Abstract
This paper describes a novel selected immobilization technique based on optical control of the sol-gel transition of thermoreversible Pluronic gel, which provides a simple, versatile, and biocompatible approach for high-resolution imaging and microinjection of Caenorhabditis elegans.
Caenorhabditis elegans (C. elegans), a free-living optically transparent nematode, is one of the prominent multicellular model organisms for biological studies including genetics, neuroscience, and developmental biology.1–3 The transparency of this small organism provides substantial opportunities for studies based on high-resolution in vivo imaging of fluorescent proteins or dynamic developmental processes. For high-resolution imaging, immobilization of this tiny but active roundworm is an essential step. Conventionally, for imaging purposes C. elegans is immobilized by anesthesis or being glued on an agar pad, but these methods are laborious and low-throughput as well as potentially perturbing of normal physiology.4, 5 There have also been several microfluidic technologies for immobilization of the worms based on a variety of approaches – tapered channel,6 membrane compression,7 cooling,8 and use of carbon dioxide.9 A few studies have also demonstrated long-term culture of C. elegans and characterized age-related phenotypic changes have been reported.10–13 Although some of these devices allow high-throughput imaging and sorting or culture of worms with automated system operation, it has been difficult to fully immobilize the worms at normal physiological conditions for long-term studies. Recently, our group has developed a novel microfluidic system for immobilization of nematodes based on the thermo-reversible sol-gel transition of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) tri-block copolymer (Pluronic) solution.14, 15 Since the Pluronic gel becomes stiff enough to prevent movement with only a small increase of temperature (~2°C), the worms can be fully immobilized under normal physiological conditions at cultivation temperatures for long-term high-resolution imaging. In addition, the critical temperature for gelation can be easily adjusted. While this method meets the needs of many applications, the microfluidic system is still complex and not simple to operate.
To address this technological need, here we report a simple and versatile method for immobilizing the worms based on optical control of the sol-gel transition of the thermo-sensitive Pluronic solution. We apply a photo-absorbing layer to the substrate for imaging to increase the temperature high enough to induce the gelation of the Pluronic gel using only a commonly found white light source (Fig. 1a). This immobilization strategy also allows us to selectively immobilize a specific worm on-demand by integrating a spatial light modulator using simple setup such as a liquid crystal display found in a commercial projector (Fig. 1a).
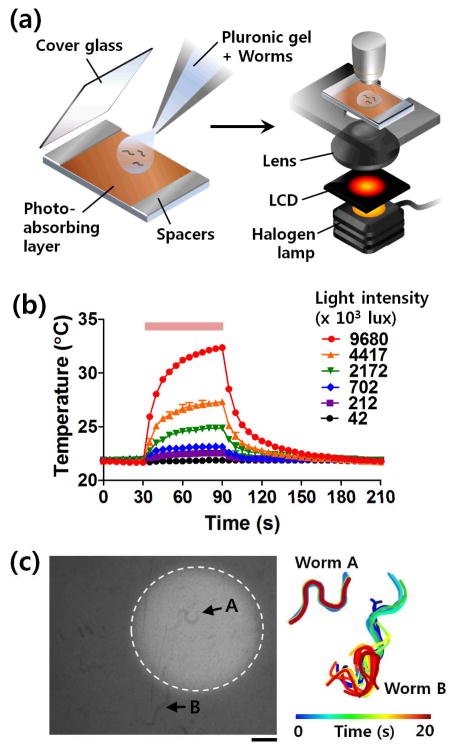
Figure 1. On-demand optical worm immobilization.
(a) Schematic diagram of a protocol for selective worm immobilization based on photothermal sol-gel transition of Pluronic F127 via conventional microscopic illumination. (b) Temperature change according to the light intensity. (c) Selective worm immobilization using a dynamic light pattern (dotted circle) generated by an LCD module. Posture of worms A and B in the illuminated area and the dark area, respectively, were tracked every 1 s and color-coded (right). Scale bar: 100 μm.
For all our experimernts, nematodes were grown on standard nematode growth media (NGM) plates with OP50 bacterial lawns at 20°C. The worms were suspended in 18% w/v Pluronic F127 solution for immobilizing and imaging. Typically a drop of the solution containing the worms was transferred onto a slide with photo-absorbing layer (1 μm-thick hydrogenated amorphous silicon), and a coverslip was added on top (Fig. 1a).
To control for the sol-gel phase transition for immobilization, we simply tune the intensity of the light source (Fig. 1b). The gelation process of the Pluronic solution begins when the temperature increases over the critical micelle temperature, at which micellar aggregates of the tri-block copolymers are formed. The critical micelle temperature can be varied by the concentration of the Pluronic in solution. Experimentally, the temperature was measured by using a thermocouple. The ambient temperature was 22°C and the 18% Pluronic F127 started to gel at ~ 25°C. With illumination of a halogen lamp found in conventional microscopes, the solution temperature increased and approached to the steady state within 90 s (Fig. 1b). We found, as expected, the steady state temperature after the 90-s illumination was determined by the light intensity. With ~4 x 106 lux light intensity (measured using a luminance meter LX1330B, Sinometer Instruments), which is typical of a hallogen lamp not at full power, ~5°C increase in the temperature could be easily achieved. The small temperature change in the normal physiological range was enough to induce gelation for fully immobilization of the worms. To decrease the time to fully immobilize animals if applications demand, we could either increase the concentration of the Pluronic solution or increase the light intensity. The limitations associated with these changes are that (1) increased concentration of Pluronic would raise the viscosity non-linearly and raise the risk of undesired gelation should room temperature flucturations occur, and (2) that there is an ultimate limit of the power output from a halogen lamp.
An advantage of our approach is selective immobilization using patterned light (Fig. 1c). This can streamline and simplify the handling of a large number of worms under examination in sequence, such that the animals will only need to be immobilized for the amount of time required for the operation. Our setup is straightforward: a liquid crystal display (LCD) module placed onto the light source of the microscope can provide a simple controllable light modulation (Fig. 1a). Figure 1c compares a worm (worm A) in the illuminated area slowed down and eventually immobilized, while a worm (worm B) in unilluminated area remained mobile. As before, this process is reversible and physiologically benign. The large advantage is that besides LCD, any light patterning methods, including diaphragm, photomasks or projectors, can be used for the selective immobilization of the animals, making this easily popularizable in any lab.
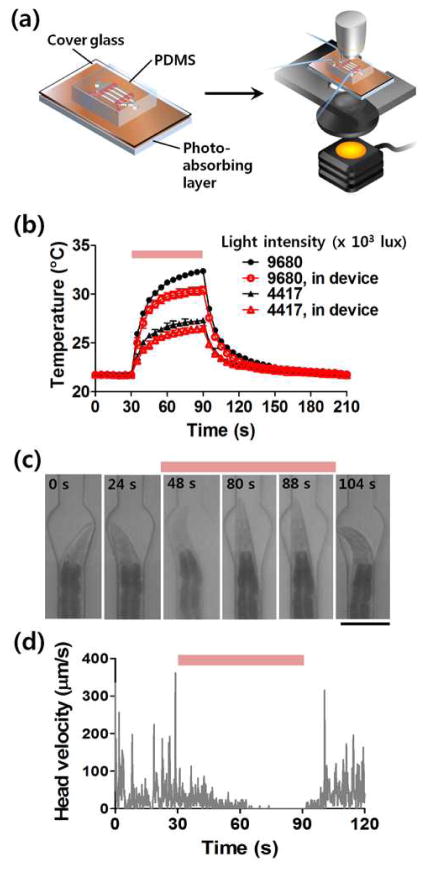
In addition to working on an open system (microscope slide), we also show here that the technology can be easily integrated with microfluidic chips (Fig. 2a). This integration offers more opportunities to conduct multi-step experiments in an automated and high-throughput manner as well as to perform additional functions, such as trapping, long-term cultivation, and stimulation. We used a standard multilayer PDMS microfluidic device on 100 μm-thick cover glass for worm trapping, under which was the photo-absorbing layer (Fig. 2a). Fig. 2b shows that while the coverslip reduces the temperature rise, the change is still significant to induce sol-gel transition for immobilization to occur. To quantify the locomotion of the worm in the microfluidic device, we tracked the displacement of the head (Figs. 2c and 2d). After 60-s illumination, the worm completely stopped moving its head. Here we show that putting the conventional PDMS–glass device on the photo-absorbing layer significantly reduces the complexity of the fabrication and allows reusability of the photothermal material, hence allowing the broad applicability of this technique. Furthermore, this immobilization process is reversible with passive and simple mechanisms. Fig. 2d shows that when the light was turned off the animal recovers movement momentarily. This reversibility provides us an opportunity to recover the animals after the steps requiring the immobilization, which is either impossible (with glue) or much more difficult (on agar pads between glass slide and coverslips).
Figure 2. Photothermal immobilization of C. elegans in a microfluidic device.
(a) Schematic diagram of a protocol for immobilization of worms in a PDMS-glass microfluidic device. (b) Temperature change in the device. (c) Time-lapsed captures showing a worm trapped and immobilized in the microfluidic channel. Scale bar: 100 μm. (d) Quantitative analysis of locomotion of the worm by tracking head position according to the time.
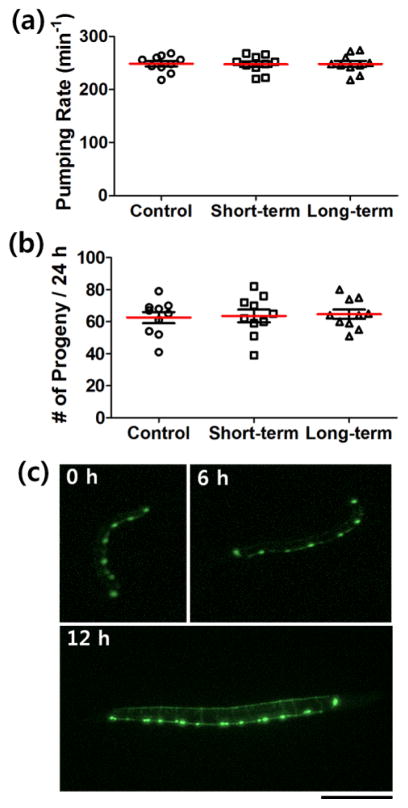
To further test the biocompatibility of the protocol, we measured pharyngeal pumping rate at young adult stage and the number of progeny in the first 24 h of egg-laying. We examined three groups of the worms: (i) worms that had never been immobilized (control); (ii) worms immobilized for 3–5 min every 30 min for 3 h from L4 stage (short-term); (iii) worms immobilized for 3–5 min every 8 h for 36 h from L1 stage (long-term). There were no significant differences among the three groups in either pumping rate or egg-laying rate (Figs. 3a and 3b). Thus it is likely that the immobilization of C. elegans by the photothermal sol-gel transition of Pluronic F127 has little or no adverse effects on their development and physiology.
Figure 3.
Biocompatibility tests and neuronal development in C. elegans. There were no significant differences in (a) pharyngeal pumping rate and (b) number of progeny in the first 24 h of egg-laying among the group which had never been immobilized (control); the group immobilized every 30 min for 3 h from L4 stage (short-term); and the group immobilized every 8 h for 36 h from L1 stage (long-term) (c) Normal development of D-type motor neurons, which express GFP, were clearly observed. The time after the first measurement (hatching) was indicated. Scale bar: 100 μm.
By applying this photothermal immobilization method, development of motor neurons in C. elegans could also be observed with a strain expressing green fluorescent protein (GFP) under the control of an unc-25 promotor in GABAergic D-type motor neurons (juIs76) (Fig. 3c). We obtained fluorescence images of the worms at various stages via complete immobilization with quality comparable to conventional methods. Here the photo-absorbing layer played another role: it absorbs and filters most low wavelength light (< 650 nm),12 which interferes with the fluorescence signal, during the white light exposure for immobilization. As shown in Fig. 3c, the ventral D neurons were generated during the L1 stage and the neural network of the D-type motor neurons was normally formed as consistent with earlier studies.16, 17 This suggests that development was normal and that the protocol is compatible with high-resolution fluorescence imaging modalities.
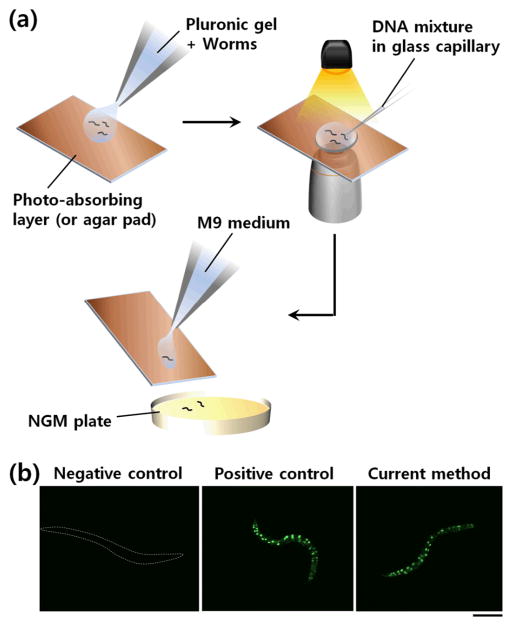
Lastly, we applied this immobilization technology for transgenesis of C. elegans. Microinjection of DNA is typically used and the conventional protocol calls for laborious and painstaking procedures of mounting, microinjection, and demounting animals. Here we simply used selected illumination to immobilize worms on an open surface and performed gonadal transformation using SUR-5::GFP DNA.15 N2 worms were mixed with the Pluronic F127 solution and put onto a substrate (Fig. 4a). After immobilizing the worms by light, the DNA mixture was injected using a conventional microinjection system. We could easily recover the animals; transformed animals were confirmed by GFP in all somatic nuclei (Fig. 4b). In comparison with traditional method, our approach has higher throughput and much simpler to perform; animals can be simply picked off the injection pad. Further, animals do not suffer from dehydration as in the traditional protocol.
Figure 4. Microinjection of DNA mixture into C. elegans nematodes immobilized in Pluronic gel.
(a) Schematic diagram showing application of photothermal worm immobilization to microinjection-based transformation of C. elegans. (b) Successful expression of microinjected DNA (SUR-5::GFP(NLS)15) in a transgenic worm. A worm immobilized but not subjected to injection (as negative control) and a worm injected in a conventional microinjection set-up (as positive control) are also shown. Scale bar: 100 μm.
Our novel selective immobilization technology provides a simple, versatile, and biocompatible method to immobilize C. elegans with no additional instrumentation requirement. Illumination at a localized area allows selective immobilization of specific worms. This technology can be applied for complete immobilization of all the developmental stages of C. elegans at normal physiological conditions. It will be useful not only for dynamic developmental studies based on long-term high-resolution imaging, but also for a variety of applications such as high-throughput microinjection of DNA, or laser surgery; this method when automated, could dramatically improve the throughput and efficiency as compared to current manual approaches.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the financial support of Human Frontier Science Program (RGP0044/2012) and the US National Science Foundation (CBET 0954578), and the US National Institutes of Health (GM088333, AG035317, and EB012803).
References
- 1.Sengupta P, Samuel ADT. Curr Opin Neurobiol. 2009;19:637–643. doi: 10.1016/j.conb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen EM, Mango SE. Nat Rev Genet. 2002;3:356–359. doi: 10.1038/nrg794. [DOI] [PubMed] [Google Scholar]
- 3.Bargmann CI. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki H, Kerr R, Bianchi L, Frøkjær-Jensen C, Slone D, Xue J, Gerstbrein B, Driscoll M, Schafer WR. Neuron. 2003;39:1005–1017. doi: 10.1016/j.neuron.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Knobel KM, Jorgensen EM, Bastiani MJ. Development. 1999;126:4489–4498. doi: 10.1242/dev.126.20.4489. [DOI] [PubMed] [Google Scholar]
- 6.Hulme SE, Shevkoplyas SS, Apfeld J, Fontana W, Whitesides GM. Lab Chip. 2007;7:1515–1523. doi: 10.1039/b707861g. [DOI] [PubMed] [Google Scholar]
- 7.Rohde CB, Zeng F, Gonzalez-Rubio R, Angel M, Yanik MF. Proc Natl Acad Sci USA. 2007;104:13891–13895. doi: 10.1073/pnas.0706513104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung K, Crane MM, Lu H. Nat Methods. 2008;5:637–643. doi: 10.1038/nmeth.1227. [DOI] [PubMed] [Google Scholar]
- 9.Chokshi TV, Ben-Yakar A, Chronis N. Lab Chip. 2009;9:151–157. doi: 10.1039/b807345g. [DOI] [PubMed] [Google Scholar]
- 10.Hulme SE, Shevkoplyas SS, McGuiga AP, Apfeld J, Fontana W, Whitesides GM. Lab Chip. 2010;10:589–597. doi: 10.1039/b919265d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying D, Zhang K, Li N, Ai X, Liang Q, Wang Y, Luo G. BioChip J. 2012;6:197–205. [Google Scholar]
- 12.Xian B, Shen J, Chen W, Sun N, Qiao N, Jiang D, Yu T, Men Y, Han Z, Pang Y, Kaeberlein M, Huang Y, Han JDJ. Aging Cell. 2013;12:398–409. doi: 10.1111/acel.12063. [DOI] [PubMed] [Google Scholar]
- 13.Wen H, Gao X, Qin J. Integr Biol. 2014;6:35–43. doi: 10.1039/c3ib40191j. [DOI] [PubMed] [Google Scholar]
- 14.Krajniak J, Lu H. Lab Chip. 2010;10:1862–1868. doi: 10.1039/c001986k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krajniak J, Hao Y, Mak HY, Lu H. Lab Chip. 2013;13:2963–2971. doi: 10.1039/c3lc50300c. [DOI] [PubMed] [Google Scholar]
- 16.White JG, Albertson DG, Anness MAR. Nature. 1978;271:764–766. doi: 10.1038/271764a0. [DOI] [PubMed] [Google Scholar]
- 17.Petersen SC, Watson JD, Richmond JE, Sarov M, Walthall WW, DMM J Neurosci. 2011;31:15362–15375. doi: 10.1523/JNEUROSCI.3181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.