Abstract
The evolution in the knowledge of tuberculosis' physiopathology allowed not only a better understanding of the immunological factors involved in the disease process, but also the development of new laboratory tests, as well as the establishment of a histological classification that reflects the host's ability to contain the infectious agent. At the same time, the increasing bacilli resistance led to alterations in the basic tuberculosis treatment scheme in 2009. This article critically examines laboratory and histological investigations, treatment regimens for tuberculosis and possible adverse reactions to the most frequently used drugs.
Keywords: Erythema induratum; Latent tuberculosis; Mycobacterium tuberculosis; Tuberculosis; Tuberculosis, cutaneous; Tuberculosis, lymph node
INTRODUCTION
Finding bacilli in a cutaneous tuberculosis (CTB) lesion is a challenge. On average, all diagnostic methods have lower sensitivity and specificity rates for cutaneous presentations compared to the pulmonary form. Considering that atypical erythema nodosum and nonspecific appearance are not uncommon, and also that histopathology may not be elucidative, physicians must resort to every possible test, so that the sum of positive elements can create the basis for diagnosis, thus reducing empirical treatments or those based solely on the positivity of tuberculin test.
Therefore, when confronted with the hypothesis of CTB, beyond mandatory tuberculin skin test (TST) and chest radiograph for all patients, it is of paramount importance to collect material for histopathological examination and also separate samples for acid-fast bacilli (AFB) detection through culture and amplification of Mycobacterium tuberculosis (Mtb) DNA by polymerase chain reaction (PCR) both in the sample as well as in the blood.
Several laboratory methods have been developed to increase the accuracy of diagnosis in cases of latent tuberculosis or when there is doubt, based on the dose intensity of interferon production and improvement of the culture medium. In cases of pulmonary tuberculosis the Ministry of Health recommends offering HIV testing to all patients. Although for cutaneous tuberculosis no such recommendation exists, it is common sense that the aforesaid measure should also be adopted. The introduction of this routine will definitely increase the number of diagnoses.
LABORATORY DIAGNOSIS
A - Tuberculin skin test (TST) - The tuberculin test identifies individuals sensitized to Mtb. The test becomes positive in 2 to 10 weeks after the infection. This technique involves intradermal injection of tuberculin purified protein derivative materials (PPD) on the front surface of the left forearm, followed by reading after 48 to 72 hours when the size of induration is measured (Mantoux technique). In Brazil the PPD-RT23 protein is used. False negative reactions can occur in children under two months old, pregnant women, patients with diabetes, renal failure, or impaired cellular immunity. On the other hand recent vaccination, particularly in children over one year and co-infection with atypical mycobacteria are causes of false positive tests.1 The patient is considered as a strong reactor when the induration is greater than or equal to 5mm for patients with AIDS and above 10 mm for those without immune deficiency, for all disease presentations. So it is vital to evaluate the seropositivity for HIV1 and 2 in all patients.
For cutaneous tuberculosis, TST has sensitivity between 33% and 96% and specificity of 62.50% with a cutoff of 10 mm. In unvaccinated populations, the sensitivity is much higher, close to 97%.2,3
Analyzing each clinical presentation separately, positivity and intensity of the tuberculin skin test also vary. Tuberculous chancre, miliary tuberculosis and tuberculosis orificialis (the latter mainly by association with AIDS), commonly show negative results. Scrofuloderma and lupus vulgaris are the forms that, more often, present strongly reactive tests. Therefore, the other forms are those with more variable results for TST.4,5
The increase in the TST cutoff value did not resulted in increased diagnosis accuracy (chance to detect cases), because while there is an increment in sensitivity, there is also a decline in specificity. In other words, the higher the TST value, the greater chance the subject has tuberculosis, but the lower the utility in screening the population because many positive individuals will not be accounted for.
This value of 10 mm may not, however, be optimal for assessing cases of cutaneous tuberculosis, especially the paucibacillary ones. The higher accuracy of TST would be between 7 and 9 mm. It would be interesting that other studies were conducted to enlighten this point, thus making it possible to determine the best TST values specifically for CTB and maybe to identify values for different paucibacillary and multibacillary forms.2
B - Interferon-gamma release assays
These are serological tests that assess latent infection by measuring interferon-gamma produced by T-cells in individuals who were exposed to Mtb antigens, resulting in fewer errors especially in endemic areas and in populations vaccinated with BCG. However, previous infections by environmental mycobacteria such as M. marinum and M. kansasii may produce false positive results.
Currently there are two tests commercially available and approved by the FDA, QuantiFERON-TB Gold (QFT-G) and T-SPOT.TB. A systematic review of 38 studies that evaluated the sensitivity and specificity of the two tests for tuberculosis in general found an average sensitivity for QFT-G of 76% (Confidence Interval of 95% (CI95%: 72%-80%) and for T-SPOT.TB of 90% (CI95%: 86%-93%). Regarding specificity, there was an inversion, with QFT-G showing the highest rate 99% (CI 95%: 98%-100%) amongst unvaccinated patients and 96% (CI95%: 94%-98%) amongst the vaccinated ones, than T-SPOT.TB 93% (CI95%: 86%-100%) in both groups.6
These values are valid for pulmonary TB cases. A prospective study using T-SPOT.TB in 45 patients suspected of cutaneous tuberculosis found a sensitivity of 91.6% (CI95%: 64.6%-98.5%), a value similar to that found in cases of pulmonary tuberculosis, but with lower reproducibility (larger CI) and even lower specificity 75.8% (CI95%: 59%-87.2%), precisely because of the false positive results due to atypical cutaneous mycobacteria.7
Despite the higher sensitivity and specificity of the method compared to TST, it may have indeterminate results in elderly patients (over 65 years old), in about 30% of cases, while in other age groups this index is 3.1%. There is a higher proportion of false negatives in this age group compared to others, 58.3% versus 25%. Other groups with high rates of indeterminate results are those patients with a history of previous severe disease requiring ICU admission, malnourished or lymphopenic individuals especially when there is low CD4 counts, those with increased Creactive protein serum levels or decreased total protein levels. Below 35 years of age, when none of these situations is present, the error rate approaches zero.3,8
It is extremely important to emphasize that most studies were conducted in countries where tuberculosis is not endemic. That is, the effect of vaccination was considered, but not the environmental exposure to Mtb. Another crucial consideration is the possibility that an influence of the population studied in the results might occur, because of the difference in the genetic pool. Ideally, there should be national studies to confirm these values in our population that has a characteristic of racial miscegenation.
The advantage of this type of testing is not limited to issues of vaccination, other utilities are the screening for latent tuberculosis in patients who are undergoing anti-TNF alpha therapy, for those with kidney disease or submitted to transplant, to aid the diagnosis of difficult situations such as in children and contacts of patients with AIDS, and to clarify the etiology of erythema induratum and erythema nodosum secondary to TB.9-12
C - Investigation of acid-fast bacilli in stained smear: this technique presents faster results than culture. Ziehl-Neelsen acid staining is the most used stain for this purpose. The diagnostic yield of smears is higher for wet or exudative lesions, because they have a higher bacterial load, as is the case with CTB by primary inoculation, scrofuloderma, tuberculosis orificialis, or metastatic tuberculous abscess.1
D - Mycobacterial culture: it is the gold standard for determining the presence of active TB infection, and it can also distinguish mycobacteria subspecies and determine antibiotic susceptibility. Culture sensitivity is much lower than its specificity, with 80-85% and 98.5% respectively in the pulmonary forms of TB. In an exclusively cutaneous presentation, positivity is even lower, around 23% in traditional media. The introduction of radiometric culture media, increased the positivity to 75% for CTB, however, these media are not accessible or known to everyone. The dermatologist must remember to request specifically for mycobacteria culture.13
E - Polymerase chain reaction (PCR): It is mainly used as a complement to clinicopathological assessment. In this test, Mtb DNA present in a sample of fresh tissues, blood or a paraffin block is amplified and it can then be identified, confirming the presence of mycobacteria.14,15
There are different manners to amplify DNA as described below:
Conventional or in-house PCR: a manual system, standardized by each laboratory, in which it a sequence of DNA previously selected is amplified through the use of oligonucleotides (primers) that mark the beginning of that sequence and DNA polymerase that helps replicate that segment.15,16
Real-time PCR: it is an automated process, wherein the selected fragment is always the same and is associated with a system that captures the fluorescent light originating from the amplification. Therefore, immediately after amplification, the light emission is used to confirm the results. The advantages over traditional amplification are decrease in equipment contamination, loss of material and operatordependent errors. Nevertheless, in other situations there seems to be no difference in sensitivity and specificity between this technique and the conventional method.15,16,17
PCR with hybridization: after amplification DNA is selected by a marker. Then the sample is placed in an ELISA plate, where there is a probe that binds to an inner portion of the fragment. The reaction between the marker and the probe produces a color that can be identified and quantified by spectrophotometer. It increases the chances of detecting DNA and reduces false negatives.15,16
In situ PCR: it is a process in which hybridization occurs in the tissue, including in paraffin slides, thus demonstrating the location of mycobacterial DNA within the cell. It has a lower sensitivity than traditional PCR. Hybridization can be done in situ, subsequently, to increase the chance of positivity.15,16
F - Genotyping: this is the study of different techniques for molecular typing of Mtb strains; it helps in the sequencing of resistance genes to assess mutations and correlate the results of molecular epidemiology with data from classical epidemiology. These data have revolutionized the understanding of tuberculosis' epidemiology. With the amplified DNA, it is also possible to separate atypical mycobacteria from Mtb, genotype mycobacteria and detect mutations that induce resistance to antibiotics.17,18 The main molecular typing methods are:
Spoligotyping (Spacer Oligonucleotide Typing): Specific test to assess the presence of polymorphism of locus DR (Direct Repeat), found exclusively in the genome of Mtb complex mycobacteria. It uses a smaller amount of DNA, which increases the sensitivity of the method. It is considered the gold standard for genotyping and indicates the strain of mycobacteria.15,17
RFLP (Restriction Fragment Length Polymorphism): Uses the insertion sequence IS6110 in the genome of Mtb strains. It is used to study outbreaks, epidemics or to study population genetics. In this method, Mtb DNA is cleaved with restriction enzymes.
MIRU-VNTR (mycobacterial interspersed repetitive units-variable number of tandem repeats): standardized technique in which 12, 15 or 24 polymorphic loci of the MIRU-VNTR family are amplified. Then, electrophoresis is performed allowing the visualization and identification of these loci, thus classifying the family of the mycobacteria.
Most authors use in situ PCR as a complementary test to histological examination, especially for undefined cases. However, the results must be understood within the context of the clinical presentation. Tan et al. studied the larger number of cases (105), which permitted even their separation by type of clinical lesions. PCR was 100% sensitive and specific in immunocompromised patients with multibacillary forms. In paucibacillary forms, there was positivity of 55% in cases of tuberculosis verrucosa and 60% in cases of lupus vulgaris. For cases of erythema induratum positivity was 54%. Considering all cases, the mean sensitivity was 73%.19 In contrast with these results, Senturk et al. observed only one case with positive result amongst 22 confirmed cases of CTB. The study was a retrospective analysis of archived blocks, possibly demonstrating a loss of sensitivity with time.20 A similar result was obtained by Hsiao et al. while evaluating only paucibacillary cases, the authors found 18 positive results in 34 cases, resulting in a sensitivity rate of 56.2%.21
More recently, Abdalla et al. in a prospective study that compared PCR with other diagnostic methods, attributed a higher sensitivity and specificity rates to it than to other methods (sensitivity 88% and specificity 83%), however, the author concluded that clinical presentation is still the main key in the process of CTB diagnosis.22
HISTOPATHOLOGY
All CTB clinical presentations have a similar histological basis, composed of lymphocytes, epithelioid histiocytes and giant cells; the histological differences observed for each clinical presentation result from the variation in the host's ability to organize the granulomatous process (Figures 1 and 2). In a didactical manner, we can divided CTB's histology in three groups in order to emphasize the concept that the intensity of the host's immune response is responsible for the clinical and pathological presentation of the disease: well-formed granulomas with absence of caseous necrosis, granulomas with caseous necrosis and the presence of poorly formed granulomas with intense caseous necrosis.1,23-25
FIGURE 1.
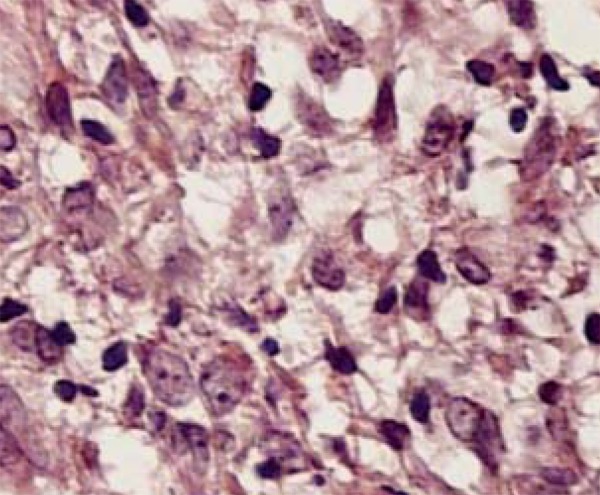
Epithelioid histiocytes and lymphocytes
FIGURE 2.
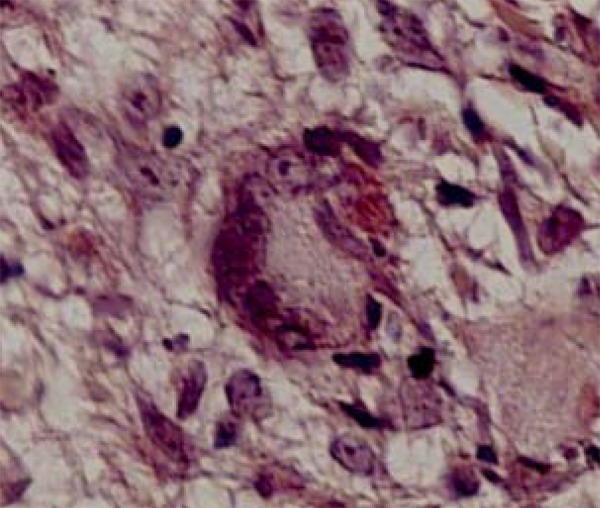
Langhans giant cells are frequentlyfound in the inflammatory infiltrate
A - Well-formed granulomas with absence of caseous necrosis
Lupus vulgaris: the epidermis may be atrophic or hypertrophic, featuring acanthosis, papillomatosis and even pseudoepitheliomatous hyperplasia. Presence of well-formed tuberculous granulomas accompanied more often by Langhans giant cells, or foreign body-like granulomas in the reticular dermis. There may be sarcoidosis-like granulomas (Figure 2). The lymphocytic infiltrate is dense and caseous necrosis is rarely present, but it will occur in small foci centrally to the granuloma. The observation of AFB is infrequent (Figure 3).
FIGURE 3.
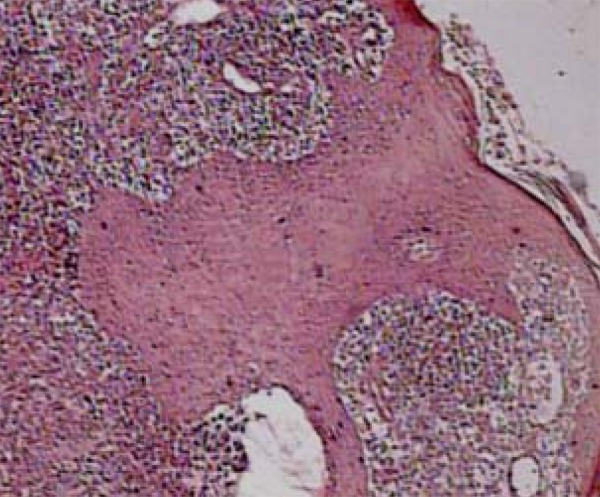
Epidermic hyperplasia accompanied by granulom atous inflammatory process with necrosis foci, without caseous, in lupus vulgaris
Lichen scrofulosorum: presence of epithelioid granulomas surrounded by lymphocytes and located more superficially in the dermis adjacent to the adnexa. Normally giant cells are absent. Caseous necrosis and acid-fast bacilli are not found (Figure 4).
FIGURE 4.
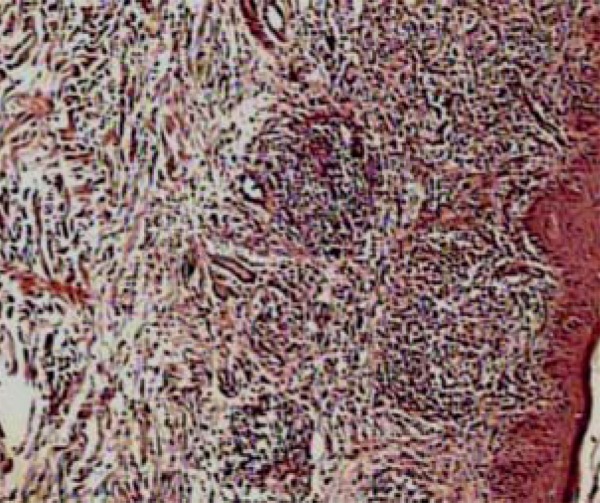
Discrete rectification of the epidermis accompanied by well-formed lymphoh istiocytic granulomas with a superficial location in lichen scrofulosorum
B - Intermediate forms: granulomas with caseous necrosis
Tuberculosis verrucosa cutis: presence of prominent epidermal changes such as hyperkeratosis, acanthosis and papillomatosis. At the same time, tuberculous granulomas with caseous necrosis of moderate intensity are seen in the dermis and bacilli can be found.
Primary cutaneous tuberculosis: it varies according to the time of inoculation; in recent lesions there is the presence of necrotizing neutrophilic infiltrate with numerous AFB. At a later stage there is organization of granulomas and decreased numbers of bacilli.
Acute miliary tuberculosis: there is a nonspecific inflammatory infiltrate, rich in lymphocytes and plasma cells; caseous necrosis, if present, is of a focal nature, and occasionally micro-abscesses are observed. Presence of bacilli varies directly with the severity of the condition.
Tuberculosis orificialis: there are tuberculoid granulomas, around a median, central, superficial ulcer accompanied by caseous necrosis in the deep dermis.
Papulonecrotic tuberculid: presence of an area of necrosis into the dermis, accompanied with granulomatous infiltrate, leukocytoclastic vasculitis, perivascular edema or follicular necrosis with suppuration.
C - Poorly formed granulomas with intense caseous necrosis
Scrofuloderma: Massive central necrosis with abscess formation and in many cases, suppuration (Figure 3). At the periphery of the lesion traces of granulomas can be observed. When present Mycobacterium tuberculosis can also be found in this location (Figure 5).
FIGURE 5.
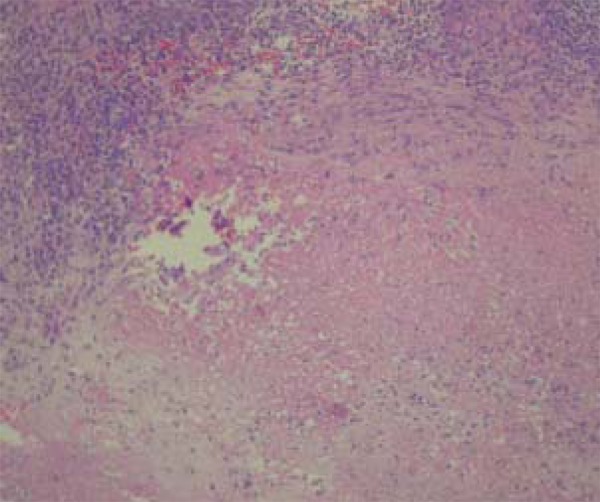
Caseous necrosis surrounded by granulomatous process in scrofuloderma
Metastatic abscesses and gumma: Central ulceration with abundant caseous necrosis, surrounded by a rim of giant cells and macrophages. AFBs are frequently detected.
Histopathological examination is essential to complement the investigation of CTB cases, however, in most samples, the bacillus cannot be observed even when special staining techniques are performed. In case series studies, the presence of AFB in samples is very low, ranging from 0 to 5% of the total samples.26-30 A single work performed in Nepal found a different percentage of observation of bacilli, 35 observations on 50 specimens, an unusual fact.29
The most common histological finding in CTB is the tuberculoid granuloma in the dermis, observed in 57% to 96% of the samples.27,29 Nevertheless, it should be remembered that other types of granuloma can also be found in CTB, such as annular-like granuloma formations, sarcoid granuloma and suppurative granuloma.30 Therefore when there is other evidence for CTB and also the presence of one of these histological elements, the diagnosis of cutaneous tuberculosis cannot be discarded.
The other two most important findings, in decreasing order of frequency, are the epidermal hyperplasia and caseous necrosis, with observation in histological examinations varying from 57% to 86% and from 11,8% to 57%.24,26,27 The first is more common in verrucous forms, and the second is observed in inflammatory forms.
Besides the absence of the bacillus, the difficulty in confirming a CTB diagnosis based on histology is also due to the fact that, characteristics considered suggestive or typical of the disease are not always found. Umaphaty et al. observed that 14% of samples from confirmed cases presented no aspect suggestive of CTB, making it impossible to predict the diagnosis solely from histology.26 This occurs because of the histological similarity between CTB, and other infectious granulomatous diseases, sarcoidosis, and granulomatous rosacea. Studies comparing the histological elements have attempted to bring some light to this issue by trying to find histological elements that can serve as a guide for difficult cases. However there are few articles on this topic, with a small number of patients and comparing tuberculosis with only one other infectious disease, which restricts the use of the data found (Chart 1).
CHART 1.
Comparative histological studies on cutaneous tuberculosis
| Author /year | Type of study | Conclusions |
|---|---|---|
| Nimala et al India, 1977 | 20 cases of CTB versus | In CTB > frequency of epidermic hyperplasia, small areas of ulceration, significant fibrosis and occasional caseous necrosis |
| 20 cases of tuberculoid leprosy | ||
| Paksoy and Hekim Turkey, 1993 | 16 cases of lupusvulgaris versus 12 cases of cutaneous leishmaniasis | Tuberculoid granulomas and giant cells are more frequent in CTB |
| Min et al South Korea, 2012 | 15 cases of CTB versus | Giant cells, tuberculoid granulomas, and caseous necrosis were significantly associated to CTB (p< 0.05). |
| 10 cases of atypical mycobacteriosis |
Due to the difficulty in finding the etiologic agent in question, the use of other complementary tests, clinical follow-up and often implementing therapeutic tests are needed to confirm many cases. A simple way to increase the sensitivity of histopathology is to add the tuberculin skin test (TST) and / or serum PCR in the fragment or in the biopsy slide. Hence, compared to clinical suspicion, a histopathological examination with suggestive aspects, plus a strong positive TST reaction is highly suggestive of CTB.
TREATMENT
Tuberculosis is curable in virtually 100% of new cases, as long as the basic principles of drug therapy and the proper management of the treatment are observed. The appropriate combination therapy, the correct doses, and the use of medications for long enough, are the basic principles to prevent bacterial persistence and the development of drug resistance, thus ensuring the patient's cure.
The recommended treatment since 1979, known as RIP (rifampicin, isoniazid, pyrazinamide) was modified in 2009 due to the preliminary results of the Second National Survey on Anti-TB drugs Resistance, in which an increase in primary isoniazid resistance, from 4.4 to 6.0%, was found. This fact led to the addition of ethambutol (E) as a fourth drug in the intensive phase of treatment (first two months). Thus, the basic treatment regimen for tuberculosis now has, mandatorily, four drugs (RIPE).
The treatment of all extrapulmonary forms (except meningoencephalitis) lasts six months, as well as the treatment of patients co-infected with HIV, regardless of the stage of evolution of viral infection. Meningoencephalitic forms are treated for nine months. Medications should be administered preferably on an empty stomach (1 hour before or two hours after breakfast) in a single intake or in case of digestive intolerance, with a meal.
A - Basic Treatment Scheme for Adults and Teenagers
Recommended for all new cases in adults and adolescents (>10 years), all forms of pulmonary and extrapulmonary tuberculosis (except meningoencephalitis), as well as all cases of relapse and return after treatment dropout (Chart 2).
CHART 2.
Treatment for TB - adult and teenagers CTB
| Regimen | Drugs | Weight Range | Unit/dose | Months |
|---|---|---|---|---|
| Intensive phase | RHZE | 20 to 35 Kg | 2 tablets | 2 Months |
| 150/75/400/275mg | 36 to 50 Kg | 3 tablets | ||
| Tablet with combined fixed-dose | >50 Kg | 4 tablets | ||
| Maintenance Phase | RH | 20 to 35 Kg | 1 capsule or tablet 300/200mg | 4 Months |
| 300/200mg or 150/100mg capsule or tablet | 36 to 50 Kg | 1 capsule or tablet 300/200mg + 1 capsule or tablet 150/100mg | ||
| >50 Kg | 2 capsules or tablets 300/200mg |
R: rifampicin; H: isoniazid; Z: pyrazinamide; E: ethambutol
* In the first months of implementation of the new scheme, the maintenance phase will continue with capsules.
B - Basic Treatment scheme for Children (2RHZ/4RH) (Chart 3)
CHART 3.
Treatment for TB - children (under 10 years-old)
| Regimen | Drugs | Patient's weight | |||
|---|---|---|---|---|---|
| Up to 20kg > mg/Kg/day | >20 to 35kg mg/Kg/ day | >35 to 45kg mg/Kg/ day | >45kg mg/Kg/ day | ||
| 2RHZ | R | 10 | 300 | 450 | 600 |
| Attack Phase | H | 10 | 200 | 300 | 400 |
| Z | 35 | 1000 | 1500 | 2000 | |
| 4RH | R | 10 | 300 | 450 | 600 |
| Maintenance Phase | H | 10 | 200 | 300 | 400 |
R: : rifampicin; H: isoniazid; Z: pyrazinamide
C - Treatment for special situations
•Pregnancy
Extrapulmonary tuberculosis does not seem to be more frequent in pregnant women. With respect to therapeutic regimens, the use of ethambutol and streptomycin are contraindicated due to their teratogenic effects. There are not, to date, evidence of teratogenic effects with the use of rifampin, isoniazid, pyrazinamide and ethambutol, allowing treatment normally during pregnancy. In pregnant women in treatment with isoniazid, pyridoxine (25mg/day) is used as a prophylactic agent against peripheral neuritis, and seizures in newborns. Breastfeeding should be encouraged due to the low concentration of these drugs in breast milk.
• Elder patients
Treatment is the same, adjusting the dose according to body weight, and knowing that changes in metabolism, morphology and homeostasis, besides concomitant use of numerous medications, favor the appearance of adverse events in the elderly.
• Renal Insufficiency
The interval or drug dosing should be adjusted in patients with creatinine clearance <30ml/min. If the creatinine clearance is> 30ml/min, the standard dose should be used, with monitoring of drug serum levels whenever possible.
• Hepatic Insufficiency
The treatment of patients with liver disease requires special considerations since rifampicin, isoniazid and pyrazinamide have great hepatotoxicity potential, increasing the likelihood of liver damage. Monitoring of drug-induced hepatitis is also hampered by higher fluctuating levels of liver enzymes secondary to the underlying condition.30 Isoniazid has a longer half-life and consequently higher serum levels in patients with liver disease and also in those over 50 years old. Ethambutol is the only drug that can be fully utilized by patients with liver insufficiency.31
Patients with transaminase values up to 3 times the normal can be treated with scheme I, but laboratory exams should be monitored monthly. If transaminase values are above that, scheme I can be replaced, usually suspending pyrazinamide, always keeping ethambutol, adding streptomycin and another drug which could be isoniazid, rifampin or ofloxacin.32
ADVERSE EVENTS
Adverse events can be divided into two major groups: (1) minor adverse events (5-20% of cases), that do not necessarily require anti-TB drug suspension, (2) major adverse events (3-8 % of cases), which typically cause treatment discontinuation. Risk factors related with the major adverse events are: age (> 30 years), alcoholism, malnutrition, previous liver disease, and AIDS.
The charts below present in a summarized form minor and major events, the drugs from the basic scheme that are most likely associated to them and the recommended conduct (Charts 4 and 5).
CHART 4.
Minor adverse events in anti-tuberculosis treatment
| Adverse events | Probable causal drug(s) | Conduct |
|---|---|---|
| Nausea, vomit, abdominal pain | Rifampicin | Reschedule time of drug administration (2h after breakfast or with breakfast); consider using symptomatic medication; assess liver function |
| Isoniazid | ||
| Pyrazinamide | ||
| Ethambutol | ||
| Sweating/red urine | Rifampicin | Advise the patient |
| Pruritus or light exanthema | Isoniazid | Prescribe antihistaminic drugs |
| Rifampicin | ||
| Articular pain | Pyrazinamide | Prescribe analgesics and Non-steroidal anti-inflammatory drugs (NSAID) |
| Isoniazid | ||
| Peripheral neuropathy | Isoniazid (common) | Prescribe pyridoxine (Vitamin B6) 50 mg/day |
| Ethambutol (uncommon) | ||
| Asymptomatic hyperuricemia | Pyrazinamide | Prescribe diet low in purine |
| Hyperuricemia with arthralgia | Pyrazinamide | Prescribe diet low in purine and medicate with allopurinol and colchicine, if necessary |
| Ethambutol | ||
| Headache, Anxiety, euphoria, insomnia | Isoniazid | Advise the patient |
| Fever | Rifampicin | Advise the patient |
| Isoniazid |
CHART 5.
Minor adverse events in anti-tuberculosis treatment
| Adverse events | Probable causal drug(s) | Conduct |
|---|---|---|
| Exanthema or moderate to sever hypersensibility | Rifampicin | Suspend treatment; reintroduce each drug separately after resolution; in severe cases or relapses replace scheme by others without the causal drug |
| Isoniazid | ||
| Pyrazinamide | ||
| Ethambutol | ||
| Streptomycin | ||
| Psychosis, seizures, toxic encephalopathy or coma | Isoniazid | Suspend Isoniazid and restart special scheme without it |
| Optical neuritis | Ethambutol | Suspend drug and restart special scheme without it. This reaction is dose-dependent, and when detected early it is reversible. It is rare to develop ocular toxicity in the first two months with the recommended doses. |
| Isoniazid | ||
| Hepatotoxicity | Pyrazinamide | Suspend treatment; wait for symptom resolution and liver enzyme levels to decrease; reintroduce drugs separately after assessing hepatic function. |
| Isoniazid | ||
| Rifampicin | ||
| Hypoacusis Vertigo, nystagmus | Streptomycin | Suspend Streptomycin and restart special scheme without it |
| Thrombocytopenia, leukopenia, eosinophilia, hemolytic anemia, agranulocytosis, vasculitis | Rifampicin | Suspend treatment and restart special scheme without the causal medication |
| Isoniazid | ||
| Interstitial nephritis | Rifampicin | Suspend treatment and restart special scheme without the causal medication |
| Pyrazinamide | ||
| Rhabdomyolysis with myoglobinuria and kidney failure | Pyrazinamide | Suspend Pyrazinamide and restart special scheme without it |
Hepatitis: the drugs used to treat tuberculosis have interactions among themselves and with other drugs, which increases the risk of hepatotoxicity. In the first two months of treatment, an asymptomatic elevation of hepatic enzymes serum levels can be observed in a small percentage of patients, followed by spontaneous normalization without any clinical manifestation and without the need to interrupt or change the therapeutic regimen.
Treatment should be stopped only if hepatic enzyme levels reach three times the normal value, with onset of symptoms, or as soon as jaundice appears. The patient should then be directed to a secondary unit of reference for clinical and laboratory evaluation, besides having an adjustment in treatment, if necessary. Assuming that, after cessation of treatment, there is a reduction in serum levels of liver enzymes and resolution of symptoms, the reintroduction of the Basic Scheme is indicated, as follows: Rifampicin + Ethambutol, followed by isoniazid and lastly, pyrazinamide, with interval of three to seven days between them.
Reintroduction of each drug should be preceded by an analysis of liver function. The length of treatment will be considered from the date on which it was possible to resume the full treatment regimen. If the levels of liver enzymes do not decrease to less than three times the normal upper limit in four weeks, or if it is a severe case of tuberculosis, the alternative scheme, as described in the text on liver disease, should be started.
QUESTIONS
1. Regarding the diagnosis of cutaneous tuberculosis, it can be stated that:
a) Histopathology is elucidative
b) Diagnostic methods have high sensitivity and specificity
c) Tuberculin skin test and chest X-ray examinations are mandatory
d) The diagnosis is based only on the positive tuberculin test
2. In which type of cutaneous tuberculosis cited below is the tuberculin test most often negative:
a) Orificial tuberculosis
b) Tuberculosis verrucosa
c) Lupus vulgaris
d) Scrofuloderma
3. It is true about AFB culture:
a) It has lost its importance with the emergence of new diagnostic methods
b) Radioimmunometric media have a higher percentage of positive results than the traditional medium
c) Traditional culture is carried out in the same manner as that of other bacteria
d) The result is not influenced by clinical form
4. PCR has gained importance in complementing the diagnosis of CTB. Select the correct answer:
a) All types of PCR have the same sensitivity
b) This test is the gold standard to diagnose paucibacillary forms
c) Spoligotyping is the gold standard for Mtb genotyping
d) In situ exam has superior results compared to the conventional technique
5. Regarding direct bacilloscopy, in which of the cutaneous tuberculoses described below is its positivity greater:
a) Tuberculous chancre
b) Tuberculosis verrucosa
c) Tuberculids
d) Metastatic tuberculous abscess
6. Mark the false alternative regarding cutaneous tuberculosis laboratory diagnosis:
a) Tuberculin test only identifies individuals who have been sensitized to TB and does not confirm active TB infection
b) Culture from a lesion tissue fragment is the gold standard for the diagnosis of cutaneous tuberculosis
c) Direct exam has a higher diagnostic yield in wet or exudative lesions that have a high bacterial load, such as primary TB inoculation, scrofuloderma, orificial tuberculosis, tuberculous or metastatic abscesses
d) Interferon-gamma release assays are serological tests performed to diagnose active infection by measuring the interferon-gamma secreted from peripheral blood polymorphonuclear cells after Mtb antigen exposure
7. The technique based on DNA polymorphism in M. tuberculosis direct repetition (DR) locus is:
a) RFLP
b) Spoligotyping
c) MIRU-VNTR
d) PCR with hybridization
8. The Mtb genotyping method that uses repetition genetic loci is:
a) Real-time PCR
b) Spoligotyping
c) RFLP
d) MIRU-VNTR
9. The genotyping method in which there is DNA cleavage with restriction enzymes is:
a) Real-time PCR
b) Spoligotyping
c) RFLP
d) MIRU-VNTR
10. An area of necrosis, accompanied by granulomatous infiltrate, leukocytoclastic vasculitis, perivascular edema and/or follicular necrosis with suppuration, is generally seen on:
a) Scrofuloderma
b) Papulonecrotic tuberculid
c) Lupus vulgaris
d) Orificial tuberculosis
11. Forty-five year old male patient presents plaques consisting of asymptomatic, indurated, grouped, follicular papules, measuring 1-5 mm, with a red-brownish color, located on the torso. Anatomopathological exam showed epithelioid granulomas, surrounded by lymphocytes and located more superficially in the dermis, adjacent to the adnexa. This is probably a case of:
a) Scrofuloderma
b) Tuberculosis verrucosa
c) Lichen scrofulosorum
d) Lupus vulgaris
12. Histopathological exam is of great importance in the diagnosis of cutaneous tuberculosis. In this context, the following statement is false:
a) In all samples one can find the typical tuberculoid granuloma, caseous necrosis and lymphocytes in varying proportions.
b) Histopathological exam reflects the host's immunological response to Mtb
c) It is possible to not distinguish between cases of cutaneous tuberculosis and other granulomatous diseases.
d) Confronted with a highly suggestive exam, the clinician must continue the investigation, with other complementary exams to increase diagnostic accuracy.
13. Besides the traditional tuberculoid granuloma, the following are granulomatous patterns that may be present in CTB, except:
a) Annular granuloma
b) Sarcoid granuloma
c) Elastolytic granuloma
d) Suppurative granuloma
14. Which is the therapeutic scheme recommended to treat cutaneous tuberculosis since 2009:
a) Rifampicin, isoniazid, pyrazinamide in the first two months; rifampicin and isoniazid for 4 more months
b) Rifampicin, isoniazid, pyrazinamide and ethambutol in the first 4 months; rifampicin and isoniazid for 2 more months
c) Rifampicin, isoniazid, pyrazinamide and ethambutol in the first two months; Rifampicin and isoniazid for 4 more months
d) Rifampicin, isoniazid, pyrazinamide and ethambutol in the first two months; Rifampicin and isoniazid for 4 more months for children and adults, regardless of age and weight
15. Which minor adverse events are caused by cutaneous tuberculosis therapeutic scheme
a) Hypoacusis, exanthema, abdominal pain
b) Exanthema, peripheral neuropathy and abdominal pain
c) Psychosis, thrombocytopenia, hepatotoxicity
d) Rhabdomyolysis, fever, seizures
16. Which medications listed below should be avoided during pregnancy:
a) Streptomycin and ethionamide
b) Ethambutol and ethionamide
c) Ethambutol and Streptomycin
d) Ofloxacin and isoniazid
17. Which of the drugs below is the least hepatotoxic:
a) Ethambutol
b) Rifampicin
c) Isoniazid
d) Pyrazinamide
18. Mark the correct alternative regarding the link between drug and adverse event:
a) Isoniazid and psychosis
b) Pyrazinamide and peripheral neuritis
c) Ethambutol and hypoacusis
d) Rifampicin and optical neuritis
19. Choose the correct alternative on hepatotoxicity caused by the tuberculosis treatment regimen:
a) Treatment for cutaneous tuberculosis should be stopped only when transaminases levels double their values or when clinical jaundice is present
b) When changes in transaminases are detected, discontinue treatment immediately and replace the scheme by less hepatotoxic drugs
c) When changes in transaminases are detected, discontinue treatment immediately and return to the scheme without ethambutol, as soon as liver enzyme levels return to normal
d) When changes in transaminases are detected, discontinue treatment immediately and reintroduce the basic scheme, as follows: Rifampicin + Ethambutol, followed by isoniazid and lastly, pyrazinamide, with an interval of three to seven days in between, as soon as the transaminases return to normal
20. Hypoacusis, nystagmus and vertigo are symptoms observed in the treatment of tuberculosis with:
a) Rifampicin
b) Isoniazid
c) Streptomycin
d) Ethambutol
Answer key.
Leprosy: review of the epidemiological, etiopathogenic, and clinical aspects - Part 2. An Bras Dermatol. 2014;89(3):389-403.
| 1) B | 6) B | 11) B | 16) C |
| 2) D | 7) C | 12) A | 17) D |
| 3) A | 8) D | 13) D | 18) D |
| 4) C | 9) B | 14) A | 19) D |
| 5) D | 10) D | 15) C | 20) B |
Papers
Information for all members: The EMC-D questionnaire is now available at the homepage of the Brazilian Annals of Dermatology: www.anaisdedermatologia.org.br. The deadline for completing the questionnaire is 30 days from the date of online publication.
Footnotes
Conflict of Interest: None.
Financial Support: None.
Work performed at the Clínica Dermatológica do Hospital das Clínicas da Universidade Federal de Pernambuco (HC/UFPE) - Recife (PE), Brazil.
How to cite this article: Santos JB, Figueiredo AR, Ferraz CE, Oliveira MH, Silva PG, Medeiros VLS. Cutaneous tuberculosis: diagnosis, histopathology and treatment - Part II. An Bras Dermatol. 2014;89(4):545-55.
REFERENCES
- 1.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância Epidemiológica . Manual de Recomendações para o Controle da Tuberculose no Brasil. Ministério da Saúde; Brasília: 2011. 284 p. (Série A. Normas e Manuais Técnicos - Series A. Standards and Technical Manuals). [Google Scholar]
- 2.Ramam M, Malhotra A, Tejasvi T, Manchanda Y, Sharma S, Mittal R, et al. How useful is the Mantoux test in the diagnosis of doubtful cases of cutaneous tuberculosis? Int J Dermatol. 2011;50:1379–1382. doi: 10.1111/j.1365-4632.2011.04971.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbagallo J, Tager P, Ingleton R, Hirsch RJ, Weinberg JM. Cutaneous tuberculosis: diagnosis and treatment. Am J Clin Dermatol. 2002;3:319–328. doi: 10.2165/00128071-200203050-00004. [DOI] [PubMed] [Google Scholar]
- 4.Marcoval J, Servitje O, Moreno A, Jucglà A, Peyrí J. Lupus vulgaris. Clinical, histopathologic, and bacteriologic study of 10 cases. J Am Acad Dermatol. 1992;26:404–407. [PubMed] [Google Scholar]
- 5.Almaguer-Cháveza J, Ocampo-Candiania, A. Rendónb. Panorama actual en el diagnóstico de la tuberculosis cutânea. Actas Dermosifiliogr. 2009;100:562–570. [PubMed] [Google Scholar]
- 6.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai CC, Tan CK, Lin SH, Liu WL, Liao CH, Huang YT, et al. Diagnostic value of an enzyme-linked immunospot assay for interferon-γ in cutaneous tuberculosis. Diagn Microbiol Infect Dis. 2011;70:60–64. doi: 10.1016/j.diagmicrobio.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Cho K, Cho E, Kwon S, Im S, Sohn I, Song S, et al. Factors Associated with Indeterminate and False Negative Results of QuantiFERON-TB Gold In-Tube Test in Active Tuberculosis. Tuberc Respir Dis (Seoul) 2012;72:416–425. doi: 10.4046/trd.2012.72.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laffitte E, Janssens JP, Roux-Lombard P, Thielen AM, Barde C, Marazza G, et al. Tuberculosis screening in patients with psoriasis before antitumour necrosis factor therapy: comparison of an interferon-gamma release assay vs. tuberculin skin test. Br J Dermatol. 2009;161:797–800. doi: 10.1111/j.1365-2133.2009.09331.x. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Velez CM. Pediatric tuberculosis: new guidelines and recommendations. Curr Opin Pediatr. 2012;24:319–328. doi: 10.1097/MOP.0b013e32835357c3. [DOI] [PubMed] [Google Scholar]
- 11.Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002;16:2285–2293. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 12.Vera-Kellet C, Peters L, Elwood K, Dutz JP. Usefulness of Interferon-release assays in the diagnosis of erythema induratum. Arch Dermatol. 2011;147:949–952. doi: 10.1001/archdermatol.2011.183. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal P, Singal A, Bhattacharya SN, Mishra K. Comparison of the radiometric BACTEC 460 TB culture system and Löwenstein-Jensen medium for the isolation of mycobacteria in cutaneous tuberculosis and their drug susceptibility pattern. Int J Dermatol. 2008;47:681–687. doi: 10.1111/j.1365-4632.2008.03675.x. [DOI] [PubMed] [Google Scholar]
- 14.Cortez MV, Oliveira CM, Monte RL, Araújo JR, Braga BB, Reis DZ, et al. HIV-associated tuberculous lymphadenitis: the importance of polymerase chain reaction (PCR) as a complementary tool for the diagnosis of tuberculosis - a study of 104 patients. An Bras Dermatol. 2011;86:925–931. doi: 10.1590/s0365-05962011000500010. [DOI] [PubMed] [Google Scholar]
- 15.Freitas FAD, Siqueira HR, Albano RM. Métodos moleculares no diagnóstico da tuberculose e resistência do Mycobacterium tuberculosis. Pulmão. 2009;18:96–101. [Google Scholar]
- 16.Barrios-Payán J, Saqui-Salces M, Jeyanathan M, Alcántara-Vazquez A, Castañon-Arreola M, Rook G, et al. Extrapulmonary locations of Mycobacterium tuberculosis DNA during latent infection. J Infect Dis. 2012;206:1194–1205. doi: 10.1093/infdis/jis381. [DOI] [PubMed] [Google Scholar]
- 17.Tortoli E, Urbano P, Marcelli F, Simonetti TM, Cirillo DM. Is real-time PCR better than conventional PCR for Mycobacterium tuberculosis complex detection in clinical samples? J Clin Microbiol. 2012;50:2810–2813. doi: 10.1128/JCM.01412-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves MG, Fukasawa LO, Oliveira RS, Salgado MM, Harrison LH, Shutt KA, et al. Fast test for assessing the susceptibility of Mycobacterium tuberculosis to isoniazid and rifampin by real-time PCR. Mem Inst Oswaldo Cruz. 2012;107:903–908. doi: 10.1590/s0074-02762012000700011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan SH, Tan HH, Sun YJ, Goh CL. Clinical utility of polymerase chain reaction in the detection of Mycobacterium tuberculosis in different types of cutaneous tuberculosis and tuberculids. Ann Acad Med Singapore. 2001;30:3–10. [PubMed] [Google Scholar]
- 20.Senturk N, Sahin S, Kocagoz T. Polymerase chain reaction in cutaneous tuberculosis: is it a reliable diagnostic method in paraffin-embedded tissues? Int J Dermatol. 2002;41:863–866. doi: 10.1046/j.1365-4362.2002.01604.x. [DOI] [PubMed] [Google Scholar]
- 21.Hsiao PF, Tzen CY, Chen HC, Su HY. Polymerase chain reaction based detection of Mycobacterium tuberculosis in tissues showing granulomatous inflammation without demonstrable acid-fast bacilli. Int J Dermatol. 2003;42:281–286. doi: 10.1046/j.1365-4362.2003.01461.x. [DOI] [PubMed] [Google Scholar]
- 22.Abdalla CM, de Oliveira ZN, Sotto MN, Leite KR, Canavez FC, de Carvalho CM. Polymerase chain reaction compared to other laboratory findings and to clinical evaluation in the diagnosis of cutaneous tuberculosis and atypical mycobacteria skin infection. Int J Dermatol. 2009;48:27–35. doi: 10.1111/j.1365-4632.2009.03807.x. [DOI] [PubMed] [Google Scholar]
- 23.Elder DE, Elenitsas R. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott; 2009. [Google Scholar]
- 24.Singal A, Bhattacharya SN. Tropical medicine rounds Lichen scrofulosorum: A prospective study of 39 patients. Int J Dermatol. 2005;44:489–493. doi: 10.1111/j.1365-4632.2005.02499.x. [DOI] [PubMed] [Google Scholar]
- 25.Jordaan HF, Van Niekerk DJ, Louw M. Papulonecrotic tuberculid. A clinical, histopathological, and immunohistochemical study of 15 patients. Am J Dermatopathol. 1994;16:474–485. [PubMed] [Google Scholar]
- 26.Umapathy KC, Begum R, Ravichandran G, Rahman F, Paramasivan CN, Ramanathan VD. Comprehensive findings on clinical, bacteriological, histopathological and therapeutic aspects of cutaneous tuberculosis. Trop Med Int Health. 2006;11:1521–1528. doi: 10.1111/j.1365-3156.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 27.Thakur BK, Verma S, Hazarika D. A clinicopathological study of cutaneous tuberculosis at Dibrugarh district, Assam. Indian J Dermatol. 2012;57:63–65. doi: 10.4103/0019-5154.92685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri N. A clinical and histopathological profiles of patients with cutaneous tuberculosis. Indian J Dermatol. 2011;56:550–552. doi: 10.4103/0019-5154.87153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dwari BC, Ghosh A, Paudel R, Kishore P. A clinicoepidemiological study of 50 cases of cutaneous tuberculosis in a tertiary care teaching hospital in Pokhara, Nepal. Indian J Dermatol. 2010;55:233–237. doi: 10.4103/0019-5154.70670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min KW, Ko JY, Park CK. Histopathological spectrum of cutaneous tuberculosis and non-tuberculous mycobacterial infections. J Cutan Pathol. 2012;39:582–595. doi: 10.1111/j.1600-0560.2012.01903.x. [DOI] [PubMed] [Google Scholar]
- 31.Arbex MA, Varella Mde C, Siqueira HR, Mello FA. Antituberculosis drugs: drug interactions, adverse effects, and use in special situations. Part 1: first-line drugs. J Bras Pneumol. 2010;36:626–640. doi: 10.1590/s1806-37132010000500016. [DOI] [PubMed] [Google Scholar]
- 32.Leitão CCSL, Campelo ARL, Dantas AT. Tuberculose. In: Filgueira NA, Costa JI Jr, Lucena VG, Leitão CCS, Kitner D, Mendes JM, et al., editors. Condutas em clínica médica. 4 ed. Rio de Janeiro: Guanabara Koogan; 2007. pp. 568–582. [Google Scholar]


