Abstract
Trigeminal neuralgia (TN), a neuropathic disorder of one or both of the trigeminal nerves, occurs most often in people over age 50. Extreme, sporadic, sudden burning or shock-like face pain in common activities greatly lowers quality of life. The precise cause of primary TN remains unknown, but it may be caused by vascular pressing on the trigeminal nerve in its root entry zone (REZ), demyelinization of trigeminal sensory fibers, or jawbone cavity. Accordingly, many treatments carry risks of adverse effects, recurrence, and complications. TN and osteoporosis have similar high-risk populations and a common influential factor – emotional stress – is also closed related to primary TN for calcitonin gene-related peptide and calcitonin. Jawbone cavity, which is a possible pathogenesis of TN, may be another form of jawbone osteoporosis. Therefore, we hypothesized that osteoporosis in jaws could be a correlative factor of primary TN. If this hypothesis is verified, it may suggest specific new ideas for the early preventive treatment of primary TN.
MeSH Keywords: Calcitonin; Calcitonin Gene-Related Peptide; Osteoporosis; Stress, Physiological; Trigeminal Neuralgia
Background
Trigeminal neuralgia (TN), a neuropathic disorder of one or both of the trigeminal nerves, occurs most often in people over age 50, and affects women more often than men. TN can be either primary trigeminal neuralgia or secondary trigeminal neuralgia, which is associated with some other diseases, with symptoms such as extreme sporadic sudden burning or shock-like face pain triggered by common activities such as eating, talking, shaving, and toothbrushing. Although TN is not a fatal disorder, it can greatly worsen patient quality of life [1]. Osteoporosis is a skeletal disease characterized by an imbalance between excessive bone resorption and inadequate bone formation and increased fracture risk. The fracture can occur in almost any bone, either in the form of cracking or collapsing. The extent of systemic osteoporosis has a positive correlation with the degree of mandibular mineral loss [2].
The precise cause of primary trigeminal neuralgia remains unknown [3]. Currently considered explanations, such as a vascular pressing on the trigeminal nerve in its root entry zone (REZ) [4,5], a demyelinization of trigeminal sensory fibers[6], and jawbone cavity[7], fail to sufficiently explain the total clinical picture [8]. Although there are fairly standard treatment approaches for TN, management with anti-epileptic drugs or surgical procedures carries risks of adverse effects, recurrence, and complications [9].
Because of the many similarities between trigeminal neuralgia and osteoporosis and some indirect evidence, we hypothesized that osteoporosis in the jaws could be a correlative factor of primary trigeminal neuralgia, which has never been clearly presented before. If this hypothesis is verified, it may suggest specific new ideas for the early preventive treatment of primary trigeminal neuralgia.
The Evidences
The similar high risk populations of trigeminal neuralgia and osteoporosis
Trigeminal neuralgia is a syndrome of intermittent, severe facial pain, and affects about 4.3 per 100 000 people per annum. It occurs most often in people over age 50 and affects females nearly twice as often as males [10]. Osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone tissue leading to enhanced bone fragility and a consequent increase in fracture incidence. It is a common disease in older adults; approximately 70% of cases are females, double the rate in males [11].
Emotional stress in trigeminal neuralgia and osteoporosis
Trigeminal neuralgia should be managed by a multidisciplinary team. It is a chronic condition and like all other chronic pain is associated with numerous co-morbidities such as depression. Often a combination of antidepressants and cognitive behavior therapy is effective. In an attack of trigeminal neuralgia, the rise of calcitonin gene-related peptide (CGRP) levels may decrease the threshold of pain in the local region. The study of CGRP in subjects with depression also shows CGRP levels were increased in subjects with depression, both in plasma and in the cerebrospinal fluid [12]. The 24-h circulating levels of CGRP are higher in women with depression compared to controls, suggesting that CGRP may be useful biological marker in women with major depressive disorder [13].
In the last decade, the accumulating evidence for the relationship of osteoporosis with psychological variables supports the conclusion that depression is associated with low BMD, with a substantially greater BMD decrease in depressed women and in cases of clinical depression. Depression should be considered as an important risk factor for osteoporosis [14].
Calcitonin gene-related peptide in trigeminal neuralgia and osteoporosis
CGRP, expressed by the calcitonin gene, is a kind of bioactive polypeptide found in various body systems: nervous, cardiovascular, digestive, respiratory, and endocrine systems. A subset of trigeminal nerve fibers containing CGRP responds to physical and chemical irritants in the environment. As the irritant transmitter or modulator, in an attack of trigeminal neuralgia, rising CGRP may decrease the threshold of pain in the local region [15].
The nerves containing CGRP are distributed throughout all body tissues, including bones [16]. Osteoblasts and osteoclasts, in response to CGRP, synthesizing and absorbing sclerostin, mainly determine the metabolism of bone. CGRP may change the release of osteoblastic cytokine (stimulating the production of the growth factor insulin-like growth factor-I and inhibiting that of the cytokine tumor necrosis factor-α) and indirectly regulate the suppressed bone resorption of osteoclasts, when injecting it into evirated rats [17]. CGRP affects the development of osteoclasts in bone marrow culture solution, as well as the differentiation and generation of osteoclastic progenitor cells [18,19]. Histochemical experiments have shown that CGRP integrates with osteoblasts, facilitating osteocytes division [20,21]. In addition, CGRP can clearly increase intra-cellular cAMP, which is relevant to osteoblastic secretion [22]. The above evidence indicates that CGRP facilitates osseous synthesis and absorption by binding to their receptors on osteoblasts and osteoclasts.
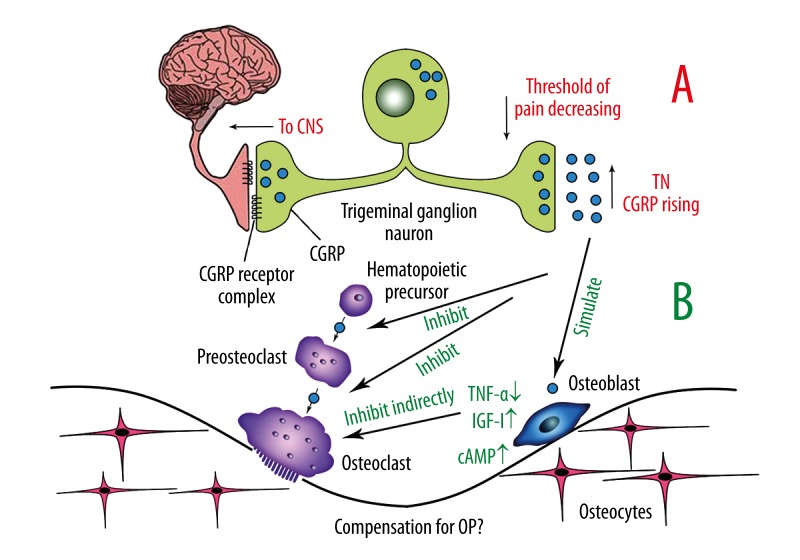
The nerve fibers containing CGRP are associated in the skeletal system with the nervous system. As a sensory nerve, they transmit osseous proprioception up to the sensory center, and at the same time, transmit neurocrine CGRP down to bone tissue. CGRP increases bone mass through affecting osteoblasts and osteoclasts. Thus, we hypothesize that the rise of CGRP level in an attack of trigeminal neuralgia is a compensation for decreased bone mass in osteoporosis (Figure 1).
Figure 1.
CGRP in TN and OP A: Indicated by the red words is the probable procedure in which CGRP affects TN. B: Indicated by the green words shows CGRP accommodates osseous synthesis.
Calcitonin in trigeminal neuralgia and osteoporosis
As a peptide hormone that lowers calcium concentration in the blood, released by thyroid cells and acting to decrease the formation and absorptive activity of osteoclasts, calcitonin, a member of the calcitonin gene-related peptide family, has been approved for the treatment of osteoporosis for over 40 years, working by inhibiting mature active osteoclasts and increasing renal calcium excretion [23]. In addition to its effect on reducing bone resorption, calcitonin has an analgesic action, the mechanism of which is still unclear, but is possibly mediated through β-endorphins and the central modulation of pain perception [24]. The analgesic activity of salmon calcitonin is effective in patients with various painful conditions, including vertebral crush fractures, adhesive capsulitis, ankylosing spondylitis, rheumatoid arthritis, metastasis, and reflex sympathetic dystrophy syndrome. Because of the current presumptions by pathologists that trigeminal neuralgia is caused by a vascular pressing on the trigeminal nerve in its REZ and a demyelinization of trigeminal sensory fibers, some scholars presume that calcitonin, the medicine used to treat osteoporosis, could also be a useful analgesic agent for trigeminal neuralgia [25].
Jawbone cavity in trigeminal neuralgia and osteoporosis
Neuralgia-inducing cavitational osteonecrosis (NICO), first defined by Ratner, served as the basis for explaining the pain syndrome with features of trigeminal neuralgia [26]. Several investigations have shown intraosseous inflammation in jawbones of trigeminal neuralgia patients, and certain treatments for these lesions (localization, decortication, and curettage) reduced or eliminated this intense pain [6,27], but this has been a controversy for several years. Changing etiologic concepts have led to confusion as well as to significant departures from the initial concept. The absence of any form of research design and approval by institutional review panels remains a weakness in terms of acceptance of the information provided in the literature said to support the stated etiology of this entity [26]. Thus, we hypothesized that the jawbone cavity in trigeminal neuralgic patients is another form of osteoporosis in maxilla and mandible.
To observe jawbone metabolism in TN patients, in our preceding study we analyzed panoramic radiographs of 59 TN patients (30 males and 29 females) and in a matched control group, sub-grouping by age and sex. The demarcated regional optical density (OD) of the mandible body was measured by software to compare their mandibular density. It was found that OD in the TN group (male 0.432±0.016; female 0.411±0.019) was significantly (P<0.01) lower than that in the control group (male 0.459±0.014; female 0.448±0.013). This suggests that abnormal jawbone metabolism and low mandibular density may be related to early-stage TN (Figure 2).
Figure 2.
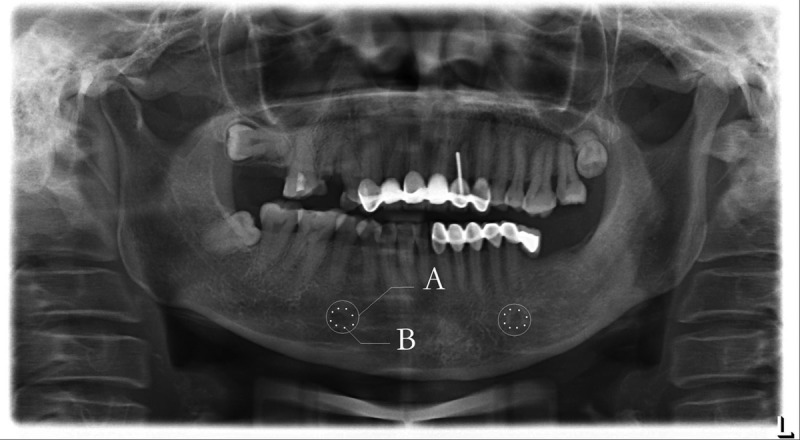
OD measurement on panoramic radiograph A: White ring-shapes are demarcated regions of mandible, centering mental foramen, dia. 1 cm. B: Eight points in each ring are random measured locations, with a mean value for final outcome.
Consequences of the Hypothesis and Discussion
It used to be widely believed that primary trigeminal neuralgia occurred potentially due to vascular pressing on the trigeminal nerve in its REZ, demyelinization of trigeminal sensory fibers, or jawbone cavity. However, we think that primary trigeminal neuralgia is also closely related to osteoporosis. Although there are fairly standard treatment approaches for TN, management with anti-epileptic drugs or surgical procedures carries risks of adverse effects, recurrence, and complications [9], with lack of certainty regarding the etiology and pathophysiology of TN. The inability to induce convincing TN in animals should not necessarily be regarded as a surprise [28]. In the future, perhaps we can survey the morbidity of TN in an osteoporosis group and in an osteoporosis-controlled group. If the difference in TN morbidity in the 2 groups is significant, the relationship between primary trigeminal neuralgia and osteoporosis may be verified further. In our opinion, osteoporosis patients should receive management in the early stage for ostalgia and increased fracture risk. We desire to see the number of people with trigeminal neuralgia decrease by prevention of osteoporosis.
Footnotes
Source of support: This study was supported by Program for New Century Excellent Talents in University (NCET-10-0597) and by Fundamental Research Funds for the Central Universities (2012SCU4A12)
Conflicts of interest statement
None of the authors have a financial or personal relationship with other people or organizations that could inappropriately influence this work.
References
- 1.Tölle T, Dukes E, Sadosky A. Patient Burden of Trigeminal Neuralgia: Results from a Cross-Sectional Survey of Health State Impairment and Treatment Patterns in Six European Countries. Pain Pract. 2006;(3):153–60. doi: 10.1111/j.1533-2500.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- 2.Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Rad. 1997;26(1):3–15. doi: 10.1038/sj.dmfr.4600226. [DOI] [PubMed] [Google Scholar]
- 3.Nurmikko TJ, Eldridge PR. Trigeminal neuralgia – pathophysiology, diagnosis and current treatment. Brit J Anaesth. 2001;87(1):117–32. doi: 10.1093/bja/87.1.117. [DOI] [PubMed] [Google Scholar]
- 4.Barker FG, Jannetta PJ, Bissonette DJ, et al. The long-term outcome of microvascular decompression for trigeminal neuralgia. New Engl J Med. 1996;334(17):1077–84. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 5.Patel NK, Aquilina K, Clarke Y, et al. How accurate is magnetic resonance angiography in predicting neurovascular compression in patients with trigeminal neuralgia? A prospective, single-blinded comparative study. Brit J Neurosur. 2003;17(1):60–64. [PubMed] [Google Scholar]
- 6.McLaughlin MR, Jannetta PJ, Clyde BL, et al. Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosur. 1999;90(1):1–8. doi: 10.3171/jns.1999.90.1.0001. [DOI] [PubMed] [Google Scholar]
- 7.Bouquot JE, Roberts AM, Person P, Christian J. Neuralgia-inducing cavitational osteonecrosis (NICO): osteomyelitis in 224 jawbone samples from patients with facial neuralgia. Oral Surg Oral Med O. 1992;73(3):307–19. doi: 10.1016/0030-4220(92)90127-c. [DOI] [PubMed] [Google Scholar]
- 8.Lang E, Naraghi R, Tanrikulu L, et al. Neurovascular relationship at the trigeminal root entry zone in persistent idiopathic facial pain: findings from MRI 3D visualisation. J Neurol Neurosur Ps. 2005;76(11):1506–9. doi: 10.1136/jnnp.2005.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spatz AL, Zakrzewska JM, Kay EJ. Decision analysis of medical and surgical treatments for trigeminal neuralgia: how patient evaluations of benefits and risks affect the utility of treatment decisions. Pain. 2007;131(3):302–10. doi: 10.1016/j.pain.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 10.Katusic S, Beard CM, Bergstralth E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol. 1990;27(1):89–95. doi: 10.1002/ana.410270114. [DOI] [PubMed] [Google Scholar]
- 11.Johnson M. Osteoporosis. In: Enna SJ, Bylund DB, editors. xPharm: The Comprehensive Pharmacology Reference. New York: Elsevier; 2007. pp. 1–5. [Google Scholar]
- 12.Mathé AA, Ågren H, Lindström L, Theodorsson E. Increased concentration ofcalcitonin gene-related peptide in cerebrospinal fluid of depressed patients. Apossible trait marker of major depressive disorder. Neurosci Lett. 1994;182(2):138–42. doi: 10.1016/0304-3940(94)90782-x. [DOI] [PubMed] [Google Scholar]
- 13.Hartman JM, Berger A, Baker K, et al. Quality of life and pain in premenopausal women with major depressive disorder: The POWER Study. Health Qual Life Out. 2006;4:2. doi: 10.1186/1477-7525-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, Magnus JH, Liu J, et al. Depression and low bone mineral density: a meta-analysis of epidemiologic studies. Osteoporosis Int. 2009;20(8):1309–20. doi: 10.1007/s00198-009-0918-x. [DOI] [PubMed] [Google Scholar]
- 15.Silver WL, Finger TE. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann NY Acad Sci. 2009;1170(1):202–5. doi: 10.1111/j.1749-6632.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 16.Irie K, Hara-Irie F, Ozawa H, Yajima T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Techniq. 2002;58(2):85–90. doi: 10.1002/jemt.10122. [DOI] [PubMed] [Google Scholar]
- 17.Valentijn K, Gutow AP, Troiano N, et al. Effects of calcitonin gene-related peptide on bone turnover in ovariectomized rats. Bone. 1997;21(3):269–74. doi: 10.1016/s8756-3282(97)00142-7. [DOI] [PubMed] [Google Scholar]
- 18.Cornish J, Callon KE, Bava U, et al. Effects ofcalcitonin, amylin, and calcitonin gene-related peptide on osteoclast development. Bone. 2001;29(2):162–68. doi: 10.1016/s8756-3282(01)00494-x. [DOI] [PubMed] [Google Scholar]
- 19.Akopian A, Demulder A, Ouriaghli F, et al. Effects of CGRP on human osteoclast-like cell formation: a possible connection with the bone loss in neurological disorders? Peptides. 2000;21(4):559–64. doi: 10.1016/s0196-9781(00)00185-6. [DOI] [PubMed] [Google Scholar]
- 20.Kawase T, Burns DM. Calcitonin gene-related peptide stimulates potassium efflux through adenosine triphosphate-sensitive potassium channels and produces membrane hyperpolarization in osteoblastic UMR106 cells. Endocrinology. 1998;139(8):3492–502. doi: 10.1210/endo.139.8.6151. [DOI] [PubMed] [Google Scholar]
- 21.Villa I, Dal Fiume C, Maestroni A, et al. Human osteoblast-like cell proliferation induced by calcitonin-related peptides involves PKC activity. Am J Physiol Endocrinol Metab. 2003;284(3):E627–33. doi: 10.1152/ajpendo.00307.2002. [DOI] [PubMed] [Google Scholar]
- 22.Michelangeli VP, Fletcher AE, Allan EH, et al. Effects ofcalcitonin gene-related peptide on cyclic AMP formation in chicken, rat, andmouse bone cells. J Bone Miner Res. 1989;4(2):269–72. doi: 10.1002/jbmr.5650040220. [DOI] [PubMed] [Google Scholar]
- 23.Zaidi M, Inzerillo AM, Moonga BS, et al. Forty years of calcitonin – where are we now? A tribute to the work of Iain Macintyre, FRS. Bone. 2002;30(5):655–63. doi: 10.1016/s8756-3282(02)00688-9. [DOI] [PubMed] [Google Scholar]
- 24.Azria M. Possible mechanisms of the analgesic action of calcitonin. Bone. 2002;30(5):80–83. doi: 10.1016/s8756-3282(02)00701-9. [DOI] [PubMed] [Google Scholar]
- 25.Qin H, Cai J, Yang FS. Could calcitonin be a useful therapeutic agent for trigeminal neuralgia? Med Hypotheses. 2008;71(1):114–16. doi: 10.1016/j.mehy.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Sciubba JJ. Neuralgia-inducing cavitational osteonecrosis: a status report. Oral Dis. 2009;15(5):309–12. doi: 10.1111/j.1601-0825.2009.01532.x. [DOI] [PubMed] [Google Scholar]
- 27.Bouquot JE, Christian J. Long-term effects of jawbone curettage on the painoffacial neuralgia. J Oral Maxil Surg. 1995;53(4):387–97. doi: 10.1016/0278-2391(95)90708-4. [DOI] [PubMed] [Google Scholar]
- 28.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18(1):4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]