Abstract
Randomized trials of α-tocopherol supplements on cognitive decline are negative whereas studies of dietary tocopherols show benefit. We investigated these inconsistencies by analyzing the relations of α- and γ-tocopherol brain concentrations to Alzheimer disease (AD) neuropathology among 115 deceased participants of the prospective Rush Memory and Aging Project. Associations of amyloid load and neurofibrillary tangle severity with brain tocopherol concentrations were examined in separate adjusted linear regression models. γ-tocopherol concentrations were associated with lower amyloid load (β= −2.10; p=.002) and lower neurofibrillary tangle severity (β= −1.16; p=0.02). Concentrations of α-tocopherol were not associated with AD neuropathology except as modified by γ-tocopherol: high α-tocopherol was associated with higher amyloid load when γ-tocopherol levels were low and with lower amyloid levels when γ-tocopherol levels were high (P for interaction=0.03). Brain concentrations of γ- and α-tocopherols may be associated with AD neuropathology in interrelated, complex ways. Randomized trials should consider the contribution of γ-tocopherol.
Key words: Nutritional, Alzheimer disease, Cohort studies
Key symbols: α-tocopherol = alpha tocopherol, γ-tocopherol=gamma tocopherol, δ-tocopherol=delta tocopherol, β= beta coefficient, r=Pearson’s r coefficient
BACKGROUND
There is an expansive literature on animal studies that demonstrate the importance of α-tocopherol to the healthy functioning of the brain, including protection against lipid peroxidation,1;2 neuron loss,3;4 β-amyloid deposition,2;5 DNA damage,6–9 mitochondrial dysfunction,10;11 and decline in memory and learning.1;12 This literature is supported by prospective epidemiological studies linking dietary intake of vitamin E or combined tocopherols to slower rate of age-related cognitive decline13–15 and lower risk of Alzheimer’s disease.15–19 It is actually γ-tocopherol that constitutes much of the vitamin E content in the US food supply.20 The vast majority of studies do not show cognitive benefit from supplements of α-tocopherol.21–27 Recent completion of several randomized controlled trials,22;23;28 all with negative results for a beneficial effect of high-dose α-tocopherol on cognitive decline, cast doubt in the scientific community on the potential beneficial effects of vitamin E. However, there are several points of issue with the design of the randomized trials that may have resulted in the negative findings.29 Among these are the trials’ focus on high-dose α-tocopherol for treatment therapy as opposed to other tocopherol forms and the lack of concern for baseline vitamin E status of the trial participants. At least one trial demonstrated a beneficial effect of α-tocopherol supplementation in the trial participants who had low dietary intakes (<6.1 mg/d).28
In this study, we examined the relations of the predominant tocopherols, α and γ, to measures of AD neuropathology in a community study of residents who were initially free of AD at enrollment. The results of the study help to shed light on the role of different levels and forms of tocopherols in human brain and in disease occurrence.
METHODS
Study Sample
The Rush Memory and Aging Project (MAP) is an ongoing clinical-neuropathological cohort study of persons living in Chicago continuous care retirement communities and subsidized housing that began in 1997.30 Volunteers are dementia free at enrollment and all agreed to annual clinical neurological evaluations and to brain autopsy at their death. Clinical diagnosis of AD is made by an expert neurologist blinded to neuropathologic findings and according to accepted criteria31 after review of annual clinical evaluations and neuropsychological performance testing as previously described.30 The average time interval between the last clinical evaluation and death was 10 months.
The ongoing tocopherol component to the MAP study began in 2004 and includes the assessment of dietary and brain tissue levels of tocopherols. Of the 1,555 individuals who have participated in MAP, 1,360 were alive and still actively participating (80 were deceased and 115 withdrew) and thus eligible to participate in the tocopherol study. Of these, 1,089 (80%) agreed to be in the tocopherol study. Since 2004, 278 of those enrolled in the tocopherol study have died, 232 autopsies have been performed (83%), and 115 have been analyzed for both brain tocopherols and neuropathology. This study reports on the first consecutive brain cases analyzed as part of the ongoing study. The study was approved by the Institutional Review Board of Rush University Medical Center.
Brain Neuropathology Analyses
Brain autopsies were performed using standard procedures, as previously described.30 Slabs from one cerebral hemisphere were placed in a −80° C freezer. This tissue was used for tocopherol analyses as described below. Slabs from the contralateral hemisphere were fixed in 4% paraformaldehyde and stored in 20% glycerol and 2% DSMO. For immunohistochemistry we used paraffin-embedded 20 micron sections from 2 or more blocks of the following regions: hippocampus CA1/subiculum, entorhinal cortex (Brodmann Area or BA28), dorsolateral prefrontal, superior frontal (BA6/8), anterior cingulate (BA24), inferior temporal (BA20), angular/supramarginal (BA39/40), and calcarine (BA17) cortices. Aggregated amyloid beta was identified using 10D5, (courtesy of Elan Pharmaceuticals, South San Francisco, CA; dilution 1:300). Immunohistochemistry was performed on an Automated Leica Bond immunostainer (Leica Microsystems Inc., Bannockborn IL).
To measure amyloid load an investigator outlined the region of interest using a Microbrightfield Stereology System (MicroBrightfield Inc., Colchester, VT) and an Olympus BX-51 microscope with a motorized stage. We used the Stereo Investigator 9.0 software program to systematically sample and then capture images. Area analysis was performed using Image J 1.42g (http://rsbweb.nih.gov/ij/). A composite measure of the percent area occupied by amyloid beta was computed by averaging the values obtained by systematic computerized sampling of amyloid load in 8 cortical regions.
Neurofibrillary tangles (NFT) were identified using Bielschowsky silver stained 6 micron sections from hippocampal, entorhinal, midfrontal, midtemporal, and inferior parietal cortices. We computed Braak NFT stage using recommended criteria32 that incorporates severity and regional progression of NFT into one score on a scale of 0 (no NFT in any region) to 6 (frequent tangles across multiple regions).
All pathologic data collection was performed blinded to the clinical and tocopherol data. Figure 1 shows examples of cases with low and high amyloid load in the neocortex and low and high Braak score in the hippocampus.
Figure 1.
Two cases, one with low (A and C) and the other with high (B and D) Alzheimer’s pathology. A and B: Hippocampal neurofibrillary tangles, Braak score 2 (A) and Braak score 5 (B); Bielschowsky silver stain, at original magnification 10X.
D and C: Dorsolateral prefrontal cortex amyloid, low(C) vs. high(D) at magnification 4X.
Brain Tocopherol Analyses
Frozen brain tissue was thawed and analyzed for tocopherol concentrations using high performance liquid chromatography coupled to electrochemical detection according to previously described methods.33;34 Tocopherol levels were measured in two cortical regions (inferior temporal and midfrontal) affected by Alzheimer disease and in two subcortical regions, the posterior putamen and ventromedial caudate, that are involved in motor function and cognitive behavior, respectively.35 For analyses, tocopherol concentrations are expressed as picomoles (pmoles) per mg protein. Extraction losses were corrected for recoveries of the internal standard, δ-tocopherol. We eliminated from the analyses two cases with extreme values (α-tocopherol >10,000 pmole/mg; γ-tocopherol>900 pmole/mg protein).
Vitamin E Supplement Use
Self-reported vitamin E supplement use (α-tocopherol) and dosage from multivitamins and individual vitamin E supplements were obtained from a modified version of the Harvard food frequency questionnaire that was validated in a sample of older Chicago residents.36 Data on α-tocopherol intake levels from supplements were available for these analyses but not information on tocopherol intakes from food sources or on non-α-tocopherol supplements. A variable for α-tocopherol vitamin supplement dose was computed by totaling the daily dosage levels from individual α-tocopherol supplements and multivitamins containing α-tocopherol as reported by participants at the last clinic visit before death that they were not demented. (Table 1) The length of time between the supplement reporting and death was a median of 1.5 years with an interquartile range of 1.9. Non-users of vitamin supplements containing α-tocopherol were assigned a value of 0. For linear correlation and regression analyses, we used the square root transformation of this highly skewed vitamin E supplement dose variable to approximate a normal distribution.
Table 1.
Characteristics of 113 deceased MAP participants with measures of brain tocopherols and neuropathology
| Model Covariates | ||
|---|---|---|
| Age at Death (mean years, ±SD*) | 88.5 ±5.9 | |
| Females (%) | 60 | |
| Education (mean years, ±SD*) | 14.9 ±2.6 | |
| Post-mortem autopsy (mean hours, ±SD*) | 6.4 ±3.2 | |
| APOE-ε4 (% with at least one ε4 allele) | 29.5 | |
| α-tocopherol vitamin supplement dose daily intake** [n (%)] | ||
| None | 33 (31) | |
| <50 IU | 43 (41) | |
| 50–250 IU | 4 (4) | |
| 300–500 IU | 21 (20) | |
| 600+ IU | 5 (5) | |
| Pathology | ||
| Amyloid load (median area %,[ IQR*]) | 2.8 [5.5] | |
| Braak score† for neurofibrillary tangle severity (mean, ±SD*) | 3.3 ±1.3 | |
| Score Range: 0–6 | ||
| Clinical AD‡ Diagnosis | ||
| No AD Dementia [n (%)] | 75 (65) | |
| AD Dementia [n (%)] | 40 (35) | |
SD=Standard deviation, 407 IQR=interquartile range
Total dose from multi-vitamins and individual α-tocopherol supplements
Higher scores represent more severe neuropathology
Clinical AD diagnosis based on 410 NINCDS-ADRDA criteria
Statistical Methods
We used linear regression models to examine the associations of the neuropathological measures of Alzheimer’s disease with brain levels of the tocopherols. All models for the primary analyses were adjusted for age at death, sex, years of education, APOE-ε4, and the time interval from death to autopsy (hours). To improve normality, we performed common log transformations (log10) on the highly skewed variables of amyloid load and the brain tocopherol variables. We modeled the brain tocopherol variables both as continuous variables and in tertiles (to examine the possibility of non-linear associations); results of both models are presented. Tocopherol associations were investigated as average levels over all four brain regions. Because of the small sample and concerns with statistical power, an a priori decision was made to examine statistical interactions in age-adjusted models. These models also included terms for the two variables of interest and their multiplicative term. Model assumptions were tested graphically and diagnostically. Due to the highly skewed distributions of the brain and supplement tocopherol variables we performed both Pearson’s linear correlations with transformed variables and Spearman correlations that do not require normally distributed variables.
RESULTS
Based on the last clinical neurological evaluation before death, approximately two thirds of the sample was not demented. (Table 1) The characteristics of the analyzed sample were not materially different from that reported on much larger samples of MAP autopsied cases.37;38 Compared to the entire MAP tocopherol sample, the analyzed deceased sample was older at the time of first FFQ completion (mean age 88.5 versus 81.6), less likely to be female (60% versus 74%), and had great likelihood of at least one APOE-ε4 allele (29% versus 23%), but had similar years of education (14.9 versus 14.8 years).
The predominant tocopherol in the brain was α-tocopherol, representing 62% to 72% of the tocopherol concentrations across the 4 regions analyzed. Levels of -tocopherol had moderate correlations across regions (r = 0.4 - 0.5) but γ-tocopherol levels were not correlated except between the ventromedial caudate and posterior putamen regions (r = 0.4, p < .001). The correlation between the brain concentrations of α- and γ-tocopherols was 0.4 (p < .0001). Vitamin E (α-tocopherol) supplement use was reported by 39 of the participants at the last visit before death. An additional 43 participants were taking a multi-vitamin containing α-tocopherol. The total supplement dose level of α-tocopherol was positively correlated with brain levels of α-tocopherol (Pearson’s r = 0.21, p = .03) and negatively correlated with brain levels of γ-tocopherol (Spearman correlation= −0.22, p=.02).
In linear regression models adjusted for age at death, α-tocopherol was not associated with amyloid load or with Braak score, a measure of the severity and regional progression of neurofibrillary tangles. (Table 2) Further adjustment for sex, education, APOE-ε4, and autopsy interval increased the beta coefficients for α-tocopherol concentrations but the estimates of association remained statistically non-significant. The results for α-tocopherol did not change with additional control for the dose level of α-tocopherol vitamin supplement intake. The summary measure of γ-tocopherol was also not associated with amyloid load or with Braak score in the age-adjusted models but when the models were adjusted for multiple confounders, the beta coefficients substantially increased indicating strong linear inverse associations with both amyloid load and Braak scores. (Table 2) These associations between γ-tocopherol and AD neuropathology did not materially change with additional adjustment for α-tocopherol vitamin supplement intake (β=−2.01, p=.04 for amyloid and β=−1.18, p=.02 for Braak score).
Table 2.
Age- and multiple-adjusted* estimates of association of brain levels of α-Tocopherol and γ-Tocopherol with measures of neuropathology based on linear regression models of 113 deceased participants of the Memory and Aging Project
| α-TOCOPHEROL TERTILES | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Log Median pmoles/mg protein | 1.8 | 2.3 | 2.6 | LINEAR TERM |
| MODEL | β (p-value) | β (p-value) | β (p-value) | β (p-value) |
| Amyloid Load | ||||
| Age-adjusted | 1.0 | −0.74 (.85) | −0.31 (.94) | −0.006 (.99) |
| Multiple-adjusted | 1.0 (referent) | −0.35 (.38) | −0.21 (.60) | −0.27 (.56) |
| Neurofibrillary Tangle Severity (Braak Score) | ||||
| Age-adjusted | 1.0 (referent) | −0.27 (.34) | −0.40 (.17) | −0.36 (.45) |
| Multiple-adjusted | 1.0 (referent) | −0.50 (.08) | −.51 (.08) | −0.55 (.12) |
| γ-TOCOPHEROL TERTILES | ||||
| 1 | 2 | 3 | ||
| Log Median pmoles/mg protein | 1.6 | 1.8 | 2.1 | LINEAR TERM |
| MODEL | β (p-value) | β (p-value) | β (p-value) | β (p-value) |
| Amyloid Load | ||||
| Age-adjusted | 1.0 (referent) | 0.11 (.77) | −0.59 (.14) | −1.14 (.09) |
| Multiple-adjusted | 1.0 (referent) | −0.03 (.94) | −1.06 (.007) | −2.10 (.002) |
| Neurofibrillary Tangle Severity (Braak Score) | ||||
| Age-adjusted | 1.0 (referent) | −0.13 (.66) | −0.34 (.24) | −0.87 (.09) |
| Multiple-adjusted | 1.0 (referent) | −0.26 (.36) | −0.60 (.04) | −1.16 (.02) |
Multiple-adjusted models adjusted for age at death (years), sex, education (years), APOE-ε4 (any ε4 versus none) and post-mortem autopsy time (hours)
Tocopherol Interactions
Based on previous evidence to suggest biological interactions among the tocopherols39 we investigated possible effect modification between levels of α-tocopherol and γ-tocopherol on the AD pathology measures. We observed a statistically significant interaction (p=.03) of the summary measures of α- and γ-tocopherols modeled as continuous variables on amyloid load but not on neurofibrillary tangle severity. Higher levels of α-tocopherol were associated with increased amyloid load except when the levels of γ-tocopherol were also high, at which point higher levels of α-tocopherol were associated with lower amyloid levels. (Figure 2) In further analyses, there was no evidence of effect modification on the brain tocopherol associations with amyloid load or neurofibrillary tangle severity by levels of APOE-ε4 allele, sex, age, or education.
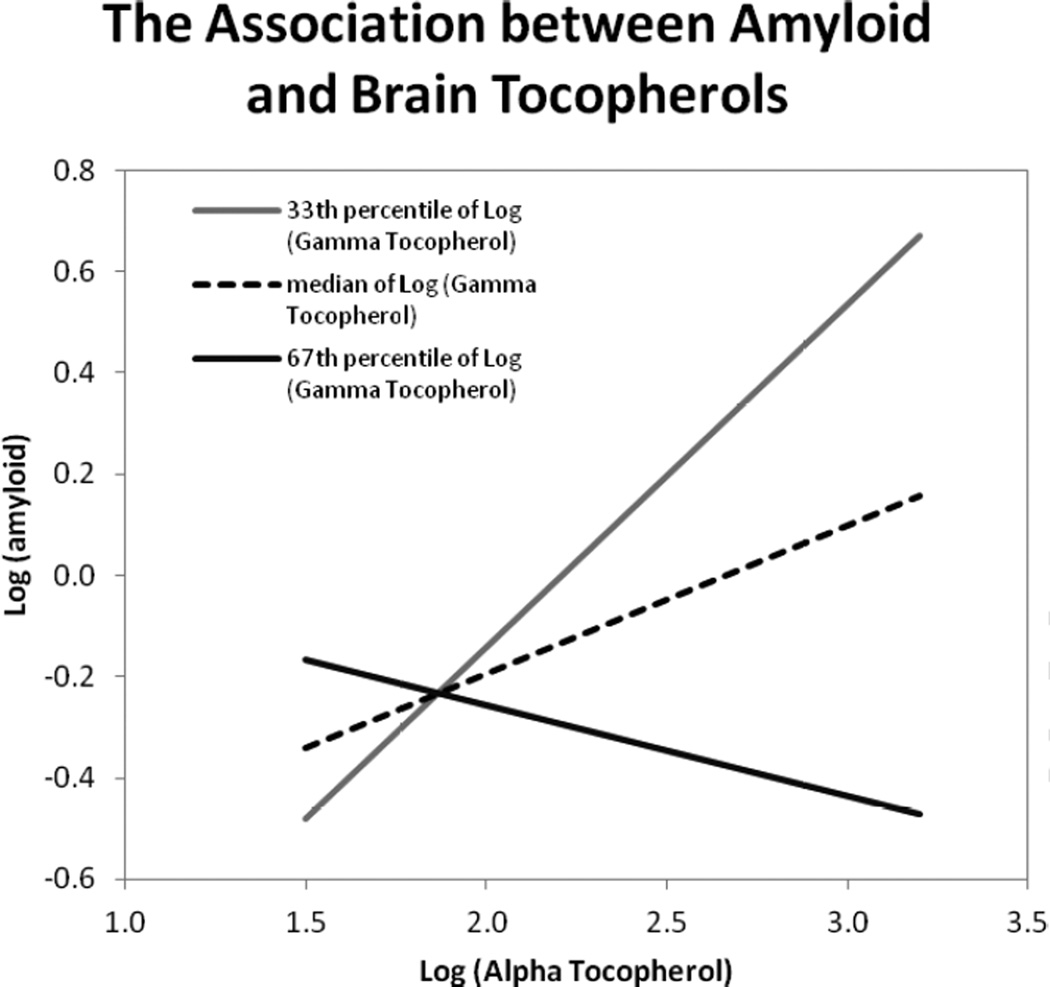
Figure 2.
Statistical interaction effect between α-tocopherol and γ-tocopherol on amyloid load based on an age-adjusted linear regression model with log10 (α-tocopherol) and log10 (γ-tocopherol) modeled as continuous variables in pmol/mg protein and their multiplicative term (p-value for interaction=0.03). The lines reflect the association between amyloid load and alpha tocopherol at three different values of gamma tocopherol: log10 =1.7 at the 33rd percentile (grey), log10 =1.8 at the 50th percentile (dotted), and log10 =1.9 at the 67th percentile (black). Higher levels of α-tocopherol were associated with increased amyloid load except when the levels of γ-tocopherol were also high, at which point higher levels of α-tocopherol were associated with lower amyloid levels.
Clinical AD Effects
In an effort to determine whether the inverse associations that were observed between brain concentrations of γ-tocopherols and neuropathology may be due to disease effects on brain nutrient levels, we re-analyzed the data in two ways: first, by controlling for clinical diagnosis of Alzheimer’s disease at the time of death, and second, by testing whether the associations were modified by clinical AD diagnosis. In models adjusted for clinical AD diagnosis, the associations of γ-tocopherol remained with amyloid load (β = −2.11, p = .001) and with Braak score (β = −1.17, p = .01). Adjustment for clinical AD diagnosis resulted in a statistically significant inverse association between α-tocopherol and Braak score (with tertile 1 as the referent, β = −0.56, p = .04 for tertile 3 and β = −0.61, p = .03 for tertile 2). However, with additional adjustment for γ-tocopherol in the model (which was positively correlated with α-tocopherol concentration), the estimated effects were diminished and became non-statistically significant (β = −0.39, p = .17 for tertile 3 and β = −0.43, p = .14 for tertile 2). The statistical interaction between α- and γ-tocopherol concentrations on amyloid load was slightly modified and became marginally statistically significant (p=.06) with adjustment of clinical AD diagnosis. There was no evidence of statistical interaction between clinical AD and either γ- or α-tocopherol on the neuropathology measures.
DISCUSSION
We are not aware of prior human studies that relate brain levels of tocopherols to AD neuropathology. Whereas, the vast majority of literature from animal studies and randomized trials has related α-tocopherol to brain function, our findings indicate that γ-tocopherol plays an important role in brain neuropathology. Higher levels of γ-tocopherol were strongly associated with lower amyloid load as well as with less severe neurofibrillary tangle pathology. Brain concentrations of α-tocopherol were not independently associated with AD neuropathology. An apparent inverse association between α-tocopherol and neurofibrillary tangle severity with statistical adjustment for clinical AD was due to confounding by γ-tocopherol.
Several randomized controlled clinical trials have tested the effects of high-dose α-tocopherol supplements on cognitive decline and AD progression with null results.22;23;28 These negative trials convinced many that the protective relations observed in the epidemiological studies for food sources of tocopherols were due to confounding bias. Findings from the current neuropathological study support the epidemiological study findings and suggest that the high-dose α-tocopherol treatment that was used in the randomized trials may not be the correct tocopherol form or dose to provide neuroprotection. Interestingly, very few epidemiological studies observed protective associations of vitamin E supplements on cognitive decline40 or dementia incidence.41 One prospective epidemiological study40 and one randomized trial28 reported neuroprotective benefit of vitamin E supplements (high-dose α-tocopherol) only among the individuals who had low vitamin E food intake.
The literature on tocopherol forms other than α-tocopherol is limited. Indeed, α-tocopherol is the only form that is considered to meet criteria for the Dietary Reference Intakes by the Institute of Medicine Food and Nutrition Board,42 and it is this form that is generally known as most biologically active. However, a growing body of literature indicates that γ-tocopherol, like α-tocopherol, has anti-inflammatory properties in addition to anti-oxidant capabilities.43 In a previous study of the Chicago Health and Aging Project, food intake levels of both γ-tocopherol and α-tocopherol were significantly associated with slower rate of cognitive decline and lower incidence of Alzheimer’s disease.15 These findings were confirmed in another prospective study that examined plasma levels of the tocopherols.17 The present study highlights the importance of γ-tocopherol to neuroprotection in the brain.
High-dose supplementation with α-tocopherol has been demonstrated to rapidly decrease circulating levels of γ-tocopherol perhaps because the tocopherol transport protein responsible for packaging tocopherol into very low density lipoproteins has a higher affinity for α-tocopherol.44 We observed a similar relation in brain tissue in that higher dose level of α-tocopherol supplement intake reported a median 1.5 years before death was positively associated with higher brain concentrations of α-tocopherol and with lower brain concentrations of γ-tocopherol. Our findings that the highest levels of α-tocopherol were associated with greater amyloid load in the brains that had intermediate or low levels of γ-tocopherol supports the notion that high-dose α-tocopherol supplementation may be contraindicated for protection against Alzheimer’s disease. This finding is also supported by the many positive findings of neuroprotection from food sources of vitamin E which provide more comparable dose levels of the tocopherol forms.15–19;45 This concept is supported by a basic principle of nutrition that states that optimum physiological function occurs within a broad intermediate range of nutrient level and that both low and high levels can result in less than optimum function or even death.46
The MAP study of brain tocopherols has a number of strengths which lend confidence in the overall findings of the study. First, the study was based on autopsied brains from participants of a cohort study who were without a known dementia at enrollment. The cohort study design minimizes selection bias that is characteristic of case-control studies. Second, both the analyses of brain tocopherols and neuropathology were conducted by single laboratories using validated, standardized methods and blinded to clinical diagnosis, thus reducing measurement bias. Third, relative to most other autopsy studies, the well-characterized analyzed sample of more than 100 brains allowed for the statistical control of the important confounding factors of AD neuropathology which helps to minimize confounding bias. Even so, the sample size was too small to lend strong confidence in the findings, particularly the tests for effect modification. The primary limitation of the study is the unpreventable cross-sectional design which makes it difficult to interpret the findings as cause or effect. However, for a number of reasons the data suggest that nutritional intake is responsible for the brain tocopherol levels rather than the effects of neurodegenerative disease. First, if the associations were due to the effects of AD on tocopherol levels then one would expect to observe associations for α-tocopherol as well. Second, AD neuropathology is present to varying degrees in many of the brains of the MAP cohort study, even the non-demented participants.38 The study design allowed us to relate the continuum of tocopherol levels to the continuum of neuropathology in a sample in which two thirds of the deceased were not clinically demented at the time of their death. And, the observed protective relations withheld even after statistical control for AD clinical diagnosis. There was also no evidence that the observed inverse effects of the tocopherols on neuropathology were largely being driven by the deceased participants who had clinical AD when we investigated for statistical interactions by AD status. And finally, the dose level of α-tocopherol supplement consumed during life was positively correlated with post-mortem brain concentrations of α-tocopherol and negatively correlated with γ-tocopherol concentrations. Tocopherols are essential nutrients, meaning that they cannot be metabolized in the human body and so must be consumed. However, the fact that we did not have available dietary intake levels of the tocopherols limited our ability to investigate the contribution of dietary contributions to the tocopherol brain levels. The laboratory method we used to analyze tocopherols detected only α- and γ-tocopherols in the brain tissue. These are just three of the eight natural forms of vitamin E, including α-,γ-,β-, and δ-tocopherols and their associated tocotrienol forms. It is possible that other laboratory methods may detect some of these other tocopherol forms in brain tissue that are now known to also have neuroprotective activity, including anti-oxidant and anti-inflammatory properties,47;48 and modulation of signaling pathways involved in neurodegeneration.49 We15 and others17 have found that the combination of dietary tocopherols rather than individual tocopherols have the strongest protective relation with the development of Alzheimer’s disease. The MAP study cohort is not a representative sample from the U.S. population, thus the ability to generalize the findings beyond the sample is limited.
This study marks the first report suggesting protective effects of tocopherols on AD neuropathology in humans. The linear inverse associations observed for γ-tocopherol, and the observed increase in amyloid load with high α-tocopherol and low to intermediate γ-tocopherol provide important information for future preventive therapies for Alzheimer’s disease. The study highlights the possible neuroprotective role of non-α-tocopherol forms of vitamin E, which is largely unexplored in human studies. The finding that high brain concentrations of α-tocopherol may be associated with increased AD neuropathology is of particular interest in light of the fact that many U.S. adults consume vitamin E supplements, usually containing only α-tocopherol in high doses.
Research in Context.
Systematic Review
Animal studies report preventive effects of vitamin E on AD neuropathology. Clinical trials and epidemiological studies of vitamin E supplements (containing only α-tocopherol) have found negative relations to AD prevention and cognitive decline. However, epidemiological studies of dietary vitamin E (containing other tocopherols) have consistently shown positive relations with AD prevention. In other studies high-dose α-tocopherol decreases the absorption of γ-tocopherol. We examined the interrelations of α- and γ-tocopherols to AD neuropathology in human brains.
Interpretation
Our findings that γ-tocopherol was associated with lower AD neuropathology, and that α-tocopherol was associated with greater neuropathology when γ-tocopherol levels were low, suggests that high-dose α-tocopherol alone may not be neuroprotective.
Future Directions
This is the first human study to examine the interactive effects of α- and γ-tocopherols on AD neuropathology, thus additional studies are needed to confirm or refute the findings with particular focus on the levels of these tocopherols.
ACKNOWLEDGEMENTS
The study was funded by the National Institute on Aging (R01AG031553 and R01AG17917).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Martha Clare Morris, Email: Martha_C_Morris@rush.edu.
Julie A Schneider, Email: Julie_A_Schneider@rush.edu.
Hong Li, Email: lena.lee.76@gmail.com.
Christy C Tangney, Email: Christy_Tangney@rush.edu.
Sukrit Nag, Email: Sukriti_Nag@rush.edu.
David A Bennett, Email: David_A_Bennett@rush.edu.
William G. Honer, Email: honer@mail.ubc.ca.
Lisa Barnes, Email: Lisa_L_Barnes@rush.edu.
Reference List
- 1.Fukui K, Omoi NO, Hayasaka T, et al. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 2.Nishida Y, Ito S, Ohtsuki S, et al. Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem. 2009;284:33400–33408. doi: 10.1074/jbc.M109.054056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annahazi A, Mracsko E, Sule Z, et al. Pre-treatment and post-treatment with alpha-tocopherol attenuates hippocampal neuronal damage in experimental cerebral hypoperfusion. Eur J Pharmacol. 2007;571:120–128. doi: 10.1016/j.ejphar.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Bostanci MO, Bas O, Bagirici F. Alpha-tocopherol decreases iron-induced hippocampal and nigral neuron loss. Cell Mol Neurobiol. 2010;30:389–394. doi: 10.1007/s10571-009-9461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang SG, Wang WY, Ling TJ, et al. alpha-Tocopherol quinone inhibits beta-amyloid aggregation and cytotoxicity, disaggregates preformed fibrils and decreases the production of reactive oxygen species, NO and inflammatory cytokines. Neurochem Int. 2010;57:914–922. doi: 10.1016/j.neuint.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 6.de Jesus Ferreira MC, Crouzin N, Barbanel G, et al. A transient treatment of hippocampal neurons with alpha-tocopherol induces a long-lasting protection against oxidative damage via a genomic action. Free Radic Biol Med. 2005;39:1009–1020. doi: 10.1016/j.freeradbiomed.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Abd Hamid NA, Hasrul MA, Ruzanna RJ, et al. Effect of vitamin E (Tri E(R)) on antioxidant enzymes and DNA damage in rats following eight weeks exercise. Nutr J. 2011;10:37. doi: 10.1186/1475-2891-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shirpoor A, Salami S, Khadem-Ansari MH, Minassian S, Yegiazarian M. Protective effect of vitamin E against ethanol-induced hyperhomocysteinemia, DNA damage, and atrophy in the developing male rat brain. Alcohol Clin Exp Res. 2009;33:1181–1186. doi: 10.1111/j.1530-0277.2009.00941.x. [DOI] [PubMed] [Google Scholar]
- 9.Crouzin N, Ferreira MC, Cohen-Solal C, Barbanel G, Guiramand J, Vignes M. Neuroprotection induced by vitamin E against oxidative stress in hippocampal neurons: involvement of TRPV1 channels. Mol Nutr Food Res. 2010;54:496–505. doi: 10.1002/mnfr.200900188. [DOI] [PubMed] [Google Scholar]
- 10.Navarro A, Bandez MJ, Lopez-Cepero JM, Gomez C, Boveris A. High doses of vitamin E improve mitochondrial dysfunction in rat hippocampus and frontal cortex upon aging. Am J Physiol Regul Integr Comp Physiol. 2011;300:R827–R834. doi: 10.1152/ajpregu.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navarro A, Gomez C, Sanchez-Pino MJ, et al. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1392–R1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- 12.Conte V, Uryu K, Fujimoto S, et al. Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury. J Neurochem. 2004;90:758–764. doi: 10.1111/j.1471-4159.2004.02560.x. [DOI] [PubMed] [Google Scholar]
- 13.Wengreen HJ, Munger RG, Corcoran CD, et al. Antioxidant intake and cognitive function of elderly men and women: the Cache County Study. J Nutr Health Aging. 2007;11:230–237. [PubMed] [Google Scholar]
- 14.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997;65:20–9. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Evans DA, Tangney CC, et al. Relation of the tocopherol forms to incident Alzheimer disease and to cognitive change. Am J Clin Nutr. 2005;81:508–514. doi: 10.1093/ajcn.81.2.508. [DOI] [PubMed] [Google Scholar]
- 16.Devore EE, Grodstein F, van Rooij FJ, et al. Dietary antioxidants and long-term risk of dementia. Arch Neurol. 2010;67:819–825. doi: 10.1001/archneurol.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangialasche F, Kivipelto M, Mecocci P, et al. High plasma levels of vitamin E forms and reduced Alzheimer's disease risk in advanced age. J Alzheimers Dis. 2010;20:1029–1037. doi: 10.3233/JAD-2010-091450. [DOI] [PubMed] [Google Scholar]
- 18.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. J Am Med Assoc. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 19.Helmer C, Peuchant E, Letenneur L, et al. Association between antioxidant nutritional indicators and the incidence of dementia: results from the PAQUID prospective cohort study. Eur J Clin Nutr. 2003;57:1555–1561. doi: 10.1038/sj.ejcn.1601724. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Q, Christen S, Shigenaga MK, Ames BN. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 21.Morris MC, Evans DA, Bienias JL, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer's disease in a biracial community study. J Am Med Assoc. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 22.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 23.Kang JH, Cook NR, Manson JE, Buring JE, Albert CM, Grodstein F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women's Antioxidant and Cardiovascular Study. Circulation. 2009;119:2772–2780. doi: 10.1161/CIRCULATIONAHA.108.816900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurin D, Foley DJ, Masaki KH, White LR, Launer LJ. Vitamin E and C supplements and risk of dementia. J Am Med Assoc. 2002;288:2266–2268. doi: 10.1001/jama.288.18.2266. [DOI] [PubMed] [Google Scholar]
- 25.Fillenbaum GG, Kuchibhatla MN, Hanlon JT, et al. Dementia and Alzheimer's disease in community-dwelling elders taking vitamin C and/or vitamin E. Ann Pharmacother. 2005;39:2009–2014. doi: 10.1345/aph.1G280. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer's disease. Arch Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 27.Gray SL, Anderson ML, Crane PK, et al. Antioxidant vitamin supplement use and risk of dementia or Alzheimer's disease in older adults. J Am Geriatr Soc. 2008;56:291–295. doi: 10.1111/j.1532-5415.2007.01531.x. [DOI] [PubMed] [Google Scholar]
- 28.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 29.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. J Am Med Assoc. 2011;305:1348–1349. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Williamson KS, Gabbita SP, Mou S, et al. The nitration product 5-nitro-gamma-tocopherol is increased in the Alzheimer brain. Nitric Oxide. 2002;6:221–227. doi: 10.1006/niox.2001.0399. [DOI] [PubMed] [Google Scholar]
- 34.Hensley K, Barnes LL, Christov A, et al. Analysis of postmortem ventricular cerebrospinal fluid from patients with and without dementia indicates association of vitamin E with neuritic plaques and specific measures of cognitive performance. J Alzheimers Dis. 2011;24:767–774. doi: 10.3233/JAD-2011-101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158:1213–1217. doi: 10.1093/aje/kwg290. [DOI] [PubMed] [Google Scholar]
- 37.Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA. Selected Findings from the Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2012;33:S397–S403. doi: 10.3233/JAD-2012-129007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 39.Liu M, Wallmon A, Olsson-Mortlock C, et al. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr. 2003;77:700–706. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 40.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Vitamin E and cognitive decline in older persons. Arch Neurol. 2002;59:1125–1132. doi: 10.1001/archneur.59.7.1125. [DOI] [PubMed] [Google Scholar]
- 41.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–88. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 42.Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Dietary Reference Intakes [serial online] 2000 Available from: National Academic Press. [PubMed] [Google Scholar]
- 43.Hensley K, Benaksas EJ, Bolli R, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Burton GW, Traber MG, Acuff RV, et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 45.Larrieu S, Letenneur L, Helmer C, Dartigues JF, Barberger-Gateau P. Nutritional factors and risk of incident dementia in the PAQUID longitudinal cohort. J Nutr Health Aging. 2004;8:150–154. [PubMed] [Google Scholar]
- 46.Mertz W. The essential trace elements. Science. 1981;213:1332–1338. doi: 10.1126/science.7022654. [DOI] [PubMed] [Google Scholar]
- 47.Taridi NM, Yahaya MF, Teoh SL, et al. Tocotrienol rich fraction (TRF) supplementation protects against oxidative DNA damage and improves cognitive functions in Wistar rats. Clin Ter. 2011;162:93–98. [PubMed] [Google Scholar]
- 48.Fukui K, Takatsu H, Koike T, Urano S. Hydrogen peroxide induces neurite degeneration: Prevention by tocotrienols. Free Radic Res. 2011;45:681–691. doi: 10.3109/10715762.2011.567984. [DOI] [PubMed] [Google Scholar]
- 49.Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. Journal of Biol Chem. 2000;275:13049–13055. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]