifetech 4458369) (in a multiplex reaction) to determine the numbers of unique Neo genes in the genome.
The wild type and modified p2ry12 alleles were identified by PCR analysis using 3 primers in a single reaction. Two of these primers (GS(E) and GS(E,T)) recognize the 4th exon of the p2ry12 gene, upstream and downstream of the modified region, while the third (NEO(T)) recognizes the NEO (neomycin) selection cassette, which was inserted into the mutant allele. By this PCR test all 3 different genotypes (wild type (+/+), heterozygous (+/−) and knockout (−/−)) could be distinguished: the GS primers (E and E,T) amplify only the wild type allele as an 541 bp fragment and the NEO(T) primer paired with the GS(E,T) primer amplify only the mutant allele as a 404 bp fragment. The primer sequences were as follows:
- GS(E)
TTCTTAGTGATGCTAAACTGGGAGC,
- Neo(T)
GGGCCAGCTCATTCCTCCCACTCAT,
- GS(E,T)
AGGGAATCCGTGCAAAGTGGAAGGG.
Measurement of mechanical sensitivity and inflammatory edema in CFA-induced inflammatory pain model
In this set of experiments peripheral inflammation was induced with CFA and the mechanical sensitivity of the hind paws was measured using a regularly calibrated electronic von Frey apparatus (Dynamic plantar esthesiometer, Ugo Basile Instruments Model 37400; Stoelting, Wood Dale, IL, USA) on each animal before and after CFA treatment as previously described (Andó et al., 2010). The extent of edema was also evaluated by measuring paw volumes using a plethysmometer (Ugo Basile 7140, Stoelting, Wood Dale, IL, USA).
Briefly, after consecutively submitting the animals (male Wistar rats, 150–250 g, 6–8/group) to both tests for baseline measurements, freshly prepared CFA (0.1 ml, 50% in saline, Sigma-Aldrich, F5881) was injected intradermally into the plantar surface of the right hind paw. 48 h after treatment with CFA, the extent of edema and mechanical sensitivity were measured on both hind paws. Animals were then treated with P2Y12R ligands or vehicle and post-drug measurements were carried out 15 or 30 min later.
Animals were subjected to intraperitoneal or intrathecal injections of several doses of the following P2Y12R antagonists: MRS2395 (0.03–1 mg/kg), clopidogrel (1–60 mg/kg), ticlopidine (3–100 mg/kg), cangrelor (0.1–3 mg/kg), PSB-0739 (0.01–1 mg/kg) or, reactive blue 2 (0.1–3 mg/kg). Each animal was injected only once. The doses of drugs were chosen based on extrapolation from previous studies (Marteau et al., 2003, Takasaki et al., 2001, Vasiljev et al., 2003). Intrathecal injection was performed following the method of Mestre et al. (1994): this method enables the injection of a drug directly to the central nervous system without anesthesia and to avoid harm to the spinal cord. Briefly, the injections were performed by holding the animal in one hand and inserting a 23 G1″ needle connected to a 250 μL Hamilton syringe with a repeating dispenser between the dorsal aspects of L5 and L6 vertebrae. A volume of 5 μL of PSB-0739 solution or saline was injected in every case.
Mechanical sensitivity of the hind paws was measured in the following way: after being placed into the observation chamber and habituated for 10 min, the animals were submitted to 5 individual consecutive measurements and the average was taken as the value for mechanical sensitivity for the paw of each animal (paw withdrawal threshold, PWT), expressed in grams.
In experiments using mice, animals were lightly anesthetized with isoflurane and received 30 μL of CFA subcutaneously in the plantar surface of the right hind paw. PWT was measured before and 48 h after the intraplantar injection on both hind paws using a dynamic plantar von Frey esthesiometer as described above.
Experimental neuropathy and measurement of mechanical hyperalgesia
In this set of experiments, male Wistar rats (150–250 g) or male wild-type and P2ry12−/− mice (20–30 g) were submitted to partial ligation of the sciatic nerve, following the method of Seltzer et al. (Andó and Sperlágh, 2013, Andó et al., 2010, Seltzer et al., 1990). Briefly, animals were deeply anesthetized with ketamine and xylazine (50 mg/kg i.p. each), and the sciatic nerve of one of the hind paws was exposed at the mid-thigh level. One-half to one-third of the nerve was then tightly ligated with siliconized silk suture (rats: 7.0; mice: 9.0, Ethicon, Johnson and Johnson, USA), the wound was closed with sutures and the animals were allowed to recover. Before and 7 days after surgery, mechanical sensitivity was measured on both paws using the same procedure as described above. 7 days after surgery, most animals showed an increased mechanical sensitivity of the operated paws as compared to pre-surgery levels. Only those animals, which showed a minimum of 30% change in mechanical sensitivity were included in the study. Subsequently, animals received intraperitoneal or intrathecal injections of P2Y12R ligands or vehicle and post-drug measurements were carried out 15 or 30 min later as described above.
Acute thermal nociception (hot plate test)
The effects of P2Y12R antagonists on acute thermal nociception were investigated using an increasing-temperature hot plate system (IITC Life Science, Woodland Hills, CA, USA). This has an advantage over the conventional (constant temperature) hot plate system, because no sensitization or desensitization occurs after repeated experiments, which enables repeated testing on the same animal (Almasi et al., 2003).
In the experiments, male Wistar rats (weighing 150–250 g) or male wild-type and P2ry12−/− mice (20–30 g) were used. On the day of testing, animals were placed in the hot-plate apparatus and after a period of habituation (ca. 10 min), the plate was heated from the starting temperature of 25 °C with a constant rate of 6 °C/minute, until the animals showed nocifensive behavior (frequent lifting and/or licking of the hind paw). Heating was then instantly stopped, the animal was removed from the cage and the plate rapidly cooled. The temperature at which the animal showed any sign of nocifensive behavior was taken as PWT, expressed in °C. 30 min later, the measurement was repeated and the average of two values was taken as the baseline thermal nociceptive threshold. After the second measurement, the animals received treatments with P2Y12R antagonists as described above and 15 or 30 min after drug administrations, post-drug nociceptive threshold was measured.
Subdiaphragmatic vagotomy (VGX)
Subdiaphragmatic vagotomy was performed according to the method described in Andrews et al. (1985). Briefly, animals were anesthetized with ketamine and xylazine (50 mg/kg i.p. each) and a 3–4-cm long midline incision was made on the ventral abdominal surface. The liver was retracted to the animal's right, and the esophageal-stomatic junction was located as a point of reference. The stomach and esophagus were gently retracted caudally. The vagus nerve was explored and visualized with the use of a surgical microscope. Both anterior and posterior segments (approximately 1.5 cm in length) of the vagus nerve were removed. The wound was closed and animals received 10 ml saline subcutaneously. Gentamicin was administered postsurgically (5 mg/kg for 5 days) and animals were separately housed for several days. All animals were monitored thoroughly during the postoperative period and wounds were cleaned when necessary. Following recovery from surgery, animals were group housed.
In order to measure the effect of subdiaphragmatic vagotomy on pain behavior PWT was measured before operation as baseline and immediately prior to intraplantar CFA injection on the tenth day after operation. Freshly prepared Complete Freund Adjuvant (CFA, 100 μl, 50%, Sigma) was then injected to the right hind paw of the animals intradermally and PWT was measured two days after treatment. Animals then received the selective P2Y12R antagonist PSB-0739 (0.3 mg/kg) or saline intrathecally and PWT was measured again.
Analysis of cellular cyclic AMP accumulation
Changes in cellular cAMP levels were determined as described previously (Algaier et al., 2008, Hoffmann et al., 2008). Briefly, CHO Flp-In cells or 1321N1 astrocytoma cells stably expressing the recombinant human P2Y12R or mock transfected cells were cultured on 24-well plates. After removal of the culture medium, cells were incubated with HBSS buffer at 36 °C. Cellular cAMP production was then accelerated by the addition of 10 μM forskolin (CHO cells) or 10 nM isoprenaline (astrocytoma cells) for 10 min. Solvent (control) or 2-methylthio-ADP was added together with forskolin or isoprenaline. When used, antagonists were given 10 to 25 min before the agonist 2-methylthio-ADP. The reaction was stopped after 10 min by removal of the reaction buffer, followed by the addition of a hot lysis solution. cAMP levels in the supernatant were then quantified by incubation of an aliquot with cAMP binding protein and [3H]-cAMP (Perkin Elmer, Rodgau, Germany) and liquid scintillation counting after removal of the unbound [3H]-cAMP by charcoal. cAMP levels per well were calculated by regression analysis from a standard curve determined for each experiment. Concentration-response data were fitted by non-linear regression using GraphPad Prism (4.03) to estimate half-maximal concentrations. Apparent pKB-values were calculated according to: pKB = log (dose ratio − 1) − log[B]. pA2-values were determined by linear regression analysis.
In our previous studies (Algaier et al., 2008, Hoffmann et al., 2008) mock transfected CHO-Flp-In cells as well as non-transfected CHO-Flp-In cells failed to respond to the agonist 2-methylthio-ADP in inhibiting cAMP levels or in a SRE-directed reporter gene assay expected with the absence of functional P2Y12-receptors in these cells. qPCR analysis of mRNA levels confirmed this view. A probe designed to detect mRNA encoding for rodent P2Y12-receptors (AGAACGAGGGGTTCAGCCAAAGC; TGAATGGATCAAGGCAGGCGTTC) showed no detectable signals (Ct values > 33) either in non-transfected CHO-Flp-In cells or in CHO-Flp-In cells stably expressing the human P2Y12-receptor. In contrast, a probe designed to detect mRNA encoding the human P2Y12-receptor (TCGACAACCTCACCTCTGCGC; CCTCATCGCCAGGCCATTTGTGA) showed a Ct-value of 28.5 ± 0.2 (n = 3) in CHO cells expressing the recombinant human P2Y12-receptor and no specific signals in non-transfected CHO cells (Ct value of the house keeping gene GAPDH 25.3 ± 0.1; Qiagen fast lane cell cDNA kit and Qiagen SYBR green PCR mix analyzed on an Applied Biosystems StepOnePlus RT-PCR system with 40 cycles consisting of 95° for 10 s and 60° for 30 s).
Measurement of the pro-inflammatory cytokine IL-1β
Animals were randomly assigned to experimental groups with 3–4 rats/mice in each group. All animals were given an intraplantar injection of sterile saline (0.9% NaCl) or CFA suspended in an oil/saline (1:1) emulsion. Inflammation was induced with CFA, injected (rats: 0.1 ml, mice: 30 μL) into the plantar surface of the right hind paw. The contralateral left hind paw of the same animal received an identical volume of saline. Mechanical hyperalgesia was measured on day 0 (i.e. prior to intraplantar injection), 48 h and 96 h after intraplantar injection of CFA or saline as described above. The selective P2Y12R antagonist PSB-0739 (0.3 mg/kg) was injected i.t. 15 min before, while the non-pro-drug-P2Y12 receptor antagonist cangrelor (3 mg/kg) was added i.p. 30 min before the post-CFA measurement of mechanical hyperalgesia. After behavioral testing at 48 h or 96 h, rats were killed by decapitation. These time points were chosen to represent acute (48 h) and subacute (96 h) phases of inflammation (Parra et al., 2002). Samples of L4-L6 lumbar spinal cord (96 h) and hind paw (48 h) were collected, frozen on dry ice/liquid nitrogen and stored at − 70 °C until mRNA and protein isolation. In a set of experiments, chemical sympathectomy was induced by intraperitoneal injections of 6-OHDA dissolved in 0.1% ascorbic acid, administered in every second day over 5 consecutive days (40 mg/kg, 60 mg/kg, 60 mg/kg) following the protocol described by Lorton et al. (1999). The final 6-OHDA treatments were followed by intraplantar injection of CFA or saline as described above. After the experiment, the monoamine content of the hind paws was analyzed by HPLC. In other experiments, the α7 nACh receptor antagonist MLA or its vehicle (saline) was administered 45 min prior to the post-CFA PWT determination.
For the IL-1β assays, samples were homogenized in 500 μl 10 mM Tris–HCl buffer containing 1 mM EGTA, 1 mM EDTA, 0.2 mM PMSF and 4 M urea per 0.1 g tissue as described previously (Csolle and Sperlagh, 2010). The initial tissue weight was 80 mg. Samples were centrifuged at 4 °C for 20 min at 15,000 g and the supernatant was collected in 500 μl 10 mM Tris–HCl buffer containing 1% BSA and 0.2% Tween 20. Levels of IL-1β (both pro and mature) production were evaluated using an ELISA kit, DuoSet IL-1β (R&D System, Minneapolis, MN, USA), specific for rat and mouse IL-1β protein, respectively, according to the manufacturer's instructions. IL-1β levels were then calculated by plotting the optical density (OD) of each sample (two-fold diluted) against the standard curve. A seven point standard curve using two-fold serial dilutions in Reagent Diluent (according the manufacturer's instructions), and high standard of 4000 pg/ml were used for the determination of IL-1β levels. Assay detection limits were < 5 pg/ml. Absorbance was measured at 450 nm, using a Perkin-Elmer Victor 3V 1420 Multilabel Counter. IL-1β level was expressed in pg/ml.
Quantitative real-time PCR experiments
Rats were treated as described for the IL-1β production assay, and animals were killed by decapitation 48 h or 4 days following CFA or saline injection. The hind paw and L4-L6 spinal cord were collected, frozen in liquid nitrogen and stored at − 70 °C until homogenization. Total RNA samples were isolated and purified from the cell lysates using the RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer's instructions. RNA (2 μl) was reverse-transcribed with RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania) as described in our previous studies (Papp et al., 2004, Sperlagh et al., 2002). Briefly, the cDNA samples were prepared by reverse transcribing 1 μg of total RNA using 1 μl of RevertAid H Minus M-MuLV reverse transcriptase in a mixture containing 5 μl of 5 × reaction buffer, 1 μl random hexamer primer (10 pmol/μl), 1 μl of RiboLock™ RNase Inhibitor (20 u/μl), and 2 μl of 10 mM dNTP mix, which was brought up to a final volume of 20 μl with 0.1% diethylpyrocarbonate-treated distilled water. The reverse transcription reaction was performed at 70 °C for 5 min, and synthesis then continued at 25 °C for 15 min followed by 60 min at 42 °C and the samples were finally stored at − 20 °C. Expression levels of the target genes were determined from the cDNA samples using quantitative real-time PCR (Rotor-Gene 3000; Corbett Research, Sydney, Australia). Real-time PCR was performed according to standard protocols using the LightCycler DNA Master SYBR Green I Kit (Roche, Indianapolis, IN, USA). PCR conditions were optimized for primers, templates, and MgCl2. The PCR cycling protocols were as follows: initial denaturation, 95 °C for 5 min; cycling, 94 °C for 1 min, 59 °C for 1 min, 72 °C for 5 min; 40 cycles. The following primers were used for P2Y12R mRNA detection: P2Y12R forward primer, CAGGTTCTCTTCCCATTGCT; reverse primer, CAGCAATGATGATGAAAACC; and 18S forward primer, 5′-GTAACCCGTTGAACCCCATT, reverse primer, 3′-CCATCCAATCGGTAGTAGCG.
Analysis of real-time PCR measurements
To ensure reaction specificity and accurate quantification, melting curve analysis was performed after each reaction, which confirmed the lack of primer–dimer artifacts or contamination in all cases. All ∆Ct values were calculated by the Rotor Gene 5 software (Corbett Research, Sydney, Australia). Expression levels of the target genes were normalized to the expression level of the reference gene, the housekeeping gene 18S rRNA. The target gene and reference gene were measured together within the same experiment. To compare expression level of target genes between the different experimental groups, the efficiency calibrated model of Pfaffl (2001) was applied. Differences in gene expression levels between experimental groups were considered significant at P < 0.05. Data are presented as mean normalized expression ratio ± SEM.
Multiplex cytokine measurement
The quantification of levels of pro-inflammatory cytokines TNF-α, IL-1β and IL-6, and the anti-inflammatory cytokine IL-10 was performed by Luminex xMAP platform assays. For the multiplex cytokine assay, the spinal cord and hind paw were homogenized in 500 μl 10mM Tris–HCl buffer containing 1 mM EGTA, 1 mM EDTA, 0.2 mM PMSF and 4 M urea per 0.1 g tissue. The samples were centrifuged at 4 °C for 20 min at 15,000g and the supernatant was collected in 500 μl 10 mM Tris–HCl buffer containing 1% BSA and 0.2% Tween 20. The bead-based Multiplex cytokine profiling on hind paw and spinal cord samples was performed in the Fluorescent Core Facility of the Semmelweis University, Budapest. Fluorokine® Multianalyte Profiling (MAP) Kits were used according to the manufacturer's instructions.
HPLC analysis
Concentrations of monoamines and their metabolites in the rat paw tissue were determined with HPLC using electrochemical detection. The native tissue was homogenized by sonication in 300 μl 0.1 M ice-cold perchloric acid which contained 10 mM theophylline as an internal standard and antioxidant, 0.1% sodium metabisulfite. The protein precipitate was removed by centrifugation at 3510 g for 10 min at 4 °C and determined according to Lowry et al. (1951). The excess of perchlorate anion of supernatant was removed by the addition of 2 M KOH and by centrifugation as described above. The liquid chromatographic system was controlled by 715 operation software (Gilson Medical Electronics inc., Middletown, and WI USA) and consisted of solvent delivery pumps, programmable injector for the automated column-switching and an auto injector (SIL-10AD Shimadzu). The effluent was connected to a BAS 400 electrochemical cell which contained a glassy carbon electrode versus Ag/AgCl reference electrode and the oxidizing potential was maintained at 0.75 V by an Eltron potentiostat.
The online enrichment and stripping of samples were carried out on a SUPELCOSIL LC-C18 (100 × 4.6 mm I.D, 5 μm particle sizes) column by “RP” buffer (0.15 mM ammonium formate buffer, 0.25 mM EDTA, the apparent pH was adjusted to 3.2). The separation was performed on SUPELCOSIL LC-C18 DB (150 × 4.6 mm I.D., 3 μm particle size) column by “RP” buffer with 0.6 ml/min flow rate. The second part of analysis was performed for 24 min with the “IP” buffer (0.15 mM ammonium formate buffer, 0.25 mM EDTA, 0.45 mM octane sulphonic acid sodium salt and 6% v/v acetonitrile, the apparent pH was adjusted to 3.2) and the flow rate increased to 0.98 ml/min. Concentrations were calculated by a two-point calibration curve using internal standard method. The data are expressed as pmol per mg protein.
Accelerating rotarod test
This study used 140–190 g drug- and test-naive male Wistar rats. Motor coordination was tested on the IITC (Woodland Hills, CA, USA) Rotarod Apparatus, which enables the simultaneous examination of five rats. The apparatus consists of five compartments separated by 8 cm diameter rotating rod, placed 25 cm above the base of the apparatus. Motor coordination of animals was tested for 300 s with linear acceleration from 5 rpm to 25 rpm. Rats were acclimatized to the rotarod in three trials (300 s) per day for 2 consecutive days before the start of the experiment. On the test day 30 min prior to drug administration baseline latencies to fall were determined and rats with baseline latencies less than 60 s were excluded from the study. The animals were then treated with sterile saline or with antagonists intraperitoneally (3 mg/kg cangrelor/saline) or intrathecally (0.3 mg/kg PSB-0739/saline). 30 min after the i.p. treatment or 15 min after i.t. treatment the falling latency was measured again in the 300 s test period. The latency time to fall off the rod was expressed in seconds.
Ex-vivo inhibition of platelet aggregation
This study used 200–250 g drug- and test-naive male Wistar rats. The animals were treated with antagonists or with an equal volume of sterile saline by intraperitoneal or intrathecal injection. 30 min after i.p. treatment or 15 min after i.t. treatment animals were anesthetized with an i.p. injection of ketamine (50 mg/kg)/xylazine (50 mg/kg), and 3 ml of blood was drawn from the heart into Vacutainer tubes and immediately mixed 1:10 with an aqueous solution of trisodium citrate (3.8%). Blood samples were centrifuged at 150 g for 8 min. Then the platelet-rich supernatant was carefully removed (platelet-rich plasma, PRP). The remaining specimen was further centrifuged at 2500 g for 8 min to obtain platelet-poor plasma (PPP). Aliquots of 450 μl PRP or PPP were placed in glass cuvettes and 50 μl of ADP (5 μM and 10 μM) was added to the PRP to induce platelet aggregation. Measurements of platelet aggregation were carried out using Carat TX-4 four channel platelet aggregometer (Carat, Hungary) using the turbidimetric method described by Born (1962). In order to eliminate individual differences, the device stores light transmission of platelet-rich plasma and platelet-poor plasma (PRP: 0%, PPP: 100%), and the induced aggregation rate was calculated from the measured difference in optical density between PPP and PRP. The light transmission of the suspension increased in parallel with the extent of aggregation, and this was displayed by the built-in program connected to the device. From the resulting curve, the maximal extent of aggregation was considered, which was expressed as percentage of maximal light transmission. During the 10-min measurement, samples were incubated at 37 °C and continuously stirred at 1000 rpm. All measurements were carried out within 2 h after blood sampling under validated conditions in the PentaCore Laboratory of the Semmelweis University, Budapest, Hungary.
Drugs
The following drugs were used: cangrelor (The Medicines Company, Parsippany, NJ USA), clopidogrel hydrochloride (Sanofi-Aventis, Budapest Hungary), 6-OHDA, MLA, MRS2395, reactive blue 2 (all from Sigma-Aldrich), morphine hydrochloride (TEVA, Gödöllő, Hungary), ticlopidine hydrochloride (Tocris), suramin hexasodium salt (Bayer, Wuppertal, Germany), and PSB-0739 (synthesized by Y. Baqi and C.E. Müller, according to described procedures (Baqi and Müller, 2007, Baqi and Müller, 2010, Baqi et al., 2009)). In in vitro experiments forskolin or isoprenaline (both from Sigma-Aldrich) was used to accelerate cellular cAMP formation and the effect of 2-methylthio-ADP trisodium salt (Sigma-Aldrich) was examined. Drugs were dissolved in sterile saline or water except MRS2395 and clopidogrel, which were dissolved in 25% DMSO + 75% PEG as a stock solution, and forskolin was dissolved in a mixture of DMSO + ethanol (1:7). Solutions were freshly prepared on the day of use.
Statistics
All data were expressed as means ± S.E.M. of n observations, where n means number of animals/well per group. For statistical comparison of multiple treatment groups one-way ANOVA followed by Neuman–Keuls (in vivo experiments) or Tukey (in vitro experiments) post-hoc test was used, and post CFA-treatment PWT values (CFA model) or postoperative PWT values of vehicle treated rats (Seltzer model) were compared to values treated with drugs. The identical statistical method was used for the multiplex cytokine measurement. For statistical analysis of the hot plate test results, corresponding baseline and post-drug data were compared using the paired t-test. For pairwise comparisons, the unpaired t-test was used, as appropriate. Mechanical hyperalgesia in experiments performed on P2ry12−/− mice and their wild-type counterparts was analyzed by a repeated measures multivariate ANOVA to identify pre-post treatment, genotype and ipsilateral–contralateral effects. In the case of the IL-β assay on mice, two-way ANOVA was used. The Fischer LSD test was used for post-hoc comparison. The minimal effective dose (mED) was determined as the lowest necessary dose to obtain significant change in the postoperative value/heat threshold as compared to the vehicle treated/baseline controls. The maximal (Emax) effect is defined as the extent of maximal effect obtained in the dose-range examined and is expressed in grams or °C, for mechanical and thermal sensitivity, respectively.
Results
P2Y12R antagonists alleviate mechanical hyperalgesia in CFA-induced inflammatory and neuropathic pain models and acute thermal nociception in rats
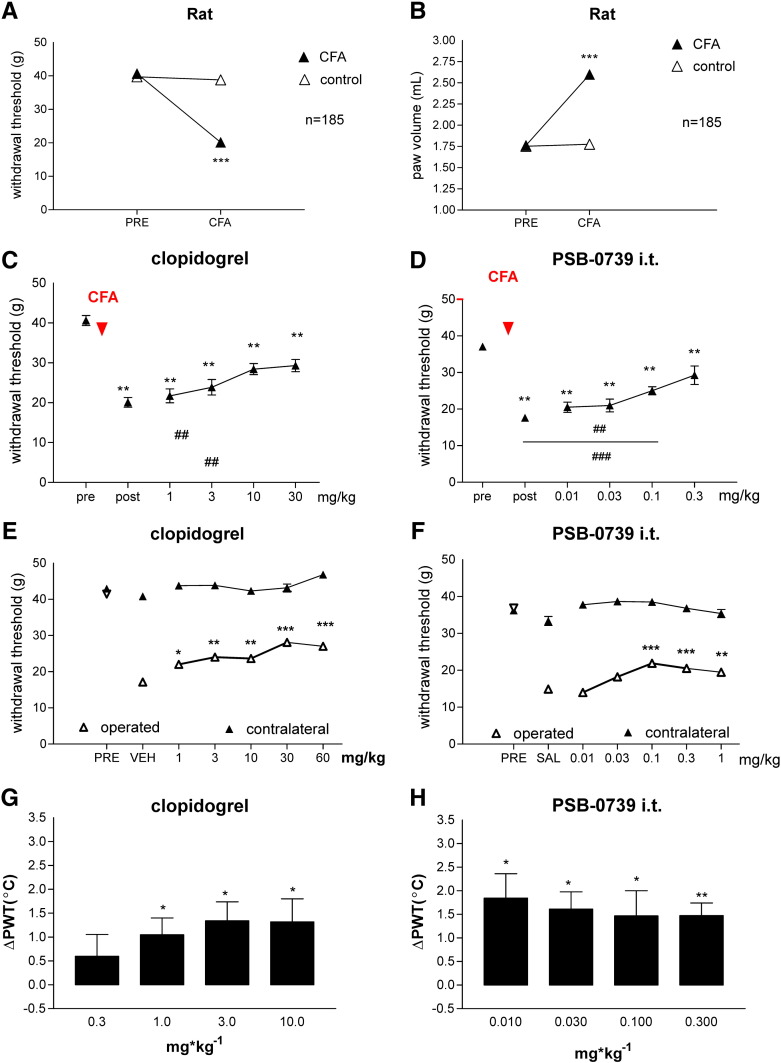
The average value of baseline PWT was 40.66 ± 0.38 g (n = 185) in rats. 48 h after intraplantar CFA injection (0.1 ml, 50%), the PWT decreased to 20.12 ± 0.33 g (n = 185), indicating the development of mechanical hyperalgesia. This change represented a statistically significant, 49% decrease (P < 0.001), compared to either the PWT values of ipsilateral pre-CFA or the contralateral (38.80 + 0.43 g, n = 185) hind paws (Fig. 1A). CFA treatment also caused edema in the treated hind paw, the extent of volumetric increase was 147%, which was significant, compared to either the pre-CFA values (pre: 1.76 ± 0.01 ml, n = 185, and post: 2.6 ± 0.01 ml, n = 185, P < 0.001) or the contralateral values (1.77 ± 0.009 ml, n = 185, P < 0.001) (Fig. 1B).
Fig. 1.
Effects of P2Y12R antagonists in rat models of inflammatory, neuropathic and acute pain. A, B. Mechanical hyperalgesia (A) and edema (B) before (PRE) after (CFA) intraplantar CFA injection in rats. CFA (100 μl, 50% in saline) was injected intradermally into the plantar surface of the right hind paw. 48 h after treatment with CFA, the extent of edema and mechanical sensitivity were measured on both hind paws. Paw withdrawal threshold values are presented in grams, whereas paw volume values are expressed in ml (mean ± S.E.M.). Injected (CFA), but not control paws demonstrated a significant, 49% decrease of pain threshold and 47% increase in paw volume. *** denotes statistical significance of P < 0.001, Student t test. C, D. Effects of clopidogrel (C) and PSB-0739 i.t. (D) on CFA mediated inflammatory pain behavior 48 h after injection in rats. The graphs show the PWT values in g corresponding to doses indicated on the abscissa. Symbols indicate significant differences from the pre-CFA treatment (**P < 0.01) and post-CFA treatment PWT values (##P < 0.01, ###P < 0.001), respectively. One-way ANOVA followed by Neuman–Keuls post-hoc test, n = 6–12/group. E, F. Effects of clopidogrel (E) and PSB-0739 i.t. (F) on neuropathic pain behavior in rats. PWT values were assessed on operated (white triangles) and contralateral hind paws (black triangles) expressed in g. Asterisks indicate significant differences from the postoperative values of saline (SAL) or vehicle (VEH) treated animals (*P < 0.05, **P < 0.01, ***P < 0.001). One-way ANOVA followed by Neuman–Keuls post-hoc test, n = 6–12/group. G, H. Effects of clopidogrel (G) and PSB-0739 i.t. (H) on acute thermal nociception in rats. Y axis values show the change in nocifensive threshold (∆PWT, °C). Animals were treated with P2Y12 receptor antagonists in doses indicated on the abscissa. Asterisks indicate significant analgesic effect, compared to the pre-treatment values, *P < 0.05, **P < 0.01, paired t-test.
We examined six P2Y12R antagonists in different doses using the experimental protocol shown in Fig. 1A. Antagonists, or their vehicle, were administered 48 h after CFA treatment, after the determination of post-CFA treatment PWT values. After 30 min, the PWT values were measured again, and the values were compared to the respective post-CFA treatment PWT values. We have evaluated the effect of clopidogrel and ticlopidine, two pro-drug P2Y12R antagonists, widely used in clinical practice; the effect of MRS2395 (compound 26 in Xu et al., 2002, and also examined by Kobayashi et al., 2008), reactive blue 2, an antagonist acting at several subtypes of P2Y receptors, cangrelor, the non-pro-drug P2Y12 receptor antagonist as well as PSB-0739, the selective and potent P2Y12R antagonist (Baqi et al., 2009).
Intraperitoneal administration of saline or vehicle (DMSO:PEG = 1:3) did not affect significantly the post-CFA treatment PWT values (19.5 ± 1.22 g, n = 8, and 22.99 ± 2.16 g, n = 6, P > 0.05 vs. pre-CFA). By contrast, all P2Y12R antagonists dose-dependently and significantly counteracted CFA-induced mechanical hyperalgesia (Figs. 1C, D, Table 1). Clopidogrel exhibited a significant effect in the range of 10–30 mg/kg and the mED value was 10 mg/kg (Fig. 1C). Ticlopidine exhibited slightly higher potency with a mED value of 3 mg/kg (Table 1). MRS2395 had a dose-dependent analgesic effect in the range of 0.1–1 mg/kg, and the greatest increase of PWT was observed at 1 mg/kg, which was significantly different from the postoperative threshold (Table 1). Reactive blue 2 had a moderate but significant effect on mechanical hyperalgesia following CFA treatment. The mED was 0.6 mg/kg (Table 1), however, in a dose higher than 1 mg/kg we could not detect significant effects. Cangrelor had significant analgesic effect in the tested range of 0.1–1 mg/kg (Table 1). The mED value was 0.1 mg/kg and the greatest effect was detected at 1 mg/kg (Table 1). Based on its highly polar chemical structure, we have assumed that PSB-0739, the selective and potent P2Y12R antagonist, does not penetrate the blood–brain barrier. Indeed, preliminary experiments revealed that it was without effect using intraperitoneal application in either pain models (data not shown). Therefore in the following experiments, we applied PSB-0739 intrathecally, which had dose-dependent and significant antihyperalgesic effect in low doses (Fig. 1D, Table 1). The minimal effective dose was 0.1 mg/kg. We found the greatest efficacy on nocifensive threshold at 0.3 mg/kg, whereas intrathecal injection of identical volume of saline did not elicit any effect (Table 1). The rank order of mED values in these experiments was the following: PSB-0739 i.t. = cangrelor > reactive blue 2 > MRS2395 > ticlopidine > clopidogrel. Of the compounds investigated, only ticlopidine counteracted paw edema (data not shown).
Table 1.
Effect P2Y12 receptor antagonists on mechanical hyperalgesia in inflammatory (CFA) and neuropathic (Seltzer) pain model as well as on acute thermal nociception.
| Drug | Dose (mg/kg) | CFA |
Seltzer |
Hot plate test |
|---|---|---|---|---|
| ΔPWT (g) | ΔPWT (g) | ΔPWT (°C) | ||
| PRE | 40.66 ± 0.38 | 41.20 ± 0.37 | n.d. | |
| POST | 20.12 ± 0.33*** | 18.48 ± 0.28*** | n.d. | |
| VEH i.p. | 22.99 ± 2.16 | 17.13 ± 1.23 | 0.39 ± 0.32 | |
| SAL i.p. | 19.5 ± 1.22 | 18.98 ± 1.71 | − 0.71 ± 0.45 | |
| SAL i.t. | 21.08 ± 1.92 | 17.20 ± 1.08 | n.d. | |
| MRS2395 | 0.03 | 23.47 ± 2.32 | 17.27 ± 1.48 | − 0.36 ± 0.34 |
| 0.1 | 19.58 ± 2.64 | 12.87 ± 0.7 | 1.33 ± 0.52 | |
| 0.3 | 22.65 ± 2.45 | 22.12 ± 0.76## | 1.51 ± 0.19** | |
| 1 | 27.82 ± 2.63# | 23.3 ± 1.4### | 2.06 ± 0.40** | |
| (±)Clopidogrel | 0.3 | n.d. | n.d. | 0.60 ± 0.45 |
| 1 | 21.72 ± 1.74 | 22.03 ± 0.77 | 1.05 ± 0.34* | |
| 3 | 23.88 ± 1.94 | 24.01 ± 1.52## | 1.34 ± 0.39* | |
| 10 | 28.43 ± 1.40## | 23.65 ± 1.33# | 1.31 ± 0.48* | |
| 30 | 29.32 ± 1.53## | 28.10 ± 1.33### | n.d. | |
| 60 | n.d. | 27.04 ± 0.61### | n.d. | |
| Ticlopidine | 3 | 24.58 ± 2.19# | 22.99 ± 1.63 | 0.23 ± 0.55 |
| 10 | 28.15 ± 2.73### | 23.93 ± 0.85 | 0.65 ± 0.18* | |
| 30 | 25.45 ± 2.18# | 28.17 ± 1.72### | 0.50 ± 0.19 | |
| 60 | 33.02 ± 1.45### | n.d. | 1.34 ± 0.46* | |
| 100 | 32.33 ± 3.97### | 37.02 ± 3.04### | 2.69 ± 0.58** | |
| PSB-0739 i.t. | 0.01 | 20.51 ± 1.39 | 13.99 ± 1.20 | 1.84 ± 0.52* |
| 0.03 | 20.99 ± 1.75 | 18.23 ± 1.78 | 1.61 ± 0.37* | |
| 0.1 | 25.00 ± 1.10## | 21.91 ± 1.80### | 1.47 ± 0.53* | |
| 0.3 | 29.25 ± 2.53### | 20.53 ± 1.36## | 1.48 ± 0.27** | |
| 1 | n.d. | 19.49 ± 1.06# | n.d. | |
| Cangrelor | 0.1 | 29.25 ± 1.09### | 23.77 ± 1.38## | 0.96 ± 0.47 |
| 0.3 | 26.86 ± 2.39 | 21.67 ± 1.22 | 0.74 ± 0.34 | |
| 0.6 | 27.07 ± 1.22# | 25.72 ± 1.31### | 0.27 ± 0.95 | |
| 1 | 32.63 ± 2.19### | 23.10 ± 2.03# | 1.48 ± 0.85 | |
| 3 | n.d. | 25.34 ± 1.91### | n.d. | |
| Reactive blue 2 | 0.1 | 21.50 ± 0.95 | 16.57 ± 0.38 | n.d. |
| 0.3 | 19.17 ± 1.97 | 17.98 ± 1.21 | 0.35 ± 0.56 | |
| 0.6 | 26.68 ± 0.82## | n.d. | n.d. | |
| 1 | 26.00 ± 0.97## | 15.37 ± 2.00 | 0.58 ± 0.43 | |
| 3 | 18.22 ± 0.79 | 16.15 ± 0.93 | − 0.73 ± 0.42 | |
| 60 | n.d. | n.d. | 0.0001 ± 0.43 |
In CFA experiments symbols indicate significant differences from the pre-CFA treatment (***P < 0.01) and post-CFA treatment PWT values (##P < 0.01, ###P < 0.001), respectively. One-way ANOVA followed by Neuman–Keuls post-hoc test, n = 6–12/group. In Seltzer experiments symbols indicate significant differences from the pre-CFA treatment (***P < 0.01) and from the postoperative values of saline (SAL) or vehicle (VEH) treated animals (#P < 0.05, ##P < 0.01, ###P < 0.001). One-way ANOVA followed by Neuman–Keuls post-hoc test, n = 6–12/group. In hot plate tests asterisks indicate significant analgesic effect, compared to the pre-treatment values, *P < 0.05, **P < 0.01, paired t-test. n.d. = not determined.
Using the Seltzer model of neuropathic pain in rats, following the protocol used in our previous studies (Ando et al., 2010), the preoperative PWT was 41.20 ± 0.37 g (n = 151). Partial ligation of the sciatic nerve decreased this value to 18.48 ± 0.28 g on the 7th postoperative day (Table 1), representing a 55% decrease (n = 151, P < 0.001) compared to either the preoperative value of the ipsilateral hind paw or the value of the contralateral hind paw (41.72 ± 034 g, n = 151).
We measured the effects of P2Y12R antagonists on mechanic hyperalgesia on the 7th day after partial sciatic nerve ligation. PWT values, determined 30 min after drug administration were compared to the postoperative PWT values of the vehicle or saline treated animals. PWT was also measured on the contralateral hind paw, however, none of the treatments significantly affected these values (e.g. Figs. 1E, F). In this test, all P2Y12R antagonists investigated had significant and dose-dependent antihyperalgesic effects except reactive blue 2 (Table 1). Clopidogrel had significant analgesic effects in the 1–60 mg/kg dose range, and the maximal effect was obtained at 30 mg/kg (Fig. 1E). Ticlopidine also had a dose-dependent effect: the mED was 30 mg/kg and at 100 mg/kg almost complete reversal of the hyperalgesia was observed (Table 1). Similar to the results obtained in the inflammatory pain model, MRS2395 had a dose-dependent analgesic effects in the range of 0.1–1 mg/kg and the mED value was 0.3 mg/kg (Table 1). Reactive blue 2 remained ineffective in this test in the dose-range of 0.1–3 mg/kg (Table 1). Cangrelor had a significant effect on the pain threshold at 0.1 mg/kg and exhibited moderate dose-dependency in higher doses (Table 1). The greatest effect was found at 0.6 mg/kg. Intrathecal administration of PSB-0739 displayed a dose-dependent inhibitory effect on mechanical hyperalgesia in the range of 0.01–0.1 mg/kg. The mED was 0.1 mg/kg (Fig. 1F, Table 1). The rank order of mED values in the neuropathic model was the following: PSB-0739 i.t. = cangrelor > MRS2395 > clopidogrel > ticlopidine.
In the hot plate test, the baseline nociceptive threshold during two consecutive measurements 30 min apart was 46.6 ± 0.12 °C and 46.84 ± 0.09 °C (n = 178), respectively. Intraperitoneal (i.p.) administration of morphine (10 mg/kg) elicited a profound increase in the thermal nociceptive threshold (50.16 ± 1.23 °C, n = 8, P < 0.05) when compared to an identical volume of i.p. saline treatment (46.83 ± 0.53 °C, n = 8). These data are similar to literature data (Almasi et al., 2003) and to our previous findings (Ando et al., 2010).
In this set of experiments four out of the six tested P2Y12R antagonists exhibited significant effect in the tested dose range (Figs. 1G–H, Table 1, clopidogrel, ticlopidine, MRS2395, PSB0739 i.t.). The pro-drugs clopidogrel (Fig. 1G) and ticlopidine (Table 1) exerted dose-dependent effects and exhibited moderate potency. MRS2395 had a dose-dependent analgesic effect within the range of 0.03–1 mg/kg with a mED of 0.3 mg/kg (Table 1). Reactive blue 2, however, which inhibits several P2Y receptors including P2Y12R, did not elicit significant analgesia in the dose-range examined (0.3–60 mg/kg, Table 1). Likewise, cangrelor, a direct competitive non-prodrug antagonist of P2Y12Rs (0.1-1 mg/kg) was also without significant effect in the hot plate test (Table 1). Finally, we have examined the potent and selective P2Y12R antagonist PSB-0739 and a significant analgesic effect was observed at all tested doses (0.01–0.3 mg/kg i.t.) (Fig. 1H, Table 1). Based on these data, the following rank order of mED values was set up among the P2Y12R antagonists: PSB-0739 i.t. > MRS2395 > clopidogrel > ticlopidine.
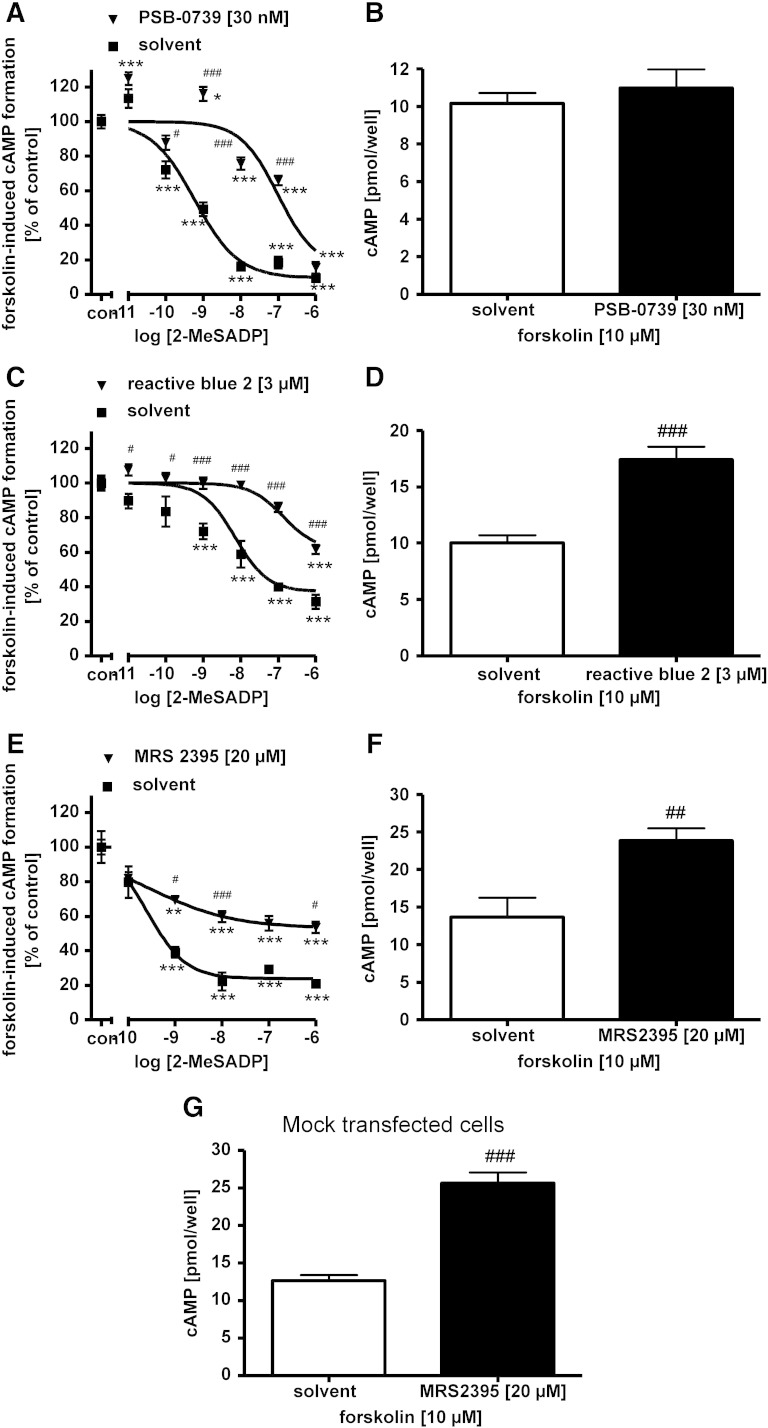
In vitro effects of P2Y12 receptor antagonists on the 2-methylthio-ADP induced inhibition of cAMP formation in cells expressing recombinant human P2Y12 receptors
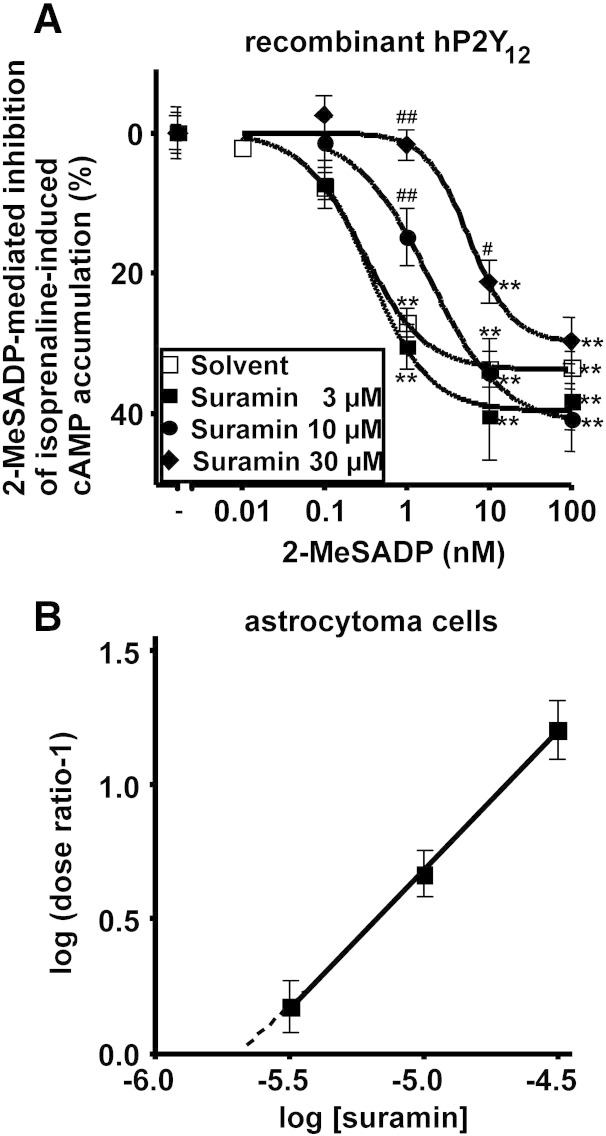
Next, we examined the in vitro efficacy of different antagonists putatively acting at human P2Y12R: the anthraquinone dye reactive blue 2, the structurally related anthraquinone PSB-0739, cangrelor, MRS2395 and suramin, a wide-spectrum P2 receptor antagonist (von Kügelgen, 2006) (Table 2). Clopidogrel and ticlopidine were not tested because they are pro-drugs and therefore not suitable for in vitro testing. The P2Y12R agonist 2-methylthio-ADP inhibited the forskolin (Fig. 2) — or isoprenaline (Fig. 3)-induced cAMP formation in cells expressing the human recombinant P2Y12R. The potent reactive blue 2-derived anthraquinone PSB-0739 (30 nM) markedly shifted the concentration-response curve of 2-methylthio-ADP to the right with a corresponding apparent pKB-value of approximately 10 (Fig. 2A). PSB-0739 elicited no change in forskolin-induced cAMP formation in CHO cells (30 nM; Fig. 2B). Table 2 summarizes apparent affinity values of antagonists at the recombinant human P2Y12R determined with the cAMP assay and a cAMP-dependent reporter-gene assay (Hoffmann et al., 2009). In CHO cells expressing the human P2Y12R, reactive blue 2 (3 μM) also shifted the concentration-response curve of 2-methylthio-ADP to the right with an apparent pKB value of 6.7 (Fig. 2C). Reactive blue 2 increased in the forskolin-induced cAMP formation by 74% (Fig. 2D). In CHO cells expressing the human P2Y12R, MRS2395 (20 μM) attenuated the effect of the agonist 2-methylthio-ADP (Fig. 2E); however, there was no change in the half-maximal concentrations of the agonist in the absence and presence of MRS2395 (amounting to 0.26 nM in both cases; see Fig. 2E). MRS2395 (20 μM) itself markedly increased the forskolin-induced cAMP formation in CHO cells expressing the human P2Y12-receptor by 75% (Fig. 2F). MRS2395 (20 μM) caused a similar increase in cellular cAMP formation induced by forskolin in mock transfected CHO cells (Fig. 2G). Suramin was tested in 1321N1 astrocytoma cells stably expressing the human P2Y12R. Suramin added at increasing concentrations of 3, 10 and 30 μM caused increasing shifts of the concentration-response curve of 2-methylthio-ADP to the right (Fig. 3A). Schild plot analysis revealed a pA2 value of 5.7 with a slope (1.029) not different from unity (Fig. 3B). Suramin (30 μM) did not change the isoprenaline-induced cAMP formation (isoprenaline alone 34.1 ± 1.4 pmol cAMP per well, n = 47; isoprenaline plus suramin 30 μM 37.7 ± 3.3 pmol cAMP per well, n = 17). Table 2 shows the rank order of potency of the antagonists acting at the recombinant human P2Y12R: PSB-0739 > cangrelor > reactive blue 2 > suramin ≈ MRS2395.
Table 2.
Apparent affinity values of P2Y12R antagonists determined in cells expressing the recombinant human P2Y12R using a cAMP assay and a cAMP-dependent reporter gene assay.
| Antagonist | cAMP assay |
Reporter gene assay |
|---|---|---|
| pKB/pA2* | pKB/pA2* | |
| PSB-0739 | 10.1 | 9.8* |
| Cangrelor | 9.2# | 8.6 |
| Reactive blue 2 | 6.7 | 7.4* |
| Suramin | 5.7* | 5.5 |
| MRS2395 | Attenuation of responses at 20 μM |
The * indicates a pA2 value; values determined in the reporter gene assay and values marked with # are taken from Hoffmann et al, 2009.
Fig. 2.
Effects of P2Y12R antagonists on the inhibition of cAMP formation mediated by the agonist 2-methylthio-ADP (2-MeSADP) in cells expressing the recombinant human P2Y12R (A, C, E) and effects of the antagonists on the cellular cAMP formation (B, D, F). cAMP formation was increased by addition of forskolin 10 μM. 2-MeSADP was added at the concentrations indicated in the absence and presence of (A) PSB-0739 30 nM, (C) reactive blue 2 3 μM and (E) MRS2395 20 μM. (G) shows effects of MRS2395 on the forskolin-induced cAMP formation in mock transfected cells. Asterisks indicate significant differences from control (con, no 2-MeSADP) (**P < 0.01, ***P < 0.001). Number signs indicate significant differences from respective values in the absence of an antagonist (#P < 0.05, ##P < 0.01, ###P < 0.001). One-way ANOVA followed by Tukey post-hoc test, n = 3–12/group.
Fig. 3.
Effects of the P2Y12R antagonist suramin on the inhibition of cAMP formation mediated by the agonist 2-methylthio-ADP (2-MeSADP) in cells expressing the recombinant human P2Y12R (A) and Schild plot analysis (B). cAMP formation was increased by addition of isoprenaline 10 nM. 2-MeSADP was added at the concentrations indicated in the absence and presence of suramin used at 3, 10 and 30 μM. Asterisks indicate significant differences from control (-, no 2-MeSADP) (**P < 0.01). Number signs indicate significant differences from respective values in the absence of suramin (#P < 0.05, ##P < 0.01). One-way ANOVA followed by Tukey post-hoc test, n = 8–45/group.
Intraplantar CFA injection elicits time-dependent upregulation of P2Y12 receptor mRNA and IL-1β protein level in rat hind paw and spinal cord
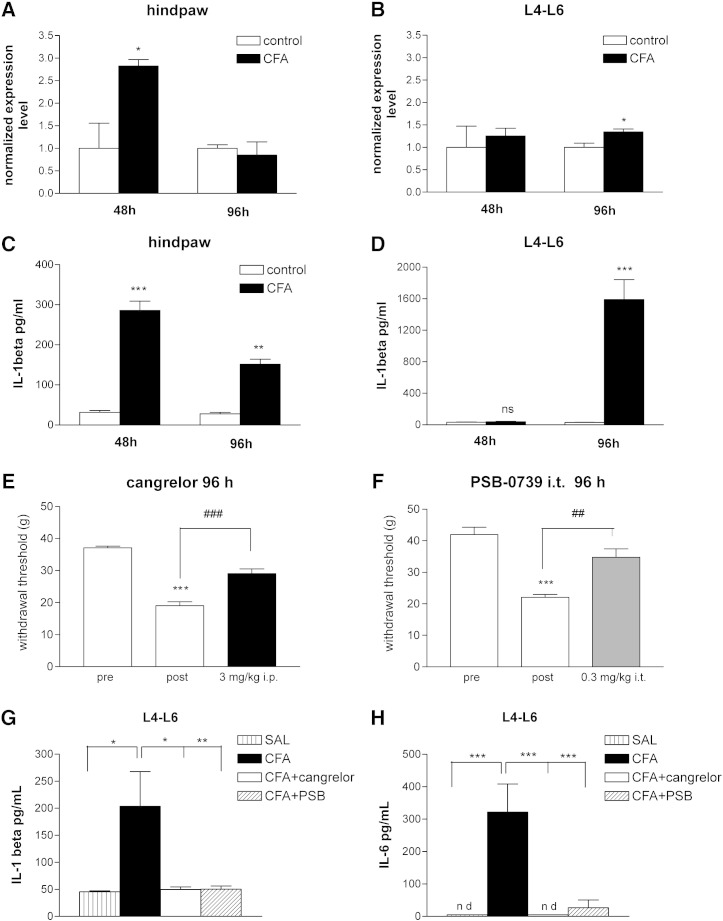
In subsequent experiments we examined the changes in the level of mRNA transcript of the P2Y12R, using real-time RT-PCR in the rat L4-L6 spinal cord and hind paw of rats after intraplantar injection of CFA (0.1 ml, 50%). Gene expression levels were evaluated 48 h and 96 h after injection and were normalized against the expression level of the 18S rRNA. Quantitative real-time PCR measurements revealed that the P2Y12R mRNA level in the rat hind paw was upregulated by 182 ± 14% of the corresponding control values (Fig. 4A; n = 4, P < 0.05) 48 h after the CFA treatment. The expression level of P2Y12R in the L4-L6 spinal cord did not change in response to CFA injection at this time point (Fig. 4B; 125 ± 17% of the corresponding control values; n = 4, P > 0.05). In contrast, a significant upregulation of P2Y12R mRNA was detected 96 h after the CFA injection in the spinal cord, but not in the hind paw of rats, when compared to saline treated animals (134.45 ± 6.25%, n = 4, P < 0.05 and 85.02 ± 29.2%, n = 4, P > 0.05 of the corresponding control values in the spinal cord and hind paw, respectively, Figs. 4B and A).
Fig. 4.
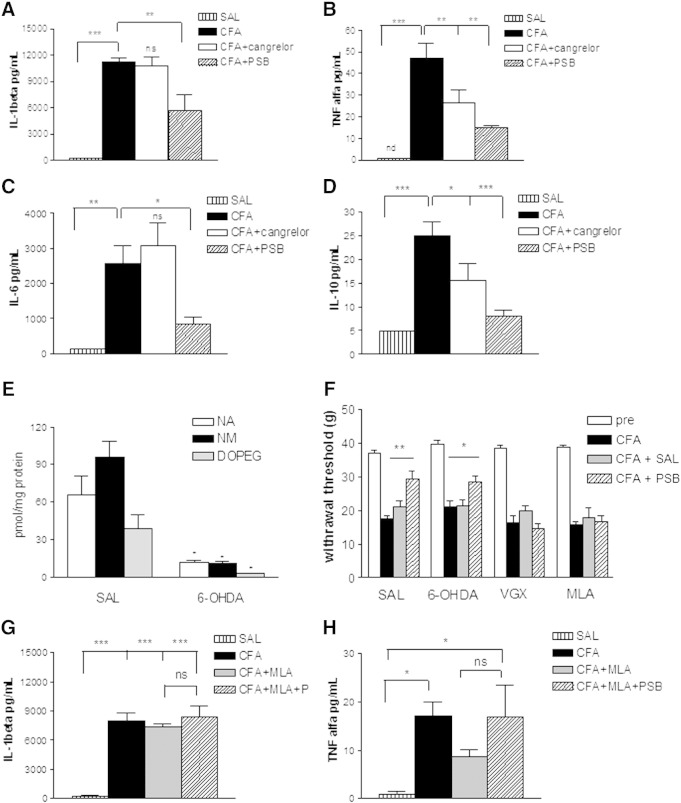
Time-dependent changes of P2Y12R mRNA expression (A, B), IL-1β protein level (C, D) in the hind paw and lumbar spinal cord (L4-L6) induced by CFA; effect of P2Y12R antagonists 96 h after CFA treatment on mechanical hypersensitivity (E, F) and on central cytokine response (G, H) in rats. A, B. Changes in mRNA expression level of P2Y12 receptor in the hind paw (A) and L4-L6 spinal cord (B). Rats received intraplantar injection of 0.1 ml 50% CFA or saline (control), and were decapitated 48 h or 96 h after treatment. Quantitative SYBR Green real-time PCR was performed by using specific primers. The experiments were repeated two times with similar results. The expression level of the P2Y12 receptors was normalized to the expression level of the distinct housekeeping gene 18S rRNA. Data are displayed as the mean ± S.E.M. Asterisks indicate significant differences from the corresponding control *P < 0.05, Student's t-test. C, D. Intraplantar injection of 0.1 ml CFA caused IL-1β production in the rat hind paw (C) and spinal cord (D) in a time-dependent manner (48 or 96 h). Data are given as the mean level of cytokines ± SEM of three independent experiments (**P < 0.01, ***P < 0.001, ns, non-significant, Student t-test). E, F. Effects of the P2Y12R antagonist cangrelor (E) and PSB-0739 (F) on CFA mediated inflammatory pain behavior 96 h after injection. The graphs show the paw withdrawal threshold values (PWT) in g corresponding to doses indicated on the abscissa. Symbols indicate significant differences from the pre-CFA treatment (***P < 0.001) and post-CFA treatment PWT values (##P < 0.01, ###P < 0.001), respectively. One-way ANOVA followed by Neuman–Keuls post-hoc test, n = 6–12/group. G. H. Effect of P2Y12R antagonists on intraplantar CFA (0.1 ml)-induced IL-1β (G) and IL-6 (H) levels in the spinal cord 96 h after treatment. The spinal inflammatory response was strongly inhibited by PSB-0739 (i.t.) and by systemic cangrelor administration. PSB-0739 (0.3 mg/kg) the selective P2Y12 receptor antagonist was injected i.t. 15 min prior post-CFA PWT determination, while cangrelor (3 mg/kg) the P2Y12/P2Y13 antagonist was added i.p. 30 min prior to post-CFA PWT determination. The levels of cytokines were quantified in the derived supernatants by multiplex cytokine assay. Data are given as the mean level of cytokines ± SEM of 5 independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Neuman–Keuls test). N.d. indicates non-detectable level, which was regarded as 10− 5 pg/ml in statistical analyses.
As shown in Fig. 4C, the level of IL-1β in the saline-treated animals 48 h after the injection was 31.4 ± 4.7 pg/ml (n = 3) in the hind paw, whereas a significant and remarkable increase was detected 48 h following CFA treatment (Fig. 4C, 286 ± 23 pg/ml; n = 3, P < 0.001 compared to saline). The IL-1β level in the spinal cord (L4-L6) of saline treated rats was not significantly different from that observed in hind paws (Fig. 4D; 33.1 ± 4.7 pg/ml, n = 3). In addition, IL-1β protein levels did not change in the spinal cord in response to CFA stimulation 48 h after the injection (Fig. 4D; 39.1 ± 5.5 pg/ml, n = 3, P > 0.05). These results suggested that there is a rapid and robust local inflammatory cytokine response following CFA administration in the hind paw, whereas the elevation of inflammatory cytokine levels in the CNS is more delayed. Therefore, in subsequent experiments IL-1β levels were evaluated 96 h after CFA administration in both regions. A remarkable increase in IL-1β levels was observed in the rat spinal cord 96 h following CFA administration when compared with the corresponding saline-treated controls at the same time point (Fig. 4D; control: 30.6 ± 2.7 pg/ml, CFA: 1591.6 ± 252.3 pg/ml, n = 4, P < 0.001). The elevation of IL-1β in the hind paw was also detected at this time point when compared to saline treated animals (Fig. 4C, control: 27.9 ± 3.5 pg/ml, n = 4, CFA: 151.6 ± 13.3 pg/ml, n = 4, P < 0.01); however the level of IL-1β in the hind paw was less than after 48 h CFA treatment (Fig. 4C; 48 h CFA treatment: 286 ± 23 pg/ml, n = 4). In order to confirm, whether anti-hyperalgesic effects of P2Y12R antagonists are sustained 96 h after CFA injection, we also evaluated mechanical hyperalgesia in saline and drug-treated rats at this time point. Indeed, significant analgesic effects of both cangrelor and PSB-0739, the two most potent P2Y12R antagonists, were observable 96 h after CFA administration, when compared to postoperative values of the same rats (Figs. 4E, F).
These findings indicated a time-dependent and parallel upregulation of P2Y12R mRNA and inflammatory cytokine response in the inflamed hind paw and spinal cord with a sustained analgesic response of P2Y12R antagonists for up to 96 h. Therefore, in the next set of experiments we evaluated the effect of cangrelor and PSB-0739 on the levels of proinflammatory cytokines 48 h (hind paws) and 96 h (L4-L6 spinal cord) after intraplantar CFA administration using a Luminex platform assay.
P2Y12R antagonists counteract CFA-induced cytokine expression in the lumbar spinal cord 96 h after CFA injection
In these experiments, rats were challenged with intraplantar injection of 0.1 ml CFA and cytokine levels evaluated 96 h after treatment in the L4-L6 spinal cord. Similarly to the results of the pilot experiments, CFA administration caused a remarkable elevation in IL-1β (Fig. 4G, saline: 45.6 ± 1.88 pg/ml, n = 5, CFA: 203.6 ± 63.3 pg/ml, n = 5, P < 0.001, 405% increase), however, this elevation was lower than at measured in the hind paw after the inflammatory stimulus (CFA central: 203.6 ± 63.3 pg/ml, n = 5, CFA peripheral: 11,198 ± 497 pg/ml, n = 5). Both P2Y12R antagonists, cangrelor (3 mg/kg i.p.) and PSB-0739 (0.3 mg/kg i.t.) completely prevented CFA-induced IL-1β production (Fig. 4G; CFA: 203.6 ± 63.3 pg/ml, n = 5, CFA + cangrelor: 50.4 ± 6.1 pg/ml, n = 5, P < 0.05, CFA + PSB: 49.8 ± 4.8 pg/ml, n = 5, P < 0.05).
The TNF-α protein was undetectable in the spinal cord 96 h after injection in the majority of saline- and CFA-treated animals suggesting that constitutive expression of TNF-α protein might be very low in lumbar spinal cord regions. Basal IL-6 was also undetectable in the majority of samples in the hind paw 48 h after saline injection. In contrast, the level of IL-6 showed an elevation in response to 4 days of systemic CFA treatment (Fig. 4H). Once again, cangrelor (3 mg/kg i.p.) and PSB-0739 (0.3 mg/kg i.t.) prevented the induction of this cytokine in the spinal cord (Fig. 4H). The CFA-induced IL-10 level in the L4-L6 spinal cord was not significantly different from that observed in rats after saline injection and P2Y12R antagonists did not change the level of IL-10 after CFA stimulus (data not shown).
The effect of P2Y12R antagonists on the CFA-induced cytokine expression in the inflamed hind paw of rats
In subsequent experiments, rats were challenged with intraplantar injection of CFA (0.1 ml) or saline, as before and the cytokine response was measured 48 h later in the inflamed hind paw. As shown in Fig. 5A, the basal level of IL-1β 48 h after the saline injection was 226 ± 42.3 pg/ml (n = 4) in the rat hind paws. Confirming the findings obtained with the single ELISA assay, a remarkable increase in IL-1β level was detected 48 h after intraplantar CFA treatment (11,198 ± 497 pg/ml, n = 5, P < 0.01). Systemic administration of cangrelor did not affect the CFA-induced elevation of the level of IL-1β. In contrast, intrathecally injected PSB-0739 elicited a 50% decrease in the CFA-induced IL-1β level in the hind paw (Fig. 5A).
Fig. 5.
The effect and mechanism of action of P2Y12R antagonists on intraplantar CFA injection (0.1 ml)-induced peripheral inflammation in rats. A, B, C. Robust elevation were detected in the levels of TNF-α, IL-1β and IL-6 in the hind paw 48 h after induction the inflammatory pain. The increase in the concentrations of cytokines was decreased by intrathecal (i.t.) PSB-0739 administration. D. Anti-inflammatory cytokine response: intraplantar injection of CFA (0.1 ml) significantly increased the level of IL-10 in the hind paw of rats 48 h after treatment. PSB-0739 (0.3 mg/kg) the selective P2Y12 receptor antagonist was injected i.t. 15 min prior post-CFA PWT determination, while cangrelor (3 mg/kg) the P2Y12/P2Y13 antagonist was added i.p. 30 min prior to post-CFA PWT determination. The levels of cytokines were quantified in the derived supernatants by multiplex cytokine assay. Data are given as the mean level of cytokines ± SEM of 5 independent experiments (* < 0.05, ** < 0.01, *** < 0.001 one-way ANOVA followed by Neuman–Keuls-test). E. The effect of 6-OHDA pretreatment (40 mg/kg + 60 mg/kg + 60 mg/kg, i.p., in every 2nd day) on endogenous monoamine levels in the hind paw. Control rats received saline treatment in an identical manner. The level of noradrenaline (NA), normetanephrine (NM) and 3,4-dihydroxyphenylglycol (DOPEG) was measured by HPLC-EC and is expressed as pmol/mg protein. (*P < 0.05, Student t test with Welch correction, n = 6/group). F. Subdiaphragmatic vagotomy (VGX) and the α7 nAChR antagonist MLA, but not 6-OHDA pretreatment occludes the antihyperalgesic effect of PSB-0739 after CFA treatment. In these experiments, the α7 nACh receptor antagonist methyllycaconitine (MLA) or its vehicle (saline, SAL) was administered 45 min, while PSB-0739 (0.3 mg/kg)/saline (SAL) was injected i.t. 15 min before the post-CFA PWT determination, respectively. 6-OHDA pretreatment was performed as described in E. Vagotomy was induced after the determination of basal PWT values, and CFA was administered 10 days after surgery (* < 0.05, ** P < 0.01, one-way ANOVA followed by Neuman–Keuls-test, n = 5–10/group). G, H. The α7 nAChR antagonist MLA prevents the inhibitory effect of PSB-0739 on IL-1β (G) and TNFα (H) induction in the inflamed hind paw 48 h following CFA treatment. Experiments were performed according to the protocols described in F. After the experiments, IL-1β and TNF-α levels were quantified in the derived supernatants by multiplex cytokine assay. Data are given as the mean level of cytokines ± SEM of 5 independent experiments. Cytokine levels are expressed as pg/ml (n = 5/group). (* < 0.05, *** P < 0.001, ns, non-significant, one-way ANOVA followed by Neuman–Keuls-test, n = 5/group).
Basal TNF-α was undetectable in the majority of samples in the hind paw 48 h after saline injection. In order to perform statistical analyses, the constitutive expression of TNF-α was regarded as 10− 5 pg/ml in samples with an undetectable level of TNF-α. TNF-α protein levels showed an increase in response to intraplantar CFA administration (47.22 ± 7.17 pg/ml; n = 5; P < 0.01 vs. saline) and both P2Y12R antagonists reduced the elevation of TNF-α levels after the inflammatory stimulus (CFA + cangrelor: 26.40 ± 6.0 pg/ml, n = 5, P < 0.05, CFA + PSB: 6.40 ± 3.4 pg/ml, n = 5, P < 0.01 vs. CFA, Fig. 5B).
A significant increase in IL-6 concentration was also observed in the rat hind paw 48 h following intraplantar CFA (0.1 ml) administration when compared with the corresponding saline-treated controls at the same time point (Fig. 5C, saline: 33.5 ± 33.5 pg/ml, n = 5, CFA: 2576 ± 505.49 pg/ml, n = 5, P < 0.01). While CFA-induced IL-6 levels were not affected by cangrelor, PSB-0739 (i.t.) alleviated the CFA-induced increase in CFA-induced IL-6 protein levels (Fig. 5C, CFA + PSB: 830 ± 210 pg/ml, n = 5, P < 0.05).
Among anti-inflammatory cytokines, we examined the changes in the level of IL-10 in the rat hind paw 48 h after saline or CFA injection. The basal level of IL-10 was 4.91 + 1.42 pg/ml (Fig. 5D; n = 5). Intraplantar CFA administration caused a remarkable increase in IL-10 levels (Fig. 5D, 25.0 ± 2.88 pg/ml, n = 5, P < 0.001, 508% increase). In the presence of cangrelor (3 mg/kg i.p.) and PSB-0739 (0.3 mg/kg i.t) the level of IL-10 decreased after CFA injection (Fig. 5D).
These experiments showed that a marked attenuation of peripheral cytokine response is detected at the periphery after central administration of PSB-0739 in parallel with its effect of decreasing inflammatory hyperalgesia. However, PSB-0739 alone hardly penetrates the blood–brain-barrier as it was ineffective via i.p. administration. Therefore in the subsequent experiments an attempt was made to identify an efferent pathway that mediates the inhibition of central P2Y12Rs to the peripheral cytokine response. At first, chemical sympathectomy was initiated by intraperitoneal injections of 6-OHDA in 0.1% ascorbic acid every second day over 5 consecutive days (40 mg/kg, 60 mg/kg, 60 mg/kg). The last 6-OHDA treatments were followed by intraplantar injection of CFA or saline as described above and mechanical hyperalgesia was measured 48 h later. To confirm the depletion of noradrenaline from sympathetic nerve terminals in response to 6-OHDA treatment in the periphery, catecholamine content of the hind paw was analyzed by HPLC after the experiments (Fig. 5E). Indeed, a substantial reduction in the level of both noradrenaline and its metabolites normetanephrine and 3,4-dihydroxyphenylglycol (DOPEG) was observed in the hind paw in response to 6-OHDA, when compared to saline treated rats (Fig. 5E). However, 6-OHDA treatment did not change the inhibitory effect of PSB-0739 (0.3 mg/kg i.t) on mechanical hyperalgesia evoked by intraplantar CFA treatment (Fig. 5F).
Because electrical stimulation of the distal vagus nerve suppresses the cytokine response in the inflamed hind paw (Borovikova et al., 2000, Pavlov et al., 2003), we next tested, whether subdiaphragmatic vagotomy relieves the effect of intrathecal application of PSB-0739 on CFA-induced mechanical hyperalgesia. The PWT values were determined before and 10 days after the vagotomy, and they did not differ significantly from each other, indicating that vagotomy by itself does not influence the mechanical sensitivity of the hind paws (38.6 ± 0.8 g, n = 10 and 37.66 ± 1.75 g, n = 10, respectively, P > 0.05). Intraplantar CFA injection was then administered, which resulted in a similar decline in the PWT values to that observed in naïve animals (Fig. 5F). In vagotomized animals, however, the inhibitory effect of PSB-0739 (0.3 mg/kg i.t) was completely absent (Fig. 5F).
Because activation of α7 nAChRs is a known mechanism for suppression of inflammatory and neuropathic hypersensitivity (Loram et al., 2012, Medhurst et al., 2008) we next examined the effect of P2Y12R antagonists in the presence of the α7 nAChR antagonist MLA (3 mg/kg i.p.), which was administered 45 min before the respective post-CFA PWT determination. When compared to identical saline treatment, MLA treatment alone did not change mechanical hyperalgesia (Fig. 5F). In contrast, the antihyperalgesic effect of PSB-0739 (0.3 mg i.t., 15 min before post-CFA measurement of mechanical hyperalgesia) was prevented by MLA pre-treatment (Fig. 5F). The effect of MLA pre-treatment on the inhibitory action of PSB-0739 on the induction of IL-1β and TNF-α 48 h after CFA injection in the hind paw (Fig. 5G, H) was also tested. In contrast to rats receiving only PSB-0739 (Figs. 5A, B), no significant change in either IL-1β (Fig. 5G) or TNF-α production (Fig. 5H) was detected in rats, which received MLA pre-treatment (3 mg/kg i.p.) prior to PSB-0739 administration (0.3 mg i.t).
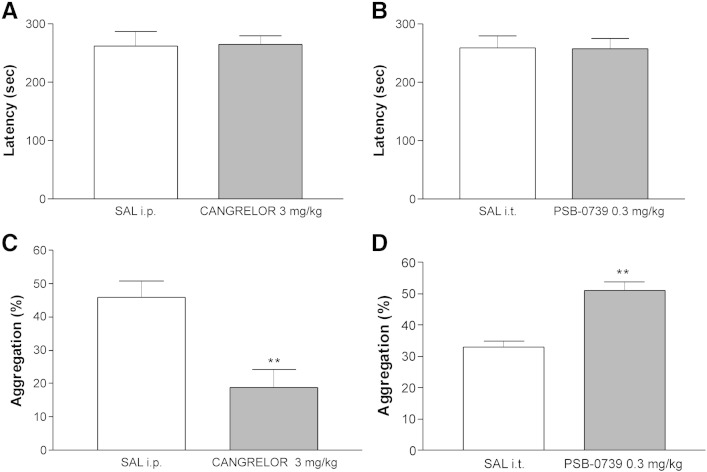
Accelerating rotarod test
Based on the results obtained in pain models, the effects of two of the most potent P2Y12R antagonists, cangrelor and PSB-0739 were examined on motor coordination in the accelerating rotarod test, using doses effective in analgesia tests (3 mg/kg i.p. for cangrelor and 0.3 mg/kg i.t. for PSB0739, respectively), in comparison with saline treatment using the identical route of administration. The falling latency values of i.p. or i.t. saline-treated animals were 262.70 ± 24.90 s (n = 10) and 259.0 ± 21.23 s (n = 9) in the 300-s test period. Neither of the two tested antagonists significantly affected the falling latency values (Figs. 6A, B).
Fig. 6.
Potential side-effects of P2Y12R antagonists. A, B. The effect of cangrelor (A) and PSB-0739 (B) treatment in accelerating rotarod test. A. Motor coordination was assessed 30 min after the injection of cangrelor (i.p.) and expressed in s, n = 10. B. PSB-0739 was administered intrathecally 15 min before testing at the dose (mg/kg) indicated on the abscissa (n = 9). C, D. The effect of cangrelor and PSB-0739 on ex vivo platelet aggregation induced by ADP (10 μM). The experimental animals were treated with saline (C: i.p., D: i.t.), cangrelor (C) and PSB-0739 (D). N = 4–9/group, **P < 0.01, Student t test. The results are expressed as percentage of maximal aggregation (%).
Ex-vivo inhibition of ADP-induced platelet aggregation
In this test the effects of cangrelor and PSB-0739 were investigated in their effective analgesic doses and with identical route of administration, and their effects on the maximal aggregation of platelets induced by ADP were evaluated.
In blood samples drawn from naive rats, ADP (5–10 μM) induced platelet aggregation in a concentration-dependent manner (data not shown); using 10 μM ADP, the maximal platelet aggregation was 49.5 ± 2.96% (n = 4). Similar values were obtained 30 min after intraperitoneal injection of saline (Fig. 6C, 45.78 ± 5.00%, n = 9, P > 0.05). Cangrelor (3 mg/kg i.p.) significantly reduced the maximal platelet aggregation induced by ADP (Fig. 6C). In contrast, in platelets from animals treated with PSB-0739 (0.3 mg/kg) intrathecally, there was an increase in the maximal platelet aggregation, when compared to platelets from i.t. saline treated animals (Fig. 6D).
Involvement of P2Y12 receptors in the regulation of CFA-induced inflammatory pain, neuropathic pain and acute thermal nociception in mice: effect of genetic deletion of P2Y receptors
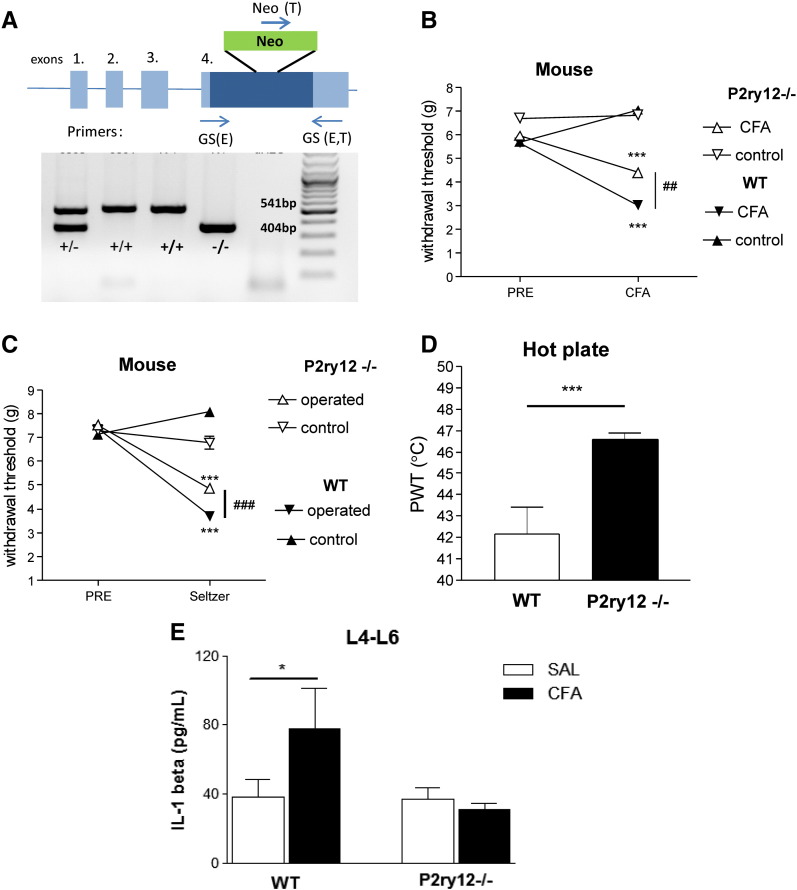
In order to further substantiate the involvement of P2Y12 receptors in the various pain modalities described above a P2Y12R deficient mouse line was also investigated. PCR analysis of genomic DNA from wild-type, heterozygous (P2ry12+/−), and homozygous (P2ry12−/−) P2Y12R deficient mice confirmed the presence of a 541 bp length product corresponding to the wild-type allele (GS(E)-GS(E,T)) in wild-type mice, whereas a 404 bp length product representing the mutant allele (GS(E,T)-NEO(T)) was detected in the P2ry12−/− mice (Fig. 7A). In case of heterozygous mice both fragments were amplified (Fig. 7A).
Fig. 7.
The effect of genetic deletion of P2Y12R in mouse models of inflammatory (B), neuropathic (C) and acute pain (D). A. The identification of wild type (+/+), heterozygote (+/−) and homozygote p2ry12 KO genotypes by PCR based analysis. With the 3 primers used in a single reaction, an 541 bp length product was amplified for the wild type allele (GS(E)-GS(E,T)) and a 404 bp length one for the mutant allele (GS(E,T)-NEO(T)). In the case of heterozygous mice both fragments were amplified. B. Mechanical hyperalgesia before (PRE) and after (CFA) intraplantar CFA injection in P2ry12−/− and wild-type (WT) mice. CFA (30 μl, 50% in saline) was injected into the plantar surface of the right hind paw. 48 h after treatment with CFA, mechanical sensitivity was measured on both hind paws. Paw withdrawal threshold values are presented in grams (mean ± S.E.M.). (***) denotes statistical significance of P < 0.001 vs. PRE values, (##) denotes statistical significance of P < 0.01 between genotypes. N = 7–10/group, multivariate ANOVA, Fischer LSD post hoc test. C. Mechanical hyperalgesia in operated and sham operated paws before (PRE) and after Seltzer operation (Seltzer) in P2ry12−/− and wild-type (WT) mice. Paw withdrawal threshold values are presented in grams (mean ± S.E.M.). (***) denotes statistical significance of P < 0.001 vs. PRE values, (###) denotes statistical significance of P < 0.001 between genotypes. N = 7/group, multivariate ANOVA, Fischer LSD post hoc test. D. Acute thermal nocifensive threshold in P2ry12−/− and wild-type (WT) mice. PWT values are expressed in °C. ***P < 0.001 vs. WT, as indicated by the horizontal bar. N = 10/group, Student t test. E. Basal and CFA induced IL-1β protein levels in the lumbar spinal cord of P2ry12−/− and wild-type (WT) mice 96 h after CFA/saline (SAL) treatment. IL-1β levels are expressed as pg/ml. N = 4–5/group, *P < 0.05, two-way ANOVA, Fischer LSD post hoc test.
In mice, the baseline PWT values were 5.65 ± 0.27 g and 5.95 ± 0.17 g in wild-type and P2ry12−/− animals, respectively; not significantly different from each other (n = 10, P = 0.32). Transdermal injection of 30 μL of CFA to the plantar surface of the right hind paw elicited a marked decrease in the PWT values of the ipsilateral hind paws of wild-type mice (Fig. 7B, multivariate ANOVA, effect of pre-post treatment comparison, F1,18 = 90.24, P < 0.001). The change in mechanical sensitivity was also significantly different when compared to the contralateral hind paws (ipsilateral vs. contralateral effect F1,18 = 86.06, P < 0.001, ipsilateral vs. contralateral effect x pre-post interaction, F1,18 = 55.69, P < 0.001). The mechanical hyperalgesia was significantly attenuated in the P2ry12−/− animals, when compared to wild-type mice (Fig. 7B). This interaction effect was significant when taking into account preoperative baseline values and contralateral hind paws (ANOVA genotype × pre–post × ipsi-contrateral effect interaction F1,18 = 9.16, P < 0.01).
Partial ligation of the sciatic nerve elicited a significant decrease in the PWT values of wild-type mice compared to either pre-operative values (Fig. 7C, pre-operative PWT: 7.37 ± 0.22 g, n = 7, postoperative PWT: 3.69 ± 0.2 g, n = 7, pre-post operative comparison F1,12 = 56.18, P < 0.001) or to the contralateral hind paw (ipsilateral vs. contralateral effect F1,12 = 58.31, P < 0.001, ipsilateral vs. contralateral effect × pre–post interaction, F1,12 = 150.40, P < 0.001). Similar to the observations in the CFA induced inflammatory pain model, in the absence of P2Y12 receptors, the mechanical hyperalgesia was attenuated in a slight but significant manner (Fig. 7C, ANOVA genotype × pre-post × ipsi-contrateral effect interaction F1,12 = 19.81, P < 0.001).
The baseline nociceptive threshold was 42.2 ± 1.3 C in wild-type mice (Fig. 7D). This value was elevated to 46.6 ± 0.3 °C in the P2ry12−/− mice (n = 8, P = 0.002).
The basal IL-β level in the lumbar spinal cord of saline-treated wild-type mice was 38.17 ± 9.98 pg/ml (n = 5, Fig. 7E). This value was increased to 77.53 ± 23.47 pg/ml (P < 0.05, n = 5) 96 h following intraplantar CFA (30 μl) injection. The IL-β level of saline treated P2ry12−/− mice was similar to that of the wild-type mice (Fig. 7E, 37.07 ± 6.50 pg/ml, n = 5, P > 0.05). In these mice, no increase in IL-β production was observed in response to CFA, 96 h after the treatment (Fig. 7E, ANOVA genotype × treatment interaction F1,34 = 42.72, P < 0.001).
Discussion
The principal novel finding of the present study is that both pharmacological blockade and genetic deficiency of central P2Y12Rs lead to the attenuation of inflammatory pain in rodents. In addition, our study confirms and extends previous investigations on the role of P2Y12Rs in neuropathic pain (Andó et al., 2010, Kobayashi et al., 2008, Tozaki-Saitoh et al., 2008) and acute thermal nociception (Ando et al., 2010). Whereas P2Y12R antagonists used in the above studies, i.e. MRS2395, the pro-drug clopidogrel and the non-pro-drug P2Y12 receptor antagonist cangrelor, have mixed or uncertain activities on human P2Y12Rs we report here for the first time that PSB-0739, a highly selective and potent P2Y12R antagonist (Baqi et al., 2009, Hoffmann et al., 2009) also reproduces these effects when administered centrally. These findings suggest that inhibition of central P2Y12Rs without action on any other pharmacological target is sufficient to elicit significant analgesic effect in neuropathic and inflammatory pain models.
To further support the involvement of P2Y12Rs in the above pain modalities, the rank order of mED doses of the antiallodynic effects of different P2Y12R antagonists correlated approximately with their potency at human P2Y12Rs (see Table 2).
There is one possible exception: MRS2395, which, showed a non-competitive mode of antagonism in cells expressing the recombinant human P2Y12R. In fact, MRS2395 did not elicit a rightward shift of the concentration-response curve of the P2Y12R agonist 2-methylthio-ADP to inhibit forskolin-induced cAMP production, but only attenuated the maximal response to 2-methylthio-ADP (Fig. 2E). Moreover, MRS2395 alone increased the cellular cAMP formation induced by forskolin. Because this effect was also observed in mock-transfected cells and the used cell lines display no or negligible endogenous P2Y12R expression (Algaier et al., 2008, Erb et al., 1995, Hoffmann et al., 2008; qPCR analysis of the present study), it seems to be independent from P2Y12R. Reactive blue 2 also increased cAMP levels, but in contrast to MRS2395, reactive blue 2 clearly shifted the concentration–response-curve of the agonist 2-methylthio-ADP to the right. MRS2395 was originally presumed to be P2Y12R antagonist, because it attenuated ADP-induced platelet aggregation, but did not antagonize P2Y1 receptor-mediated responses (Xu et al., 2002). The present data indicate a non-competitive mode of antagonistic action of MRS2395 on hP2Y12R. A portion of that antagonistic effect of MRS2395 may be mediated indirectly by an effect on cellular cAMP concentrations. Interestingly, not all P2Y12R antagonists reproduced the effect of genetic deletion and their effect depended on the specific experimental model. In the hot plate test, clopidogrel, ticlopidine, MRS2395 and PSB-0739 elicited significant analgesic effects, whereas cangrelor and reactive blue 2 remained ineffective in doses that produced significant antihyperalgesic effects in the CFA model. Reactive blue 2 was also ineffective in the tested range in the neuropathic pain model. A potential explanation to this discrepancy is that, in contrast to PSB-0739, cangrelor and reactive blue 2 also inhibit the P2Y13 receptor in addition to P2Y12R (cf. von Kügelgen, 2006), which may confound the effect. Previously, we found that activation of P2Y13R, but not P2Y12Rs inhibits the stimulation-evoked release of glutamate from acute spinal cord slices (Heinrich et al., 2008), indicating that the activation, but not the inhibition of the P2Y13 receptor conveys acute antinociceptive action in the spinal cord.
As a confirmation of the findings obtained on rats, we also report here that mice genetically deficient in P2Y12R display elevated baseline nociceptive threshold, and attenuated mechanical hyperalgesia in the respective neuropathic and inflammatory pain models. Although heterozygous mice were not assessed in this study previous studies revealed that the phenotype of P2ry12+/− mice was either similar to wild-type or transitional between P2ry12+/+ and P2ry12−/− mice, depending on the experimental conditions (André et al., 2003). For instance, partly relevant to our conditions, in the case of in vitro platelet aggregation and inhibition of cAMP production by ADP, the values of heterozygotes were closed to the wild-type, while they displayed a significantly elongated blood vessel occlusion time in vivo.
Next, we have attempted to identify the mechanism of action of P2Y12R antagonists for alleviating CFA-induced mechanical hyperalgesia in rats. Previous studies indicated an upregulation of P2Y12R mRNA in microglia cells of the spinal cord in neuropathic models using either semi-quantitative RT-PCR (Kobayashi et al., 2008) or in situ hybridization histochemistry (Kobayashi et al., 2008, Kobayashi et al., 2012). In our experiments, a massive upregulation of P2Y12R mRNA was detected 48 h after CFA injection in the hind paw in parallel with the robust induction of the proinflammatory cytokine IL-1β, indicating a rapid cytokine response at the periphery. Interestingly, however, neither P2Y12R mRNA expression nor IL-1β production was changed significantly at this time point in the spinal cord, only later, suggesting a time-dependent reactivity of P2Y12R-mediated events locally and at a distance from the site of inflammation. Indeed, a profound elevation of IL-1β production was detected 96 h after CFA injection in the spinal cord, coincidently with the upregulation of P2Y12R mRNA and the sustained antihyperalgesic response of both cangrelor and PSB-0739. Both P2Y12R antagonists almost completely abolished the elevation of IL-1β protein levels in response to CFA application in the spinal cord. These findings were also confirmed by using P2Y12R-deficient mice, in which no increase in IL-1β production was detected in response to CFA treatment. IL-1β is regarded as a mediator of central sensitization mechanisms leading to enhanced pain sensitivity by the augmentation of spontaneous miniature excitatory postsynaptic currents (sEPSCs) with a simultaneous inhibition of spontaneous miniature inhibitory postsynaptic currents (sIPSCs) in Lamina II neurons of spinal cord slices and by the induction of CREB phosphorylation and subsequent long-term plasticity events in spinal nociceptive neurons (Kawasaki et al., 2008). Therefore, the inhibition of spinal IL-1β production is one potential signaling pathway that mediates the antihyperalgesic action of P2Y12R antagonists in inflammatory pain. However, it is probably not the only one, as P2Y12R antagonists were already effective in the alleviation of CFA-induced inflammatory pain 48 h after CFA injection, i.e. before the induction of IL-1β in the spinal cord.
Moreover, we detected the profound induction of proinflammatory cytokines at the periphery, i.e. in the inflamed hind paw at this time point, and PSB-0739 effectively counteracted the elevation of IL-1β, TNF-α and IL-6. The contribution of peripheral cytokines to the pain sensitization is well documented (Ren and Dubner, 2010, Scholz and Woolf, 2007); therefore it is reasonable to suggest that peripheral IL-1β, TNF-α and IL-6 mediate the antihyperalgesic action of PSB-0739 at this time point. Upregulation of P2Y12Rs in the hind paw, detected in our study may contribute to this action. P2Y12R is expressed not only on the peripheral sensory nerve terminals (Malin and Molliver, 2010), but also probably elsewhere as well, such as on keratinocytes and dendritic cells, which also participate in cutaneous-neuro-immune interactions and known to express various other subtypes of P2 receptors (Dussor et al., 2009). Nevertheless, the mechanism leading from central administration of PSB-0739 to the alleviation of peripheral cytokine response still requires explanation. Although we cannot entirely exclude the possibility that the compound itself reached the periphery, increased permeability of the blood–brain barrier is observable only 3–5 days after CFA administration (Raghavendra et al., 2004), and the same dose of PSB-0739 was ineffective when administered intraperitoneally, which suggests a primary central target site of its action. A potential efferent neuronal pathway would be sympathetic postganglionic nerves originating in the lumbar spinal cord (Sandkühler, 2009), which express P2Y12R (Lechner et al., 2004). 6-OHDA pretreatment, however did not counteract the effect of PSB-0739 in alleviating inflammatory hyperalgesia. In contrast, following subdiaphragmatic vagotomy or systemic treatment with the α7 nAChR antagonist MLA, PSB-0379 no longer influenced mechanical hypersensitivity and the peripheral cytokine response. Because the penetration of systemic MLA to the CNS is negligible (Medhurst et al., 2008, Turek et al., 1995), these findings suggest that the vagus nerve mediates this effect from the spinal cord to the periphery with the involvement of α7 nAChRs. This is consistent with the inhibitory effect of the distal vagus nerve, on the cytokine response in the inflamed hind paw (Borovikova et al., 2000, Pavlov et al., 2003) and with the presence of α7 nAChRs on peripheral immune cells. The activation of α7 nAChRs is a well-documented mechanism for the suppression of inflammatory and neuropathic pain (Loram et al., 2012, Medhurst et al., 2008). It was assumed, therefore, that endogenous activation of α7 nAChRs on immune cells recruited at the site of inflammation would be responsible for this effect. However, early mediators of the inflammatory response, such as different chemokines or other, yet unidentified supraspinal mechanisms might also participate in the effect of central P2Y12R on the local peripheral cytokine response at this time point (Kiguchi et al., 2012, Ransohoff, 2009). Previous findings, showing that activation of P2Y12R is involved in the chemotaxis of microglia (Honda et al., 2001) as well as in the engulfment of nerve axons by microglia (Maeda et al., 2010) also point in this direction.
A major limitation of the current therapy used in inflammatory and neuropathic pain is not only the lack of efficacy but also the occurrence of untoward side-effects in the therapeutic dose-range (Negus et al., 2006). Importantly, using doses higher than the mED of the respective compounds, we did not detect any acute effect on motor coordination by the most potent P2Y12R antagonists used in the study. On the other hand, i.p. application of an analgesic dose of cangrelor, but not i.t. application of PSB-0739 had a significant inhibitory effect on ex vivo platelet aggregation, which is unsurprising considering the well-known inhibitory effects of P2Y12R antagonists on this process (Schumacher et al., 2007). This effect, however, could also be equally considered as a protective effect on cardiovascular risk rather than a side effect, given that the P2Y12R occupancy in the blood shows close correlation with the formation of arterial thrombi. The finding that PSB-0739 did not inhibit but augmented platelet aggregation indicates that the analgesic effect of P2Y12R antagonists could be enhanced by the adequate penetration into the CNS without increasing the risk of bleeding.
In conclusion, our findings provide a rationale to target central P2Y12Rs as a potential therapeutic approach in inflammatory and neuropathic pain.
Acknowledgments
This study was supported by research grants from the Hungarian Research and Development Fund (Grants NN79957 and NN107234 to B.S.), the Hungarian Office of Science and Technology (Grant TÉT_10-1-2011-0050 to B.S.), the European Research Council (Grant 294313-SERRACO to B.S.), the Hungarian Brain Research Program [KTIA_13_NAP-A-III/1] and the Richter Gedeon Plc (4700136840). The P2ry12−/− mouse line has been funded by the Wellcome Trust. The authors thank Ádám Dénes for helpful discussions, Ed Beamer for editing the manuscript, and Petra Spitzlei (Department of Pharmacology and Toxicology, University of Bonn, Bonn, Germany) for expert technical assistance.
References
- Algaier I., Jakubowski J.A., Asai F., von Kügelgen I. Interaction of the active metabolite of prasugrel, R-138727, with cysteine 97 and cysteine 175 of the human P2Y12 receptor. J. Thromb. Haemost. 2008;6:1908–1914. doi: 10.1111/j.1538-7836.2008.03136.x. [DOI] [PubMed] [Google Scholar]
- Almasi R., Petho G., Bolcskei K., Szolcsanyi J. Effect of resiniferatoxin on the noxious heat threshold temperature in the rat: a novel heat allodynia model sensitive to analgesics. Br. J. Pharmacol. 2003;139:49–58. doi: 10.1038/sj.bjp.0705234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andó R.D., Sperlágh B. The role of glutamate release mediated by extrasynaptic P2X7 receptors in animal models of neuropathic pain. Brain Res. Bull. 2013;93:80–85. doi: 10.1016/j.brainresbull.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Andó R.D., Mehesz B., Gyires K., Illes P., Sperlágh B. A comparative analysis of the activity of ligands acting at P2X and P2Y receptor subtypes in models of neuropathic, acute and inflammatory pain. Br. J. Pharmacol. 2010;159:1106–1117. doi: 10.1111/j.1476-5381.2009.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre P., Delaney S.M., LaRocca T., Vincent D., DeGuzman F., Jurek M., Koller B., Phillips D.R., Conley P.B. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J. Clin. Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P.L., Rothwell N.J., Stock M.J. Effects of Subdiaphragmatic vagotomy on energy-balance and thermogenesis in the rat. J. Physiol. London. 1985;362:1–12. doi: 10.1113/jphysiol.1985.sp015658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqi Y., Müller C.E. Rapid and efficient microwave-assisted copper(0)-catalyzed ullmann coupling reaction: general access to anilinoanthraquinone derivatives. Org. Lett. 2007;9:1271–1274. doi: 10.1021/ol070102v. [DOI] [PubMed] [Google Scholar]
- Baqi Y., Müller C.E. Synthesis of alkyl- and aryl-amino-substituted anthraquinone derivatives by microwave-assisted copper(0)-catalyzed Ullmann coupling reactions. Nat. Protoc. 2010;5:945–953. doi: 10.1038/nprot.2010.63. [DOI] [PubMed] [Google Scholar]
- Baqi Y., Atzler K., Köose M., Glänzel M., Müller C.E. High-affinity, non-nucleotide-derived competitive antagonists of platelet P2Y(12) receptors. J. Med. Chem. 2009;52:3784–3793. doi: 10.1021/jm9003297. [DOI] [PubMed] [Google Scholar]
- Born G.V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Borovikova L.V., Ivanova S., Nardi D., Zhang M., Yang H., Ombrellino M., Tracey K.J. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Krügel U., Abbracchio M.P., Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog. Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Coddou C., Yan Z.H., Obsil T., Huidobro-Toro J.P., Stojilkovic S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csolle C., Sperlagh B. Peripheral origin of IL-1 beta production in the rodent hippocampus under in vivo systemic bacterial lipopolysaccharide (LPS) challenge and its regulation by P2X(7) receptors. J. Neuroimmunol. 2010;219:38–46. doi: 10.1016/j.jneuroim.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Debnath B., Al-Mawsawi L.Q., Neamati N. Are we living in the end of the blockbuster drug era? Drug News Perspect. 2010;23:670–684. doi: 10.1358/dnp.2010.23.10.1506088. [DOI] [PubMed] [Google Scholar]
- Dussor G., Koerber H.R., Oaklander A.L., Rice F.L., Molliver D.C. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res. Rev. 2009;60:24–35. doi: 10.1016/j.brainresrev.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb L., Garrad R., Wang Y., Quinn T., Turner J.T., Weisman G.A. Site-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J. Biol. Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- Gum R.J., Wakefield B., Jarvis M.F. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal. 2012;8:S41–S56. doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S.E., Hollopeter G., Yang G., Kurpius D., Dailey M.E., Gan W.B., Julius D. The P2Y(12) receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heinrich A., Kittel A., Csolle C., Vizi E.S., Sperlagh B. Modulation of neurotransmitter release by P2X and P2Y receptors in the rat spinal cord. Neuropharmacology. 2008;54:375–386. doi: 10.1016/j.neuropharm.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Hoffmann K., Sixel U., Di Pasquale F., von Kügelgen I. Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y(12)-receptor. Biochem. Pharmacol. 2008;76:1201–1213. doi: 10.1016/j.bcp.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Hoffmann K., Baqi Y., Morena M.S., Glänzel M., Müller C.E., von Kügelgen I. Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y(12) receptor. J. Pharmacol. Exp. Ther. 2009;331:648–655. doi: 10.1124/jpet.109.156687. [DOI] [PubMed] [Google Scholar]
- Hollopeter G., Jantzen H.M., Vincent D., Li G., England L., Ramakrishnan V., Yang R.B., Nurden P., Nurden A., Julius D., Conley P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Honda S., Sasaki Y., Ohsawa K., Imai Y., Nakamura Y., Inoue K., Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through G(i/o)-coupled P2Y receptors. J. Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M.F. The neural–glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y., Zhang L., Cheng J.K., Ji R.R. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1 beta, interleukin-6, and tumor necrosis factor-beta in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiguchi N., Kobayashi Y., Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr. Opin. Pharmacol. 2012;12:55–61. doi: 10.1016/j.coph.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Yamanaka H., Fukuoka T., Dai Y., Obata K., Noguchi K. P2Y12 receptor upregulation in activated microglia is a gateway of p38 signaling and neuropathic pain. J. Neurosci. 2008;28:2892–2902. doi: 10.1523/JNEUROSCI.5589-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Yamanaka H., Yanamoto F., Okubo M., Noguchi K. Multiple P2Y subtypes in spinal microglia are involved in neuropathic pain after peripheral nerve injury. Glia. 2012;60:1529–1539. doi: 10.1002/glia.22373. [DOI] [PubMed] [Google Scholar]
- Koles L., Leichsenring A., Rubini P., Illes P. P2 receptor signaling in neurons and glial cells of the central nervous system. Adv. Pharmacol. 2011;61:441–493. doi: 10.1016/B978-0-12-385526-8.00014-X. [DOI] [PubMed] [Google Scholar]
- Lechner S.G., Dorostkar M.M., Mayer M., Edelbauer H., Pankevych H., Boehm S. Autoinhibition of transmitter release from PC12 cells and sympathetic neurons through a P2Y receptor-mediated inhibition of voltage-gated Ca2 + channels. Eur. J. Neurosci. 2004;20:2917–2928. doi: 10.1111/j.1460-9568.2004.03760.x. [DOI] [PubMed] [Google Scholar]
- Loram L.C., Taylor F.R., Strand K.A., Maier S.F., Speake J.D., Jordan K.G., James J.W., Wene S.P., Pritchard R.C., Green H., Van Dyke K., Mazarov A., Letchworth S.R., Watkins L.R. Systemic administration of an alpha-7 nicotinic acetylcholine agonist reverses neuropathic pain in male Sprague Dawley rats. J. Pain. 2012;13:1162–1171. doi: 10.1016/j.jpain.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorton D., Lubahn C., Klein N., Schaller J., Bellinger D.L. Dual role for noradrenergic innervation of lymphoid tissue and arthritic joints in adjuvant-induced arthritis. Brain Behav. Immun. 1999;13:315–334. doi: 10.1006/brbi.1999.0564. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maeda M., Tsuda M., Tozaki-Saitoh H., Inoue K., Kiyama H. Nerve injury-activated microglia engulf myelinated axons in a P2Y12 signaling-dependent manner in the dorsal horn. Glia. 2010;58:1838–1846. doi: 10.1002/glia.21053. [DOI] [PubMed] [Google Scholar]
- Malin S.A., Molliver D.C. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol. Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau F., Le Poul E., Communi D., Communi D., Labouret C., Savi P., Boeynaems J.M., Gonzalez N.S. Pharmacological characterization of the human P2Y(13) receptor. Mol. Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- Medhurst S.J., Hatcher J.P., Hille C.J., Bingham S., Clayton N.M., Billinton A., Chessell I.P. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J. Pain. 2008;9:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- Mestre C., Pelissier T., Fialip J., Wilcox G., Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Negus S.S., Vanderah T.W., Brandt M.R., Bilsky E.J., Becerra L., Borsook D. Preclinical assessment of candidate analgesic drugs: Recent advances and future challenges. J. Pharmacol. Exp. Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Papp L., Balazsa T., Kofalvi A., Erdelyi F., Szabo G., Vizi E.S., Sperlagh B. P2X receptor activation elicits transporter-mediated noradrenaline release from rat hippocampal slices. J. Pharmacol. Exp. Ther. 2004;310:973–980. doi: 10.1124/jpet.104.066712. [DOI] [PubMed] [Google Scholar]
- Parra M.C., Nguyen T.N., Hurley R.W., Hammond D.L. Persistent inflammatory nociception increases levels of dynorphin 1–17 in the spinal cord, but not in supraspinal nuclei involved in pain modulation. J. Pain. 2002;3:330–336. doi: 10.1054/jpai.2002.125185. [DOI] [PubMed] [Google Scholar]
- Pavlov V.A., Wang H., Czura C.J., Friedman S.G., Tracey K.J. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V., Tanga R.Y., DeLeo J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Raju N.C., Eikelboom J.W., Hirsh J. Platelet ADP-receptor antagonists for cardiovascular disease: past, present and future. Nat. Clin. Pract. Cardiovasc. 2008;5:766–780. doi: 10.1038/ncpcardio1372. [DOI] [PubMed] [Google Scholar]
- Ransohoff R.M. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K., Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Scholz J., Woolf C.J. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Schumacher W.A., Bostwick J.S., Ogletree M.L., Stewart A.B., Steinbacher T.E., Hua J., Price L.A., Wong P.C., Rehfuss R.P. Biomarker optimization to track the antithrombotic and hemostatic effects of clopidogrel in rats. J. Pharmacol. Exp. Ther. 2007;322:369–377. doi: 10.1124/jpet.106.119156. [DOI] [PubMed] [Google Scholar]
- Seltzer Z., Dubner R., Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Sperlagh B., Kofalvi A., Deuchars J., Atkinson L., Milligan C.J., Buckley N.J., Vizi E.S. Involvement of P2X(7) receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- Takasaki J., Kamohara M., Saito T., Matsumoto M., Matsumoto S.I., Ohishi T., Soga T., Matsushime H., Furuichi K. Molecular cloning of the platelet P2T(AC) ADP receptor: pharmacological comparison with another ADP receptor, the P2Y(1) receptor. Mol. Pharmacol. 2001;60:432–439. [PubMed] [Google Scholar]
- Tozaki-Saitoh H., Tsuda M., Miyata H., Ueda K., Kohsaka S., Inoue K. P2Y12 receptors in spinal microglia are required for neuropathic pain after peripheral nerve injury. J. Neurosci. 2008;28:4949–4956. doi: 10.1523/JNEUROSCI.0323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T., Salter M.W. P2X4 purinoceptor signaling in chronic pain. Purinergic Signal. 2012;8:621–628. doi: 10.1007/s11302-012-9306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T., Beggs S., Salter M.W. ATP receptors gate microglia signaling in neuropathic pain. Exp. Neurol. 2012;234:354–361. doi: 10.1016/j.expneurol.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Tozaki-Saitoh H., Inoue K. Purinergic system, microglia and neuropathic pain. Curr. Opin. Pharmacol. 2012;12:74–79. doi: 10.1016/j.coph.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Turek J.W., Kang C.H., Campbell J.E., Arneric S.P., Sullivan J.P. A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J. Neurosci. Methods. 1995;61:113–118. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- Vasiljev K.S., Uri A., Laitinen J.T. 2-Alkylthio-substituted platelet P2Y(12) receptor antagonist reveal pharmacological identity between the rat brain G(i)-linked ADP receptors and P2Y(12) Neuropharmacology. 2003;45:145–154. doi: 10.1016/s0028-3908(03)00142-4. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I., Harden T.K. Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv. Pharmacol. 2011;61:373–415. doi: 10.1016/B978-0-12-385526-8.00012-6. [DOI] [PubMed] [Google Scholar]
- Xu B., Stephens A., Kirschenheuter G., Greslin A.F., Cheng X., Sennelo J., Cattaneo M., Zighetti M.L., Chen A., Kim S.A., Kim H.S., Bischofberger N., Cook G., Jacobson K.A. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J. Med. Chem. 2002;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]