Abstract
Orteronel (also known as TAK-700) is a novel hormonal therapy that is currently in testing for the treatment of prostate cancer. Orteronel inhibits the 17,20 lyase activity of the enzyme CYP17A1, which is important for androgen synthesis in the testes, adrenal glands and prostate cancer cells. Preclinical studies demonstrate that orteronel treatment suppresses androgen levels and causes shrinkage of androgen-dependent organs, such as the prostate gland. Early reports of clinical studies demonstrate that orteronel treatment leads to reduced prostate-specific antigen levels, a marker of prostate cancer tumor burden, and more complete suppression of androgen synthesis than conventional androgen deprivation therapies that act in the testes alone. Treatment with single-agent orteronel has been well tolerated with fatigue as the most common adverse event, while febrile neutropenia was the dose-limiting toxicity in a combination study of orteronel with docetaxel. Recently, the ELM-PC5 Phase III clinical trial in patients with advanced-stage prostate cancer who had received prior docetaxel was unblinded as the overall survival primary end point was not achieved. However, additional Phase III orteronel trials are ongoing in men with earlier stages of prostate cancer.
Keywords: 17, 20 lyase; CYP17A1; orteronel; prostate cancer; TAK-700
Prostate cancer is the most common cancer among men in the USA, with over 220,000 new cases of prostate cancer predicted for 2013 [1]. Prostate cancer is also the second most lethal cancer in the USA, and over 28,000 men are predicted to die of this disease this year [1]. Nearly all prostate cancer deaths are due to metastatic tumors that have progressed despite androgen deprivation therapies (ADTs) that work principally by suppressing androgen synthesis in the testes. The target of ADT is the androgen receptor (AR), a nuclear hormone receptor activated by androgen ligands. The AR is critical for both proliferation and the differentiation state of prostate cancer cells.
ADT with luteinizing hormone-releasing hormone (LHRH) agonists or LHRH antagonists is the first-line treatment for metastatic prostate cancer. While most patients initially respond to ADT, tumor progression termed ‘castration resistance’ is universal. This castration-resistant state is defined by a continued rise in prostate-specific antigen (PSA) or progression of metastases in the setting of castrate serum levels of testosterone (<50 ng/dl). However, despite the fact that serum levels of testosterone are reduced with ADT, androgens persist within tumors from men with castration-resistant prostate cancer (CRPC) [2]. In addition, androgen synthetic enzymes are frequently upregulated in CRPC tumors, and AR overexpression and AR mutations are also common, demonstrating the continued importance of AR signaling [2–4].
These results led to the development of newer, more potent forms of ADT. These drugs include the CYP17A inhibitor abiraterone acetate and the novel AR antagonist enzalutamide. Treatment with both agents interferes with persistent intratumoral androgens and suppresses CRPC cell growth in preclinical studies [5,6]. Furthermore, treatment with abiraterone acetate or enzalutamide in Phase III clinical trials in men with metastatic CRPC leads to clinical benefit, including significant prolongation of both progression-free and overall survival with the exception of the abiraterone Phase III clinical trial in men with metastatic CRPC who had not received prior docetaxel chemotherapy [7–9]. That trial only showed a significant improvement in progression-free survival [8]. While abiraterone acetate and enzalutamide are effective in many CRPC patients, these treatments are noncurative. Thus, novel therapies are required to provide more durable tumor control in patients with CRPC.
Overview of the market
Abiraterone acetate was the first novel hormonal therapy approved for the treatment of metastatic CRPC in the past 2 years. Abiraterone acetate inhibits CYP17A1 (both the 17α-hydroxylase and 17,20 lyase activities of this enzyme) in the steroid biosynthesis pathway. Suppression of 17α-hydroxylase leads to reduced cortisol synthesis and compensatory overproduction of mineralocorticoids. Therefore, prednisone is administered concomitantly with abiraterone acetate to suppress the adrenocorticotropic hormone overdrive that results in hypertension, hypokalemia and edema and to prevent adrenal insufficiency. Currently, abiraterone acetate is approved for metastatic CRPC patients both prior to and after treatment with docetaxel chemotherapy based on the results of two recent Phase III clinical trials [7,8]. Enzalutamide is a novel AR antagonist that prevents androgens from binding to and activating the AR. Treatment with enzalutamide in a Phase III clinical trial in patients who had received prior docetaxel demonstrated improved overall survival and progression-free survival [9]. A Phase III enzalutamide clinical trial in patients with metastatic CRPC who had not yet received docetaxel chemotherapy was recently unblinded after interim analysis showed a significant improvement in overall survival and progression-free survival. Sipuleucel-T [10], a form of immunotherapy, docetaxel chemotherapy [11], cabazitaxel chemotherapy [12], and the α-emitting radiopharmaceutical radium-223 [13] have all demonstrated improved overall survival in Phase III clinical trials with men with CRPC. These drugs are the other approved treatment options for men with metastatic CRPC.
Introduction to the compound
Orteronel is a novel, nonsteroidal inhibitor androgen synthesis with greater specificity for 17,20 lyase versus 17α-hydroxylase. Orteronel is manufactured by Millennium: The Takeda Oncology Company (MA, USA) (Figure 1).
Figure 1.
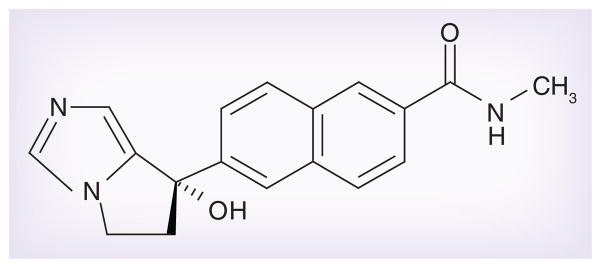
Orteronel (TAK-700).
Chemistry
Like abiraterone acetate, orteronel is an inhibitor of the steroidogenic enzyme CYP17A1. However, orteronel has higher specificity for the 17,20 lyase activity of CYP17A1, while abiraterone acetate potently inhibits both CYP17A1’s 17,20 lyase and 17α-hydroxylase activities [14]. The increased specificity of orteronel inhibition potentially means less disruption to the glucocorticoid biosynthesis pathway (Figure 2) and less inhibition of other CYP enzymes, many of which are important for drug metabolism. Despite this, many of the orteronel clinical trials have included prednisone coadministration. Possible explanations for this are that the placebo arm of these trials included prednisone or owing to the concern of the risk of adrenal insufficiency with prolonged orteronel treatment.
Figure 2. Pathway of androgen production in castration-resistant prostate cancer.
The dashed line indicates potent inhibition by orteronel and the dotted line indicates weaker inhibition by orteronel. AR: Androgen receptor; DHEA: Dehydroepiandrosterone; DHT: Dihydrotestosterone.
Structurally, the fused imidazole ring system within orteronel makes it an attractive ligand for the catalytic heme iron that is found in many CYP enzymes. Orteronel’s selectivity for CYP17A1’s 17,20 lyase activity over 17α-hydroxylase and other CYP enzymes, such as CYP3A, is attributed to its conformational rigidity and low ClogP index (a measure of lipiphilicity) [15].
Preclinical studies
Pharmacokinetic characteristics
Preclinical studies demonstrate that orteronel has high bioavailability and a favorable area under the curve profile with oral administration [14,16]. Orteronel levels in the testes and adrenal glands in rats treated with orteronel peaked at 6 h with similar clearance kinetics across all tissue types tested [16]. Examination of orteronel’s molecular structure along with clearance kinetics suggest that CYP17A1 inhibition with orteronel is reversible, unlike abiraterone acetate [15,16].
Pharmacodynamics
Orteronel was 5.4-times more potent for inhibition of human 17,20 lyase activity versus 17α-hydroxylase activity in a cell-free enzyme assay [14]. These results were further validated in culture using human adrenal tumor cells treated with varying concentrations of orteronel or abiraterone acetate and monitored for the downstream 17,20 lyase metabolite dehydroepiandrosterone (DHEA) and the 17α-hydroxylase metabolite cortisol. Orteronel treatment led to potent suppression of DHEA – 27-fold more than cortisol suppression. Conversely, in the same assay, abiraterone acetate led to more similar suppression of DHEA and cortisol, highlighting orteronel’s specificity for 17,20 lyase inhibition. Similarly, in another study when rat testicular cells were treated with orteronel in culture, testosterone was potently inhibited with little effect on corticosterone [16].
In experiments with uncastrated rats, orteronel treatment significantly suppressed serum testosterone levels and decreased the overall weight of several androgen-dependent organs [16]. Furthermore, twice daily (b.i.d.) administration of orteronel in cynomolgus monkeys significantly reduced serum DHEA and testosterone levels compared with vehicle treatment [14,15]. Importantly, when orteronel treatment was combined with castration in these animals, testosterone levels were further suppressed versus castration alone, and this suppression was sustained throughout the duration of treatment. These results provided the rationale to test orteronel in patients with advanced prostate cancer.
Clinical studies
Phase I trials with orteronel in metastatic CRPC
The results of a Phase I/II dose-escalation study in 26 patients with metastatic CRPC were presented at the 2010 American Society of Clinical Oncology annual meeting [17]. That study evaluated b.i.d. orteronel at five dose levels: 100, 200, 300, 400 and 600 mg. An additional six patients received orteronel 400 mg b.i.d. plus prednisone 5 mg b.i.d. Six patients discontinued therapy due to adverse events (AEs), four owing to radiographic progression, two owing to PSA progression, two owing to patient request and one for ‘other reasons’. In total, 92% of patients experienced a drug-related AE, including 54% with grade ≥3 AEs. The most common treatment-emergent AEs were fatigue (65%), nausea (42%), constipation (38%), anorexia (35%) and vomiting (27%). At 4 weeks, testosterone levels decreased from 5.5 ng/dl to a median level of 0.6 ng/dl and DHEA decreased from 50.0 μg/dl to ‘unquantifiable levels’ in all patients. Of the cohorts that received ≥300 mg of orteronel for three cycles and had PSA levels measured at 3 months, 80% of patients had a ≥50% PSA decline and 27% had a ≥90% PSA decline (Tables 1 & 2).
Table 1.
Orteronel (TAK-700) Phase I, II and III clinical trials.
| Study (year) | Trial details | Outcome | Ref. |
|---|---|---|---|
| Dreicer et al. (2010) | Phase I/II in 26 men with metastatic CRPC | 300 mg b.i.d. is well tolerated and lowers PSA and androgen levels | [17] |
| Petrylak et al. (2012) | Phase I with docetaxel in 14 men with metastatic CRPC (≤1 prior cycle of chemotherapy) | 200 or 400 mg b.i.d. plus docetaxel/prednisone is safe and tolerable. 400 mg b.i.d. has androgen-lowering activity and was the recommended dose for a Phase II trial | [18] |
| Petrylak et al. (2013) | Phase II with docetaxel in 24 men with metastatic CRPC (chemotherapy naive) | Results for 400 mg b.i.d. plus docetaxel/prednisone at 3 months: ≥50% PSA decline: 72% ≥90% PSA decline: 28% Objective response rate: 56% Median time to PSA progression: 6.1 months Median time to radiographic progression: not reached |
[19] |
| Agus et al. (2012) | Phase I/II in 97 men with metastatic CRPC (chemotherapy naive) | Four dose cohorts ≥300 mg b.i.d. effective with similar efficacy in PSA response, androgen-lowering activity and reduction in CTCs ≥50% PSA decline at 12 weeks: 63% (300 mg b.i.d.) 50% (400 mg b.i.d. plus prednisone) 41% (600 mg b.i.d. plus prednisone) 60% (600 mg q.d.) Objective response rate: 19% |
[20] |
| George et al. (2012)† | Phase II of 38 men with nonmetastatic CRPC | 300 mg b.i.d. without steroids reduces testosterone and PSA levels | [21] |
| Hussain et al. (2013)† | Phase II of 38 men with nonmetastatic CRPC | ≥50% PSA decline at 3 months: 76% ≥50% PSA decline at 6 months: 45% Median PSA declined 83% Median time to PSA progression and progression-free survival: 13.8 months 1- and 2-year metastasis-free survival: 94 and 69%, respectively |
[22] |
| Dreicer et al. (2014) | Phase III of 1099 men randomized 2:1 to orteronel/prednisone vs placebo/prednisone | Orteronel does not improve overall survival (HR: 0.886 [95% CI: 0.739–1.062]; p = 0.1898) Orteronel does improve radiographic progression-free survival (HR: 0.76 [95% CI: 0.653–0.885]; p = 0.00038) |
[23] |
Same trial.
b.i.d.: twice daily; CRPC: Castrate-resistant prostate cancer; CTC: Circulating tumor cell; HR: Hazard ratio; PSA: Prostate-specific antigen; q.d.: Once daily.
Table 2.
Safety outcomes in clinical trials
| Study (year) | Study details | Toxicities | Ref. |
|---|---|---|---|
| Dreicer et al. (2010) | Orteronel Phase I/II | Most common TEAEs were fatigue, nausea, constipation, anorexia and vomiting | [17] |
| Petrylak et al. (2012) | Orteronel plus docetaxel Phase I | DLT was grade 3 febrile neutropenia at 400 mg b.i.d. Most common grade ≥3 AEs were neutropenia, hyperglycemia, febrile neutropenia, infusion reaction and hyponatremia | [18] |
| Petrylak et al. (2013) | Orteronel plus docetaxel Phase II | Most common grade ≥3 AEs were neutropenia, fatigue, leukopenia and dehydration Two on-study deaths unrelated to treatment |
[19] |
| Agus et al. (2012) | Orteronel Phase I/II | Most common AEs were fatigue, nausea and constipation. Most common grade ≥3 AEs were fatigue and hypokalemia | [20] |
| George et al. (2012)† | Orteronel Phase II | Most common grade ≥3 AEs were hypertension, dyspnea, fatigue, hypokalemia and pneumonitis | [21] |
| Hussain et al. (2013)† | Orteronel Phase II | Most common grade ≥3 AEs were hypertension, dyspnea, fatigue, hypokalemia and pneumonitis | [22] |
| Dreicer et al. (2014) | Orteronel Phase III | Most common AEs were nausea, vomiting and fatigue Most common grade ≥3 AEs were increased lipase, increased amylase and fatigue |
[23] |
Same trial.
AE: Adverse event; b.i.d.: Twice daily; DLT: Dose-limiting toxicity; TEAE: Treatment-emergent adverse event.
Petrylak et al. presented a Phase I study of docetaxel in combination with orteronel at the 2012 American Society of Clinical Oncology annual meeting [18]. In this study, orteronel was given b.i.d. with prednisone (5 mg b.i.d.) starting on day 8 of cycle one. A 3 + 3 dose escalation was used with a starting orteronel dose of 200 mg b.i.d. The dose was increased to 400 mg b.i.d.; a total of 14 men with metastatic CRPC were treated in this study. The only dose-limiting toxicity was grade 3 febrile neutropenia. A total of 11 men, including both dose groups, had a treatment-related grade ≥3 AE, including neutropenia (50%), hyperglycemia (21%), febrile neutropenia (14%), infusion reaction (14%) and hyponatremia (14%). The 400-mg dose also had the unique AE of hypophosphatemia. Five of 14 men had serious drug-related AEs. The most common serious drug-related AE was febrile neutropenia in two patients. At 3 months, 86% of patients had a ≥50% PSA decline, while 36% had a ≥90% PSA decline. Median testosterone levels declined to ≤0.2 ng/dl. The addition of orteronel to docetaxel appeared to have no evidence of AE potentiation, and orteronel 400 mg b.i.d. was the chosen dose for the subsequent Phase II study.
Phase II studies with orteronel in metastatic CRPC
Petrylak et al. subsequently performed the Phase II study using orteronel 400 mg b.i.d. plus docetaxel/prednisone [19]. The primary end points were: safety, pharmacokinetics and PSA response. Secondary objectives included: time to PSA progression and/or radiographic progression and radiographic response using Response Evaluation Criteria In Solid Tumors 1.1. At the time of that report, 24 men had been treated, and 12 remained on study. Nine men discontinued treatment due to AEs. All men experienced at least one grade ≥3 AE, and 23 were reported to be treatment-related. The most common treatment-related grade ≥3 AEs were neutropenia (38%), fatigue (21%), leukopenia (13%), and dehydration (13%). Two patients died in the study from causes that were felt to be unrelated to the treatment (disease progression and respiratory failure). At 3 months, 18 men were evaluable for PSA response. In total, 72% of men experienced a ≥50% PSA decline and 28% experienced a ≥90% PSA decline. Median testosterone levels decreased from 6.8 to ≤0.2ng/dl at 3 months. The overall objective response rate was 56%. Median time to PSA progression was 6.1 months, and median time to radiographic progression was not reached. There was no evidence of drug–drug interactions between orteronel and docetaxel/prednisone.
Agus et al. presented results from a Phase I/II study at the 2012 Genitourinary American Society of Clinical Oncology annual meeting. That trial evaluated four orteronel dose cohorts (300 mg b.i.d., 400 mg b.i.d. plus prednisone 4 mg b.i.d., 600 mg b.i.d. plus prednisone 4 mg b.i.d. and 600 mg daily [q.d.]) in 97 subjects with metastatic CRPC who had no prior chemotherapy exposure [20]. A total of 19% of subjects were withdrawn from the study owing to AEs. The most common AEs were fatigue (76%), nausea (47%) and constipation (38%). The most common grade ≥3 AEs were fatigue (12%) and hypokalemia (8%). PSA response rates (≥50% PSA decline) at 12 weeks were 63, 50, 41 and 60% in the 300 mg b.i.d., 400 mg b.i.d. plus prednisone, 600 mg b.i.d. plus prednisone and 600 mg q.d. groups, respectively. Testosterone and DHEA levels decreased on study in all dose cohorts, and mean circulating tumor cell levels decreased from 16.6 per 7.5 ml of blood to 3.9 at 12 weeks.
Phase II studies with orteronel in nonmetastatic CRPC
Two abstracts presented data from a Phase II study evaluating the efficacy of orteronel 300 mg b.i.d. without concomitant steroids in 38 men with nonmetastatic CRPC [21,22]. The primary end point was the percentage of men who achieved a PSA ≤0.2 ng/ml at 3 months. Secondary end points included: safety, PSA kinetics, progression-free survival, time to PSA progression, time to metastases, changes in endocrine and bone markers, changes in bone mineral density and circulating tumor cell counts. The median duration of therapy was 12.4 months. A total of 12 patients discontinued the study owing to AEs, including two cases of possible adrenal insufficiency. The most common grade ≥3 AE was hypertension in seven patients [22]. In total, 14 patients experienced other grade ≥3 AEs, including dyspnea (8%), fatigue (5%), hypokalemia (5%) and pneumonitis (5%) [21]. At 3 months, median testosterone declined by 89% to 0.78 ng/dl, median adrenocorticotropic hormone increased by 171%, and median cortisol declined by 21% but remained within normal range. A total of 18% of patients had a PSA ≤0.2 ng/ml at 3 months [21,22] with 76 and 32% of men achieving a ≥50% PSA decline or ≥90% PSA decline, respectively [21]. At 6 months, 45% of men experienced a ≥50% PSA decline, and 21% of men experienced a ≥90% PSA decline [21]. The median time to PSA progression and progression-free survival were both 13.8 months. The 1-year metastasis-free survival was 94%, and the 2-year metastasis-free survival was 69% [22].
Phase III orteronel studies
There are currently five ongoing Phase III studies evaluating the role of orteronel in various stages of prostate cancer.
Phase III studies with orteronel in metastatic CRPC after docetaxel chemotherapy
ELM-PC 5 was a Phase III multicenter, randomized, double-blind, placebo-controlled study evaluating orteronel 400 mg b.i.d. plus prednisone 5 mg b.i.d. versus placebo plus prednisone (randomized 2:1) in 1099 men with metastatic CRPC who had radiographic evidence of progression within 6 months of receiving a cumulative dose of ≥225 mg/m2 of docetaxel. The primary end point was overall survival. Secondary end points included: radiographic progression-free survival, PSA response rate, pain response, safety, time to PSA progression, circulating tumor cell and endocrine marker levels, and patient-reported outcomes. At the prespecified interim analysis, orteronel plus prednisone compared with the control arm was unblinded after orteronel failed to meet the primary end point of improved overall survival. The median overall survival was 17.0 months (95% CI: 15.2–19.9) in the orteronel arm versus 15.2 months (95% CI: 13.5–16.9) in the placebo arm (HR: 0.886 [95% CI: 0.739–1.062]; p = 0.1898) [23]. However, radiographic progression-free survival was significantly improved with a median of 8.3 months for orteronel treatment versus 5.7 months with placebo (HR: 0.76 [95% CI: 0.653–0.885]; p = 0.00038) [23]. On 25 July 2013, Takeda Pharmaceutical Company Limited (Osaka, Japan) made the decision to unblind the study and allow patients to continue taking orteronel at the discretion of their treating physicians. It will be important to determine the proportion of patients in each arm who went on to receive other life-extending therapies after withdrawal from the study. Subsequent treatment of patients from the placebo arm with drugs, such as abiraterone acetate or enzalutamide, may explain why this study demonstrated an improvement in progression-free survival without an improvement in overall survival.
SAKK 08/11 is a Phase III Swiss multicenter, randomized, double-blind, placebo-controlled study designed to determine the role of maintenance orteronel. A total of 192 men with metastatic CRPC who have stable disease (based on PSA and imaging) after receiving a cumulative dose of ≥300 mg/m2 of docetaxel will be enrolled on this trial. Orteronel 300 mg b.i.d. plus best supportive care will be compared with placebo plus best supportive care. The primary end point is event-free survival. Secondary end points include: safety, PSA response, time to PSA progression, radiographic progression-free survival, quality of life, pain response and overall survival.
Phase III studies with orteronel in metastatic CRPC prior to docetaxel chemotherapy
ELM-PC 4 is a Phase III study evaluating orteronel 400 mg b.i.d. plus prednisone 5 mg b.i.d. versus placebo plus prednisone in chemotherapy-naive patients [24]. Planned enrollment is 1454 patients. Patients who require opiates for bone pain or who have received ‘adrenal-targeting therapy’ or chemotherapy in the past 2 years are excluded. The primary end points include: radiographic progression-free survival and overall survival. Secondary end points include: PSA response rate (≥50% decline) at 12 weeks, time to pain progression and change in circulating tumor cell numbers. Two interim analyses are planned, but results have yet to be released.
Phase III study with orteronel in metastatic ADT-naive prostate cancer
SWOG-1216 is an on-going Phase III multicenter, randomized, controlled study evaluating orteronel versus the AR antagonist bicalutamide as part of a combined androgen blockade with an LHRH agonist in men with newly diagnosed, ADT-naive metastatic prostate cancer. A total of 1486 men will be randomized to LHRH agonist plus orteronel 300 mg b.i.d. versus LHRH agonist plus bicalutamide 50 mg q.d. Patients must not have received chemotherapy, abiraterone acetate, ketoconazole or enzalutamide for metastatic disease, but are allowed to have received neoadjuvant or adjuvant chemotherapy for curative intent if these were given ≥2 years prior to enrollment. The primary end point is overall survival.
Phase III study with orteronel in high-risk localized prostate cancer
RTOG-1115 is a Phase III multicenter, randomized, controlled study evaluating radiation therapy plus combined androgen blockade (LHRH agonist plus an AR antagonist) with or without the addition of 2 years of orteronel treatment b.i.d. The sample size is 900, and this trial focuses on men with high-risk localized prostate cancer. The primary end point is overall survival. Secondary end points include: safety, prevention of biochemical (PSA) recurrence, progression-free survival, prostate cancer-specific survival, quality of life measures, testosterone levels and clinical survivorship end points.
Safety & tolerability
Overall, orteronel appears to be well tolerated in doses ranging from 200 to 600 mg b.i.d. (Table 2). In Phase I clinical studies, the only dose-limiting toxicity was grade 3 febrile neutropenia with orteronel 400 mg b.i.d. in combination with docetaxel chemotherapy [18]. In Phase II studies, the most common AEs were fatigue, nausea and constipation [18,20]. Grade ≥3 AEs included neutropenia (with orteronel in combination with docetaxel), fatigue, hypokalemia, dyspnea, pneumonitis and hypertension [17–18,20–21]. Two out of 38 men treated with orteronel without steroids developed adrenal insufficiency [21,22]. The addition of orteronel to docetaxel/prednisone reportedly had no evidence of AE potentiation or drug–drug interactions [18,19].
In the ELM-PC 5 Phase III study of orteronel plus prednisone versus placebo plus prednisone, the most common drug-related AEs (any grade) included: nausea (30% orteronel, 16% placebo), vomiting (23% orteronel, 8% placebo) and fatigue (17% orteronel, 11% placebo) [23]. The most common grade ≥3 drug-related AEs included: elevated lipase (12% orteronel, <1% placebo), increased amylase (8% orteronel, <1% placebo) and fatigue (3% orteronel, 3% placebo) [23].
Ongoing Phase III studies are currently evaluating orteronel 300 or 400 mg b.i.d. doses. We await the safety results with these doses in these larger trials.
Conclusion
Orteronel is a novel CYP17A1 inhibitor that effectively suppresses androgen synthesis and PSA values in single agent Phase I and II studies [17,20–22], and in studies in combination with docetaxel chemotherapy [18,19]. As orteronel has greater effects on 17,20-lyase versus 17-α-hydroxylase, it may be possible to avoid concomitant corticosteroid administration. Indeed, orteronel regimens without steroids have been safely administered with only rare cases of adrenal insufficiency [21,22]. Overall, orteronel is well tolerated with the most common reported AEs of: fatigue, neutropenia, hypokalemia, nausea, vomiting, constipation, hypertension, dyspnea and pneumonitis [17–22]. An interim analysis of a Phase III clinical trial with orteronel in patients with metastatic CRPC who had received prior docetaxel chemotherapy did not achieve its primary end point of overall survival [23]. This is in contrast to Phase III trials of other novel hormonal agents, abiraterone acetate and enzalutamide, that did demonstrate improved overall survival in a similar patient group [7,9]. Additional Phase III studies are currently underway to determine if orteronel improves survival for CRPC patients in the prechemotherapy setting, where abiraterone acetate is already approved and where it is likely that enzalutamide will soon be approved based on a recent analysis of the PREVAIL trial. We await the results of this orteronel trial and trials of orteronel in even earlier disease states to determine whether orteronel will be added to an expanded arsenal of treatments to combat advanced prostate cancer.
EXECUTIVE SUMMARY.
Mechanism of action
Orteronel is a novel, oral, nonsteroidal, selective inhibitor of CYP17A1 that suppresses androgen production.
Pharmacokinetic/pharmacodynamic properties
Orteronel has high bioavailability as both an oral and intravenous agent.
Orteronel is a more potent inhibitor of CYP17A1’s 17,20 lyase activity compared with its 17α-hydroxylase activity.
Clinical efficacy
Orteronel is an investigational drug under study in the treatment of metastatic prostate cancer (both androgen deprivation therapy [ADT]-naive and castrate-resistant prostate cancer), as well as newly diagnosed, high-risk localized prostate cancer.
Phase II studies are promising with evidence of androgen suppression and prostate-specific antigen responses in doses ≥300 mg twice daily ± prednisone.
Interim analysis of one Phase III trial (ELM-PC 5) did not meet the primary end point of improved overall survival in the postdocetaxel setting and was unblinded.
Additional Phase III studies evaluating the role of orteronel in chemotherapy-naive metastatic castrate-resistant prostate cancer, ADT-naive metastatic prostate cancer and ADT-naive, high-risk localized prostate cancer are ongoing.
Safety & tolerability
Fatigue and nausea were the most common adverse event in single-agent orteronel studies while the dose-limiting toxicity of orteronel in combination with docetaxel was febrile neutropenia. Severe adverse effects include: pneumonitis, febrile neutropenia (orteronel in combination with docetaxel) and dehydration. Overall, the most common adverse effects include: nausea, vomiting, fatigue, neutropenia (orteronel in combination with docetaxel), hypokalemia, anorexia and constipation.
There are no reported specific drug–drug interactions at this time.
Orteronel treatment appears to be well tolerated at doses of 300 and 400 mg twice daily.
Footnotes
Financial & competing interests disclosure
This publication was made possible with support by the Pacific Northwest Prostate Cancer SPORE/National Cancer Institute (P50CA097186; JJ Alumkal), a Stand Up to Cancer Dream Team Award (7465sc; JJ Alumkal), a Department of Defense Synergistic Idea Award (W81XWH-13-1-0420; JJ Alumkal), a Wayne D Kuni and Joan E Kuni Foundation Kuni Scholar Award (JJ Alumkal), and a Prostate Cancer Foundation Young Investigator Award (JJ Alumkal). JJ Alumkal has previously received an honorarium for consulting from Millennium Pharmaceuticals. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
For reprint orders, please contact: reprints@futuremedicine.com
Information resources
Yamaoka et al. describes the effects of orteronel treatment both in vitro and in vivo in preclinical studies [14].
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holzbeierlein J, Lal P, Latulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164(1):217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 5.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17(18):5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, Smith MR, De Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Tannock IF, De Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 12.De Bono JS, Oudard S, Ozguroglu M. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 13.Parker C, Sartor O. Radium-223 in prostate cancer. N Engl J Med. 2013;369(17):1659–1660. doi: 10.1056/NEJMc1310231. [DOI] [PubMed] [Google Scholar]
- 14.Yamaoka M, Hara T, Hitaka T, et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol. 2012;129(3–5):115–128. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Kaku T, Hitaka T, Ojida A, et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg Med Chem. 2011;19(21):6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 16.Hara T, Kouno J, Kaku T, et al. Effect of a novel 17,20-lyase inhibitor, orteronel (TAK-700), on androgen synthesis in male rats. J Steroid Biochem Mol Biol. 2013;134:80–91. doi: 10.1016/j.jsbmb.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Dreicer R, Agus DB, Macvicar GR, Wang J, Maclean D, Stadler WM. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostrate cancer: a Phase I/II, open-label study. J Clin Oncol. 2010;28(15 Suppl):Abstract 3084. [Google Scholar]
- 18.Petrylak DP, Gandhi JG, Clark WR, et al. Phase I results from a Phase I/II study of orteronel, an oral, investigational, nonsteroidal 17,20-lyase inhibitor, with docetaxel and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2012;30(15 Suppl):Abstract 4656. [Google Scholar]
- 19.Petrylak D, Gandhi JG, Clark WR, et al. A Phase I/II study of safety and efficacy of orteronel (TAK-700), an oral, investigational, nonsteroidal 17,20-lyase inhibitor, with docetaxel and prednisone (DP) in metastatic castration-resistant prostate cancer (mCRPC): updated Phase II results. J Clin Oncol. 2013;31(6 Suppl):Abstract 59. [Google Scholar]
- 20.Agus DB, Stadler WM, Shevrin DH, et al. Safety, efficacy, and pharmacodynamics of the investigational agent orteronel (TAK-700) in metastatic castration-resistant prostate cancer (mCRPC): updated data from a Phase I/II study. J Clin Oncol. 2012;30(5 Suppl):Abstract 98. [Google Scholar]
- 21.George DJ, Corn PG, Michaelson MD, et al. Safety and activity of the investigational agent orteronel (ortl) without prednisone in men with nonmetastatic castration-resistant prostate cancer (nmCRPC) and rising prostate-specific antigen (PSA): updated results of a Phase II study. J Clin Oncol. 2012;30(15 Suppl):Abstract 4549. [Google Scholar]
- 22.Hussain M, Corn PG, Michaelson MD, et al. Safety, efficacy, and health-related quality of life (HRQoL) of the investigational single agent orteronel (ortl) in nonmetastatic castration-resistant prostate cancer (nmCRPC) J Clin Oncol. 2013;31(15 Suppl):Abstract 5076. [Google Scholar]
- 23.Dreicer R, Jones R, Oudard S, et al. Results from a Phase 3, randomized, double-blind, multicenter, placebo-controlled trial of orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel-based therapy (ELM-PC 5 trial) J Clin Oncol. 2014;32(Suppl 4):Abstract 7. doi: 10.1200/JCO.2014.56.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad F, Akaza H, Eisenberger MA, et al. A Phase III, randomized study of the investigational agent TAK-700 plus prednisone for patients with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2011;29(15 Suppl):Abstract TPS184. [Google Scholar]



