Abstract
Background
In Ukraine, HIV-infection, injection drug use, and incarceration are syndemic; however, few services are available to incarcerated people who inject drugs (PWIDs). While data are limited internationally, within-prison drug injection (WP-DI) appears widespread and may pose significant challenges in countries like Ukraine, where PWIDs contribute heavily to HIV incidence. To date, WP-DI has not been specifically examined among HIV-infected prisoners, the only persons that can transmit HIV.
Methods
A convenience sample of 97 HIV-infected adults recently released from prison within 1–12 months was recruited in two major Ukrainian cities. Post-release surveys inquired about WP-DI and injection equipment sharing, as well as current and prior drug use and injection, mental health, and access to within-prison treatment for HIV and other comorbidities. Logistic regression identified independent correlates of WP-DI.
Results
Complete data for WP-DI were available for 95 (97.9%) respondents. Overall, 54 (56.8%) reported WP-DI, among whom 40 (74.1%) shared injecting equipment with a mean of 4.4 (range 0–30) other injectors per needle/syringe. Independent correlates of WP-DI were recruitment in Kyiv (AOR 7.46, p=0.003), male gender (AOR 22.07, p=0.006), and active pre-incarceration opioid use (AOR 8.66, p=0.005).
Conclusions
Among these recently released HIV-infected prisoners, WP-DI and injection equipment sharing were frequent and involved many injecting partners per needle/syringe. The overwhelming majority of respondents reporting WP-DI used opioids both before and after incarceration, suggesting that implementation of evidence-based harm reduction practices, such as opioid substitution therapy and/or needle/syringe exchange programs within prison, is crucial to addressing continuing HIV transmission among PWIDs within prison settings. The positive correlation between Kyiv site and WP-DI suggests that additional structural interventions may be useful.
Keywords: HIV, Incarceration, Drug Injection, Substitution Therapy, Eastern Europe, Ukraine
Introduction
Ukraine’s HIV epidemic is Europe’s most severe, with adult prevalence exceeding 1.0% (Bobrovskyy et al., 2012). While sexual transmission is increasing, suggesting a transitional epidemic, HIV transmission among people who inject drugs (PWIDs) accounts for 70% of cumulative HIV infections (UNAIDS, 2010). With 290,000 PWIDs (Balakiryeva, Bondar, Sereda, & Sazonova, 2012) and HIV prevalence among them ranging between 21.5% – 41.8% (Balakiryeva et al., 2012; Bobrovskyy et al., 2012; Mathers et al., 2008), PWIDs continue to bear a disproportionate share of the epidemic, thus constituting a priority target for treatment and prevention efforts.
Worldwide, incarceration, substance use disorders (SUDs), and HIV/AIDS are syndemic, comorbid phenomena; being affected by one increases the risk for and/or compounds the effects of the other two. Thus, effective HIV prevention and treatment must address all three problems simultaneously (Altice, Kamarulzaman, Soriano, Schechter, & Friedland, 2010). An estimated 56% to 90% of PWIDs are imprisoned during their lifetime (Jürgens, Csete, Amon, Baral, & Beyrer, 2010; Mathers et al., 2008), which partly explains why HIV prevalence among prisoners greatly exceeds that in the general population (Dolan et al., 2007; Stephenson et al., 2006; Wodak & McLeod, 2008). In Ukraine, 15% of all inmates are incarcerated for drug-related offenses, excluding crimes committed to finance their drug use (Bewley-Taylor, Hallam, & Allen, 2009). Estimates of HIV prevalence within Ukraine’s prisons have ranged from 13.6% to 25.4% (Azbel, Wickersham, Grishaev, Dvoryak, & Altice, 2013; Bobrovskyy et al., 2012; Dolan et al., 2007), with HIV prevalence among incarcerated PWIDs exceeding 20% (Bobrovskyy et al., 2012). In one Ukrainian region, PWIDs accounted for only 30% of the prison population but 83% of HIV-infected inmates (Dolan et al., 2007). A more recent national survey suggested that 47% of prisoners transitioning to the community were PWIDs (Azbel et al., 2013).
Studies of drug injection within prison estimate that between 3–53% of all inmates inject at some point while incarcerated (Jürgens, Ball, & Verster, 2009; Kinner, Jenkinson, Gouillou, & Milloy, 2012), a sobering consequence of the high concentration of PWIDs and untreated SUDs in prison settings. Evidence suggests that PWIDs inject less frequently in prisons than they do in community settings, but HIV transmission risks are markedly elevated within prisons because a scarcity of injection equipment leads to increased high-risk sharing (Darke, 1998; Jürgens et al., 2009). This may in part contribute to findings that prior incarceration is independently associated with HIV infection (Booth, Kwiatkowski, Brewster, Sinitsyna, & Dvoryak, 2006).
Most inmates eventually return to the community, a transition that for PWIDs often involves relapse to drug and alcohol use and increased HIV transmission risk behaviours (Milloy et al., 2008; Stephenson et al., 2006; Wood et al., 2005). This has been underscored by data showing recent incarceration to be a risk-factor for syringe lending by HIV-infected PWIDs (Milloy et al., 2013). For those receiving HIV treatment, poor antiretroviral therapy (ART) adherence and impaired viral suppression are common post-release (Baillargeon et al., 2009; Springer et al., 2004; Stephenson et al., 2005; Westergaard, Kirk, Richesson, Galai, & Mehta, 2011), with prior incarceration itself identified as a risk factor for uncontrolled viral replication—often due to inconsistent access to ART—among HIV-infected PWIDs (Milloy et al., 2013). Not only do such outcomes jeopardize these individuals’ health, they also raise the risk of HIV transmission to others in the community. Moreover, those who acquire HIV while incarcerated may not be aware of their infection, adding to the danger of unwitting viral transmission. Despite frequent HIV testing of prisoners, recent data from Ukraine suggests that over half of HIV-infected prisoners are unaware of being HIV-infected at the time of release (Azbel et al., 2013). For these reasons, understanding and ultimately addressing injection-related behaviour within prisons is crucial not only to HIV prevention and treatment success among prisoners, but also for the general population and overall public health.
While there are a number of studies examining WP-DI, data from low- and middle-income countries, including those of the Former Soviet Union, are scarce. Moreover, while several studies have simultaneously examined HIV seroprevalence and risk behaviour in prison, the numbers of HIV-infected respondents have generally been small (Ford et al., 2000; Rotily et al., 2001) and, to our knowledge, no study of risk behaviour within prison has focused specifically on the behaviour of HIV-infected individuals—those who can actually transmit the virus. Our aim here is to identify the prevalence and explore correlates of within-prison drug injection (WP-DI) and injection equipment sharing among recently released HIV-infected prisoners in Ukraine in order to guide further development of drug policies and treatment protocols that will appropriately address Europe’s fastest growing HIV epidemic.
Methods
Ethics Statement
This study was performed in accordance with international ethical standards on human subjects research. Institutional Review Boards at the Yale University School of Medicine and the Ukrainian Institute on Public Health Policy provided oversight and approval of all procedures, including oral informed consent.
Patient Population and Study Design
From November 2010 through January 2011, a convenience sample of 97 HIV-infected individuals was recruited using the following criteria: 1) age ≥18 years; 2) HIV-infected by self-report (supported further by questions inquiring about diagnosis and treatment either before or during the most recent prison term); and 3) released from prison with the past 1–12 months. Potential respondents were screened without prior knowledge of these inclusion criteria and ineligible participants were not informed of the reason for exclusion in order to reduce recruitment bias. Eligible individuals were recruited in Kyiv and Odessa, two major Ukrainian cities with high burdens of HIV. In each city, a non-governmental organization serving former inmates assisted with recruitment. Modified snowball recruitment was used in Odessa, while individuals seeking assistance at a single site providing post-release services were recruited in Kyiv. No eligible recruit refused further participation, consistent with previous experiences recruiting PWIDs in Ukraine (Booth, Lehman, Dvoryak, Brewster, & Sinitsyna, 2009). Because staff collected no identifying information, respondents only provided oral consent to participate. Each consenting respondent received a copy of the consent form as a means of verifying and recording consent before participating in a 60-minute survey conducted in a private room. No government representatives were present during the interviews. After completing the survey, participants were paid UAH 50 ($8.25 USD) for their time and travel to the survey site.
Study Measures
Surveys were initially created in English, translated into Russian and Ukrainian and back-translated into English using previously described methods (Bullinger et al., 1998). Bilingual Ukrainian research staff verified integrity of the translation and conducted a brief pilot. To assess the primary outcomes, all respondents were asked, “During your most recent incarceration, did you inject any drugs?” Those who responded yes were also asked the following question: “You stated that you injected drugs during your most recent incarceration. Approximately how many people used this same needle or syringe that you used to inject?”
Demographic measures included age and gender. Income was divided at or below poverty for respondents earning 888UAH (~$110) or less monthly based on national 2010 poverty estimates. Relationship status was defined as either having a stable partner or no partner, with stable partner including either a spouse or “girlfriend/boyfriend”. Housing was stratified into “stable” or “unstable” using previously defined groupings (Chen et al., 2011), with the former category including living in one’s own apartment, that of a relative or partner, a rented apartment, or a long-term treatment facility. Unstable housing included residency in a brothel or shelter, or self-reported homelessness. Education was categorized as either completing or not completing secondary school, the highest compulsory level in Ukraine. Criminal justice measures included details about most recent and lifetime incarcerations, total number of months in prison, lifetime non-prison detentions, and age of first incarceration.
Measures related to substance use and addiction screening included two standardized screening tools validated in Russian: the Alcohol Use Disorders Identification Test (AUDIT) (Nilssen et al., 2005) and the 10-item Drug Abuse Severity Test (DAST-10) (Uusküla et al., 2012)—and one recently developed and translated specifically for the purposes of this study: the rapid opioid dependence scale, which has high specificity and sensitivity for meeting DSM-IV criteria (Wickersham, Azar, Cannon, Alticle, & Springer, n.d.). Standardized cut-offs for hazardous drinking using the AUDIT were ≥8 for men and ≥4 for women (J. P. Allen, Litten, Fertig, & Babor, 1997; Meyer, Qiu, Chen, Larkin, & Altice, 2012; Neumann et al., 2004), while for alcohol dependence, scores of ≥15 and ≥13 were used for men and women, respectively (Rubinsky, Kivlahan, Volk, Maynard, & Bradley, 2010). A cut-off of ≥3 was used on the DAST-10 because of its correlation with moderate to severe drug addiction (Skinner, 1982). Other drug-use measures included an inventory of drugs used during the thirty days prior to the most recent incarceration, including those common in Ukraine. Drugs were categorized as stimulants, illegal opioids, alcohol, and poly-substance. Illegal opioids included heroin, morphine, opium, and homemade poppy straw extract, the most commonly injected opioid in Ukraine. Stimulants included amphetamines, ecstasy, cocaine, and homemade stimulants. Poly-substance use was defined as use of more than one of the listed substances in a single day. “Active use” of any of the above was defined as any use in the 30 days prior to incarceration. Measures of injection and sharing behaviour in the 30 days prior to incarceration included days of injection and average injections per day, as well as binary variables representing any injection and any equipment sharing or use of liquid drugs from a common container. High-risk injection was defined as participating in either type of behaviour. For comparison, data on injection behaviour during the post-incarceration period of 30 days immediately prior to interview were also obtained. Injection-related morbidity was defined as abscess, skin-infection, or overdose resulting from drug injection occurring within 12 months prior to incarceration.
Several measures focused on access to care before, during and after imprisonment, including HIV diagnosis and receipt of ART. HIV care (excluding HIV testing) was reported for both the period within prison and before incarceration. Self-reported co-morbidities and treatment utilization included HCV, tuberculosis, and mental illness.
Data Analysis
Analyses for this study were conducted using SPSS Version 19.0 Advanced Statistics Package (SPSS Inc., Chicago, Il, USA). An initial bivariate analysis assessed for any significant relationships between covariates and the primary dependent variable, namely within-prison drug injection (WP-DI), using chi-square tests for binary variables and t-tests or Mann-Whitney U tests (depending on response distribution) for continuous variables. An initial association of p<0.10 was used to identify potentially significant variables, which were then entered into a backwards-stepwise likelihood-ratio logistic regression to determine inclusion into a final multivariate model to describe correlates of WP-DI. To control for admitted variable bias, we relied on prior studies as a theoretical basis for the addition of variables that were not initially retained, including age and pre-incarceration injection patterns using a combined three-category variable (no injection, injection with no reported high-risk injection, and injection inclusive of reported high-risk injection) to describe injection in the month before incarceration. The variable for site was retained in the final model in order to control for some of the possible variation between respondents recruited at the two sites when examining other correlates of interest (see table 4). Akaike Information Criterion (AIC) was used to ensure best possible fit from among final model candidates.
Table 4.
Independent correlates of within prison drug injection
| Covariate | UOR | 95% CI | p-value | AOR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Recruitment in Kyiv | 5.02 | 2.07 – 12.21 | <0.001 | 7.46 | 1.99 – 27.92 | 0.003 |
| Age | 0.96 | 0.92 – 1.01 | 0.140 | 0.95 | 0.93 – 1.07 | 0.080 |
| Male gender | 17.10 | 2.09 – 140.01 | 0.008 | 22.07 | 1.69 – 155.38 | 0.006 |
| Opioid use in the 30 days before prison | 7.42 | 2.42 – 22.68 | <0.001 | 8.67 | 1.91 – 39.44 | 0.005 |
| Injection behavior in the 30 days before prison | ||||||
| No injection | Ref | -- | Ref | -- | ||
| Injection, no high- risk injection | 6.75 | 0.93 – 49.23 | 0.060 | 2.83 | 0.16 – 47.35 | 0.417 |
| High-risk injection | 7.39 | 1.50 – 36.71 | 0.014 | 2.95 | 0.15 – 15.21 | 0.293 |
| AIC = 81.93 |
Legend: UOR=unadjusted odds ratio; AOR=adjusted odds ratio; 95% CI=confidence interval; AIC=Akaike Information Criterion
Results
Baseline Characteristics
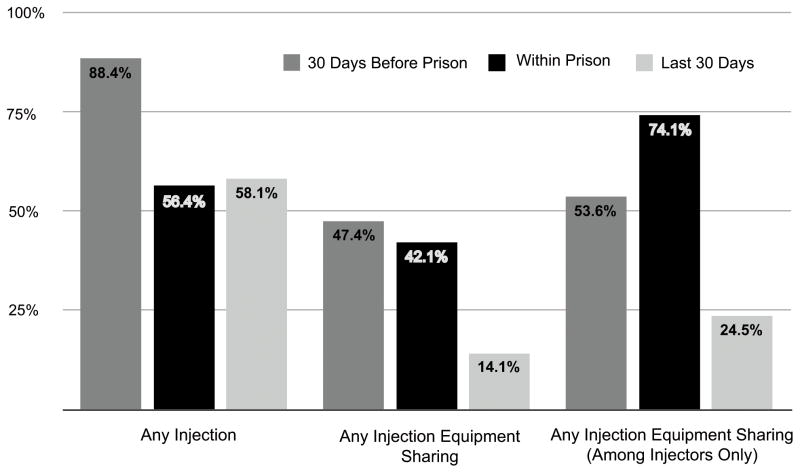
Of 97 subjects recruited, 95 provided a response for the main outcome and comprised the final analytic sample. Figure 1 shows the prevalence of WP-DI and injection equipment sharing in prison, as well as in the 30 days prior to incarceration and the 30 days prior to interview (post-release period). Over half (54.6%) of the respondents reported WP-DI during the most recent incarceration. Nearly three quarters (74.1%) of within-prison drug injectors (WP-DIs) reported injection equipment sharing in prison, representing a markedly higher percentage of injectors sharing compared to the pre-incarceration and post-release periods. These individuals shared their equipment with a mean of 4.4 other injecting partners (range 0–30). No correlation was observed between length of prison term and WP-DI (p=0.768) or injection equipment sharing (p=0.258).
Figure 1. Overview of injection behaviour before incarceration, within prison, and in the last thirty days.
This figure illustrates the reported prevalence of drug injection, injection equipment sharing among the entire study group, and injection equipment sharing among injectors over three time periods: the 30 days prior to prison, within prison, and in the last 30 days (N=95).
Tables 1 and 2 describe individual characteristics and bivariate associations for the primary outcome, while table 3 lists those characteristics that differed significantly by recruitment site. Briefly, the sample was equally divided between Kyiv (48.4%) and Odessa (51.6%), mostly male (88.4%), and stably housed (61.7%). Regarding the most recent prison term, the average duration was 49.5 months and the mean time since release was 5.8 months. The majority of respondents had most recently been imprisoned on a drug-related charge (76.8%).
Table 1.
Bivariate demographic, criminal justice and medical associations with within-prison drug injection
| Characteristic | N | Total n (%) | Injected in prison n (%) | Did not inject in prison n (%) | p-value |
|---|---|---|---|---|---|
| Recruited in Kyiv | 95 | 46 (48.4) | 35 (64.8) | 11 (26.8) | <0.001 |
| Mean age (SD) | 95 | 35.4 (±8.2) | 34.3 (±8.4) | 36.9 (±7.9) | 0.116 |
| Mean days since release (SD) | 93 | 169.2 (±70.7) | 172.7 (±63.0) | 164.6 (±79.9) | 0.523 |
| Mean length last prison term, months (SD) | 93 | 49.5 (±27.2) | 49.0 (±22.8) | 50.0 (32.2) | 0.768 |
| Male gender | 95 | 84 (88.4) | 53 (98.1) | 31 (75.6) | 0.001 |
| Stable relationship partner | 91 | 59 (64.8) | 33 (63.5) | 26 (66.7) | 0.751 |
| Income Below Poverty | 94 | 57 (60.0) | 33 (61.1) | 24 (60.0) | 0.913 |
| Did not complete secondary education | 95 | 35 (36.8) | 22 (40.7) | 13 (31.7) | 0.366 |
| Stable housing | 94 | 58 (61.7) | 32 (60.4) | 26 (63.4) | 0.709 |
| Mean age first incarcerated (SD) | 95 | 21.1 (±6.2) | 19.6 (±3.4) | 23.2 (±8.3) | 0.143 |
| Mean lifetime prison terms (SD) | 95 | 2.9 (±2.0) | 3.1 (±2.2) | 2.6 (±1.6) | 0.209 |
| Mean lifetime police detentions (SD) | 91 | 11.1 (±11.5) | 12.4 (±12.3) | 9.5 (±10.5) | 0.126 |
| Knew HIV status before last prison term | 90 | 46 (48.4) | 22 (43.1) | 22 (56.4) | 0.212 |
| Accessed HIV care in 30 days prior to prison | 91 | 8 (8.8) | 5 (9.6) | 3 (7.7) | 0.749 |
| Accessed HIV care within prison | 91 | 60 (65.9) | 41 (78.8) | 19 (48.7) | 0.003 |
| Received ART in 30 days prior to prison | 91 | 13 (14.3) | 10 (19.2) | 3 (7.7) | 0.120 |
| Received ART within prison | 91 | 17 (18.7) | 13 (24.5) | 4 (10.5) | 0.091 |
| TBD: Ever diagnosed | 90 | 54 (60.0) | 32 (64.0) | 22 (55.0) | 0.386 |
| TBD: Treated in 6 months before prison | 89 | 21 (23.6) | 15 (31.3) | 6 (14.6) | 0.066 |
| TBD: Treated in prison | 90 | 46 (51.1) | 31 (63.3) | 15 (36.6) | 0.019 |
| HCV: Ever diagnosed | 89 | 37 (41.6) | 22 (44.9) | 15 (37.5) | 0.481 |
| HCV: Treated 6 months before prison: | 85 | 10 (11.8) | 6 (13.6) | 4 (9.8) | 0.579 |
| HCV: Treated in prison | 89 | 18 (20.2) | 14 (29.2) | 4 (9.8) | 0.023 |
| Mental illness: Ever diagnosed | 83 | 16 (19.3) | 8 (18.2) | 8 (20.5) | 0.788 |
| Mental illness: Treated in 6 months before prison | 82 | 4 (4.9) | 2 (4.7) | 2 (5.1) | 0.920 |
| Mental illness: Treated in prison | 71 | 2 (2.8) | 1 (2.9) | 1 (2.7) | 0.952 |
Legend: SD=standard deviation; ART=antiretroviral therapy; TBD = tuberculosis disease, HCV = hepatitis C virus
Table 2.
Bivariate drug and alcohol use associations with within-prison drug injection d sharing of injection equipment
| Characteristic | N | Total n (%) | Injected in prison n (%) | Did not inject in prison n (%) | p-value |
|---|---|---|---|---|---|
| Last incarceration drug-related | 95 | 73 (76.8) | 44 (81.5) | 29 (70.7) | 0.219 |
| Met screening criteria for opioid dependence | 95 | 80 (84.2) | 49 (90.7) | 31 (75.6) | 0.045 |
| Moderate to severe drug-use severity | 95 | 88 (92.6) | 51 (94.4) | 37 (90.2) | 0.438 |
| Met screening criteria for any hazardous drinking | 87 | 54 (62.1) | 27 (54.0) | 27 (73.0) | 0.071 |
| Met screening criteria for alcohol dependence | 87 | 24 (27.6) | 10 (20.0) | 14 (37.8) | 0.066 |
| On OST at time of last incarceration | 92 | 17 (18.5) | 11 (21.6) | 6 (14.6) | 0.394 |
| Opioid use 30 days prior to prison | 92 | 70 (73.7) | 48 (90.6) | 22 (56.4) | <0.001 |
| Stimulant use 30 days before prison | 85 | 45 (52.9) | 26 (56.5) | 19 (48.7) | 0.473 |
| Alcohol Use 30 days before prison | 91 | 58 (63.7) | 32 (65.3) | 24 (60.0) | 0.606 |
| Poly-substance use 30 days before prison | 86 | 57 (66.3) | 35 (74.5) | 22 (56.4) | 0.078 |
| Any drug injection during 30 days before prison | 95 | 84 (88.4) | 52 (96.3) | 32 (78.0) | 0.008 |
| Mean injection days for those injecting (SD) | 84 | 20.5 (±7.9) | 20.1 (±7.1) | 21.3 (±9.2) | 0.350 |
| Mean injections per day for those injecting (SD) | 73 | 3.1 (±4.5) | 3.3 (±4.7) | 2.7 (±4.2) | 0.213 |
| Any high-risk injection 30 days before prison | 95 | 75 (78.9) | 46 (85.2) | 29 (70.7) | 0.087 |
| Injection equipment sharing 30 days before prison | 95 | 45 (47.4) | 29 (53.7) | 16 (39.0) | 0.156 |
| Common liquid drug container 30 days before prison | 95 | 73 (76.8) | 46 (85.2) | 27 (65.9) | 0.027 |
| Injection-related morbidity 12 months before prison | 94 | 64 (68.1) | 44 (81.5) | 20 (50.0) | 0.001 |
Legend: SD=standard deviation; OST=opioid substitution therapy
Table 3.
Bivariate comparisons of baseline characteristics differing significantly by recruitment site
| Characteristic | N | Kyiv n (%) | Odessa n (%) | p-value |
|---|---|---|---|---|
| Mean Age (SD) | 95 | 32.2 (±5.7) | 38.5 (±9.1) | <0.001 |
| Mean length of last incarceration in months (SD) | 93 | 59.1 (±22.8) | 40.8 (±28.0) | <0.001 |
| Mean age of first incarceration (SD) | 95 | 19.4 (±4.2) | 22.7 (±7.4) | 0.003 |
| Received HIV care within prison | 91 | 39 (92.9) | 21 (42.9) | <0.001 |
| Received ART within 30 days before prison | 91 | 11 (26.2) | 2 (4.1) | 0.005 |
| Received ART in prison | 91 | 14 (32.6) | 3 (6.2) | 0.001 |
| Met screening criteria for any hazardous drinking | 87 | 23 (50.0) | 31 (75.6) | 0.014 |
| Met screening criteria for alcohol dependence | 87 | 8 (17.4) | 16 (39.0) | 0.024 |
| On OST at time of last incarceration | 92 | 16 (36.4) | 1 (2.1) | <0.001 |
| Used common liquid drug container for injection in 30 days before prison | 95 | 27 (58.7) | 18 (36.7) | 0.032 |
| Injection-related morbidity 12 months before prison | 94 | 41 (89.1) | 23 (47.9) | <0.001 |
Legend: SD=standard deviation; ART=antiretroviral therapy; OST=opioid substitution therapy
Nearly half of respondents (48.4%) were diagnosed with HIV before their most recent incarceration, with the remainder diagnosed during incarceration. Approximately one sixth (14.3%) of respondents had been prescribed ART before incarceration, a proportion that was not appreciably higher (18.7%) during incarceration.
Drug Use
Opioids were the most commonly used drugs in the 30 days leading up to incarceration (73.7%), though stimulant (54.9%) and alcohol (63.7%) use were also common. Two-thirds reported polysubstance use (66.3%). Opioids were the only drugs for which pre-incarceration use was significantly higher among WP-DIs compared to those who did not inject in prison (90.6% vs. 56.4% p<0.001).
The overwhelming majority (88.4%) of participants reported active injection immediately before incarceration, including 10 of 11 women surveyed. The average PWID injected three times per day on just over 20 of 30 days. High-risk injection was common, with nearly half (47.4%) reporting pre-incarceration injection equipment sharing and over three-quarters (76.8%) reporting drawing drugs from a “common” liquid container of homemade poppy straw. Compared to those who did not inject in prison, WP-DIs were also more likely to report injection (96.3% vs. 78.0%, p=0.009) using a common liquid container (85.2% vs. 65.9%, p=0.027) in the 30 days prior to incarceration, and to report injection-related morbidity in the year prior to prison (81.5% vs. 50.0%, p=0.001). Nearly all (92.6%) respondents met criteria for moderate to severe addiction severity, while a substantial majority met criteria for opioid dependence (84.2%) and for an alcohol use disorder (60.9%).
Multivariate correlates of within prison drug injection
Table 4 shows unadjusted and adjusted correlates associated with WP-DI. Significant independent correlates included recruitment in Kyiv (AOR=7.46, p=0.003), male gender (AOR=22.07, p=0.006), and active opioid use in the 30 days before prison (AOR=8.67, p=0.005). An additional logistic regression model (data not shown) for within-prison injection equipment sharing (females were excluded because none reported sharing within prison) revealed similar independent covariates (active opioid use in the 30 days before incarceration AOR=6.50, p=0.025; recruitment in Kyiv (AOR=27.89, p<0.001).
Discussion
While WP-DI has been reported as prevalent and geographically widespread (Jürgens et al., 2009), this is to our knowledge the first study to examine the prevalence of WP-DI and injection equipment sharing within prison specifically among individuals with known HIV infection. This study is also one of only a few examining risk behaviour in prison within a middle-income country, specifically Ukraine, home to Europe’s worst HIV epidemic (Hurley, 2010). Findings from this survey suggest that WP-DI in Ukraine may significantly contribute to HIV transmission, raising important implications for intervention.
Alarmingly, over half of respondents reported injecting drugs within prison, a number that is high but not inconsistent with the range of findings from prison populations elsewhere (Clarke, Stein, Hanna, Sobota, & Rich, 2001; Jürgens et al., 2009; Kinner et al., 2012). Nearly 75% of those injecting within prison reported sharing of injection equipment with several other individuals, likewise reflecting prior findings that incarcerated PWIDs generally share needles and syringes far more than their counterparts in the community, a trend that is generally attributed to a scarcity of injection equipment within prison settings (Calzavara et al., 2003; Carvell & Hart, 1990; Kinner et al., 2012; Malliori et al., 1998). Even more disturbingly, respondents in our study who did share injection equipment on average reported doing so with a substantial number of other inmates, all of who would be at risk for acquiring HIV infection if not already infected.
The “perfect storm” of a high prevalence of HIV-infected inmates injecting within prison and widespread injection equipment sharing involving multiple individuals using a single needle or syringe points to an extraordinarily high-risk environment where repeated opportunities for transmission of HIV and other blood-borne infections lead to the possibility of one individual infecting many others. Such a scenario, documented by our data, supports the hypothesis that prisons significantly contribute to overall HIV incidence in Ukraine, a notion bolstered by the finding that prior incarceration is an independent HIV risk factor there (Booth et al., 2006). This situation is at least in part a result of two critical structural issues, both of which should be addressed urgently. The first is a criminal justice policy that punishes the possession of illicit drugs, resulting in tremendous numbers of PWIDs with lengthy prison sentences. The second is a lack of any meaningful drug treatment or harm reduction options for incarcerated PWIDs, especially those who are HIV-infected. While much of our discussion focuses on the latter, reforming core drug policies to shift away from criminalization of minor drug crimes—as has been done in Portugal, for example—has the potential for a durable and far-reaching positive impact on HIV transmission and other harms related to drug use (Hughes & Stevens, 2012; Russoniello, 2013).
Our data document a highly significant positive correlation between recent pre-incarceration opioid use and WP-DI, affirming data from one previous study (Calzavara et al., 2003). While our study did not independently assess the types of drugs injected in prison, such a correlation between pre-incarceration opioid use and WP-DI itself suggests respondents were likely injecting opioids in prison. This hypothesis is further supported by a variety of other findings including the extraordinarily high prevalence of opioid dependence in our own sample, observations from the Ukrainian community (Booth et al., 2009), other studies on WP-DI (Calzavara et al., 2003; Malliori et al., 1998), and a recent bio-behavioural survey that found opioid dependence to be the most common underlying substance use disorders among prisoners transitioning to the community (Azbel et al., 2013), with widespread pre-release concerns about relapse to drug use among prisoners (Morozova et al., 2013). It remains likely, however, that some WP-DI involved other drugs as well.
Despite international guidelines recommending its provision, evidence-based treatment for opioid dependence, including opioid substitution therapy (OST) using methadone or buprenorphine, is only rarely available in prisons (Bruce & Schleifer, 2008). OST serves two key purposes, distinct but closely related. First, it prevents withdrawal symptoms, an imperative for both human rights—given the severe physical and psychological discomfort associated with withdrawal, particularly from opioids—and for public health—given that avoidance of withdrawal symptoms is one motivating factor leading individuals to inject. Second, long-term OST effectively treats opioid dependence, a chronic and relapsing medical condition (Altice et al., 2010), thus preventing continuing injection or relapse, both during and after release (Kinlock, Gordon, Schwartz, Fitzgerald, & O’Grady, 2009) and bolstering retention in OST (Wickersham, Marcus, Kamarulzaman, Zahari, & Altice, 2013a; Wickersham, Zahari, Azar, Kamarulzaman, & Altice, 2013b).
Recent research has demonstrated that prison-based OST programs are effective at reducing both drug injection and equipment sharing within prisons, the two main outcomes of interest here (Jürgens et al., 2009; Larney, 2010; Stöver, 2003). Moreover, when linked with effective transitional coordination and community-based treatment, prison-based OST can help reduce drug injection and relapse upon release (Kinlock et al., 2009). Put into context, our data make a powerful case for providing OST to Ukrainian prisoners, an intervention with the potential to reduce HIV transmission to others within prison and to improve post-release health outcomes related to both HIV and injection drug use (Wickersham, Marcus, Kamarulzaman, Zahari, & Altice, 2013a; Wickersham, Zahari, Azar, Kamarulzaman, & Altice, 2013b).
In addition to OST, a variety of other structural interventions have the potential to help reduce HIV transmission within prisons. For example, both OST and needle-syringe exchange programs (NSEPs) reduce HIV transmission and other injection-related harms (Jürgens et al., 2009), are effective interventions for people who inject any class of drugs, are safe, and can be introduced inexpensively and quickly, For these reasons, conversations around interventions to reduce within-prison HIV-transmission in Ukraine must include NSEPs and other harm-reduction services. Such programs are already widely available in community settings in Ukraine.
Despite all of our respondents being HIV-infected and over half reporting being diagnosed while incarcerated, only two-thirds of respondents accessed any HIV care in prison, and less than one-fifth actually received ART. The proportion prescribed ART in this sample is higher (6%) than reported among a national sample of Ukrainian prisoners (Azbel et al., 2013), perhaps because this sample was receiving post-release social services or had been incarcerated in larger, more central prisons with better-established HIV care. Aside from the health implications for those failing to receive necessary therapy, there are public-health implications as well. The “seek, test, treat, and retain” strategy for HIV prevention, which calls for identification and prompt treatment of HIV-infected individuals, has gained favour based on evidence showing effective viral suppression through ART treatment results in impressive reductions in HIV transmission among HIV serodiscordant couples (M. S. Cohen et al., 2011); more recent data point to a similar effect among PWIDs (McNairy, Deryabina, Hoos, & El-Sadr, 2013; Milloy, Marshall, Montaner, & Wood, 2012).
One failed “structural” intervention to reduce HIV transmission in prison deployed in some settings, including the US, is physical segregation of HIV-infected prisoners in congregate housing units. While it may be seductive to consider this approach as an alternative to providing evidence-based drug treatment services, such policies harm PWIDs in numerous ways by increasing the risk of devastating TB outbreaks among immunocompromised inmates (Al-Darraji, Razak, Ng, & Altice, 2013; Mayer et al., 2002) and subjecting HIV-infected inmates to profound stigmatization and other human rights violations. Moreover, there is no evidence that such policies reduce the transmission of blood-borne viruses (Jürgens, Nowak, & Day, 2011).
An unexpected finding from our analysis was that recruitment in Kyiv was highly correlated with WP-DI. While potentially interesting, this finding is difficult to interpret from these data. No prison-based harm-reduction services are available throughout Ukraine, meaning differential access to services could not account for the observed correlation. An alternative explanation could be that WP-DI was facilitated by corruption, increased flow of drugs, or less stringent enforcement within Kyiv prisons; however, we cannot document a priori that any of these is the case. Also possible, though unable to be substantiated, is that variability in the recruitment approach itself may have resulted in subpopulations that were different from each other. We may speculate that the single-site recruitment approach in Kyiv was more likely to attract individuals actively seeking services, reflecting a higher level of perceived poor health or perhaps greater self-efficacy. Factors that differed significantly by site, such as greater access to HIV services and pre-prison OST in the Kyiv group, may reflect a more “linked-in” scenario for those recruited in Kyiv; however, such differences do not readily account for WP-DI. Further research using a representative sampling approach and exploring contextual factors is recommended.
Limitations of this study
While the amount of WP-DI and injection equipment sharing by Ukrainian prisoners is highly concerning, this study does have several limitations. First, because of the illicit and covert nature of drug injection and equipment sharing within prisons, data on these outcomes were collected by self-report, which may have led to underreporting of the main outcome due to fear of repercussions or social desirability bias. Nonetheless, the disturbingly high prevalence of HIV-infected prisoners reporting this behaviour suggests that when interviewed in a non-coercive setting during the early post-release period, many participants were willing to report sensitive, stigmatized injection practices. Moreover, drug-related risk behaviours are more impervious to disclosure bias than sexual risk behaviours (Ghanem, Hutton, Zenilman, Zimba, & Erbelding, 2005). Second, recall bias may have been introduced as some respondents had been released from prison for up to 12 months and the mean duration of incarceration was long; however, it should be noted that the median time since release was less than six months. Third, convenience samples from only two Ukrainian cities do not fully provide a representative sample for all prison-based risk behaviours in Ukraine. This approach was deployed, however, to recruit only HIV-infected released prisoners–a relatively small, specific, highly marginalized, and difficult to access population in a manner most appropriate to resources available at each site. Fourth, we identified that individuals shared injection equipment with a large number of other individuals, but we did not specify whether this occurred during a single episode or over time. Since it is common for injection equipment to be hidden and shared repeatedly, our estimate of sharing partners may be conservative. Finally, respondents’ self-reported HIV status leaves open the possibility that some were not HIV-infected while incarcerated (or at any time thereafter). We attempted to constrain inaccurate self-report of HIV status by withholding eligibility criteria during screening procedures and asking collateral questions regarding site of HIV diagnosis and related treatment issues. Moreover, payment for participation was low and HIV-positive individuals are highly stigmatized in Ukraine, reducing the likelihood that participants would falsely report HIV-infection. Notwithstanding these limitations, the sample of 97 HIV-infected participants who were recently released from prison represents the largest and only sample to date to assess the risk of WP-DI among the only group of inmates that can transmit HIV.
Conclusions
Harsh punitive drug policies associated with high rates of incarceration and the limited availability of evidence-based OST, ART, and harm reduction services within prisons are likely contributing to a rapidly growing HIV epidemic in Ukraine specifically and in Eastern Europe and Central Asia generally (Atun & Olynik, 2008; Barcal et al., 2005; J. Cohen, 2010; UNAIDS, 2010; Vagenas et al., 2013; Wolfe, Carrieri, & Shepard, 2010). From 2000–2009, new HIV cases in this region increased by 24%, even while declining by 19% throughout the rest of the world (UNAIDS, 2010). Our data underscore the idea that any successful effort to effectively reverse this epidemic must address the challenges facing PWIDs who interface with prison settings, and importantly must include efforts to reduce drug-related incarceration itself (Maru, Basu, & Altice, 2007). While the findings from this study arguably lend support to growing calls for changes to core drug policies that criminalize drug use and possession, at a minimum they indicate that evidence-based treatment for substance use disorders and HIV-infection, in particular OST and ART, should be scaled up within the prison system to ensure widespread coverage throughout incarceration and continued into the high-risk post-release period with optimal transitional coordination.
Acknowledgments
Funding
Funding for this project was provided in part by the International Renaissance Foundation, Kyiv, Ukraine as well grants from the National Institute on Drug Abuse for research (R01 DA033679 and R01 DA029910) and for career development (K24 DA017072) and the Yale University School of Medicine’s Office of Student Research and Yale University Global Health Initiative. No funding source had any role in study design, collection or analysis of data, writing or review of the manuscript, or decision to submit this paper for publication.
We would like to acknowledge the assistance of Tiara Winn, Maua Herme, and Artem Kopelev at the Yale University AIDS Program for data management, organizational and translation assistance of the staff of the Ukrainian Institute on Public Health Policy, and the operational involvement of the staff of the Future Without AIDS foundation, Odessa, Ukraine. We also wish to thank the study participants for giving generously of their time.
Footnotes
Declaration of Conflicts of Interest
Regarding the manuscript entitled, “Within-Prison Drug Injection among HIV-Infected Ukrainian Prisoners: Prevalence and Correlates of an Extremely High-Risk Behaviour,” all authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Darraji H, Razak H, Ng KP, Altice FL. The Diagnostic Performance of a Single GeneXpert MTF/RIB Assay in an Intensified Tuberculoisis Case Finding Survey among HIV-infected Prisoners in Malaysia. PloS One. 2013 doi: 10.1371/journal.pone.0073717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcoholism, Clinical and Experimental Research. 1997;21(4):613–619. [PubMed] [Google Scholar]
- Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376(9738):367–387. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atun R, Olynik I. Resistance to implementing policy change: the case of Ukraine. Bulletin of the World Health Organization. 2008;86(2):147–154. doi: 10.2471/BLT.06.034991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PloS One. 2013;8(3):e59643–e59643. doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, Paar DP. Accessing antiretroviral therapy following release from prison. JAMA : the Journal of the American Medical Association. 2009;301(8):848–857. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakiryeva OM, Bondar TV, Sereda YV, Sazonova YO. Analytical Report. International HIV/AIDS Alliance in Ukraine. 2012:1–129. Retrieved from http://www.aidsalliance.org.ua/ru/library/our/2012/me/idu_en_2011.pdf.
- Barcal K, Barcal K, Schumacher JE, Schumacher JE, Dumchev K, Dumchev K, et al. A situational picture of HIV/AIDS and injection drug use in Vinnitsya, Ukraine. Harm Reduction Journal. 2005;2(1):16. doi: 10.1186/1477-7517-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley-Taylor D, Hallam C, Allen R. The Incarceration of drug offenders: an overview. Beckley Foundation/International Centre for Prison Studies; 2009. http://www.idpc.net/php-bin/documents/Beckley_Report_16_2_FINAL_EN.pdf Accessed. [Google Scholar]
- Bobrovskyy O, Bochkova L, Eschenko O, Hayovych Y, Kobyschcha Y, Kruhlov Y, et al. In: Ukraine Harmonized AIDS Respose Progress Report. Izotov V, Kravchenko O, Yakubovskyi D, translators; Bezimenna S, Rachkevych M, editors. Kyiv, Ukraine: International HIV/AIDS Alliance in Ukraine; 2012. pp. 1–241. [Google Scholar]
- Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV sero-status among drug injectors at three Ukraine sites. AIDS (London, England) 2006;20(17):2217–2223. doi: 10.1097/QAD.0b013e328010e019. [DOI] [PubMed] [Google Scholar]
- Booth RE, Lehman WEK, Dvoryak S, Brewster JT, Sinitsyna L. Interventions with injection drug users in Ukraine. Addiction (Abingdon, England) 2009;104(11):1864–1873. doi: 10.1111/j.1360-0443.2009.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Schleifer RA. Ethical and human rights imperatives to ensure medication-assisted treatment for opioid dependence in prisons and pre-trial detention. The International Journal on Drug Policy. 2008;19(1):17–23. doi: 10.1016/j.drugpo.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullinger M, Alonso J, Apolone G, Leplège A, Sullivan M, Wood-Dauphinee S, et al. Translating health status questionnaires and evaluating their quality: the IQOLA Project approach. International Quality of Life Assessment. Journal of Clinical Epidemiology. 1998;51(11):913–923. doi: 10.1016/s0895-4356(98)00082-1. [DOI] [PubMed] [Google Scholar]
- Calzavara LM, Burchell AN, Schlossberg J, Myers T, Escobar M, Wallace E, et al. Prior opiate injection and incarceration history predict injection drug use among inmates. Addiction (Abingdon, England) 2003;98(9):1257–1265. doi: 10.1046/j.1360-0443.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- Carvell AL, Hart GJ. Risk behaviours for HIV infection among drug users in prison. BMJ (Clinical Research Ed) 1990;300(6736):1383–1384. doi: 10.1136/bmj.300.6736.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NE, Meyer JP, Avery AK, Draine J, Flanigan TP, Lincoln T, et al. Adherence to HIV Treatment and Care Among Previously Homeless Jail Detainees. AIDS and Behavior. 2011 doi: 10.1007/s10461-011-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JG, Stein MD, Hanna L, Sobota M, Rich JD. Active and Former Injection Drug Users Report of HIV Risk Behaviors During Periods of Incarceration. Substance Abuse : Official Publication of the Association for Medical Education and Research in Substance Abuse. 2001;22(4):209–216. doi: 10.1080/08897070109511463. [DOI] [PubMed] [Google Scholar]
- Cohen J. Late for the epidemic: HIV/AIDS in Eastern Europe. Science (New York, NY) 2010;329(5988):160, 162–4. doi: 10.1126/science.329.5988.160. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England Journal of Medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S. Self-report among injecting drug users: a review. Drug and Alcohol Dependence. 1998;51(3):253–63. doi: 10.1016/s0376-8716(98)00028-3. discussion 267–8. [DOI] [PubMed] [Google Scholar]
- Dolan K, Kite B, Black E, Aceijas C, Stimson GV Reference Group on HIV AIDS Prevention and Care among Injecting Drug Users in Developing and Transitional Countries. HIV in prison in low-income and middle-income countries. The Lancet Infectious Diseases. 2007;7(1):32–41. doi: 10.1016/S1473-3099(06)70685-5. [DOI] [PubMed] [Google Scholar]
- Ford PM, Pearson M, Sankar-Mistry P, Stevenson T, Bell D, Austin J. HIV, hepatitis C and risk behaviour in a Canadian medium-security federal penitentiary. Queen’s University HIV Prison Study Group. QJM : Monthly Journal of the Association of Physicians. 2000;93(2):113–119. doi: 10.1093/qjmed/93.2.113. [DOI] [PubMed] [Google Scholar]
- Ghanem KG, Hutton HE, Zenilman JM, Zimba R, Erbelding EJ. Audio computer assisted self interview and face to face interview modes in assessing response bias among STD clinic patients. Sexually Transmitted Infections. 2005;81(5):421–425. doi: 10.1136/sti.2004.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CE, Stevens A. A resounding success or a disastrous failure: Re-examining the interpretation of evidence on the Portuguese decriminalisation of illicit drugs. Drug and Alcohol Review. 2012;31(1):101–113. doi: 10.1111/j.1465-3362.2011.00383.x. [DOI] [PubMed] [Google Scholar]
- Hurley R. How Ukraine is tackling Europe’s worst HIV epidemic. BMJ (Clinical Research Ed) 2010;341:c3538. doi: 10.1136/bmj.c3538. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Ball A, Verster A. Interventions to reduce HIV transmission related to injecting drug use in prison. The Lancet Infectious Diseases. 2009;9(1):57–66. doi: 10.1016/S1473-3099(08)70305-0. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Csete J, Amon JJ, Baral S, Beyrer C. People who use drugs, HIV, and human rights. Lancet. 2010;376(9739):475–485. doi: 10.1016/S0140-6736(10)60830-6. [DOI] [PubMed] [Google Scholar]
- Jürgens R, Nowak M, Day M. HIV and incarceration: prisons and detention. Journal of the International AIDS Society. 2011;14:26. doi: 10.1186/1758-2652-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O’Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. Journal of Substance Abuse Treatment. 2009;37(3):277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner SA, Jenkinson R, Gouillou M, Milloy M-J. High-risk drug-use practices among a large sample of Australian prisoners. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Larney S. Does opioid substitution treatment in prisons reduce injecting-related HIV risk behaviours? A systematic review. Addiction (Abingdon, England) 2010;105(2):216–223. doi: 10.1111/j.1360-0443.2009.02826.x. [DOI] [PubMed] [Google Scholar]
- Malliori MM, Sypsa VV, Psichogiou MM, Touloumi GG, Skoutelis AA, Tassopoulos NN, et al. A survey of bloodborne viruses and associated risk behaviours in Greek prisons. Addiction (Abingdon, England) 1998;93(2):243–251. doi: 10.1046/j.1360-0443.1998.9322438.x. [DOI] [PubMed] [Google Scholar]
- Maru DSR, Basu S, Altice FL. HIV control efforts should directly address incarceration. The Lancet Infectious Diseases. 2007;7(9):568–569. doi: 10.1016/S1473-3099(07)70190-1. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Spaulding A, Stephenson B, Macalino G, Ruby W, Clarke JG, Flanigan TP. Human immunodeficiency virus in correctional facilities: a review. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2002;35(3):305–312. doi: 10.1086/341418. [DOI] [PubMed] [Google Scholar]
- McNairy ML, Deryabina A, Hoos D, El-Sadr WM. Antiretroviral therapy for prevention of HIV transmission: Potential role for people who inject drugs in Central Asia. Drug and Alcohol Dependence. 2013;132(Suppl 1):S65–S70. doi: 10.1016/j.drugalcdep.2013.06.034. [DOI] [PubMed] [Google Scholar]
- Meyer JP, Qiu J, Chen NE, Larkin GL, Altice FL. Emergency department use by released prisoners with HIV: an observational longitudinal study. PloS One. 2012;7(8):e42416. doi: 10.1371/journal.pone.0042416.t004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Kerr T, Salters K, Samji H, Guillemi S, Montaner J, Wood E. Incarceration is associated with used syringe lending among active injection drug users with detectable plasma HIV-1 RNA: a longitudinal analysis. BMC Infectious Diseases. 2013;13:565. doi: 10.1186/1471-2334-13-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Marshall BDL, Montaner J, Wood E. Housing status and the health of people living with HIV/AIDS. Current HIV/AIDS Reports. 2012;9(4):364–374. doi: 10.1007/s11904-012-0137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milloy MJ, Wood E, Small W, Tyndall M, Lai C, Montaner J, Kerr T. Incarceration experiences in a cohort of active injection drug users. Drug and Alcohol Review. 2008;27(6):693–699. doi: 10.1080/09595230801956157. [DOI] [PubMed] [Google Scholar]
- Morozova O, Azbel L, Grishaev Y, Dvoryak S, Wickersham JA, Altice FL. Ukrainian Prisoners and Community Reentry Challenges: Implications for Transitional Care. Int Journal of Prisoner. 2013 doi: 10.1108/17449201311310760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann T, Neuner B, Gentilello LM, Weiss-Gerlach E, Mentz H, Rettig JS, et al. Gender differences in the performance of a computerized version of the alcohol use disorders identification test in subcritically injured patients who are admitted to the emergency department. Alcoholism, Clinical and Experimental Research. 2004;28(11):1693–1701. doi: 10.1097/01.alc.0000145696.58084.08. [DOI] [PubMed] [Google Scholar]
- Nilssen O, Averina M, Brenn T, Brox J, Kalinin A, Archipovski V. Alcohol consumption and its relation to risk factors for cardiovascular disease in the north-west of Russia: the Arkhangelsk study. International Journal of Epidemiology. 2005;34(4):781–788. doi: 10.1093/ije/dyi078. [DOI] [PubMed] [Google Scholar]
- Rotily M, Weilandt C, Bird SM, Käll K, Van Haastrecht HJ, Iandolo E, Rousseau S. Surveillance of HIV infection and related risk behaviour in European prisons. A multicentre pilot study. European Journal of Public Health. 2001;11(3):243–250. doi: 10.1093/eurpub/11.3.243. [DOI] [PubMed] [Google Scholar]
- Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug and Alcohol Dependence. 2010;108(1–2):29–36. doi: 10.1016/j.drugalcdep.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russoniello K. Decriminalization of drugs in Portugal: Lessons for public health. 141st APHA Annual Meeting; November 2- ….2013. [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7(4):363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2004;38(12):1754–1760. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Reports (Washington, DC : 1974) 2005;120(1):84–88. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson BL, Wohl DA, McKaig R, Golin CE, Shain L, Adamian M, et al. Sexual behaviours of HIV-seropositive men and women following release from prison. International Journal of STD & AIDS. 2006;17(2):103–108. doi: 10.1258/095646206775455775. [DOI] [PubMed] [Google Scholar]
- Stöver H. Ten years of experience with needle and syringe exchange programmes in European prisons. International Journal of Drug Policy 2003 [Google Scholar]
- UNAIDS. Global Report (Pap/Chrt) Joint United Nations Program on HIV/AIDS. 2010:360. Retrieved from http://www.unhcr.org/refworld/docid/4cfca9c62.html.
- Uusküla A, Laisaar K-T, Raag M, Smidt J, Semjonova S, Kogan J, et al. Antiretroviral therapy (ART) adherence and correlates to nonadherence among people on ART in Estonia. AIDS Care. 2012 doi: 10.1080/09540121.2012.672724. [DOI] [PubMed] [Google Scholar]
- Vagenas P, Azbel L, Polonsky M, Kerimi N, Mamyrov M, Dvoryak S, Altice FL. A review of medical and substance use co-morbidities in Central Asian prisons: Implications for HIV prevention and treatment. Drug and Alcohol Dependence. 2013 doi: 10.1016/j.drugalcdep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard RP, Kirk GD, Richesson DR, Galai N, Mehta SH. Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America. 2011;53(7):725–731. doi: 10.1093/cid/cir491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Azar M, Cannon CM, Alticle FL, Springer SA. Validation of a brief measure of opioid dependence: The rapid opioid dependence screen. Journal of Psychoactive Drugs. n.d doi: 10.1177/1078345814557513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Marcus R, Kamarulzaman A, Zahari MM, Altice FL. Implementing methadone maintenance treatment in prisons in Malaysia. Bulletin of the World Health Organization. 2013a;(91):124–129. doi: 10.2471/BLT.12.109132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham JA, Zahari MM, Azar MM, Kamarulzaman A, Altice FL. Methadone dose at the time of release from prison significantly influences retention in treatment: Implications from a pilot study of HIV-infected prisoners transitioning to the community in Malaysia. Drug Alcohol Depend. 2013b doi: 10.1016/j.drugalcdep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodak A, McLeod L. The role of harm reduction in controlling HIV among injecting drug users. AIDS (London, England) 2008;22(Suppl 2):S67–S79. doi: 10.1097/01.aids.0000327439.20914.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376(9738):355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- Wood E, Li K, Small W, Montaner JS, Schechter MT, Kerr T. Recent incarceration independently associated with syringe sharing by injection drug users. Public Health Reports (Washington, DC : 1974) 2005;120(2):150–156. doi: 10.1177/003335490512000208. [DOI] [PMC free article] [PubMed] [Google Scholar]