Abstract
Psoriasis is a chronic inflammatory, immune-mediated disease that affects 1% to 2% of the world's population. Immunobiological medications are prescribed for certain patients with severe forms of psoriasis, however, these drugs increase the risk of reactivation of viral diseases such as hepatitis B. We report the case of a patient with severe psoriasis with positive serology for the Hepatitis B virus, who received ustekinumab (a human monoclonal antibody against interleukin 12 and 23). In this patient, the use of ustekinumab did not reactivate the Hepatitis B virus. Given the high prevalence of chronic viral infections in patients who are candidates for biologic therapy, as well as the potential for reactivate chronic viral illness, randomized controlled studies are needed to assess the risks and benefits of such therapy in these populations.
Keywords: Hepatitis B, Hepatitis B virus, Psoriasis
INTRODUCTION
Psoriasis is a chronic inflammatory, immunemediated, systemic disease that afflicts 1% to 2% of the world's population.1,2
Treatments with biologics have been shown to improve the lives of many patients with psoriasis.3 Issues concerning the potential risks of reactivating the hepatitis B virus (HBV) arise when the use of biologic agents is imperative in patients with concurrent psoriasis and HBV infection.4 The reactivation of HBV during immunosuppressive therapy has even been reported in HBsAg negative patients, who are anti-HBc positive with or without anti-HBs antibodies.5
CASE REPORT
We report the case of a 53-year-old Brazilian black man who presented with a 27-year history of recalcitrant severe psoriasis. Over the last 14 years, he had been treated at a specialized dermatology department, where he received standard topical and systemic treatment for psoriasis. PUVA, methotrexate, cyclosporine and acitretin had to be discontinued because of inefficacy, intolerance or toxicity. He had a history of pulmonary tuberculosis, which was duly treated 29 years ago. With this background, a PASI score of 61.2, BSA 81%, and a poor quality of life, treatment with ustekinumab was indicated, a fully human monoclonal antibody directed against interleukin 12 (IL-12) and interleukin 23 (IL-23) (Figure 1).
FIGURE 1.
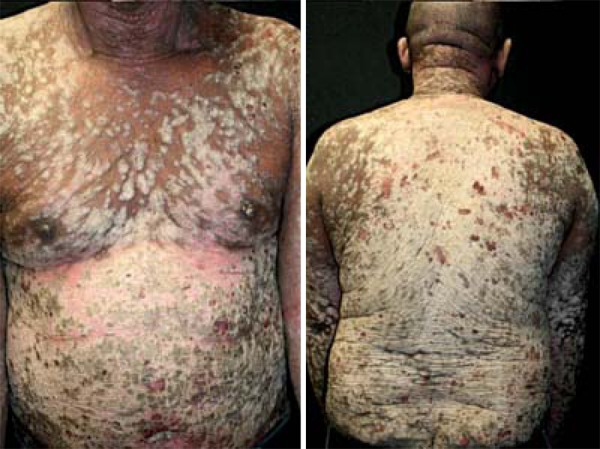
Severe psoriasis and a poor quality of life in a patient with history of pulmonary tuberculosis and positive HBV serology
Before treatment, HBV serology revealed the following results: anti-HBc IgG positivity; low titer anti-HBs (2 mIU/ml), and negativity for anti-HBc IgM, HBsAg, HBeAg and anti-HBe. The viral load was undetectable for HBV. Anti-HCV and anti-HIV were also negative. There were no respiratory symptoms, while the chest X-ray and Mantoux test were normal.
The patient was prophylactically treated with isoniazid for 6 months before starting the biologic treatment, and vaccinated against HBV, achieving anti-HBs positivity. Lamivudine (75mg per day) was started on the same day as the ustekinumab induction dose (45mg), lasting throughout the whole treatment period.
Lesions improved significantly 3 weeks after the first dose of ustekinumab (PASI 18 / BSA 47%). The patient responded excellently, maintaining PASI 3.8 / BSA 13%, normal liver tests, with no adverse effects in a three-year follow-up (Figure 2).
FIGURE 2.
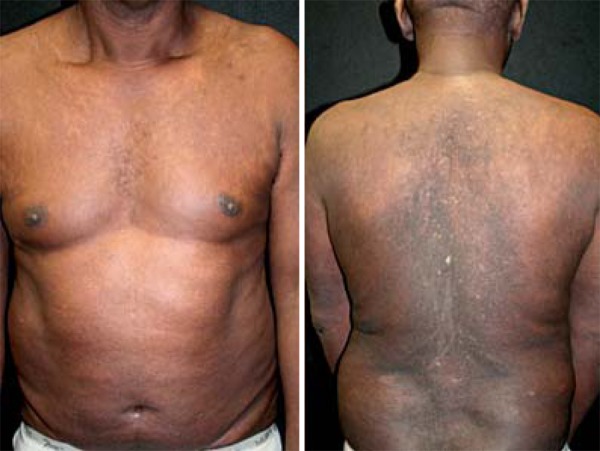
The patient obtained excellent response with anti-IL12/23 and lamivudine in a two year follow-up with no adverse effects
DISCUSSION
There are several reports of HBV reactivation after immune suppression. It is known that the HBV integrates with the host genome and can remain indefinitely in the nucleus of hepatocytes in the form of ccc-DNA. This material serves as a genomic template for HBV replication and sets the stage for future HBV reactivation whenever immunologic control of the virus is disrupted.1 The drugs most associated with HBV recurrence are those used either in the treatment of hemato-oncological malignancies, or the control of autoimmune diseases such as rituximab (anti-CD20) and tumor necrosis factor-α antagonists.5
The prophylactic use of antiviral drugs like lamivudine during and after anti-TNF-α and ustekinumab therapy is an important issue that has yet to be properly defined in patients with HBV infection. Lamivudine is a reverse transcriptase inhibitor commonly prescribed in patients with chronic HBV infection receiving cancer chemotherapy or undergoing transplantation.4 Antiviral prophylaxis appears to minimize the risk of viral reactivation in patients undergoing biologics therapy with concurrent psoriasis and HBV. However, some medical entities do not reimburse anti-viral therapy for non-cancer patients receiving biologics.6
We presented a patient that probably developed immunity to HBV following contact with this virus, although anti-HBs titer was low. The use of ustekinumab did not reactivate HBV, possibly because his HBsAg was negative, his anti-HBs titers increased before treatment and an antiviral with activity against HBV replication was initiated concomitantly.
Recently, reports were made of 2 cases of acute hepatitis B infection in patients treated with ustekinumab. Although the serological profiles of both patients corroborate the definition of a typical acute infection, a potential reactivation of an undetected, latent infection cannot be discounted in either case.7 Some observations suggest that ustekinumab may effectively neutralize IL-12/23 mediated functional responses, without affecting immune responses stimulated through other cytokines or cellular activities. While it is not possible to generate definitive conclusions about the roles of IL-12/23 in host immunity toward HBV based on these 2 cases, ustekinumab did not appear to impact acute hepatitis B immunity.7
Given the high prevalence of chronic viral infections in patients who are candidates for therapy with biologic products, as well as the potential for these agents to reactivate chronic viral illness, randomized controlled studies are needed to assess the risks and benefits of such therapy in these populations, whether one anti-TNFa agent is safer than the others, and when or if to initiate antiviral therapy.1,4,8 It remains uncertain whether ustekinumab therapy affects the risk of HBV.6
Nevertheless, close surveillance of the patients' HBV status before and during therapy with any biologic agent is strongly recommended. In addition, laboratory tests are fundamental in monitoring liver function for early detection of viral reactivation.
Footnotes
Conflict of interest: None
Financial funding: None
Work performed at the Complexo Hospitalar Santa Casa de Misericórdia de Porto Alegre - Porto Alegre (RS), Brazil.
How to cite this article: Steglich RB, Meneghello LP, Carvalho AVE, Cheinquer H, Müller FM, Reginatto FP. The use of ustekinumab in a patient with severe psoriasis and positive HBV serology. An Bras Dermatol. 2014;89(4):652-4.
REFERENCES
- 1.Abramson A, Menter A, Perrillo R. Psoriasis, hepatitis B, and the tumor necrosis factor-alpha inhibitory agents: a review and recommendations for management. J Am Acad Dermatol. 2012;67:1349–1361. doi: 10.1016/j.jaad.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 2.Arruda LHF, Campbell GAM, Takahashi MDF. Psoriasis. An bras Dermatol, 2001;76:141–167. [Google Scholar]
- 3.Uhlenhake EE, Mehregan DA. Ustekinumab: differential use in psoriasis. Clin Cosmet Investig Dermatol. 2011;4:93–99. doi: 10.2147/CCID.S17917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fotiadou C, Lazaridou E, Ioannides D. Safety of anti-tumour necrosis factor-a agents in psoriasis patients who were chronic hepatitis B carriers: a retrospective report of seven patients and brief review of the literature. J Eur Acad Dermatol Venereol. 2011;25:471–474. doi: 10.1111/j.1468-3083.2010.03754.x. [DOI] [PubMed] [Google Scholar]
- 5.Koskinas J, Tampaki M, Doumba PP, Rallis E. Hepatitis B virus reactivation during therapy with ustekinumab for psoriasis in an HBsAg negative anti-HBs positive patient. Br J Dermatol. 2013;168:679–680. doi: 10.1111/bjd.12120. [DOI] [PubMed] [Google Scholar]
- 6.Chiu HY, Chen CH, Wu MS, Cheng YP, Tsai TF. The safety profile of ustekinumab in the treatment of patients with psoriasis and concurrent hepatitis B or C. Br J Dermatol. 2013;169:1295–1303. doi: 10.1111/bjd.12461. [DOI] [PubMed] [Google Scholar]
- 7.Opel D, Economidi A, Chan D, Wasfi Y, Mistry S, Vergou T, et al. Two cases of Hepatitis B in patients with moderate to severe psoriasis treated with Ustekinumab. J Drugs Dermatol. 2012;11:1498–1501. [PubMed] [Google Scholar]
- 8.Calabrese LH, Zein N, Vassilopoulos D. Safety of antitumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis. 2004;63:ii18–ii24. doi: 10.1136/ard.2004.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]


