Abstract
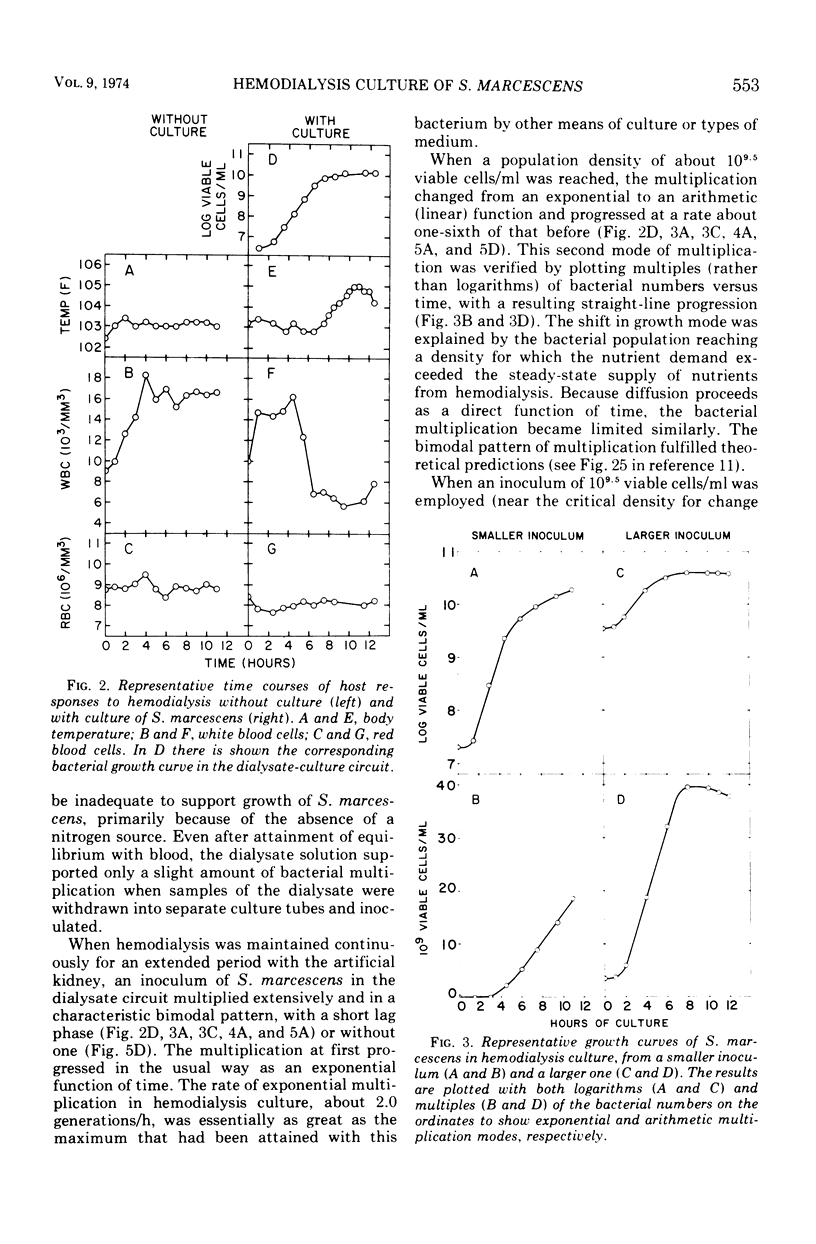
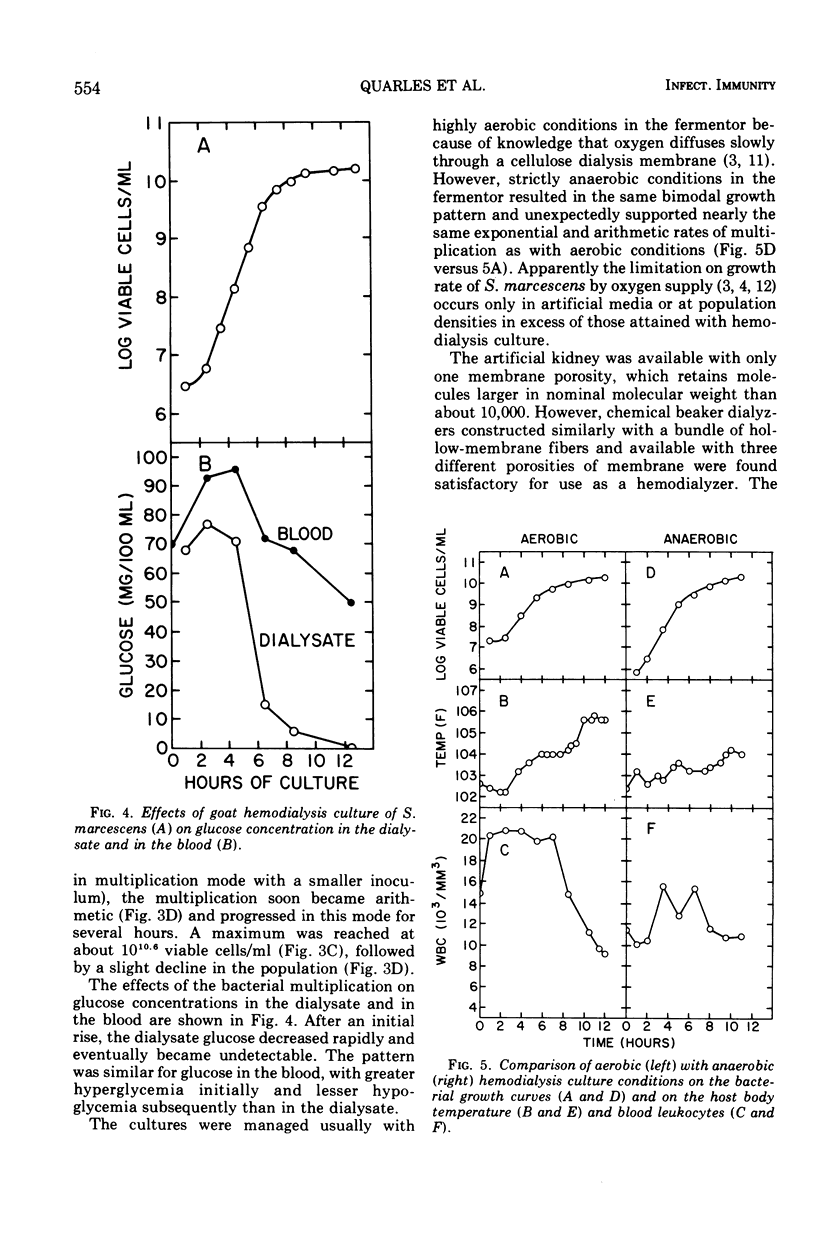
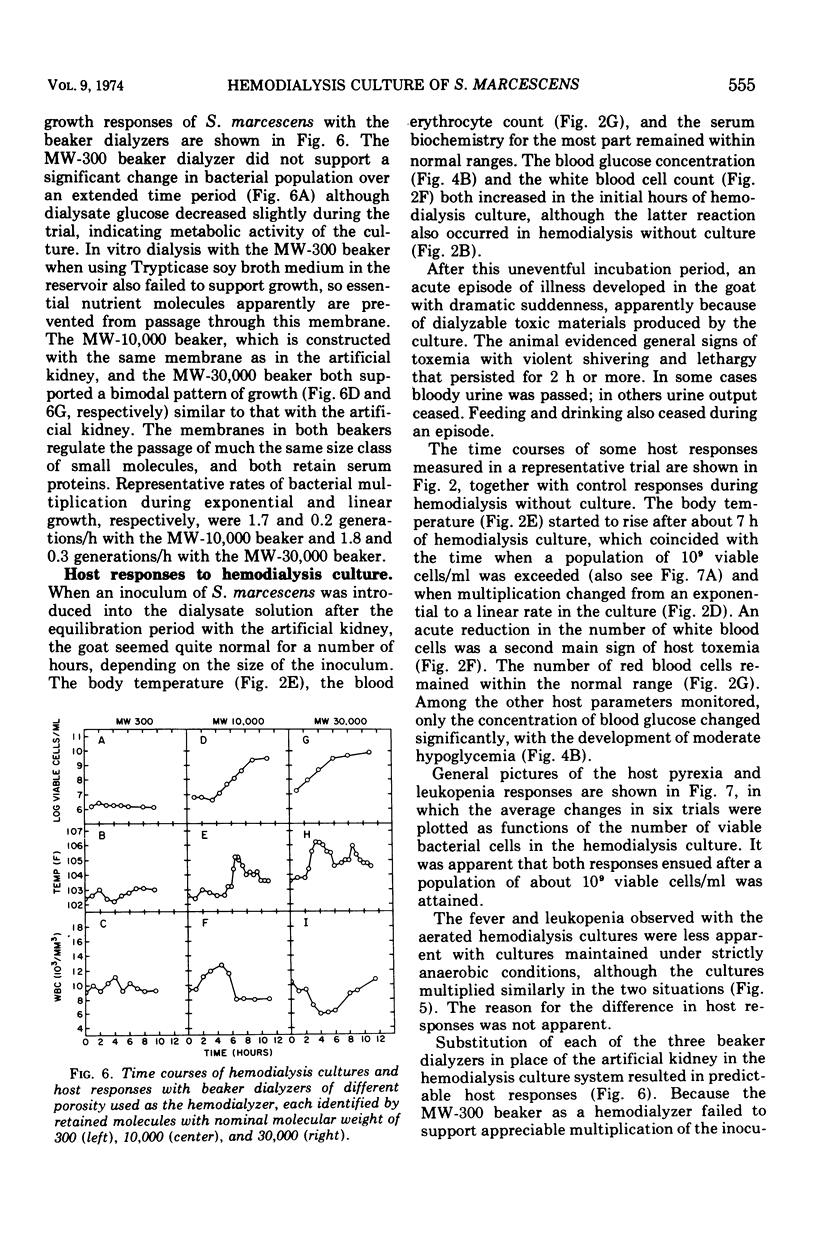
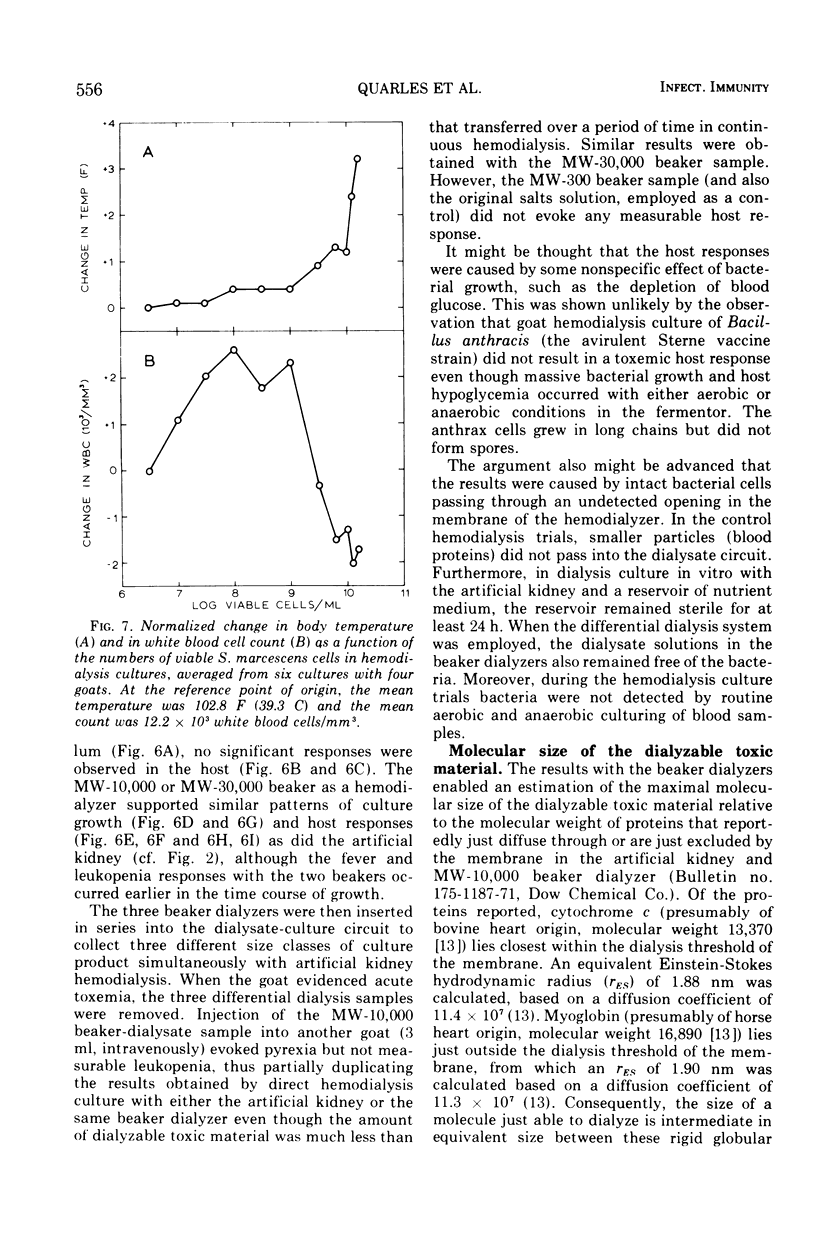
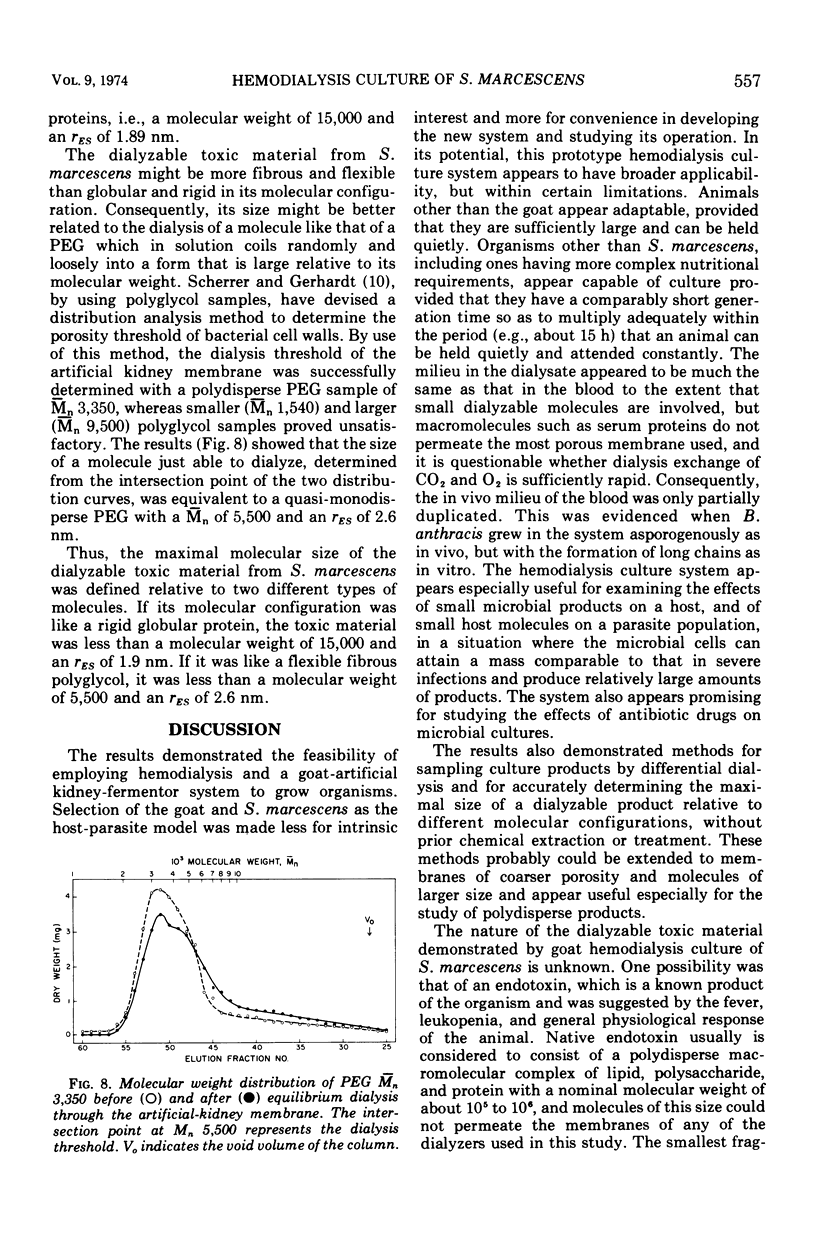
Hemodialysis was employed to simulate in vivo conditions for growth in mammalian blood, but without phagocytosis, by using the goat and Serratia marcescens as a host-parasite model. The blood stream was shunted surgically via prosthetic tubing from a carotid artery through the hollow-fiber membranes in an artificial kidney hemodialyzer and back into a jugular vein. The dialysate solution concurrently was pumped from a modular fermentor through the hemodialyzer jacket outside of the membranes and back into the fermentor. Hemodialysis between the two circuits was maintained continuously. When equilibrium was attained, bacteria inoculated into the dialysate circuit multiplied first exponentially at the maximal rate and then arithmetically at a lesser rate equally well under aerobic or anaerobic conditions. When a population of about 109 viable bacteria/ml was exceeded, the goat reacted acutely with signs of general toxemia, pyrexia, and leukopenia, apparently because of dialyzable toxic material produced by the culture. The maximal molecular size of the toxic material was defined relative to a rigid globular protein of 15,000 in molecular weight and 1.9 nm in hydrodynamic radius or to a flexible fibrous polyglycol of 5,500 in molecular weight and 2.6 nm in hydrodynamic radius, based on determinations of the membrane porosity threshold for dialysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows W. Cholera toxins. Annu Rev Microbiol. 1968;22:245–268. doi: 10.1146/annurev.mi.22.100168.001333. [DOI] [PubMed] [Google Scholar]
- FLETCHER W. S., ROGERS A. L., DONALDSON S. S. THE USE OF THE GOAT AS AN EXPERIMENTAL ANIMAL. Lab Anim Care. 1964 Apr;14:65–90. [PubMed] [Google Scholar]
- GERHARDT P., GALLUP D. M. DIALYSIS FLASK FOR CONCENTRATED CULTURE OF MICROORGANISMS. J Bacteriol. 1963 Nov;86:919–929. doi: 10.1128/jb.86.5.919-929.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup D. M., Gerhardt P. Dialysis Fermentor Systems for Concentrated Culture of Microorganisms. Appl Microbiol. 1963 Nov;11(6):506–512. doi: 10.1128/am.11.6.506-512.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner K. C., Finkelstein R. A. Bioassay of endotoxin: correlation between pyrogenicity for rabbits and lethality for chick embryos. J Infect Dis. 1966 Dec;116(5):529–536. doi: 10.1093/infdis/116.5.529. [DOI] [PubMed] [Google Scholar]
- QUINTON W. E., DILLARD D. H., COLE J. J., SCRIBNER B. H. Possible improvements in the technique of long-term cannulation of blood vessels. Trans Am Soc Artif Intern Organs. 1961;7:60–77. [PubMed] [Google Scholar]
- QUINTON W., DILLARD D., SCRIBNER B. H. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960 Apr 10;6:104–113. [PubMed] [Google Scholar]
- SMITH C. G., JOHNSON M. J. Aeration requirements for the growth of aerobic microorganisms. J Bacteriol. 1954 Sep;68(3):346–350. doi: 10.1128/jb.68.3.346-350.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. S., Gerhardt P. Dialysis culture of microorganisms: design, theory, and results. Bacteriol Rev. 1969 Mar;33(1):1–47. doi: 10.1128/br.33.1.1-47.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by acetic acid hydrolysis of endotoxin from Serratia marcescens 08. Eur J Biochem. 1971 Apr;19(3):357–367. doi: 10.1111/j.1432-1033.1971.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Wober W., Alaupović P. Studies on the protein moiety of endotoxin from gram-negative bacteria. Characterization of the protein moiety isolated by phenol treatment of endotoxin from Serratia marcescens 08 and Escherichia coli 0 141:K85(B). Eur J Biochem. 1971 Apr;19(3):340–356. doi: 10.1111/j.1432-1033.1971.tb01323.x. [DOI] [PubMed] [Google Scholar]




