Abstract
Sirolimus (rapamycin) is an immunosuppressive agent commonly used in transplant recipients. Although sirolimus has less renal toxicity than calcineurin inhibitors, its use has been limited by its side effects. The most common cutaneous pathologies associated with sirolimus are inflammatory acneiform eruptions, lymphedema and aphthous ulcers. We present a novel cutaneous manifestation of sirolimus therapy that limited its use in at least one transplant recipient. Upon commencing sirolimus therapy, four solid organ transplant recipients developed tender, nonpruritic palmoplantar peeling within the first month of therapy. The peeling clinically resembled a mild form of hand-foot syndrome, yet none of the patients had been treated with chemotherapeutics. Desquamation presented on the palms and soles with dry vesicles and minor peeling extending to the dorsal aspects of the hands and feet. Histologically, the lesions were noninflammatory; the epidermis showed subtle separation between keratinocytes, suggesting either spongiosis or a defect in intercellular adhesion. One patient opted to discontinue treatment because of the tenderness associated with the palmoplantar peeling, which resulted in complete resolution within 2 weeks.
Keywords: Acral, desquamation, palmoplantar, peeling, rapamycin, sirolimus
Introduction
Acral skin peeling is a condition found in an array of dermatologic conditions. These include genetic syndromes like acral peeling skin syndrome and keratolysis exfoliativa; infections such as tinea pedis and scarlet fever; and iatrogenic rashes secondary to chemotherapeutics, epidermal growth factor receptor inhibitors and tyrosine multikinase inhibitors. In most of these conditions, it is not well understood why certain systemic exposures or genes will cause only the acral skin to peel. We report four patients who presented to our transplant dermatology clinic for palmoplantar peeling after initiating sirolimus therapy. This report draws attention to a previously unreported side effect of sirolimus and reviews potential etiologic mechanisms of acral skin peeling.
Sirolimus (rapamycin) is an immunosuppressive agent commonly prescribed for transplant recipients because it does not elicit many of the complications caused by the calcineurin inhibitors cyclosporine and tacrolimus, such as nephrotoxicity, neurotoxicity, hypertension and diabetes mellitus (1–3). In addition to its use as a prophylaxis for organ rejection in transplant patients, sirolimus has antiangiogenic and antineoplastic properties (4). Because of these characteristics, sirolimus has been implicated in several dermatologic applications, including treatment of Kaposi’s sarcoma, psoriasis, dermatitis and keloids (5).
Although sirolimus possesses a lower toxicity profile in comparison to its older immunosuppressive cousins, it has several potent dermatologic side effects. The most common cutaneous pathologies associated with sirolimus are inflammatory acneiform eruptions on the trunk and face, lymphedema and aphthous ulcers (2,6). Herein we describe a novel cutaneous side effect of systemic sirolimus therapy in four patients who developed palmoplantar epidermal peeling secondary to sirolimus therapy, review the literature on palmoplantar desquamation and propose mechanisms of etiology.
Cases
All cases are summarized in Table 1
Table 1.
Patient characteristics
| Age | Sex | DOS | Immunosuppression | Organ | Reason for transplant | Days post-SRL to rash | Time to resolution | Pain | D/c SRL? | PMH |
|---|---|---|---|---|---|---|---|---|---|---|
| 55 | M | 5-2010 | Prednisone 10 mg QD Mycophenolate mofetil 500 mg BID |
Heart | Cardiomyopathy HTN |
5 | <3 weeks | Yes | Yes restarted 2 years later | HTN Chronic renal insuff DM HLD Chronic allograft rejection, cardiomyopathy |
| 67 | M | 6-2010 | Mycophenolate mofetil 1500 mg BID | Heart | Ischemic Cardiomyopathy | 3–4 | <6 months | Yes | No | TB Cystic acne CAD CABG, PCI HTN HLD BPH CKD |
| 61 | F | 2-2012 | Cyclosporine 125 mg BID Prednisone 5 mg QD |
Heart | CHF | 14 | >4 months | No | Yes, due to dyspnea | Cardiomyopathy DM HTN Migraines intermittent vertigo |
| 69 | F | 2-2012 | Prednisone 5 mg QD Tacrolimus 3 mg QD |
Kidney | ESRD T2DM and HTN |
14 | 3 months | Yes | No | NMSC arthritis CAD/CABG (2007) AS HTN PVD HLD DM Gout Uterine CA monoclonal B cell lymphocytosis/CLL |
M, male; F, female; AS, aortic stenosis; BPH, benign prostate hypertrophy; CA, cancer; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; DM, diabetes mellitus; D/c, discontinue; DOS, date of service; ESRD, end-stage renal disease; HLD, hyperlipidemia; HTN, hypertension; NMSC, nonmelanoma skin cancer; PCI, percutaneous coronary intervention; PMH, past medical history; PVD, peripheral vascular disease; SRL, sirolimus; TB, tuberculosis.
Case 1
A 55-year-old Peruvian male who underwent cardiac transplantation presented to our clinic for 10 days of asymptomatic peeling skin over the palms and soles that began 5 days after adding sirolimus to his immunosuppressive regimen of mycophenolate mofetil 500 mg twice daily. Twice daily application of ketoconazole cream 2% prescribed by his transplant physician did not resolve his palmoplantar peeling. Review of systems did not reveal symptoms suggestive of underlying systemic disease. On examination, there was peeling scale over all 10 fingers and all 10 toes (Figure 1A). The mucous membranes, hair and nails were clear. There was no lymphadenopathy in the cervical, axillary or inguinal chains. His peeling did not resolve with petroleum jelly or Aquaphor, but did resolve after he discontinued sirolimus 1 month later due to the discomfort from the palmoplantar peeling. One year later, sirolimus was once again added to the patient’s immunosuppressive regimen, and the patient did not experience palmoplantar desquamation or tenderness.
Figure 1.
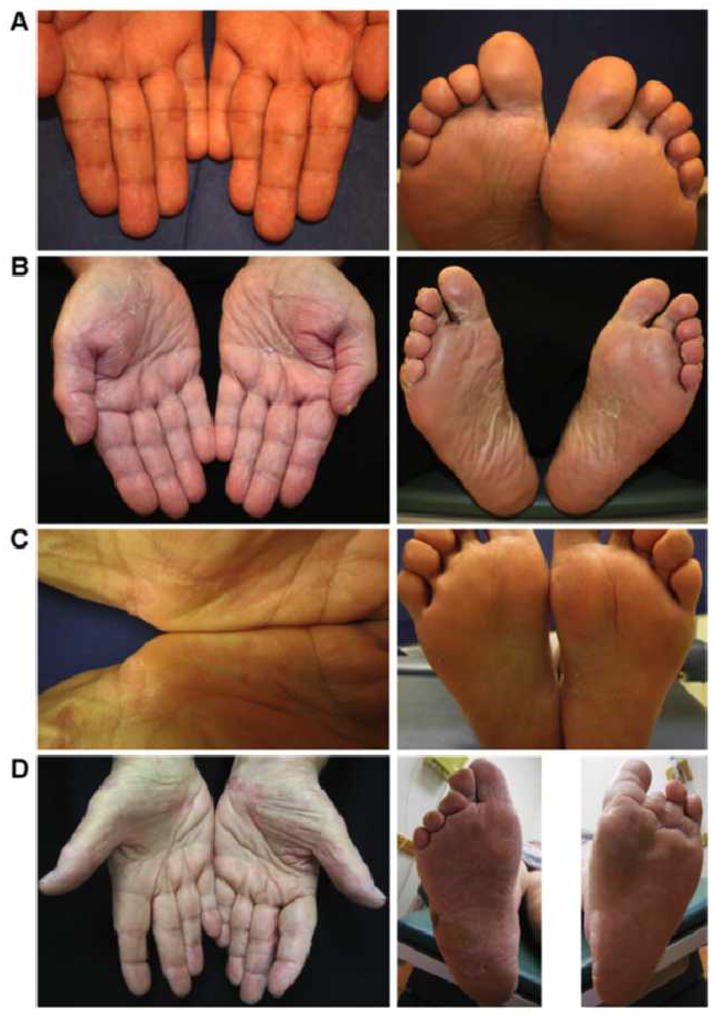
(A) Case 1: superficial peeling scale without erythema over fingers and toes. (B) Case 2: superficial peeling scale without erythema over the palms and soles. (C) Case 3: superficial peeling scale over the palms and soles. (D) Case 4: superficial peeling scale with mild erythema over the palms and soles.
Case 2
A 67-year-old male auto mechanic who underwent an orthotopic cardiac transplant presented to our transplant dermatology clinic 2 weeks after adding sirolimus 1 mg daily to his immunosuppressive regimen of mycophenolate mofetil 1500 twice a day. He noted palmoplantar tenderness 3–4 days after starting sirolimus. The tenderness improved over time, but the palms and soles began peeling (Figure 1B). Histologic examination of the desquamated stratum corneum revealed orthokeratosis and parakeratosis; a Periodic acid–Schiff (PAS) stain was negative for fungal organisms. Our patient opted to continue taking his sirolimus, and his hands had stopped peeling 6 months later.
Case 3
A 61-year-old Puerto Rican housewife presented to our clinic 2 years after her orthotopic cardiac transplant. She had initiated sirolimus therapy 5 months prior and had been on sirolimus for 4 months prior to discontinuing it in favor of mycophenolate mofetil due to an episode of dyspnea for which she was hospitalized. She presented to our clinic with peeling of her palms and medial insoles that had developed within 2 weeks of starting sirolimus (Figure 1C). The palmoplantar peeling was asymptomatic at the time of examination, and resolved 1 week after her initial clinic visit.
Case 4
A 69-year-old female former swimming instructor with an extensive history of skin cancer underwent renal transplantation. While on her posttransplant immunosuppressive regimen of prednisone 5 mg daily and tacrolimus 3 mg daily, the patient’s skin cancer tumor burden had progressed to a level requiring revision of immunosuppression. Her nephrologist replaced her tacrolimus with sirolimus 3mg daily, and within 2 weeks, she began to notice palmoplantar tenderness and peeling (Figure 1D). It was advised that she try applying baby oil and petroleum jelly to the affected areas. She also decreased her dose of sirolimus from 3 mg daily to 3 mg 3 days/week and 2 mg 4 days/week. After 4 months her palmoplantar desquamation and tenderness had resolved.
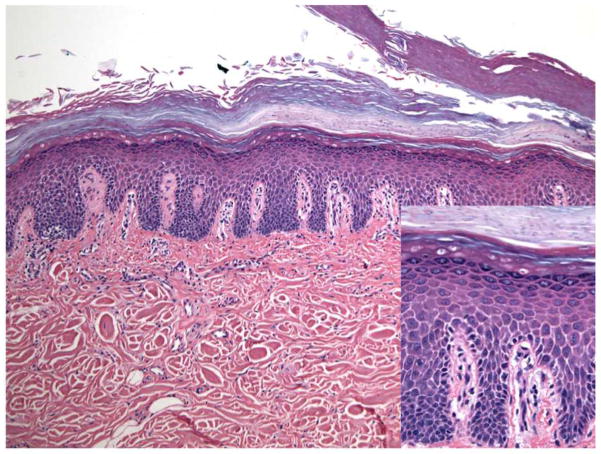
Histologic examination via punch biopsy revealed modest intercellular spaces between keratinocytes in the lower epidermis, resembling spongiosis (Figure 2). Inflammation was negligible, and interface dermatitis and necrotic keratinocytes were not detected. A PAS stain was negative for fungal hyphae.
Figure 2. Case 4: There are modest intercellular spaces between keratinocytes in the lower epidermis, resembling spongiosis.
The granular cell layer was retained, and there were layers of parakeratin alternating with thick orthokeratin on the surface of the biopsy. Clefts were apparent in the orthokeratotic layer. Inflammation was negligible, and interface dermatitis and necrotic keratinocytes were not detected.
Discussion
In the four cases presented, sirolimus immunosuppressive therapy was attributed to the pathogenesis of palmoplantar epidermal peeling for two reasons: (i) the peeling started within 2 weeks of initiating sirolimus therapy in all four cases, and (ii) the first case completely resolved less than 3 weeks after discontinuation of sirolimus therapy. None of these patients were on medications that would contribute to skin peeling like oral retinoids. To date, this is the first report of sirolimus-associated palmoplantar epidermal peeling in the literature. A review of the common literature on Medline in June 2013 using the search terms “sirolimus, rapamycin, mTOR” crossed with the search terms “desquamation, peel, blister, keratolysis, flake, flaking, acral, palmoplantar, erythema, exfoliation and dyskeratosis” did not reveal any previously reported case of palmoplantar skin peeling associated with sirolimus therapy.
In recent years, several independent groups have confirmed that sirolimus has adverse effects on the skin, predominantly inflammatory acne and edema of the limbs, which has led to drug termination in several affected patients. Table 2 outlines the main cutaneous adverse effects attributed to sirolimus therapy. The underlying mechanism by which sirolimus produces desquamation of the palms and soles is unclear. Although it is unlikely that the palmoplantar desquamation is due to any insult other than sirolimus treatment, the cutaneous presentation described in this report closely resembles those found in bacterial toxin-mediated palmoplantar desquamation following scarlatiniform rash; chemotherapy-mediated hand-foot syndrome (HFS); and the hereditary diseases acral peeling skin syndrome and keratolysis exfoliativa.
Table 2.
Previously reported cutaneous adverse effects associated with sirolimus
| Aphthous ulceration |
| Inflammatory acne on the face and trunk that is worse in men than in women |
| Edema: angioedema (11), lymphedema (12) |
| Xerosis |
| Viral infections: herpes simplex virus, human papilloma virus (common warts) |
| Skin fragility |
| Folliculitis |
| Seborrheic keratosis |
| Dermatophyte infections |
| Dermatitis |
| Nonmelanoma skin cancer: basal cell carcinoma, squamous cell carcinoma, Bowen’s disease, actinic keratosis |
Accordingly, our cases may represent a mild form of HFS, which is also referred to as palmoplantar erythrodysesthesia and is now thought to be within the spectrum of toxic erythema of chemotherapy (7). This phenomenon is typically associated with chemotherapy regimens, especially with targeted agents such as tyrosine multi-kinase inhibitors, and has not yet been reported in relation to sirolimus therapy. Clinically, HFS presents as erythema of the palms and soles, tender deep intact bullae, followed by desquamation and thinning of the epidermis that is more severe in the flexural digital creases. Unlike typical HFS, our cases did not present with erythema, manifested as superficial desquamation, and did not favor the flexural digital creases. The precise mechanism is not clear, but in 1986, Cox et al (8) suggested that the HFS results from a cytoxic affect of chemotherapeutics on basal keratinoctyes. There are numerous sweat glands on acral sites, and if drugs accumulate in sweat, there may be a particularly high concentration of drugs in the skin of palms and soles, with subsequent damage to the epidermis at these sites. Recent articles have restated this theory as well (9,10). The histologic findings in classic cases of HFS usually include some degree of interface damage. That was lacking in our cases, suggesting either a mild form of disease or a different mechanism underlying the acral peeling.
Our cases draw attention to a potential new cutaneous side effect associated with sirolimus therapy in transplant patients, which is supported by four patients who developed asymptomatic bilateral peeling of the hands and feet that began within 2 weeks of sirolimus initiation, and the palmoplantar peeling and tenderness spontaneously resolved in 3–6 months in cases 2 and 4 and resolved after 3– 5 weeks in cases 1 and 3 after sirolimus discontinuation. However, rechallenge of sirolimus in two patients did not elicit palmoplantar peeling. More rigorous investigation is needed to confirm a causal relationship between sirolimus therapy and palmoplantar peeling. Although this condition is not life-threatening, it may cause anxiety and functional impairment. One of our patients opted to terminate sirolimus treatment because of the discomfort and anxiety he was experiencing, which resulted in resolution of his hand-foot peeling. Although the pathophysiology has not been elucidated, it is important for physicians to recognize that sirolimus therapy can be associated with palmoplantar peeling so that patient anxiety and discomfort can be addressed.
Abbreviations
- CI
calcineurin inhibitor
- EGFR
epidermal growth factor receptor
- FDA
Federal Drug Administration
- HFS
hand-foot syndrome
- mTOR
mammalian target of rapamycin
- PAS
Periodic acid–Schiff
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Mahe E, Morelon E, Lechaton S, et al. Acne in recipients of renal transplantation treated with sirolimus: Clinical, microbiologic, histologic, therapeutic, and pathogenic aspects. J Am Acad Dermatol. 2006;55:139–142. doi: 10.1016/j.jaad.2005.11.1072. [DOI] [PubMed] [Google Scholar]
- 2.Mahe E, Morelon E, Lechaton S, et al. Cutaneous adverse events in renal transplant recipients receiving sirolimus-based therapy. Transplantation. 2005;79:476–482. doi: 10.1097/01.tp.0000151630.25127.3a. [DOI] [PubMed] [Google Scholar]
- 3.Di Benedetto F, Di Sandro S, De Ruvo N, et al. Sirolimus monotherapy in liver transplantation. Transplant Proc. 2007;39:1930–1932. doi: 10.1016/j.transproceed.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Stucki L, Piguet V. The paradox of sirolimus-induced immunosuppression and tumor control. Dermatology. 2006;213:3–5. doi: 10.1159/000092829. [DOI] [PubMed] [Google Scholar]
- 5.Paghdal KV, Schwartz RA. Sirolimus (rapamycin): From the soil of Easter Island to a bright future. J Am Acad Dermatol. 2007;57:1046–1050. doi: 10.1016/j.jaad.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald AS. A worldwide, phase III, randomized, controlled, safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71:271–280. doi: 10.1097/00007890-200101270-00019. [DOI] [PubMed] [Google Scholar]
- 7.Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: A useful clinical term. J Am Acad Dermatol. 2008;59:524–529. doi: 10.1016/j.jaad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Cox GJ, Robertson DB. Toxic erythema of palms and soles associated with high-dose mercaptopurine chemotherapy. Arch Dermatol. 1986;122:1413–1414. [PubMed] [Google Scholar]
- 9.Horn TD. Antineoplastic chemotherapy, sweat, and the skin. Arch Dermatol. 1997;133:905–906. [PubMed] [Google Scholar]
- 10.Lipworth AD, Robert C, Zhu AX. Hand-foot syndrome (hand-foot skin reaction, palmar-plantar erythrodysesthesia): Focus on sorafenib and sunitinib. Oncology. 2009;77:257–271. doi: 10.1159/000258880. [DOI] [PubMed] [Google Scholar]
- 11.Mahe E, Morelon E, Lechaton S, Kreis H, de Prost Y, Bodemer C. Angioedema in renal transplant recipients on sirolimus. Dermatology. 2007;214:205–209. doi: 10.1159/000099584. [DOI] [PubMed] [Google Scholar]
- 12.Desai N, Heenan S, Mortimer PS. Sirolimus-associated lymphoedema: Eight new cases and a proposed mechanism. Br J Dermatol. 2009;160:1322–1326. doi: 10.1111/j.1365-2133.2009.09098.x. [DOI] [PubMed] [Google Scholar]