Abstract
Cocaine- and amphetamine-regulated transcript (CART) is a neuropeptide implicated in addiction to drugs of abuse. Several studies have characterized the role of CART in addiction to psychostimulants, but few have examined the role of CART in alcohol use disorders including alcoholism. The current study utilized a CART knockout (KO) mouse model to investigate the role of CART in ethanol appetitive behaviors. A two-bottle choice, unlimited-access paradigm was used to compare ethanol appetitive behaviors between CART wild type (WT) and KO mice. The mice were presented with an ethanol solution (3%-21%) and water, each concentration for four days, and their consumption was measured daily. Consumption of quinine (bitter) and saccharin (sweet) solutions were measured following the ethanol preference tests. In addition, ethanol metabolism rates and ethanol sensitivity were compared between genotypes. CART KO mice consumed and preferred ethanol less than their WT counterparts in both sexes. This genotype effect could not be attributed to differences in bitter or sweet taste perception or ethanol metabolism rates. There was also no difference in ethanol sensitivity in male mice; however, CART KO female mice showed a greater ethanol sensitivity than the WT females. Taken together, these data demonstrate a role for CART in ethanol appetitive behaviors and as a possible therapeutic drug target for alcoholism and abstinence enhancement.
Keywords: addiction, alcoholism, CART, Ethanol, Knock out, mouse
Introduction
Cocaine- and amphetamine-regulated transcript is a putative neuropeptide transmitter found in the brain and gut. Initially identified as an up regulated striatal mRNA transcript in response to systemic administration of cocaine or amphetamine (Douglass et al., 1995), CART has been shown to modulate a number of biological processes including appetite regulation, metabolism, nociception, anxiety/depression, and ion channel modulation (see Rogge et al., 2008 for review).
The role of CART in drug reinforcement, while first suggested by its responsivity to psychostimulant administration, is further implicated by its localization to and interactions with numerous brain regions known to be critically important in drug responses (Couceyro et al., 1997; Dallvechia-Adams et al., 2002; Koylu et al., 1997). Furthermore, changes in CART gene expression in human cocaine abusers (Albertson et al., 2004) and overdose victims have been repeatedly observed (Tang et al., 2003). CART prominently interacts with the mesolimbic dopamine system (for review, see Hubert et al., 2008); for instance, icv infusions of CART appear to increase DOPAC & HVA levels in the shell of the nucleus accumbens (NAc) (Shieh, 2003; Yang et al., 2004). Conversely, the expression of CART mRNA in the NAc is decreased by dopamine receptor agonists and antagonists (Hunter et al., 2006; Salinas et al., 2006), and behavioral studies suggest that intracranial injections of CART into the ventral tegmental area produce psychostimulant-like increases in locomotor activity (Kimmel et al., 2000) and a conditioned place preference (Kuhar et al., 2005). Injections of CART into the NAc following systemic cocaine or amphetamine also blunt the drug-induced increases of locomotor activity (Jaworski et al., 2003; Kim et al., 2003). Taken together, these studies strongly suggest that CART may function to significantly modulate the mesolimbic dopamine system and thereby contribute to the development or expression of aberrant drug-seeking behaviors.
Two CART KO mice lines have been generated and used to further investigate the role of CART in psychostimulant drug related behaviors. The first line, generated by Asnicar et al. (2001), removed the first two exons of the CART gene and utilized a ES-129/SvJ bacterial artificial chromosome clone injected into 129 SvJ mouse ES cells. The subsequent cells were injected into C57BL6 blastocysts. The resultant male offspring were crossed with C57BL6 wild type females and backcrossed to a C57BL6J background. These mice appeared normal and had no gross physical abnormalities except that at approximately twenty weeks of age their body weights were greater than their wild type counterparts. CART KO mice from the second line generated by Wierup et al. (2005) also displayed an increase in body weight when fed a regular diet except that the differences were not apparent until 40 weeks of age. The Wierup CART KO mouse line was generated by removing all three of the CART gene exons and was maintained on an outbred, black swiss x 129SvJ background. Both of these mouse strains have been used in studies examining responses to psychostimulant drugs of abuse (see Moffett et al., 2006 for review). The results are mixed; one study (Steiner et al., 2006) showed no genotype differences in cocaine-induced locomotor sensitization or cocaine self administration. However, a review by Moffett et al., (2006) which contained new data found a reduction in cocaine-induced, but not amphetamine-induced, locomotor activity in CART KO mice relative to their WT counterparts. A third study comparing CART KO and WT mouse psychostimulant-related behaviors found that CART KO mice had diminished acute amphetamine-induced vertical activity and grooming responses relative to their WT counterparts (Couceyro et al., 2005). The CART KO mice did, however, display an increase in locomotor activity in response to acute amphetamine. With repeated amphetamine administration, dose-dependent increases in locomotor activity (sensitization) were observed in CART WT, but not CART KO mice. The authors also tested two doses (0.3 & 1.0 mg/kg) of amphetamine in a conditioned place preference (CPP) paradigm and found that CART KO mice failed to acquire a CPP response to the low dose of amphetamine. Finally, Couceyro et al. (2005) also found that cocaine self-administration was reduced in CART KO mice relative to their WT counterparts; WT mice had greater intake of and responding for cocaine at the majority of doses tested. Overall, it appears that the results from the CART KO mouse studies reflect a decrease in the reinforcing/rewarding properties of psychostimulant drugs in CART KO mice. This conclusion is somewhat controversial, though when taken together with the CART literature in WT mice mentioned previously one can generally conclude that CART is involved in the reinforcing properties of psychostimulant drugs of abuse.
In contrast to the numerous studies which document the role of CART in psychostimulant drug reinforcement, our knowledge of CART in alcohol-seeking behaviors and alcoholism is limited. Jung et al. (2004) first provided strong evidence of a role for CART in human alcoholism when they documented a correlation between a polymorphism in intron 1 of the CART gene with alcoholism in a population of Korean men. Subsequent work from our lab demonstrated a direct link between ethanol and CART expression. We found that high doses of acute ethanol increase CART mRNA and protein in the NAc in a dose-dependent manner. Furthermore, we demonstrated that this increase is dependent on both D1 and D2/D3 dopamine receptors (Salinas et al., 2006). Work by Dayas et al. (2008) found that exposure to stimuli previously linked with ethanol availability activates CART-containing neurons in the arcuate nucleus as measured by c-fos immunoreactivity. A role for CART in ethanol withdrawal has also been shown in two studies: the first demonstrated that ethanol withdrawal induced anxiety was partially mediated by increases in central amygdala CART levels (Dandekar et al., 2008a); whereas the second study found that CART levels in specific hypothalamic nuclei were increased during acute withdrawal from 15 days of a liquid ethanol diet (Dandekar et al., 2008b). Furthermore, King et al. (2010) demonstrated that icv infusions of CART inhibit context-induced reinstatement of alcohol seeking behaviors; thereby strongly suggesting a role for CART in mediating the rewarding or motivational properties of ethanol.
The present study sought to continue to directly examine the role of CART in ethanol preference and consumption. Specifically, we examined ethanol appetitive behaviors in a two bottle choice, unlimited ethanol access paradigm in a CART KO mouse model generated by (Asnicar et al., 2001). We then examined several factors involved in mediating ethanol appetitive behaviors including bitter/sweet taste perception, ethanol metabolism, and ethanol sensitivity.
Materials and methods
CART KO mice
Three breeding trios of CART KO mice were obtained from Eli Lilly (Indianapolis, IN). Their generation has been previously described (Asnicar et al., 2001). The progeny of these trios were bred with C57BL6J wild type mice to produce CART heterozygotes. The resulting heterozygotes were further crossed to a C57BL6J WT background for three generations. Heterozygotes were then crossed to produce litters with CART WT, heterozygote, and KO mice. At weaning age, mice were separated by sex with 2-5 mice per cage. Within one week of weaning, mice were ear labeled and tail biopsies collected for genotyping. Genomic DNA was isolated from the tail biopsies using the Promega Wizard gDNA kit (Madison, WI). The purified gDNA was then used to genotype the mice with primers previously described by Asnicar et al. Briefly, three CART primers were used; one targeting the WT allele (5′-AAG GTA GCA GTA GCA GCA GG-3′), one targeting a region in the mutant/CART KO allele (5′-GAA AAT GGC CGC TTT TCT GG-3′), and the third targeting a common fragment (5′-TAT GTG TAC ACG AGT GCA GG -3′) in both alleles. The predicted product sizes obtained were 880 bp and 658 bp, respectively, for the WT and mutant/CART KO alleles. A second set of actin primers (5′-GCT CGT CGT CGA CAA CGG CTC-3′ & 5′-CAA ACA TGA TCT GGGT CAT CTT CTC-3′; 353 bp expected product) was included in each reaction to serve as an internal control. All of primers used were synthesized by Integrated DNA Technology (Coralville, IA). The JumpStart REDTaq Readymix (Sigma-Aldrich, St. Louis, MO) was used according to the manufacturer's protocol. To minimize non-specific products in the multiplex reaction, a touchdown PCR protocol was used. The cycling parameters consisted of an initial 95°C denaturation cycle for 4 minutes. Then 30 cycles of a 30 second 95°C denaturation step, a 60°C 30 second hybridization step (decreasing 0.5°C every subsequent cycle), and a one minute extension step at 72°C. An additional 30 cycles consisting of a 30 second 95°C denaturation step, a 30 second 45°C hybridization step, and one minute 72°C extension step. A final 72°C extension step for seven minutes ended the protocol. The resultant products were run on 2% agarose/TBE –ethidium bromide gels. The PCR was repeated for every sample to ensure the genotypes were correct. Only WT and KO littermates were used in all experiments. At 2-3 months of age, the mice were individually housed under a reversed 12:12 hour light schedule (lights on at 8 p.m.) for 3 weeks before beginning any experiments. Food and water were available ad libitum. All of the experiments were approved by the Institutional Animal Care and Use Committee.
Ethanol consumption & preference
A continuous two-bottle choice paradigm was used to compare ethanol preference between WT and KO mice of both sexes. During the acclimation period to the reversed light schedule and individual housing, the mice were presented with two bottles containing only water. At the onset of the ethanol preference test, one of the water bottles was replaced with a 3% ethanol solution for four days. After the fourth day, the ethanol solution was replaced with a 6% ethanol solution for four days, followed by 9, 12, 15, 18, & 21% ethanol solutions, each following the same paradigm. The bottles were removed only briefly for daily measurements of the total amounts of ethanol and water. In addition, the position of the ethanol and water bottles in each cage were alternated daily to avoid a bottle position preference. Mouse body weights were measured every other day.
The amount of daily ethanol consumption was determined (in g/kg of body weight) for each animal and averaged over the four day period for each ethanol concentration. Two control tubes (one with water and the other with the corresponding ethanol solution) were used to estimate evaporation and spillage and the consumption amounts obtained for experimental animals were adjusted accordingly. Preference was determined by dividing the volume of ethanol solution consumed by the total volume of ethanol and water consumed. Total intake of ethanol and water consumption was also calculated for each subject.
Bitter/Sweet tastant preference
To control for any differences in bitter or sweet tastant preferences, consumption of quinine and saccharin solutions was assessed following the ethanol preference tests. After the final day of ethanol consumption, the mice were given two water tubes for two weeks. The mice were then once again offered water and a non-alcohol tastant tube containing either a bitter (quinine) or sweet (saccharin) tastant as before (four days, escalating concentrations). Quinine hemisulfate (Sigma-Aldrich) was prepared as 0.03 and 0.06 mM solutions. Saccharin was prepared as 0.033 and 0.066% solutions. As before, the mice were weighed every other day, the consumption of each solution was determined daily, and the bottle position was alternated daily to control for any possible bottle position preference. There was a two-week water only period between the quinine and saccharin tastants.
Ethanol metabolism
Ethanol naïve mice were given an i.p. injection of ethanol (2.5 g/kg, 20% solution in sterile PBS) between 1 p.m. and 2 p.m. Three blood samples were collected from each mouse at one of the 30, 60, 120, 180, 240, or 300 minute post injection time points. The blood samples were collected in heparinized capillary tubes, centrifuged at 5K rpm for two to three minutes (to clear the serum), and immediately used to determine blood ethanol content with the Pointe Scientific (Canton, MI) enzyme assay.
Ethanol Sensitivity/Loss or Righting Reflex
Ethanol naïve male and female mice of both genotypes were tested for their sensitivity to ethanol with a loss of righting reflex (LORR) test. Briefly, mice were injected (i.p.) with either 3.4 or 3.8 g/kg ethanol (20% solution in sterile PBS). The doses were selected because they were high enough to induce a loss of the righting reflex without inducing an excessively long sleep time (Blednov et al., 2006). Fifteen to twenty minutes after the injection all mice were tested to ensure the righting reflex (defined here as the ability of the mouse to right itself three times in a thirty second period when placed in the supine position) was lost. Every ten minutes after the initial loss of the righting reflex, the animals were tested until they had fully recovered. One mouse failed to lose the righting reflex (most likely due to a misplaced injection) and was excluded from this study. The experimenter testing the mice was not aware of the genotypes of the mice throughout the study. All of the experiments were carried out at room temperature with the ethanol injection taking place between 1 p.m. and 2 p.m.
Immunohistochemistry
At the conclusion of the experiment, the mice were intracardially perfused and processed for CART-immunoreactivity (CART-ir) to confirm, on the protein level, the completion of the knockout. Briefly, the mice were anesthetized with chloral hydrate, the thoracic cavity was opened and the heart freed from the pericardium, the descending aorta was clamped and 0.5 mL of 5,000 U/mL Heparin (Sigma-Aldrich, St. Louis, MO) in phosphate buffered saline (PBS) was injected into the left ventricle to prevent clot formation in the vasculature. To prevent recirculation, the right atrium was cut and a small incision into the left ventricle allowed the insertion of the perfusion catheter. Saline (0.9% NaCl) containing 0.2% NaNO2 as a vasodilator was perfused for two minutes to clear the vasculature of any remaining blood. This step was followed by 4% paraformaldehyde for 12 minutes. The brains were then quickly excised and post fixed in 4% paraformaldehyde for two hours before being transferred to a 30% sucrose/PBS solution for at least two days. Following this step, the brains were sectioned on a rotary microtome (Leica Microsystems, Bannockburn, IL) equipped with a freezing stage (Physitemp Instruments, Clifton, NJ). The brains were sectioned at 40 μms and collected sequentially in three series. The tissue was then stored at -20°C in a cryoprotectant solution (Watson et al., 1986) to maintain peptide immunoreactivity and tissue morphology until immunohistochemistry could be performed.
Free floating tissue sections were rinsed six times for ten minutes with tris buffered saline (TBS) to remove the cryoprotectant. To remove residual aldehydes, the sections were pretreated with a 1% sodium borohydrite/TBS solution for ten minutes. This step was followed by a one hour preincubation step in a 20% Normal Goat Serum (NGS), 1% hydrogen peroxide, 0.3% Triton-X 100 solution (to block nonspecific labeling, quench endogenous peroxide activity, and permeabilize, respectively) at room temperature. The sections were then incubated in a 1/4,000 dilution of Rabbit anti CART (Phoenix Pharmaceuticals, Burlingame, CA) in 2% NGS, 0.3% Triton-X 100 for 48 hours at 4°C with gentle agitation. After primary antibody incubation, the sections were rinsed with TBS four times for five minutes and then incubated in a biotinylated secondary antibody raised in goat directed against rabbit antibodies at a 1/200 dilution in 2% NGS, 0.3% Triton-X 100 solution for two hours at room temperature with agitation. After washing as described before, a one hour tertiary incubation with the Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA) was performed according to manufacturer's protocol. The sections were washed again before labeling with a 3,3′-diaminobenzidine (DAB; 0.8 mg/mL), 0.05% hydrogen peroxide, TBS solution for five minutes. The labeling reaction was halted by rinsing the sections with TBS as described before. The labeled sections were mounted onto subbed slides and allowed to dry overnight. The slides were then dehydrated in an increasing gradient of ethanols, cleared with xylenes, and coverslipped with permount. The slides were allowed to dry for at least three days. The slides were then coded before image acquisition. DAB stained images were captured using an Olympus IX-70 microscope with a Hamamatsu camera and Simple PCI 6 image acquisition software (Leeds Instruments, Irving, TX).
Statistics
All data are reported as the mean ± SEM. All data were compiled into excel and exported to R 2.11.1 for statistical analysis. A repeated measures, two-factor ANOVA was conducted to examine group differences in the ethanol drinking data. Two-factor ANOVAs were conducted to examine group differences in the remaining experiments.
Results
Genotyping & Immunohistochemistry
Since this study is the first by our lab utilizing the CART KO mice, and given that the genetic knockout of the CART gene is incomplete (see Asnicar et al. (2001) for details), we chose to verify the complete knockout of the CART protein using immunohistochemistry with an antibody directed against the full length (55-102) CART protein. Our PCR genotyping results were verified in a random sample of mice used in the study. We observed no CART protein expression in any brain region in the CART KO mice (NAc is shown in Fig. 1). The WT mice displayed a CART-ir distribution similar to that previously described (Koylu et al., 1998) (Fig. 1B).
Figure 1.
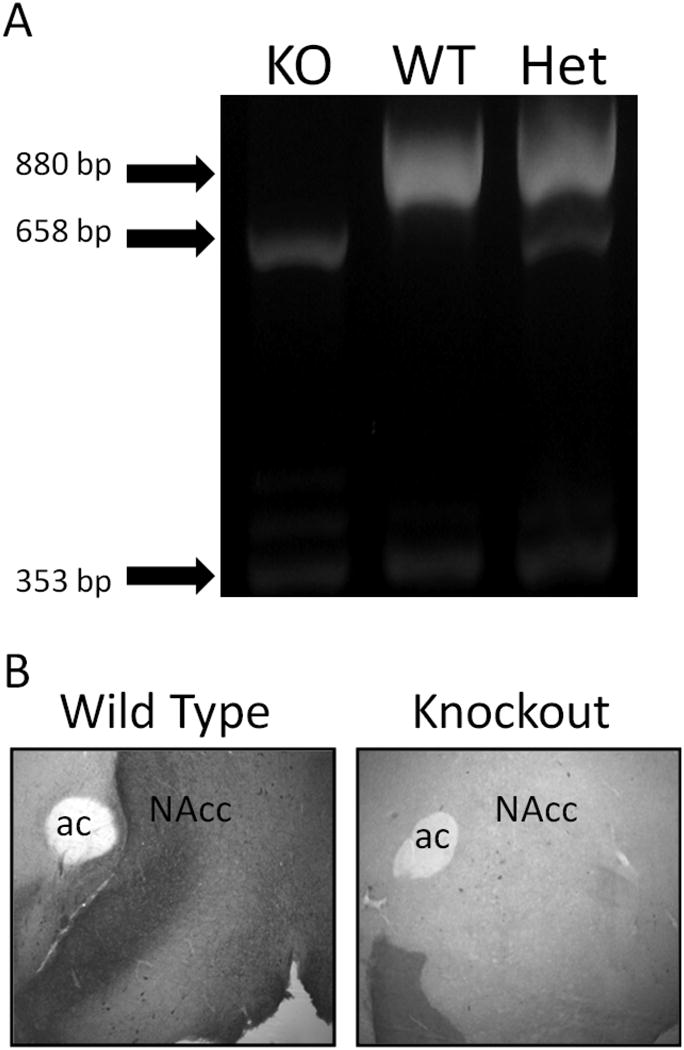
PCR genotyping results and immunohistochemistry confirmation. (A) Typical example of PCR genotyping results indicate the presence (or absence) of the wild type & CART knockout alleles, as well as, the actin internal control bands (880, 658, & 353 bps, respectively). Periodically, the PCR genotyping results were verified with immunohistochemistry in brain tissues using a CART antibody (B). The nucleus accumbens region of the brain expresses CART in the wild type sample, but not in the CART knockout sample. NAc – nucleus accumbens, ac – anterior commissure.
Ethanol consumption, preference, and total intake
We observed a main effect of genotype on ethanol consumption (F(1,21)=8.4, p<0.01 and F(1,27)=11.7, p<0.01, for males & females, respectively) such that, CART KO mice (of both sexes) consumed less ethanol than their WT counterparts (Figure 2A & B). Total intake did not differ between genotypes (Figure 2C & D). Ethanol preference was altered in the CART KO mice such that they also preferred ethanol less than their WT counterparts (F(1,21)=6.2, p<0.01 and F(1,27)=4.4, p<0.01, for males and females, respectively; Figure 2E & F).
Figure 2.
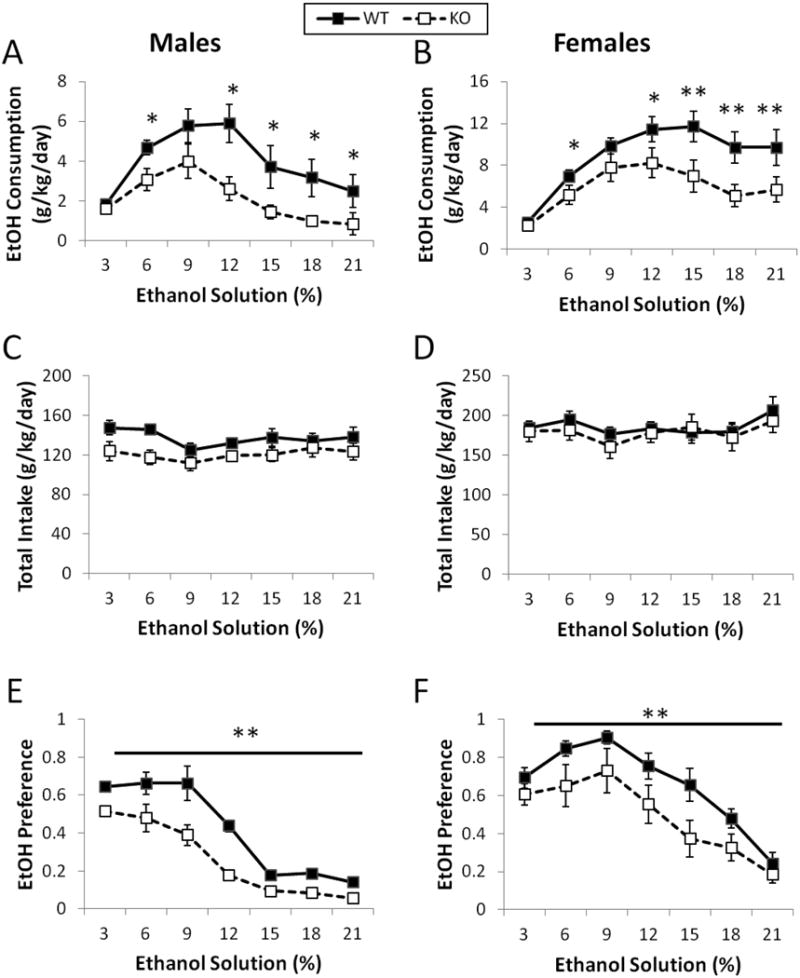
CART KO mice display reduced consumption and preference of ethanol. Male CART KO mice consumed less ethanol than their wild type counterparts (A & E, respectively), but did not differ in their total liquid intake (C). Female CART KO mice also consumed and preferred less ethanol than their wild type counterparts (B & F, respectively) and had similar levels of total liquid intake to the wild type mice (D). n=11 per genotype for males and n=14 per genotype for females. *, p<0.05; **, p<0.01.
Bitter/Sweet tastant preference, ethanol metabolism, and sensitivity
There was no effect of concentration or genotype on saccharin (Figure 3A & B) or quinine consumption (Figure 3C & D). There was no effect of sex or genotype on ethanol metabolism. Both male (Figure 4A) and female (Figure 4B) mice reached a peak BEC of approximately 60 mM within the first hour after the injection. Five hours after the injection, the BEC of the mice returned to basal levels. There was no main effect of ethanol dose on ethanol sensitivity, as measured by the duration of the LORR. There was also no genotype effect in male mice (Figure 5A); however, in female mice, a genotype effect was observed such that CART KO mice had a longer duration of the LORR at both of the ethanol doses (F(1,27)=2.9, p<0.01; Figure 5B).
Figure 3.
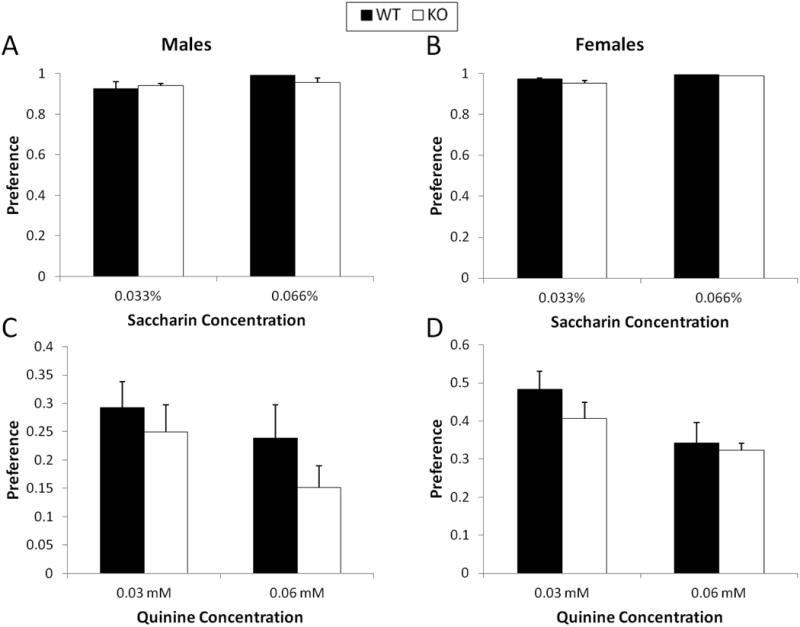
Removal of the CART gene does not alter taste preference for saccharin (A & B, respectively, for males and females) or quinine (C & D, respectively for males and females) tastants. n=11 per genotype for males and n=14 per genotype for females.
Figure 4.
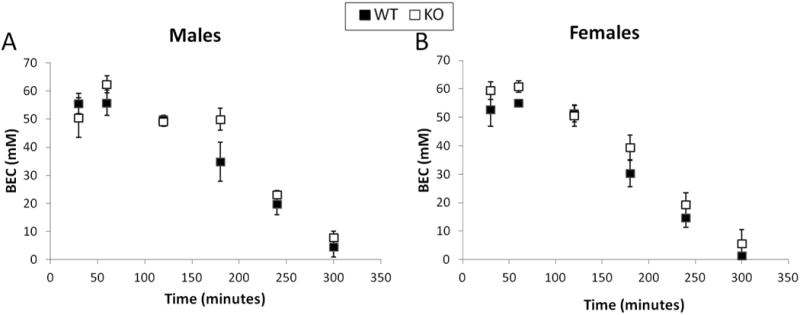
The metabolism of ethanol did not differ between CART KO and wild type mice in male (A) or female (B) mice. n=7 per genotype for males and n=8 per genotype for females.
Figure 5.
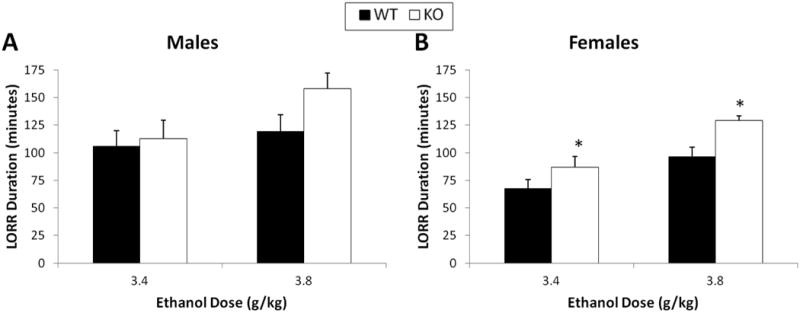
There was no difference in ethanol sensitivity between CART KO and wild type male mice (A). In females, however, the CART KO mice had an increased LORR duration compared to their wild type counterparts (B). n=6 per genotype for males and n=7 per genotype for females. *, p<0.05.
Discussion
This constitutes the first report that removal of CART protein is accompanied with a decrease in ethanol consumption and preference behaviors that cannot be attributed to differences in taste perception, ethanol metabolism, or ethanol sensitivity. Most notably, these data are in direct accord with previous work that reduction of CART expression reduces cocaine self-administration, but not of sweet tastants (Couceyro et al., 2005). In light of the fact that non-drug rewards are not affected by removal of the CART gene, this consistent response to both ethanol and psychostimulants is strongly suggestive that CART expression is functionally significant in a variety of drug-seeking states. Behavioral and pharmacological studies examining the role of CART in drug reinforcement indicate that CART itself is reinforcing. Kimmel et al. (2000) and Kuhar et al. (2005) found that intra-VTA injections of CART produce psychostimulant-like behavioral effects (including dose-dependent increases in locomotor activity and conditioned place preference) and increase dopamine release in the NAc. It is therefore possible that without this agonist-like activity of CART in the VTA (which would be increased in response to drug administration), the reinforcing properties of drugs of abuse are mitigated or occluded, resulting in the observed decrease of drug and ethanol consumption in CART KO mice reported here and previously (Couceyro et al., 2005).
The reduced consumption and preference for ethanol observed in CART KO mice of both sexes supports the aforementioned theory. The genotype difference in preference and consumption cannot be attributed to differences in total liquid intake between genotypes. Therefore, other factors known to contribute to ethanol appetitive behaviors were examined. It is possible that the palatability or taste preference of ethanol differs between CART KO and wild type mice; however, in our studies, we observed no differences in the consumption of saccharin or quinine solutions, suggesting that there were no differences in taste preference between genotypes. We next compared the metabolism rates in CART KO and wild type mice to assess whether or not the differences in ethanol consumption and preference could be accounted for by a decrease in ethanol metabolism rate in CART KO mice. Mice of both sexes and genotypes received a 2.5 g/kg i.p. dose of ethanol and blood samples were collected and assayed for blood ethanol content across a number of time points. There was no difference in the time-matched blood ethanol concentrations between genotypes, suggesting no differences in ethanol metabolism rates. Given the known role of CART in feeding, metabolism, and energy homeostasis, the lack of a CART KO effect on the ethanol metabolism rate was surprising (see Rogge et al. (2008) for review). It is also noteworthy that contrary to previous reports, no significant differences in body weight were observed between genotypes in any of the experiments. The lack of a body weight difference could be attributed to the fact that these studies were all conducted on mice that were at most 25 weeks old. Previous studies of genetically modified mice have shown a negative relationship between the latency to recover the righting reflex and ethanol consumption (Thiele et al., 1998). We therefore examined the ethanol sensitivity of CART KO and wild type mice using the loss-of-righting-reflex assay. A reduced consumption of ethanol could be attributed to a relative increase in ethanol sensitivity; indeed, in female mice we observed a genotype effect such that CART KO mice had an increased sensitivity to the doses of ethanol tested here. However, we observed no such difference in ethanol sensitivity between male CART KO and wild type mice.
Another CART-rich brain region shown to be important for ethanol consumption, the Edinger-Westphal nucleus (EWcp) (Bachtell et al., 2004), was recently shown to have a significantly reduced expression of CART in the low alcohol drinking DBA/2J mouse strain relative to the high alcohol drinking C57BL6J mouse strain (Giardino et al., 2012). These results further support a role for CART in ethanol consumption and strengthen the main finding of the current study that null (or low) levels of CART are associated with reduced ethanol consumption.
It should be noted that the use of global CART knockout mice leaves open the possibility for unforeseen compensatory effects in CART-related neurotransmitter systems that could account for the differences in ethanol consumption and preference observed. Throughout this study, no gross abnormal physiology or behaviors were observed in any of the CART KO mice. Additionally, a preliminary study (Couceyro PR, 2005) using a second CART KO mouse reported results similar to those described in the current study with two exceptions, the CART KO mice in that study were on a different background strain than the mice in this study and had a greater total fluid intake than their wild type counterparts. Nonetheless, the overall finding that CART KO mice consumed and preferred ethanol less than their WT counterparts is consistent with the findings in our study.
An alternative (or additional) role for CART suggested by the literature posits that CART acts as a homeostatic mechanism to curtail excessive dopamine signaling (in response to psychostimulant administration) in the NAc (Hubert et al., 2008; Jaworski and Jones, 2006; Jaworski et al., 2003; Kim et al., 2003). These studies have focused on the mesolimbic dopamine system; however, other studies have investigated the role of thalamic and hypothalamic CART in regulating NAc dopamine signaling and drug related behaviors. The arcuate nucleus (ARC) is a CART-neuron rich region that projects to the neurons of the paraventricular thalamus (PVT) (Kirouac et al., 2006) which in turn project to the NAc shell (Parsons et al., 2006). Stimulation of the PVT (and its glutamatergic afferents) has been shown to increase NAc shell dopamine levels independent of VTA activity, presumably by activating presynaptic dopamine terminals in the NAc (Parsons et al., 2007). It has been previously reported that in a reinstatement model of relapse, stimuli previously linked to ethanol activated CART neurons in the ARC (Dayas et al., 2008). Given that CART neurons in the ARC project to and appose PVT neurons, and that PVT neurons subsequently project to and modulate dopamine levels in the NAc, it is not unexpected that CART in the PVT could modulate drug-related behaviors. Indeed, James et al., (2010) found that injections of CART into the PVT dose-dependently decreased active lever pressing for cocaine in a drug-primed reinstatement model. Taken together, a common mechanism for CART as an inhibitor of aberrant dopamine signaling in response to drugs of abuse begins to form. Thus, one would expect that CART KO mice may readily engage in uncontrolled drug intake (without a negative or inhibitory feedback signaling system in response to high or binge doses of drugs). However, this is not what we observed in the current study. It is likely that the role of CART in mediating the reinforcing/rewarding effects of drugs is more complex than behavioral studies in rodents would seem to indicate and warrants further investigation.
In summary, the present study demonstrates a role for CART in ethanol consummatory behaviors; moreover, we demonstrated that removal of the CART gene reduced volitional ethanol consumption in mice in a manner that cannot be attributed to altered intake levels, taste preference, ethanol metabolism, or ethanol sensitivity (at least in males). Further research aimed at exploring and understanding exactly how the CART system is involved in addiction (and alcoholism) and how the removal of CART was able to reduce ethanol consumption is necessary. Future behavioral, biochemical, and electrophysiological studies will elucidate the mechanism(s) by which CART exerts its effects on ethanol consumption and its potential role in mediating the reinforcing effects of drugs of abuse. The findings of this study imply a role for CART in drug reinforcement and as a potential therapeutic drug target that may reduce volitional ethanol intake or enhance abstinence.
Acknowledgments
The authors would like to thank Tavanna Porter for expert technical assistance, Angela R. Ozburn for advice on the study design, and the Waggoner Center for Alcohol & Addiction Research. This work was supported by an individual predoctoral NRSA (NIAAA F31AA017834) to A.G.S. and NIAAA R01AA15167 and the Integrative Initiative on Alcoholism – West U01AA16651 to R.A.M.
Footnotes
Authors Contribution: AGS contributed to the study design, conducted all of the animal experiments, analyzed data and prepared the manuscript for submission. CTQN and DAT assisted with the genotyping and immunohistochemistry experiments. RAM contributed to the study design and preparation of the manuscript. All authors reviewed the manuscript before submission for publication.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Koster A. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–4400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur J Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Harris RA. Reduced alcohol consumption in mice lacking preprodynorphin. Alcohol. 2006;40:73–86. doi: 10.1016/j.alcohol.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couceyro PREC, D'Alfonso TM, Richards WG, Bannon AW. Program No 56014 2005 Abstract Viewer. Washington, DC: Society for Neuroscience; 2005. Reduced ethanol preference and consumption in CART peptide-deficient mice. 2005. [Google Scholar]
- Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, White FJ, Douglass J, Richards WG, Bannon AW. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–1100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Dallvechia-Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol. 2002;448:360–372. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Singru PS, Kokare DM, Lechan RM, Thim L, Clausen JT, Subhedar NK. Importance of cocaine- and amphetamine-regulated transcript peptide in the central nucleus of amygdala in anxiogenic responses induced by ethanol withdrawal. Neuropsychopharmacology. 2008a;33:1127–1136. doi: 10.1038/sj.npp.1301516. [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Singru PS, Kokare DM, Subhedar NK. Transient up-regulation of cocaine- and amphetamine-regulated transcript peptide (CART) immunoreactivity following ethanol withdrawal in rat hypothalamus. Brain Res. 2008b;1240:119–131. doi: 10.1016/j.brainres.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Cote DM, Li J, Ryabinin AE. Characterization of Genetic Differences within the Centrally Projecting Edinger-Westphal Nucleus of C57BL/6J and DBA/2J Mice by Expression Profiling. Front Neuroanat. 6:5. doi: 10.3389/fnana.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ. CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochem Pharmacol. 2008;75:57–62. doi: 10.1016/j.bcp.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Jones D, Vicentic A, Hue G, Rye D, Kuhar MJ. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–864. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Jones DC. The role of CART in the reward/reinforcing properties of psychostimulants. Peptides. 2006;27:1993–2004. doi: 10.1016/j.peptides.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jung SK, Hong MS, Suh GJ, Jin SY, Lee HJ, Kim BS, Lim YJ, Kim MK, Park HK, Chung JH, Yim SV. Association between polymorphism in intron 1 of cocaine- and amphetamine-regulated transcript gene with alcoholism, but not with bipolar disorder and schizophrenia in Korean population. Neurosci Lett. 2004;365:54–57. doi: 10.1016/j.neulet.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- King BJ, Furlong TM, McNally GP. Cocaine and amphetamine related transcript (CART) inhibits context induced reinstatement of reward seeking. Behav Neurosci. 124:423–427. doi: 10.1037/a0019540. [DOI] [PubMed] [Google Scholar]
- Kirouac GJ, Parsons MP, Li S. Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol. 2006;497:155–165. doi: 10.1002/cne.20971. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine-regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. AAPS J. 2005;7:E259–265. doi: 10.1208/aapsj070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett M, Stanek L, Harley J, Rogge G, Asnicar M, Hsiung H, Kuhar M. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides. 2006;27:2037–2045. doi: 10.1016/j.peptides.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol. 2007;500:1050–1063. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas A, Wilde JD, Maldve RE. Ethanol enhancement of cocaine- and amphetamine-regulated transcript mRNA and peptide expression in the nucleus accumbens. J Neurochem. 2006;97:408–415. doi: 10.1111/j.1471-4159.2006.03745.x. [DOI] [PubMed] [Google Scholar]
- Shieh KR. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of central dopaminergic neurons. Neuropharmacology. 2003;44:940–948. doi: 10.1016/s0028-3908(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Steiner RC, Hsiung HM, Picciotto MR. Cocaine self-administration and locomotor sensitization are not altered in CART knockout mice. Behav Brain Res. 2006;171:56–62. doi: 10.1016/j.bbr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahren B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129:203–211. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Yang SC, Pan JT, Li HY. CART peptide increases the mesolimbic dopaminergic neuronal activity: a microdialysis study. Eur J Pharmacol. 2004;494:179–182. doi: 10.1016/j.ejphar.2004.05.018. [DOI] [PubMed] [Google Scholar]


