Abstract
Background:
To overcome limitations of cytology, biopsy needles have been developed to procure histologic samples during EUS.
Objective:
To compare 22-gauge (G) FNA and 22G biopsy needles (FNB) for EUS-guided sampling of solid pancreatic masses.
Design:
Randomized trial.
Setting:
Tertiary-care medical center.
Patients:
This study involved 56 patients with solid pancreatic masses.
Intervention:
Sampling of pancreatic masses by using 22G FNA or 22G FNB devices.
Main Outcome Measurements:
Compare the median number of passes required to establish the diagnosis, diagnostic sufficiency, technical performance, complication rates, procurement of the histologic core, and quality of the histologic specimen.
Results:
A total of 28 patients were randomized to the FNA group and 28 to the FNB group. There was no significant difference in median number of passes required to establish the diagnosis (1 [interquartile range 1-2.5] vs 1 [interquartile range 1-1]; P = .21), rates of diagnostic sufficiency (100% vs 89.3%; P = .24), technical failure (0 vs 3.6%; P = 1.0), or complications (3.6% for both) between FNA and FNB needles, respectively. Patients in whom diagnosis was established in passes 1, 2, and 3 were 64.3% versus 67.9%, 10.7% versus 17.9%, and 25% versus 3.6%, respectively, for FNA and FNB cohorts. There was no significant difference in procurement of the histologic core (100% vs 83.3%; P = .26) or the presence of diagnostic histologic specimens (66.7% vs 80%; P = .66) between FNA and FNB cohorts, respectively.
Limitations:
Only pancreatic masses were evaluated.
Conclusion:
Diagnostic sufficiency, technical performance, and safety profiles of FNA and FNB needles are comparable. There was no significant difference in yield or quality of the histologic core between the 2 needle types. (Clinical trial registration number: AQ:NCT01394159.) (Gastrointest Endosc 2012;76:321-7.)
Pancreatic cancer is associated with a poor prognosis, and the median survival after diagnosis of metastatic disease is only 3 to 5 months.1 Therefore, rapid and accurate assessment of a pancreatic mass is important to direct patient management.2
EUS-guided FNA (EUS-FNA) is the current standard of care for sampling pancreatic mass lesions, with reported sensitivity of 64% to 95%, specificity of 75% to 100%,3,4 and diagnostic accuracy of 78% to 95%.4,5 However, EUS-FNA has some limitations. The diagnostic accuracy is impacted by the availability of a cytopathologist to render on-site diagnosis,6,7 and its sensitivity for diagnosing malignancy is low in the setting of associated chronic pancreatitis.8 Also, certain neoplasms such as stromal cell tumors and lymphomas may be difficult to diagnose without histologic samples because their tissue architecture and morphology are essential for accurate pathologic assessment and histochemical studies.9 Moreover, in a recent study, the false-positive rate occurring with FNA cytology was reported to be 5% to 7%, which is higher than the originally reported rates of 0% to 1%.10,11
In order to overcome some of these limitations and to improve diagnostic accuracy, a Trucut needle biopsy (EUS-TNB) was developed to procure larger amounts of tissue with conserved architecture that would enable histologic analysis.12 Although the EUS-TNB technique was more accurate than FNA for diagnosing lymphomas and stromal tumors, the rigidity induced by its 19-gauge (G) caliber and the mechanical friction of the firing mechanism produced by the torqued echoendoscope limited its use for evaluating pancreatic head masses and duodenal lesions.9 Also, the disadvantages of the biopsy specimen, unlike cytology samples, is the lack of instant on-site diagnosis, the requirement for more processing time, the need for a repeat procedure in nondiagnostic cases, and the consequent delay in patient management. The ideal needle, therefore, would be one that provides both histologic core samples and cytology aspirates and was easily maneuverable.
A new, 19G EUS-FNB device with ProCore reverse-bevel technology (Wilson-Cook Medical, Winston-Salem, NC) was developed recently to enable the acquisition of core specimens for histologic analysis. In a large, prospective study from Europe,13 histologic samples were obtained successfully with this new 19G FNB needle in the majority of patients, with diagnostic accuracy of more than 90%. However, because technical difficulties were encountered with this needle when transduodenal passes were performed, the same FNB device was developed in a 22G platform.
The objective of this randomized study was to assess the capability of the new 22G EUS-FNB device to obtain cytology specimens and to compare its performance with the 22G FNA system. We also compared the ability of both needle systems to yield histologic core tissue. Furthermore, because the pancreas is the most challenging organ to sample during EUS, the study was limited to patients with solid pancreatic mass lesions.
Box 1. Take-home Message.
The 22-gauge biopsy needle procured diagnostic cytologic specimens in 89.3% of patients and histologic specimens in 80% of patients with solid pancreatic mass lesions.
There was no significant difference in technical performance or diagnostic yield between the 22-gauge biopsy and 22-gauge aspiration needles in this study.
PATIENTS AND METHODS
Patients
A prospective study of patients with solid pancreatic mass lesions referred for EUS was performed. All pancreatic masses were previously diagnosed by using CT at outside facilities or patients were referred after non-diagnostic EUS-FNA. Patients were excluded if a mass lesion was not seen at EUS, if the mass had a cystic component, or if the coagulation parameters were abnormal. The study was approved by the University of Alabama Institutional Review Board, and written informed consent was obtained from all patients for participation in the study.
Procedural technique
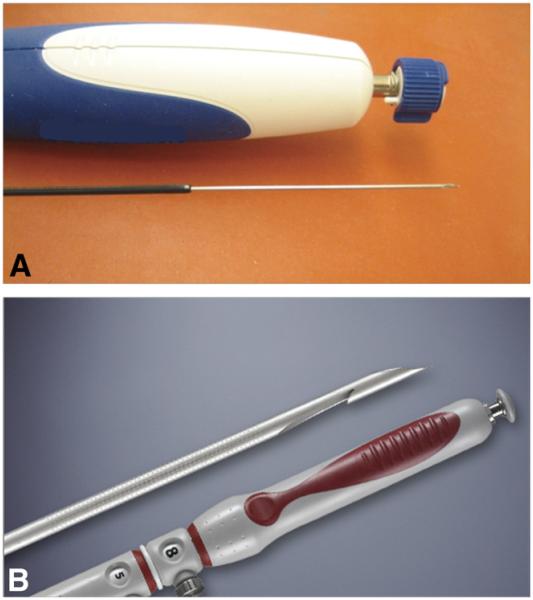
Computer-generated randomization assignments were placed in sealed envelopes and opened by the nurse during the procedure when patients met criteria for study inclusion. Patients were then randomized to undergo EUS-guided sampling of the pancreatic mass lesion with either the 22G FNA (Expect; Boston Scientific, Natick, Mass) or the 22G FNB device (Echotip ProCore; Cook Endoscopy, Bloomington, IN). The FNA device is made of cobalt chromium to enhance flexibility of the needle and has an outer diameter of 0.72 mm and an inner diameter of 0.52 mm (Fig. 1A). The FNB device is made of stainless steel and has a 5.2F shaft with a beveled tip (Fig. 1B). The reverse-bevel length is 4 mm. All procedures were performed by using a linear array echoendoscope (Olympus UCT140; Olympus America, Center Valley, Pa), with patients in the left lateral decubitus position under conscious sedation. All pancreatic head and uncinate masses were accessed via the duodenum and all pancreatic body and tail masses via the stomach.
Figure 1.
A, An image of the 22-gauge Expect FNA needle that is made of cobalt chromium to enhance flexibility. B, An image of the 22-gauge ProCore biopsy needle with a side hole at its tip for tissue acquisition during puncture.
Technique for FNA
After we punctured the mass, the stylet was removed, and the needle was moved to-and-fro within the lesion 12 to 16 times. Suction was not applied, and the stylet was not deployed after the first pass. Tissue material was expressed onto slides by advancing the stylet within the needle assembly.
Technique for FNB
After we punctured the mass, the stylet was removed, and the needle was moved to-and-fro within the lesion 4 times. Suction was applied by using a 10-mL syringe for 20 seconds and was released before the needle was withdrawn from the mass lesion. The specimen was then expressed onto slides by flushing air into the needle assembly. The stylet was not used for subsequent passes.
After the initial pass, the specimen was processed on site by an attending pathologist who was blinded to the needle type used for tissue procurement. Three maximum passes were performed by using the original needle type, and if there was diagnostic failure (defined as failure to obtain sufficient diagnostic material after 3 passes) or technical failure (defined as malfunction of the needle before we reached a diagnosis), the patient underwent crossover to the alternative needle. However, if a definitive diagnosis was established after the initial attempt, the procedure was terminated, and the number of passes performed was documented. In the crossover cohort, 3 maximum passes were attempted by using the alternate needle until sufficient diagnostic material was obtained or the needle technically failed. If no diagnosis was established in the crossover cohort, the procedure was terminated, and the patient was rescheduled for a repeat EUS on a different day. If on-site analysis warranted more tissue for further studies, 1 or 2 additional passes were made, and the specimen was collected in Hank buffered salt solution (HBSS; Invitrogen, Grand Island, NY). Also, 2 dedicated passes were carried out for histologic assessment by cell block preparation.
Preparation of specimen for on-site analysis
Air-dried and alcohol-stained smears were prepared on site after individual passes. Air-dried smears were stained with Diff-Quick stain (Dade Diagnostics, Miami, Fla) and immediately reviewed by a cytopathologist to ascertain sample adequacy and diagnosis. Alcohol-stained smears were prepared by using Papanicolaou stain.
Preparation of cell block for histologic analysis
In the laboratory, a 10-mL vial of HBSS containing the collected specimen was placed into the centrifuge, counter-balanced, and spun for 5 minutes. If the specimen quantity was sufficient, the supernatant was removed, and 3 drops of plasma and thrombin were added to the sediment. On formation of a clot, the cell button was removed intact, enclosed in a Tissue-Loc HistoScreen cassette (Microm International, Walldorf, Germany), and fixed in formalin. The cassette was processed, embedded in paraffin, and then prepared in hematoxylin and eosin to be evaluated by one pathologist, who was blinded to the randomization sequence, for the presence of a histologic core. If the histologic core was present, the specimen was graded as optimal or suboptimal. Optimal specimens were those in which the procured material enabled satisfactory assessment of histologic architecture that either did not change the original diagnosis or yielded additional findings. Suboptimal specimens were those in which the quality of the histologic core was unsatisfactory for assessment of histologic architecture. When required, immunohistochemical or special staining was performed for differentiation of morphologically challenging lesions.
Outcome measures
The primary objective was to elucidate the median number of passes required to establish on-site diagnosis. The secondary outcome measures were rates of diagnostic sufficiency, technical failure, complications, presence of histologic core, and quality of histologic specimens. Diagnostic sufficiency was defined as the proportion of patients in whom an on-site diagnosis was established within 3 passes. Complications were defined as any deviation from the clinical course after EUS-guided sampling as observed by the endosonographer or recovery suite nurse or as reported by patients. Excessive bleeding at the site of puncture, perforation, hypotension, and need for reversal medication were documented. For patients with abdominal pain, serum amylase and lipase levels were initially checked; an abdominal CT scan was performed if the symptoms persisted. Acute pancreatitis was defined as abdominal pain associated with nausea or vomiting coupled with a 3-fold elevation of serum amylase or lipase level. Immediate complications were documented at the time of the procedure, and late complications were documented via telephone follow-up at 72 hours. All patients were followed for a mean duration of 6 months.
Statistical analysis
A 2-tailed sample size calculation was performed with the type I error rate (α) set at 0.05 to attain 90% power for detecting a median effect size of 1 pass for the number of passes needed to acquire a diagnosis. This produced target sample sizes of 26 for the FNA group and 26 for the FNB group.
Baseline characteristics of the patient population, pancreatic mass lesions, and technical details were calculated. For comparison of categorical data, chi-square or Fisher exact tests were used as indicated. For comparison of continuous data, a 2-sample t test was performed if normal distribution was likely (such as the patient’s age), and the Wilcoxon rank sum test was carried out if normality could not be demonstrated (median number of passes needed for diagnosis and pancreatic mass size). Statistical significance was taken as P < .05. Although there was multiple testing of outcome data arising from individual patients, no corrections to P values were made because the purpose of the research was to highlight any potential differences. It is also noted that there were no instances of statistical testing where correction by the Bonferroni method would have removed significance from a finding. Datasets were compiled by using Microsoft Excel, and all statistical analyses were performed by using Stata 10 (StataCorp LP, College Station, Tex).
RESULTS
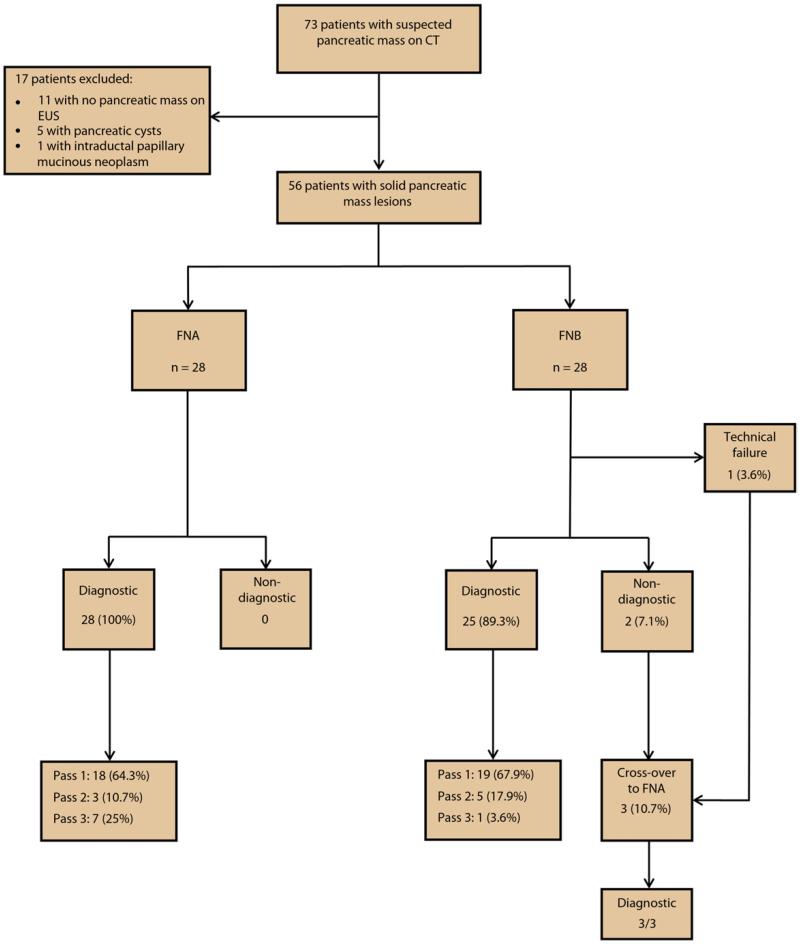
Of 73 patients enrolled for participation in this study between June and September 2011, 17 were excluded for the following reasons: a pancreatic mass lesion was not visualized at EUS in 11 patients, pancreatic cyst lesions were present in 5 patients, and intraductal papillary mucinous neoplasm was diagnosed in 1 patient. The remaining 56 patients with solid pancreatic mass lesions constituted the study cohort, where 28 patients were randomized to undergo EUS-FNA and 28 patients to undergo EUS-FNB.
The demographic characteristics of patients and pancreatic mass lesions are shown in Table 1 and technical outcomes in Table 2. In the FNA group, 24 of 28 pancreatic mass lesions were adenocarcinoma (85.7%), 1 was a neuroendocrine tumor (3.6%), and 3 were chronic pancreatitis (10.7%). In the FNB group, 22 of 28 pancreatic mass lesions were adenocarcinoma (78.6%), 2 were neuroendocrine tumors (7.1%), 1 was a pancreatic spindle cell tumor (3.6%), and 3 were chronic pancreatitis (10.7%).
TABLE 1.
Patient demographic and pancreatic mass characteristics
| Type of needle |
|||
|---|---|---|---|
| Characteristic | FNA (n = 28) |
FNB (n = 28) |
P value |
| Age, mean (SD), y | 65.4 (11.1) | 65.0 (15.4) | .898* |
| Median | 68 | 65 | |
| IQR | 57-74 | 58-77 | |
| Range | 40-82 | 18-87 | |
| Sex, no. (%) | |||
| Male | 16 (57.1) | 15 (53.6) | .788† |
| Female | 12 (42.9) | 13 (46.4) | |
| Prior EUS performed, no. (%) |
0 | 3 (10.7) | .236‡ |
| Size of mass on EUS, mm |
|||
| Mean (SD) | 33.7 (7.2) | 32.5 (9.0) | |
| Median | 35 | 30 | .625§ |
| IQR | 25-40 | 30-40 | |
| Range | 20-45 | 10-50 | |
| Tumor location, no. (%) |
|||
| Head/uncinate | 20 (71.4) | 20 (71.4) | 1.000† |
| Body/tail | 8 (28.6) | 8 (28.6) | |
| Final diagnosis, no. (%) |
|||
| Pancreatic tumor | 25 (89.3) | 25 (89.3) | 1.000‡ |
| Other | 3 (10.7) | 3 (10.7) | |
FNB, Fine-needle biopsy; SD, standard deviation; IQR, interquartile range.
Two sample t test.
Chi-square test.
Fisher exact test.
Wilcoxon rank sum (Wilcoxon-Mann-Whitney) test.
TABLE 2.
Technical characteristics and outcomes of EUS-FNA/FNB
| Type of needle |
|||
|---|---|---|---|
| Characteristic | FNA (n = 28) |
FNB (n = 28) |
P value |
| Access route, no. (%) | |||
| Transgastric | 8 (28.6) | 8 (28.6) | 1.000* |
| Transduodenal | 20 (71.4) | 20 (71.4) | |
| Diagnosis achieved, no. (%) | 28 (100) | 25 (89.3) | .236† |
| No. of passes for diagnosis | |||
| Mean (SD) | 1.61 (0.88) | 1.28 (0.54) | |
| Median | 1 | 1 | .209‡ |
| IQR | 1-2.5 | 1-1 | |
| Range | 1-3 | 1-3 | |
| Pass 1, no. (%) | 18 (64.3) | 19 (67.9) | – |
| Pass 2, no. (%) | 3 (10.7) | 5 (17.9) | – |
| Pass 3, no. (%) | 7 (25) | 1 (3.6) | – |
| Failure to achieve diagnosis, no. (%) | |||
| Total | 0 | 3 (10.7) | .236† |
| Diagnostic failure | 0 | 2 (7.1) | .491† |
| Technical failure | 0 | 1 (3.6) | 1.000† |
| Crossover, no. (%) | 0 | 3 (10.7) | .236† |
| Complications, no. (%) | 1 (3.6) | 1 (3.6) | 1.000† |
FNB, Fine-needle biopsy; SD, standard deviation; IQR, interquartile range; –, P value not calculated.
Chi-square test.
Fisher exact test.
Wilcoxon rank-sum (Wilcoxon-Mann-Whitney) test.
There was no significant difference in the median number of passes required to establish on-site diagnosis, rates of diagnostic accuracy, or technical failure between the FNA and FNB cohorts, respectively (Table 2). Three of the 28 patients undergoing EUS-FNB (10.7%) underwent crossover to the FNA cohort: 2 patients because of diagnostic failure despite 3 passes and 1 after technical failure because the stylet cap became detached from the needle apparatus. All 3 of these patients were subsequently diagnosed with adenocarcinoma by EUS-FNA. No technical difficulties were encountered with either needle when transduodenal passes were performed. An overview of the results is shown in Figure 2. Only 2 patients in the entire cohort had complications: 1 patient (3.6%) in the FNA group had postprocedural abdominal pain that was managed conservatively on an outpatient basis, and 1 patient (3.6%) in the FNB cohort developed mild acute pancreatitis that required hospitalization for 2 days.
Figure 2.
A flow diagram of the study results.
There was no significant difference between the 2 cohorts in the proportion of samples in which histologic core tissue was present (FNA 100% vs FNB 83.3%, P = .26). Histologic core of optimal quality was present in 66.7% of FNA specimens and 80% of FNB specimens (P = .66). In the remaining patients, the specimen quality was suboptimal for further analysis.
At a mean and median follow-up of 6 and 5.5 months (interquartile range = 4-8 months), respectively, 44 of 46 patients with pancreatic adenocarcinoma were undergoing chemoradiation, and 2 had surgical resection. Of the 3 patients with neuroendocrine tumors, 2 underwent surgical resection and 1 was managed conservatively because of an underlying comorbidity. One patient with pancreatic spindle cell tumor underwent distal pancreatectomy. All 6 patients with chronic pancreatitis were doing well without disease progression.
DISCUSSION
This study demonstrates that the diagnostic yield of the new 22G FNB system is comparable to that of an FNA assembly. The technical performance and safety profile of both needles were comparable, and on-site cytologic diagnosis was established with the biopsy needle in nearly 90% of patients. However, the yield of histologic core tissue was unsatisfactory with the biopsy needle, and the quality of specimen obtained was no better than that procured with the FNA system.
EUS-FNA of solid pancreatic mass lesions is safe, with diagnostic accuracy of greater than 90% when an on-site pathologist is available.14 Nevertheless, several attempts have been made to procure core biopsy specimens that may obviate the need for an on-site pathologist and enable the diagnosis of challenging lesions that cannot be evaluated by cytology alone. In a recent study by Larghi et al,15 a standard 19G FNA needle with modified suction technique was used to procure tissue for histologic analysis. With the use of this technique, the authors reported a diagnostic accuracy of 93.2%; however, this study did not include patients who required transduodenal FNAs, which is an obvious limitation of the 19G FNA needle. Others have advocated using a Quick-Core needle to perform EUS-TNB for evaluating suspicious lesions at various sites in the body, with overall diagnostic accuracy of 75% to 84%16,17 and 61% to 67.5% for pancreatic masses.17,18 Also, in a report of 3 cases, EUS-TNB but not EUS-FNA could correctly diagnose autoimmune or chronic pancreatitis.19 This advantage was, however, offset by technical limitations of the device, which made transduodenal sampling very difficult.20
In the study by Iglesias-Garcia et al,13 the 19G FNB needle was used to evaluate 114 lesions in 109 patients, and the achieved rates of technical success, sample adequacy for histology, and diagnostic accuracy were 98.2%, 89.5%, and 92.9%, respectively. The authors reported 2 failures when performing transduodenal passes (2 of 35 transduodenal FNAs [5.7%]), where the removal of the stylet proved to be impossible. Also, in many cases, puncture from the duodenum was difficult, which necessitated advancement of the FNB needle out of the echoendoscope while in the stomach before the scope could be passed into the duodenum to perform the biopsy. We encountered no technical difficulties when performing transgastric or transduodenal passes with the 22G FNB device in this study, and the needle exited the sheath with relative ease in all cases. In 1 patient, it was not possible to remove the stylet from the needle assembly when we performed transduodenal FNB, which has also been reported with the 19G FNB device.13
Specimens procured by using the FNB device were suitable for cytopathologic analysis in the majority of patients, and the diagnostic accuracy of the FNB and FNA devices were comparable at 89.3% and 100%, respectively. A definitive diagnosis was established on pass 1 in only 67.9% of patients who underwent FNB, which was similar to the 64.3% first-pass accuracy rate achieved with the FNA needle. Cumulative diagnostic sufficiency of 90% or greater was possible only after 3 passes for both needle types, and if patients randomized to the 22G FNB device were to undergo only 1 pass, it is likely that a diagnosis would not have been reached in almost one-third of these patients.
When each cell block was evaluated for histologic core, 100% of FNA specimens contained histologic material, compared with 83.3% for FNB specimens. Although 80% of FNB specimens were optimal for histologic analysis in this study, the rate of optimal specimens certainly was lower than the 92.9% reported with the 19G FNB device.13 Although the small-caliber 22G FNB device procures adequate tissue for cytologic assessment, the quantity and quality of acquired samples appear to be suboptimal for histologic analysis. Although we did not quantify the bloodiness of samples, the amount of blood tended to be more when we used the FNB device because of the beveled needle design and use of suction for tissue sampling. Another explanation could be that the quality of specimens collected for cell block was unsatisfactory because of the preceding passes that were performed for on-site analysis.
The safety profile of the FNB device was comparable to that of the 22G FNA device, with only 2 minor complications encountered in the entire cohort. This is in line with the earlier study evaluating the 19G FNB system in which none of the 109 patients experienced procedure-related complications.13
One of the limitations of the current study is the lack of standard criteria for reference. Although none of the patients with benign disease demonstrated disease progression at follow-up, we could not obtain further tissue confirmation for ethical reasons. Additionally, we did not evaluate other organ systems or lesions for which the FNB device could be useful, and specimens obtained with the FNB needle underwent only cell block analysis for histologic assessment. Nevertheless, studies have shown that cell block is a valid technique for performing histologic assessments, and it can improve the diagnostic accuracy of smears.21-23 Finally, it was not possible to blind the endoscopist to the type of device used for sampling pancreatic masses, which could have introduced bias into our study.24 However, this may not be a major limitation because the pathologist was blinded to the type of accessory used for tissue procurement.
In summary, the diagnostic sufficiency, technical performance, and complication rates of the new 22G FNB needle were comparable to the 22G FNA needle. Although it was possible to obtain adequate tissue for cytologic analysis in nearly 90% of patients by using the FNB device, histologic sampling was unsatisfactory.
Abbreviations
- EUS-FNA
EUS-guided FNA
- FNB
fine-needle biopsy
- HBSS
Hank buffered salt solution
- TNB
Trucut needle biopsy
Footnotes
DISCLOSURE: S. Varadarajulu is a consultant for Boston Scientific. No other financial relationships relevant to this publication were disclosed.
REFERENCES
- 1.Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008;10:58–62. doi: 10.1080/13651820701883148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun SS, Remotti H, Vazquez MF, et al. Endoscopic ultrasound-guided biopsies of pancreatic masses: comparison between fine needle aspiration and needle core biopsies. Diagn Cytopathol. 2007;35:276–82. doi: 10.1002/dc.20621. [DOI] [PubMed] [Google Scholar]
- 3.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshinaga S, Suzuki H, Oda I, et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23(suppl 1):29–33. doi: 10.1111/j.1443-1661.2011.01112.x. [DOI] [PubMed] [Google Scholar]
- 5.Itoi T, Sofuni A, Itokawa F, et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23(suppl 1):17–21. doi: 10.1111/j.1443-1661.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 6.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 7.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 8.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 9.Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc. 2005;62:417–26. doi: 10.1016/j.gie.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Gleeson FC, Kipp BR, Caudill JL, et al. False positive endoscopic ultrasound fine needle aspiration cytology: incidence and risk factors. Gut. 2010;59:586–94. doi: 10.1136/gut.2009.187765. [DOI] [PubMed] [Google Scholar]
- 11.Hawes RH. The evaluation of endoscopic ultrasound: improved imaging, higher accuracy for fine needle aspiration and the reality of endoscopic ultrasound-guided interventions. Curr Opin Gastroen. 2010;26:436–44. doi: 10.1097/MOG.0b013e32833d1799. [DOI] [PubMed] [Google Scholar]
- 12.Levy MJ. Endoscopic ultrasound-guided Trucut biopsy of the pancreas: prospects and problems. Pancreatology. 2007;7:163–6. doi: 10.1159/000104240. [DOI] [PubMed] [Google Scholar]
- 13.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Varadarajulu S, Desai S, et al. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg. 2007;11:813–9. doi: 10.1007/s11605-007-0151-x. [DOI] [PubMed] [Google Scholar]
- 15.Larghi A, Verna EC, Ricci R, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: a prospective study. Gastrointest Endosc. 2011;74:504–10. doi: 10.1016/j.gie.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Ginès A, Wiersema MJ, Clain JE, et al. Prospective study of a Trucut needle for performing EUS-guided biopsy with EUS-guided FNA rescue. Gastrointest Endosc. 2005;62:597–601. doi: 10.1016/j.gie.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 17.Thomas T, Kaye PV, Ragunath K, et al. Efficacy, safety and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584–91. doi: 10.1038/ajg.2008.97. [DOI] [PubMed] [Google Scholar]
- 18.Larghi A, Verna EC, Stavropoulos SN, et al. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185–90. doi: 10.1016/s0016-5107(03)02538-0. [DOI] [PubMed] [Google Scholar]
- 19.Levy MJ, Reddy RP, Wiersema MJ, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467–72. doi: 10.1016/s0016-5107(04)02802-0. [DOI] [PubMed] [Google Scholar]
- 20.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge Trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 21.Kung ITM, Chan S, Lo ESF. Application of the immunoperoxidase technique to cell block preparations from fine needle aspirates. Acta Cytol. 1990;34:297–303. [PubMed] [Google Scholar]
- 22.Mayall F, Chang B, Darlington A. A review of 50 consecutive cytology cell block preparations in a large general hospital. J Clin Pathol. 1997;50:985–90. doi: 10.1136/jcp.50.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopelman Y, Marmor S, Ashkenazi I, et al. Value of EUS-FNA cytological preparations compared with cell block sections in the diagnosis of pancreatic solid tumours. Cytopathology. 2011;22:174–8. doi: 10.1111/j.1365-2303.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- 24.Hennekens CH, Buring JE. Intervention studies. In: Mayrent SL, editor. Epidemiology in medicine. Lippincott Williams & Wilkins; London: 1987. pp. 178–212. [Google Scholar]