Abstract
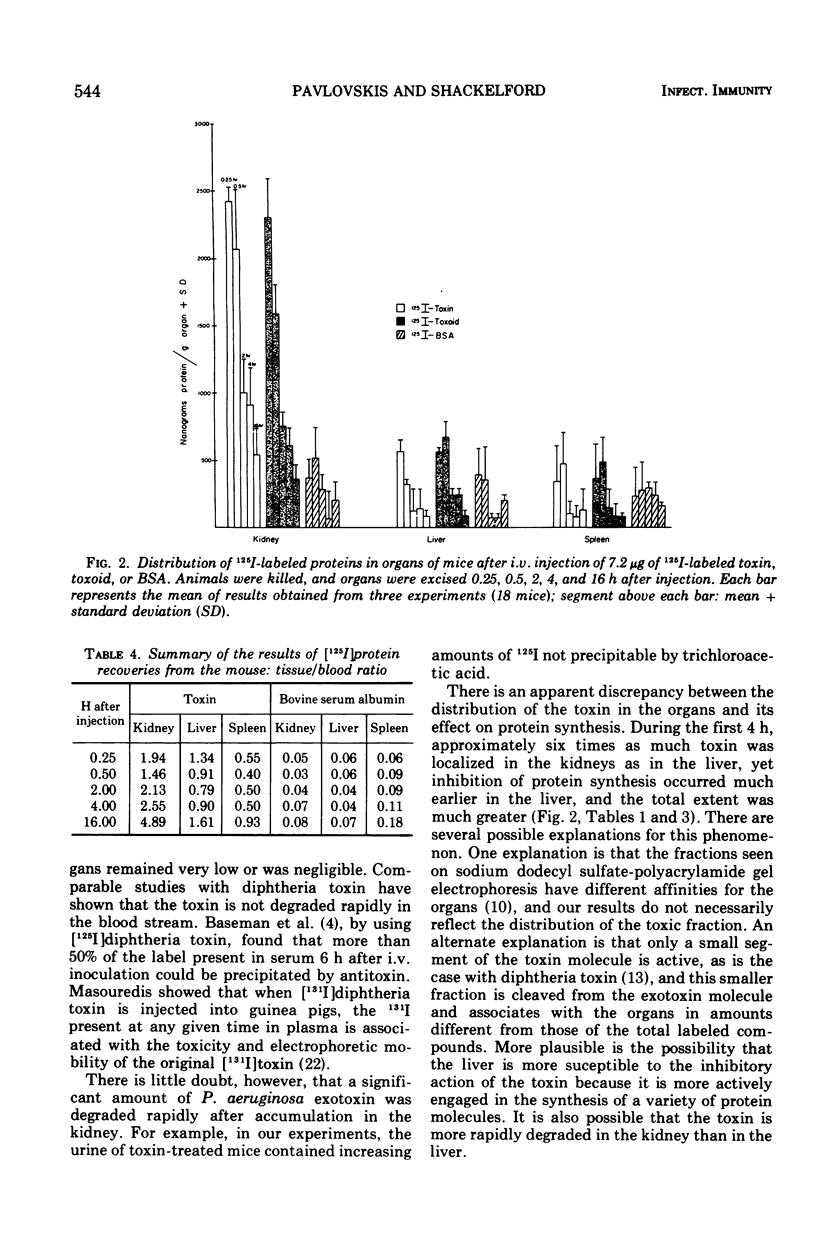
We studied the fate of 125I-labeled Pseudomonas aeruginosa (PA 103) exotoxin injected intravenously into mice and the effect of the exotoxin on the protein synthesis of various organs. After 2 h, only 5% of the injected label remained in the blood, in comparison with 30% of the injected control, consisting of [125I]bovine serum albumin. The highest concentration of radioactivity was consistently observed in the kidneys of toxin-treated mice. Lesser amounts of the label were found in the liver and the spleen. The heart, pancreas, lung, and brain showed very little uptake. Approximately 30% of the label (non-trichloroacetic acid precipitable) was recovered from the urine within 2 h after the injection. The behavior of [125I]toxoid was similar to that of the [125I]toxin. In contrast, in [125I]bovine serum albumin-treated mice, the label was uniformly distributed among the organs examined, and the concentration was low. After intravenous administration of the exotoxin, a 50% inhibition of protein synthesis was observed in the liver within 4 h, and virtually complete inhibition was observed shortly before the time of death. Kidney and spleen displayed slight reduction of protein synthesis at 2 to 4 h and approximately 50% reduction during the terminal stage. It appeared that the highest concentration of the toxin occurred in the kidney, where it was degraded, but its greatest toxic effect took place in the liver.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong A. V., Stewart-Tull D. E., Roberts J. S. Characterisation of the Pseudomonas aeruginosa factor that inhibits mouse-liver mitochondrial respiration. J Med Microbiol. 1971 May;4(2):249–262. doi: 10.1099/00222615-4-2-249. [DOI] [PubMed] [Google Scholar]
- Atik M., Liu P. V., Hanson B. A., Amini S., Rosenberg C. F. Pseudomonas exotoxin shock. A preliminary report of studies in dogs. JAMA. 1968 Jul 15;205(3):134–140. doi: 10.1001/jama.205.3.134. [DOI] [PubMed] [Google Scholar]
- BERK R. S. PARTIAL PURIFICATION OF THE EXTRACELLULAR HEMOLYSIN OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1964 Sep;88:559–565. doi: 10.1128/jb.88.3.559-565.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Garcia M. The lethal events in experimental Pseudomonas aeruginosa infection of mice. J Infect Dis. 1968 Apr;118(2):165–172. doi: 10.1093/infdis/118.2.165. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Pappenheimer A. M., Jr, Gill D. M., Harper A. A. Action of diphtheria toxin in the guinea pig. J Exp Med. 1970 Dec 1;132(6):1138–1152. doi: 10.1084/jem.132.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Studies on the mode of action of diphtheria toxin. I. Protein synthesis in guinea pig tissues. J Exp Med. 1966 Dec 1;124(6):1107–1122. doi: 10.1084/jem.124.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Saelinger C. B., Imhoff J. G. Studies on the effect of diphtheria toxin on protein synthesis in mice. J Med Microbiol. 1973 May;6(2):169–176. doi: 10.1099/00222615-6-2-169. [DOI] [PubMed] [Google Scholar]
- Bonventre P. F., Saelinger C. B. Inhibition of protein synthesis after intravenous or intramuscular challenge with diphtheria toxin. Infect Immun. 1972 Sep;6(3):418–421. doi: 10.1128/iai.6.3.418-421.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. G., Bonventre P. F. Studies on the mode of action of diphtheria toxin. III. Effect on subcellular components of protein synthesis from the tissues of intoxicated guinea pigs and rats. J Exp Med. 1970 Apr 1;131(4):659–674. doi: 10.1084/jem.131.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLAHAN W. S., BEYERLEIN B., MULL J. D. TOXICITY OF PSEUDOMONAS AERUGINOSA SLIME. J Bacteriol. 1964 Sep;88:805–806. doi: 10.1128/jb.88.3.805-806.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Purification and characterization of Pseudomonas aeruginosa exotoxin. Infect Immun. 1974 Jan;9(1):113–118. doi: 10.1128/iai.9.1.113-118.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. A., Jones R. J. Biological and immunochemical properties of culture filtrates of virulent and avirulent strains of Pseudomonas aeruginosa. Br J Exp Pathol. 1968 Oct;49(5):395–410. [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Kubota Y., Liu P. V. An enterotoxin of Pseudomonas aeruginosa. J Infect Dis. 1971 Jan;123(1):97–98. doi: 10.1093/infdis/123.1.97. [DOI] [PubMed] [Google Scholar]
- Kusama H., Suss R. H. Vascular permeability factor of Pseudomonas aeruginosa. Infect Immun. 1972 Mar;5(3):363–369. doi: 10.1128/iai.5.3.363-369.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P. V., ABE Y., BATES J. L. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. J Infect Dis. 1961 Mar-Apr;108:218–228. doi: 10.1093/infdis/108.2.218. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levin J., Bang F. B. Clottable protein in Limulus; its localization and kinetics of its coagulation by endotoxin. Thromb Diath Haemorrh. 1968 Mar 31;19(1):186–197. [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- MASOUREDIS S. P. Behavior of intravenously administered I 131 diphtheria toxin in the guinea pig. J Immunol. 1959 Apr;82(4):319–327. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke G., Barum J., Rosenberg B., Berk R. In Vivo Studies with the Partially Purified Protease (Elastase) from Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):583–589. doi: 10.1128/iai.2.5.583-589.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke G., Berk R. S. In vivo studies with a toxic fraction of Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1970 Nov;135(2):360–363. doi: 10.3181/00379727-135-35051. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R. Pseudomonas aeruginosa exotoxin: effect on cellular and mitochondrial respiration. J Infect Dis. 1972 Jul;126(1):48–53. doi: 10.1093/infdis/126.1.48. [DOI] [PubMed] [Google Scholar]
- Rugstad H. E. Characterization of a plasma kinin-inactivating enzyme produced by Pseudomonas aeruginosa. Br J Pharmacol Chemother. 1967 Jun;30(2):425–435. doi: 10.1111/j.1476-5381.1967.tb02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



