Abstract
Background
Patients with hormone receptor-positive breast cancer typically show favorable survival. However, identifying individuals at high risk of recurrence among these patients is a crucial issue. We tested the hypothesis that [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) scans can help predict prognosis in patients with hormone receptor-positive breast cancer.
Methods
Between April 2004 and December 2008, 305 patients with hormone receptor-positive breast cancer who underwent FGD-PET were enrolled. Patients with luminal B subtype were identified by positivity for human epidermal growth factor receptor-2 (HER2) or high Ki67 (≥14%) according to criteria recently recommended by the St. Gallen panelists. The cut-off value of SUVmax was defined using the time-dependent receiver operator characteristic curve for recurrence-free survival (RFS).
Results
At a median follow up of 6.23 years, continuous SUVmax was a significant prognostic factor with a hazard ratio (HR) of 1.21 (p = 0.021). The cut-off value of SUVmax was defined as 4. Patients with luminal B subtype (n = 82) or high SUVmax (n = 107) showed a reduced RFS (p = 0.031 and 0.002, respectively). In multivariate analysis for RFS, SUVmax carried independent prognostic significance (p = 0.012) whereas classification with immunohistochemical markers did not (p = 0.274). The Harell c-index was 0.729. High SUVmax was significantly associated with larger tumor size, positive nodes, HER2 positivity, high Ki67 (≥14%), high tumor grade, and luminal B subtype.
Conclusions
Among patients with hormone receptor-positive breast cancer, FDG-PET can help discriminate patients at high risk of tumor relapse.
Introduction
In patients with hormone receptor (HR)-positive breast cancer, which accounts for approximately 75% of breast cancers [1], endocrine therapies targeting the estrogen receptor (ER) or estrogen synthesis have reduced annual recurrences by 41% and deaths by 34%. Nevertheless, treatment failure still occurs in 30% of patients who are treated with tamoxifen [2], therefore identifying patients with a poor prognosis among those with HR-positive breast cancer has become a critical issue in clinical field. Recent advances in molecular studies based on gene expression profiling have classified subtypes of breast cancer and suggest that HR-positive breast cancer is a clinically and biologically heterogeneous entity [3], [4]. These studies identified at least two major groups of HR-positive tumors, known as luminal A and B. Identification of luminal B tumors among HR-positive cancers using immunohistochemical (IHC) markers has become accepted in clinical practice [5], [6], however, debate against current classification based on IHC markers still remains.
[18F] fluorodexoyglucose positron emission tomography (FDG-PET) is a well-established imaging tool for the diagnosis and staging of various malignancies [7]. In addition, the degree of FDG uptake can reflect the biologic characteristics of a tumor and is a validated prognostic factor in various malignancies [8], [9]. In breast cancer, it is well known that ER-positive tumors are characterized by rather low FDG uptake. Previous studies suggest that high FDG uptake is correlated with negative estrogen receptor (ER) expression, negative progesterone receptor (PR) expression, positive human epidermal growth factor receptor-2 (HER-2) expression, high histologic grade, and high proliferative marker such as Ki67 [10]–[12].
Therefore, among ER-positive breast cancer, tumors with increased FDG uptake can be considered to show more aggressive behavior than tumors with decreased uptake. Here, we tested the hypothesis that FDG-PET can help predict prognosis in patients with hormone receptor-positive breast cancer.
Materials and Methods
Patient selection
Between January 2004 and December 2008, 835 consecutive women underwent surgery for breast cancer. Of these 835 patients, 682 had undergone preoperative FDG-PET. Patients were excluded on the basis of the following criteria: known bilateral breast cancer (n = 28); preoperative chemotherapy, because chemotherapy can affect tumor characteristics related with FDG uptake (n = 65); ductal carcinoma in situ (n = 83); and distant metastases at initial assessment (n = 41). Among these patients, 309 women with ER-positive and/or PR-positive tumors were identified.
These HR-positive patients were classified as two intrinsic subtypes according to criteria recently recommended by the St. Gallen panelists [5], [6]. The Ki67 cut-off value of 14% also adhered to these criteria. Two subtypes were defined as follows: luminal A (ER-positive and/or PR-positive, HER2-negative and Ki-67<14%); or luminal B (ER-positive and/or PR-positive, HER2-negative, and Ki-67≥14%, or ER-positive and/or PR-positive and HER2-positive, irrespective of Ki67 index). Patients missing data for any IHC marker were excluded (n = 2). Patients with an IHC score of 2+ for HER2 but without fluorescence in situ hybridization (FISH) results for HER2 amplification were also excluded (n = 2). Data for the remaining 305 patients were entered into the analysis (Figure S1).
For IHC evaluation of four markers, formalin-fixed paraffin-embedded tissue sections obtained from surgical specimens were stained with appropriate antibodies for ER (Novocastra, Newcastle upon Tyne, UK), PR (Novocastra), HER2 (Ventana Medical Systems, Tucson, AZ, USA), and Ki-67 (MIB-1; Dako, Glostrup, Denmark). ER and PR were determined by nuclear staining, which was graded from 0 to 8 using the Allred score [13]. The results were categorized as positive when the total score, expressed as the sum of the proportion score and intensity score, was 3 or greater. For HER2 evaluation, membranous staining was graded as 0, 1+, 2+, or 3+ [14]. HER2 status was deemed to be positive for a score of 3+ and negative for a score of 0 or 1+. Tumors with a score of 2+ were sent for FISH analysis using the PathVysion HER2 DNA Probe Kit (Abbott-Vysis, Des Plaines, IL, USA).
Staging was performed according to the American Joint Committee on Cancer (AJCC), 7th edition. The modified Scarf-Bloom-Richardson grading system was used for tumor grading. Adjuvant systemic therapy and/or radiotherapy were considered according to the standard guidelines based on patient age, primary tumor characteristics, and axillary lymph node status. Endocrine therapy was delivered to all patients. The institutional review board (IRB) of Gangnam Severance Hospital, Yonsei University, Seoul, Korea, approved the study in accordance with good clinical practice guidelines and the Declaration of Helsinki. The IRB waived the need for written informed consent from the participants because of the retrospective design.
FDG-PET method
Prior to FDG-PET, each patient fasted for a minimum of 8 hours and blood glucose level was controlled to lower than 130 mg/dl. Patients received an intravenous injection of 0.14 mCi MBq 18F-FDG in the arm contralateral to the primary tumor. At 60 min after injection of 18F-FDG, whole-body emission scans were obtained using a Philips Allegro PET camera (Philips Medical Systems, Cleveland, Ohio, USA). All patients were studied in the supine position with their arms raised. Attenuation-corrected transaxial images were reconstructed with an iterative transmission algorithm called a row-action maximum likelihood 3D protocol using a 3D image filter into a 128×128 matrix. For semi-quantitative evaluation, SUVmax was calculated by measuring the absorption of 18F-FDG by tumors in the region of interest (ROI) as follows:
SUVmax = [maximal radioactivity concentration in ROI (µCi/g)/injected dose (µCi)/patient’s weight (kg)].
Statistical analysis
Age was presented as median value with range and compared by Mann–Whitney U test. Discrete variables were compared by chi-square test. The cut-off point of SUVmax was defined using the time-dependent ROC curve for recurrence-free survival (RFS).
The primary end-point was RFS. RFS was measured from the date of the first curative surgery to the date of the first loco-regional recurrence or distant metastasis. The Kaplan-Meier method was used to estimate the RFS, and the estimated survival curves were compared using the log-rank test. Significant prognostic factors associated with recurrence-free survival were selected using Harrell c-statistic [15] and a Cox proportional hazard regression model was applied for multivariate survival analysis. Student’s t-tests were conducted to compare SUVmax according to subtype or prognostic factors. All analyses were performed using SPSS version 18 (SPSS; Chicago, IL) and R (http://www.r-projet.org). Statistical significance was defined by a P-value<0.05 or a 95% confidence interval (CI) that did not include 1.
Results
Patients’ characteristics
Table 1 shows patient characteristics according to the intrinsic subtypes (luminal A and luminal B). The two groups did not differ significantly in T stage, N stage, and AJCC stage, representing tumor burden, or in ER and PR status. In contrast, median age of the luminal A subgroup was significantly higher than that of the luminal B subgroup whereas histologic grade was significantly higher in luminal B than in luminal A. Furthermore, median SUVmax was significantly higher in luminal B than in luminal A (4.7 vs. 2.6, respectively). There were no significant differences in adjuvant treatments except for adjuvant endocrine treatment. The higher rate of tamoxifen use in the luminal B subgroup suggested that more premenopausal women were classified as luminal B and was concordant with the lower median age of patients with the luminal B subtype.
Table 1. Baseline characteristics of patients according to intrinsic subtypes.
| Characteristics | All patients (n = 305) | Luminal A (n = 223) | Luminal B (n = 82) | P-valuea |
| Age years, median (range) | 48 (25–80) | 48 (28–80) | 45 (25–80) | 0.019b |
| T stage | 0.127 | |||
| T1 | 174 (57) | 135 (60) | 39 (48) | |
| T2 | 128 (41) | 86 (39) | 42 (51) | |
| T3 | 3 (2) | 2 (1) | 1 (1) | |
| N stage | 0.367 | |||
| N0 | 201 (66) | 146 (65) | 55 (67) | |
| N1 | 81 (27) | 63 (28) | 18 (22) | |
| N2 | 17 (5) | 11 (5) | 6 (7) | |
| N3 | 6 (2) | 3 (1) | 3 (4) | |
| AJCC stage | 0.284 | |||
| I | 126 (41) | 98 (44) | 28 (34) | |
| II | 155 (51) | 109 (49) | 46 (56) | |
| III | 24 (8) | 16 (7) | 8 (10) | |
| Histologic grade c | <0.001 | |||
| 1 | 129 (42) | 112 (50) | 17 (27) | |
| 2 | 117 (38) | 83 (37) | 34 (41) | |
| 3 | 26 (9) | 0 (0) | 26 (32) | |
| Estrogen receptor | 0.507 | |||
| Positive | 277 (91) | 204 (91) | 73 (89) | |
| Negative | 28 (9) | 19 (9) | 9 (11) | |
| Progesterone receptor | 0.331 | |||
| Positive | 266 (87) | 197 (88) | 69 (84) | |
| Negative | 39 (13) | 26 (12) | 13 (16) | |
| HER2 | <0.001 | |||
| Positived | 47 (15) | 0 (0) | 47 (57) | |
| Negative | 258 (85) | 223 (100) | 35 (43) | |
| Ki67 | <0.001 | |||
| High | 33 (11) | 0 (0) | 33 (40) | |
| Low | 272 (89) | 223 (100) | 49 (60) | |
| SUVmax | <0.001b | |||
| Median (range) | 3.1 (0.8–12.8) | 2.6 (0.8–12.8) | 4.7 (1.0–11.2) | |
| Adjuvant endocrine therapy | 0.030 | |||
| Tamoxifen | 129 (42) | 85 (38) | 44 (54) | |
| Toremifene | 62 (20) | 44 (20) | 18 (22) | |
| Letrozole | 61 (20) | 49 (22) | 12 (15) | |
| Anastrozole | 53 (18) | 45 (20) | 8 (9) | |
| Adjuvant chemotherapy | 0.146 | |||
| Yes | 187 (61) | 131 (59) | 56 (68) | |
| No | 118 (39) | 92 (41) | 26 (32) | |
| Adjuvant radiotherapy | 0.999 | |||
| Yes | 119 (39) | 87 (39) | 32 (39) | |
| No | 186 (61) | 136 (61) | 50 (61) |
AJCC, American Joint Committee on Cancer; SUVmax, maximum standardized uptake value; HER2, human epidermal growth factor receptor-2.
Chi-square test, except for bMann–Whitney U test.
Data with missing values.
HER-2 positivity was defined by 3+ score on immunohistochemistry or amplification on fluorescence in situ hybridization.
Survival analysis with SUVmax
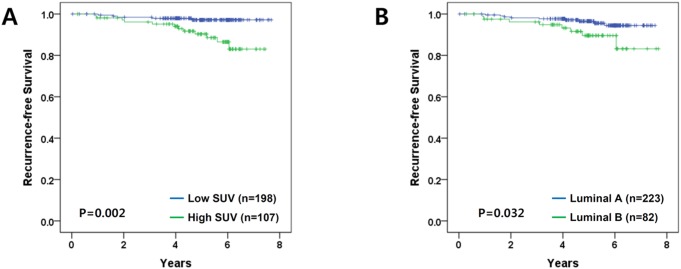
At a median follow up of 6.12 years there were 17 recurrences: 5 loco-regional and 12 distant metastases. First, we performed univariate analysis using continuous SUVmax. This analysis showed that continuous SUVmax was a significant prognostic factor with a hazard ratio (HR) of 1.21 (P = 0.018; 95% CI, 1.03–1.42). Next, we determined the cut-off point of SUVmax using the time-dependent ROC curve in relation to RFS, which yielded an area under the curve (AUC) of 0.721 (95% CI, 0.594–0.884; Fig. 1). Youden’s index was highest for SUVmax of 4.1. With consideration of clinical application, we defined the cut-off for SUVmax as 4, which gave an AUC for RFS of 0.731 (95% CI, 0.592–0.902). Subsequently, high and low SUVmax groups were defined by a cut-off value of 4. Based on univariate analysis, the RFS time differed significantly between groups stratified by SUVmax (SUVmax<4 versus≥4; P = 0.002; Fig. 2A).
Figure 1. Time-dependent ROC curve for recurrence-free survival (n = 305).
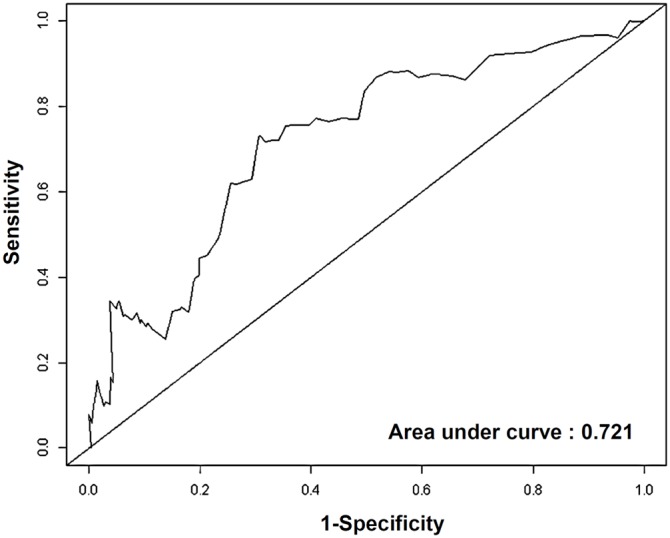
Figure 2. Kaplan-Meier plots for recurrence-free survival.
All P-values are calculated by the log-rank test (A) Stratification by SUVmax (P = 0.002) (B) Stratification by the intrinsic subtypes (P = 0.032).
In univariate analyses using other characteristics (Table S1), RFS differed significantly between groups stratified by age (≤35 vs. >35 years; P<0.001), intrinsic subtype (luminal A vs. luminal B; P = 0.031; Fig. 2B), and PR (positive vs. negative; P = 0.003).
SUVmax versus intrinsic subtypes
Kaplan-Meier plots comparing groups stratified by SUVmax and intrinsic subtype are presented in Fig. 3. RFS did not differ significantly according to subtype within the group of low SUVmax (group 1 vs. group 2; P = 0.315, log-rank test) or the group of high SUVmax (group 3 vs. group 4; P = 0.060, log-rank test). In contrast, within the group of luminal B, RFS significantly differed according to SUVmax (group 2 vs. group 4; P = 0.018, log-rank test). Within the group of luminal A, RFS did not differ significantly according to SUVmax (group 1 vs. group 3; P = 0.280, log-rank test).
Figure 3. Kaplan-Meier plots stratified by combined factors with SUVmax and the intrinsic subtype: Group 1, low SUVmax and luminal A (n = 163); Group 2, low SUVmax and luminal B (n = 35); Group 3, high SUVmax and luminal A (n = 60); Group 4, high SUVmax and luminal B (n = 47).
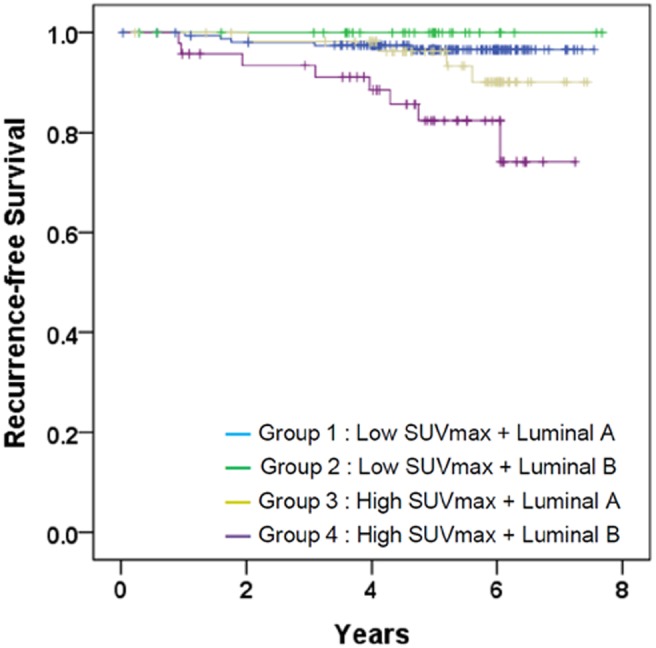
P-value calculated by the log-rank test was 0.001.
Multivariate analysis
Multivariate analysis using a Cox regression hazard model suggested that high SUVmax (adjusted HR 4.09; 95% CI 1.36–12.31) was an independent prognostic factor for RFS, and age less than 35 (adjusted HR 7.09; 95% CI 2.56–19.60) and negative PR status (adjusted HR 4.47; 95% CI 1.61–12.44) were associated with an increased risk of tumor recurrence (Table 2). However, the intrinsic subtype was not a prognostic factor in multivariate analysis (P = 0.240). For this model, the Harrell c-index was 0.729. In addition, among patients who received adjuvant chemotherapy (n = 187), dichotomized SUVmax was repeatedly shown to be an independent prognostic factor for RFS (adjusted HR 5.96; 95% CI 1.49–23.95; Table S2). The Harrell c-index was 0.741 in this model.
Table 2. Multivariate analysis using Cox regression hazard model for recurrence-free survival.
| Characteristicsa | P-value | Adjusted HR | 95% confidence interval |
| Age, years | <0.001 | ||
| >35 (n = 27) | reference | ||
| ≤35 (n = 278) | 7.09 | 2.56–19.60 | |
| Progesterone receptor | 0.004 | ||
| Positive (n = 266) | reference | ||
| Negative (n = 39) | 4.47 | 1.61–12.44 | |
| Intrinsic subtype | 0.274 | ||
| Luminal A (n = 223) | reference | ||
| Luminal B (n = 82) | 1.77 | 0.64–4.90 | |
| SUVmax | 0.012 | ||
| 4< (n = 198) | reference | ||
| ≥4 (n = 107) | 4.09 | 1.36–12.31 |
HR, hazard ratio; SUVmax, maximum standardized uptake value.
Presented variables are selected using Harrell c-statistic. In this analysis, Harrell c-index was 0.729.
SUVmax according to tumor characteristics
The mean SUVmax in the two groups was compared after stratification by tumor characteristics (Table 3). The mean SUVmax was significantly higher for the following prognostic factors: large tumor size (P<0.001), positive lymph node (P = 0.011), positive HER2 (P<0.001), high Ki67 (P = 0.011), and high histologic grade (P<0.001), all of which are considered risk factors. In addition, the mean SUVmax of the luminal B subtype was significantly higher than that of luminal A.
Table 3. Comparisons of SUVmax according to tumor characteristics.
| Characteristics | Mean SUVmax±SD | P-valuea |
| Age, years | 0.224 | |
| >35 (n = 27) | 3.63±2.31 | |
| ≤35 (n = 278) | 4.43±3.28 | |
| Tumor size | <0.001 | |
| ≤2 cm (n = 174) | 4.72±2.60 | |
| >2 cm (n = 131) | 2.92±1.94 | |
| Lymph node status | 0.011 | |
| Negative (n = 201) | 3.44±2.36 | |
| Positive (n = 104) | 4.18±2.41 | |
| Estrogen receptor status | 0.928 | |
| Positive (n = 277) | 3.69±2.43 | |
| Negative (n = 28) | 3.74±2.33 | |
| Progesterone receptor status | 0.394 | |
| Positive (n = 266) | 3.74±2.47 | |
| Negative (n = 39) | 3.43±2.00 | |
| HER2 | <0.001 | |
| Negative (n = 258) | 3.44±2.33 | |
| Positive (n = 47) | 5.12±2.40 | |
| Ki67 | 0.011 | |
| <14% (n = 272) | 3.57±2.35 | |
| ≥14% (n = 33) | 4.70±2.76 | |
| Histologic grade b | <0.001 | |
| 1 and 2 (n = 246) | 5.85±2.84 | |
| 3 (n = 26) | 3.58±2.27 | |
| Subtype | <0.001 | |
| Luminal A (n = 223) | 3.22±2.15 | |
| Luminal B (n = 82) | 4.98±2.64 |
HER2, human epidermal growth factor receptor-2.
Student’s t-tests.
Data with missing values.
Discussion
In this genomic era, molecular profiling of breast cancer has enhanced our understanding of the complexity and heterogeneity of breast cancer [4]. To translate this understanding of intrinsic subtypes into clinical practice, IHC expression patterns have been widely investigated as surrogate markers. The experts of the St. Gallen’s panel recommended optimal guidelines for intrinsic subtyping based on IHC markers [5], [6]. In our study, we used these criteria to identify the intrinsic subtypes of HR-positive tumors.
In terms of prognostic discrimination of endocrine-responsive breast cancer, our results suggested that SUVmax is superior to classification based on surrogate IHC markers. SUVmax demonstrated independent prognostic significance in multivariate analysis whereas classification with IHC markers did not. Analysis revealed that our model including SUVmax had good predictive value for RFS, with a Harell c-index of 0.729. These findings suggest a potential role for FDG-PET in better predicting groups with poor and good prognosis among patients with HR-positive breast cancer.
It is known that HR-positive tumors show lower SUVmax than HR-negative tumors [10]–[12]. Regarding intrinsic subtypes, SUVmax was lowest in the luminal A subtype and higher in HER2 and triple negative subtypes [16], [17]. The increased accumulation of FDG observed in the high SUVmax tumors reflects highly proliferating and poorly differentiated cancer. Therefore, HR-positive tumors with high SUVmax might have aggressive behavior and show a propensity for high proliferation. Indeed, our findings suggested that SUVmax is associated with high Ki67, HER2 positivity, and higher histologic grade, even in HR-positive cancer (Table 3).
To evaluate tumor proliferation based on PET scans, PET imaging using 18F-fluorothymidine (FLT) has been investigated in breast cancer [18], [19]. FLT-PET is known to be closely associated with Ki67 expression in breast cancer. Although high SUVmax of FDG-PET correlated with high Ki67 in our study, FLT-PET is recognized as a better tool than FDG-PET in terms of evaluation of tumor proliferation. FLT-PET was not available for our study, but it will be interesting to compare the prognostic significance of FDG-PET with that of FLT-PET in HR-positive tumors.
Interestingly, in the analysis with combined factor using SUVmax and intrinsic subtypes (Figure 3), we noted that Group 4 had higher proportion of HER2-positive tumors than group 2 (Figure S2). As suggested in Table 3, SUVmax was higher in HER2-positive tumor, thus it seems reasonable that luminal B tumors with high SUVmax (Group 4) had a higher HER2-positive rate than luminal B tumors with low SUVmax (Group 2). It is concordant with previous studies that most of HER2-positive luminal B had a higher chance of tumor recurrence among hormone receptor-positive tumors [20], [21]. In addition, it is noteworthy that there might be tumors with good prognosis even in HER2-positive luminal B tumors and those tumors can be identified by SUVmax.
Recent studies suggested that PR status should be considered as a critical prognosticator in HR-positive women [22]–[24]. Prognostic power of PR is also recognized by the panels of St. Gallen in 2013 [5]. Our data confirm the prognostic significance of PR. The reproducibility of previous findings implied that our cohort showed a reliable outcome and the quality of IHC markers was well controlled. Furthermore, our data suggest that SUVmax remains a good prognostic marker for HR-positive breast cancer independent of PR.
The limitations of our study reflect its retrospective nature and the heterogeneity of the patient population. One important factor is the inability to control for variations in adjuvant treatments that may influence survival outcomes. Therefore, to minimize the confounding effect of adjuvant treatment, we performed the same analyses in patients with adjuvant chemotherapy. These analyses revealed that SUVmax still carried prognostic significance for RFS in patients receiving both adjuvant chemotherapy and endocrine therapy.
Despite these limitations, our study highlights the prognostic impact of FDG-PET for patients with HR-positive breast cancer. The prognostic implications of SUVmax observed in our study warrant further investigation in prospective studies.
In conclusion, among patients with hormone receptor-positive breast cancer, FDG-PET can help discriminate patients at high risk of tumor relapse.
Supporting Information
Consort chart showing the patients identified in our study.
(TIF)
HER2-positive rates among the groups classified with IHC markers and SUVmax.
(TIF)
Univariate analyses according to tumor characteristics.
(DOCX)
Multivariate analysis using Cox regression hazard model for recurrence-free survival in patients receiving adjuvant chemotherapy (n = 187).
(DOCX)
Data used in this study.
(XLS)
Acknowledgments
The authors thank Mr. Dong-Su Jang, Research Assistant, Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea, for his help with the figures.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Relevant data are included within the Supporting Information files.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (grant 2013R1A1A2007759). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Millar EK, Graham PH, McNeil CM, Browne L, O’Toole SA, et al. (2011) Prediction of outcome of early ER+ breast cancer is improved using a biomarker panel, which includes Ki-67 and p53. Br J Cancer 105: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365: 1687–1717. [DOI] [PubMed] [Google Scholar]
- 3. Oh DS, Troester MA, Usary J, Hu Z, He X, et al. (2006) Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol 24: 1656–1664. [DOI] [PubMed] [Google Scholar]
- 4. Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. (2000) Molecular portraits of human breast tumours. Nature 406: 747–752. [DOI] [PubMed] [Google Scholar]
- 5. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, et al. (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24: 2206–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, et al. (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22: 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, et al. (2008) Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 49: 480–508. [DOI] [PubMed] [Google Scholar]
- 8. Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA (2005) The maximum standardized uptake values on positron emission tomography of a non-small cell lung cancer predict stage, recurrence, and survival. J Thorac Cardiovasc Surg 130: 151–159. [DOI] [PubMed] [Google Scholar]
- 9. Seo S, Hatano E, Higashi T, Hara T, Tada M, et al. (2007) Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res 13: 427–433. [DOI] [PubMed] [Google Scholar]
- 10. Osborne JR, Port E, Gonen M, Doane A, Yeung H, et al. (2010) 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med 51: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanli Y, Kuyumcu S, Ozkan ZG, Isik G, Karanlik H, et al. (2012) Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med 26: 345–350. [DOI] [PubMed] [Google Scholar]
- 12. Shimoda W, Hayashi M, Murakami K, Oyama T, Sunagawa M (2007) The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer 14: 260–268. [DOI] [PubMed] [Google Scholar]
- 13. Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11: 155–168. [PubMed] [Google Scholar]
- 14. Moeder CB, Giltnane JM, Harigopal M, Molinaro A, Robinson A, et al. (2007) Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray based assessment of outcome. J Clin Oncol 25: 5418–5425. [DOI] [PubMed] [Google Scholar]
- 15. Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 16. Basu S, Chen W, Tchou J, Mavi A, Cermik T, et al. (2008) Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer 112: 995–1000. [DOI] [PubMed] [Google Scholar]
- 17. Humbert O, Berriolo-Riedinger A, Riedinger JM, Coudert B, Arnould L, et al. (2012) Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol 23: 2572–2577. [DOI] [PubMed] [Google Scholar]
- 18. Chalkidou A, Landau DB, Odell EW, Cornelius VR, O’Doherty MJ, et al. (2012) Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur J Cancer 48: 3499–3513. [DOI] [PubMed] [Google Scholar]
- 19. Smyczek-Gargya B, Fersis N, Dittmann H, Vogel U, Reischl G, et al. (2004) PET with [18F] fluorothymidine for imaging of primary breast cancer: a pilot study. Eur J Nucl Med Mol Imaging 31: 720–724. [DOI] [PubMed] [Google Scholar]
- 20. Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, et al. (2010) Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 7: e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, et al. (2009) Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101: 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohsin SK, Weiss H, Havighurst T, Clark GM, Berardo M, et al. (2004) Progesterone receptor by immunohistochemistry and clinical outcome in breast cancer: a validation study. Mod Pathol 17: 1545–1554. [DOI] [PubMed] [Google Scholar]
- 23. Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, et al. (2013) Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol 31: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consort chart showing the patients identified in our study.
(TIF)
HER2-positive rates among the groups classified with IHC markers and SUVmax.
(TIF)
Univariate analyses according to tumor characteristics.
(DOCX)
Multivariate analysis using Cox regression hazard model for recurrence-free survival in patients receiving adjuvant chemotherapy (n = 187).
(DOCX)
Data used in this study.
(XLS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Relevant data are included within the Supporting Information files.