Abstract
Environmental insults, such as exposure to toxicants or nutritional abnormalities, can lead to epigenetic changes that are in turn related to increased susceptibility to disease. The focus of this review is on the transgenerational inheritance of such epigenetic abnormalities (epimutations), and how it is that these inherited epigenetic abnormalities can lead to increased disease susceptibility, even in the absence of continued environmental insult. Observations of environmental toxicant specificity and exposure specific disease susceptibility are discussed. How epimutations are transmitted across generations and how epigenetic changes in the germline are translated into an increased disease susceptibility in the adult is reviewed in regards to disease etiology.
Keywords: Epigenetic, Transgenerational, Non-Genetic Inheritance, Disease Etiology
Epigenetic Transgenerational Inheritance Defined
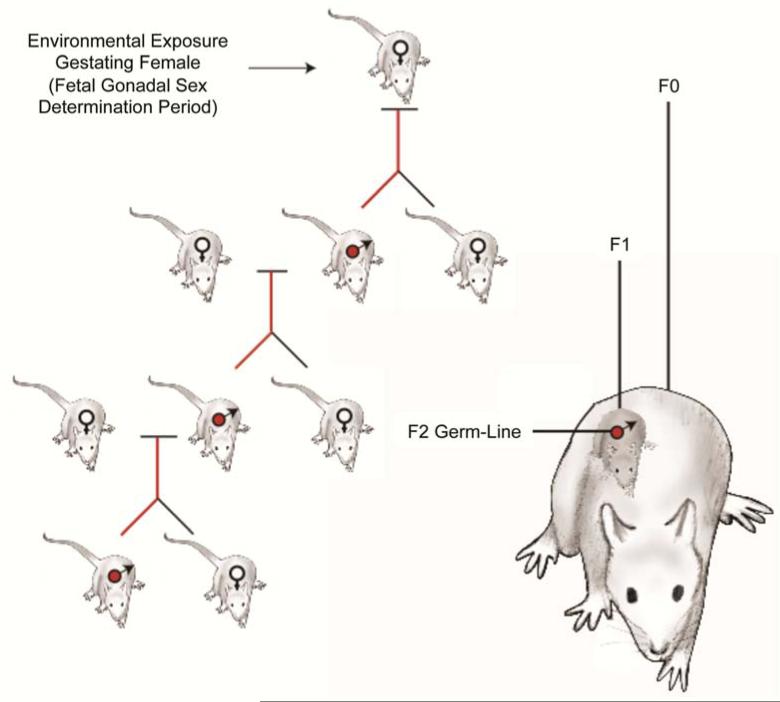
The definition of epigenetic transgenerational inheritance is “germline mediated inheritance of epigenetic information between generations that leads to phenotypic variation in the absence of direct environmental influences” [1]. One of the initial reports of environmental epigenetic transgenerational inheritance involved agriculturally used toxicants [2]. In these studies pregnant rats were exposed to the agricultural fungicide vinclozolin and pesticide methoxychlor, with the goal of examining the effects these environmental toxicants had on gonadal development and function in the offspring (F1 generation). A serendipitous observation was made when F1 generation animals were mistakenly bred to generate the F2 generation offspring. The vast majority of the testes in the F2 generation carried a spermatogenic cell defect of increased apoptosis. This increased apoptosis persisted into the transgenerational F3 and F4 generations, as well as in outcrossed offspring, exhibiting non-Mendelian genetic inheritance, and affecting 90% of the male population. Since the gestating female F0 generation rats were exposed to the toxicants at the time that their embryos were undergoing sex determination, the F1 generation animals were directly exposed as a fetus (Figure 1). In addition, the germ cells present in the developing fetus are directly exposed. These exposed germ cells created the F2 generation (grand-offspring). Therefore, the first generation without direct environmental exposure is the F3 generation (great-grand-offspring), and this is the first generation said to exhibit transgenerational inheritance of disease susceptibility. In these studies ancestral exposure to vinclozolin resulted in epigenetic changes in sperm DNA methylation at specific sites [2].
Figure 1.
Schematic of environmentally induced epigenetic transgenerational inheritance in F3 generation. Direct exposure is shown in the F0, F1, and F2 generations.
In contrast, when an F0 generation male is exposed to an environmental insult, his sperm will be directly exposed. These sperm will generate the F1 generation. Therefore the first unexposed generation would be the F2 (grand-offspring) (Figure 1). Transgenerational effects in humans have been documented as passing through the male line after men were exposed to famine conditions early in life. These dietary exposures were correlated with longevity and disease in the grandchildren (i.e. the transgenerational F2 generation) of these men [3]. More recently similar observations in rodents with effects on behavior and brain development have been observed [4].
There are many examples in the literature of epigenetically mediated multi-generational inheritance that is not transgenerational, but due to direct exposure [5-16]. A well-characterized model of this utilizes the Agouti mouse. Pregnant Agouti mice when exposed to the presence of a methyl donor in their diet will have increased DNA methylation of an allele of the Agouti gene, leading to a change in coat color in offspring [17]. However, this DNA methylation change is not successively passed to subsequent generations [18]. Rather, the normal processes of DNA de-methylation and re-methylation that occur during germ cell specification and fertilization reset the DNA methylation state of these alleles [19]. A human example of epigenetic multi-generational inheritance is the fetal actions of diethylstilbesterol (DES). The children and grandchildren of women treated with DES during pregnancy show abnormalities or increased risk of disease. In animal model systems these abnormalities are associated with epigenetic changes [20-22]. However, negligible abnormalities have been shown for the F3 (great-grandchildren) generation in humans [23].
Germline Epimutations and Toxicant Specificity
Environmentally induced epigenetic transgenerational inheritance requires epimutations to be present in the germline, as it is only the germ cells (sperm and egg) that are passed on to form the next generation. The best-characterized molecular mechanism for epigenetic changes to be transmitted through the germ cells involve changes in DNA methylation. These differential DNA methylation regions (DMRs) appear to become ‘imprinted-like’ [19], such that they are not reset during germ cell specification and fertilization [2, 19, 24-30]. True imprinted genes have programmed epigenetic marks and are defined as genes with “parent-of-origin allelic transmission with monoallelic gene expression.” While the differential DMRs associated with transgenerational inheritance of disease susceptibility do often exhibit parent-of-origin allelic transmission [2, 19], the monoallelic gene expression has not been investigated and may in fact not be a salient feature of these differential DMRs. Therefore, the abnormally methylated DNA sites found in germ cells transgenerationally are termed “imprinted-like” [30]. Although other epigenetic marks such as histone modifications [31] and non-coding RNA [32] will likely have important roles in transgenerational phenomena, their mechanisms remain to be elucidated.
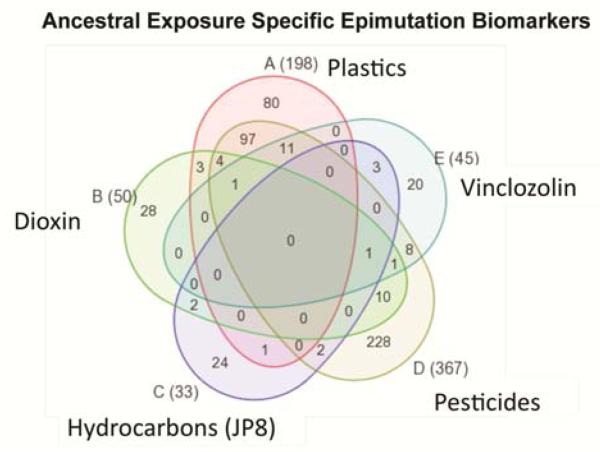
The effects of exposure to several environmental toxicants have now been investigated to assess exposure specificity. Increased rates of disease and sperm epimutations have been demonstrated transgenerationally after exposure of the F0 generation gestating female to vinclozolin [2, 24, 33-35], dioxin [25, 36, 37], a pesticide and insect repellent mix (permethrin and N,N-diethyl-m-toluamide (DEET)) [27], plastics (bisphenol A (BPA) and phthalates) [38], and hydrocarbons (jet fuel JP8) [39]. Interestingly, each toxicant induced a unique set of epitmutations in sperm, with negligible overlap in the sperm epimutations between the different ancestral exposures [40] (Figure 2). This raises the possibility that there are epigenetic signatures of specific ancestral exposures. In the future epigenetic testing may uncover what toxicant exposures and associated disease risks are in a person’s ancestry [40]. Other laboratories have also shown transgenerational inheritance of disease succeptibility to a variety of exposures including nutrition [41], stress [42], and other toxicants [43, 44].
Figure 2.
Venn diagram of transgenerational sperm epimutations associated with different exposure groups showing a number of common epimutations in the F3 generation of rats due to ancestral exposure of F0 generation gestating females to vinclozolin, dioxin, pesticide, plastics or hydrocarbons. Modified from [40].
Diseases Inherited Transgenerationally and Phenotypic Variation
The initial observations of epigenetic transgenerational inheritance of disease susceptibility documented an increase in spermatogenic cell apoptosis after ancestral exposure to vinclozolin [2, 34]. Other diseases and pathologies seen transgenerationally at increased rates after ancestral exposure to various toxicants include prostate disease [25, 27, 33, 35, 38-40], kidney disease [25, 27, 33, 35, 38-40], mammary tumor development [33], immune abnormalities [33, 39], behavioral effects related to anxiety [45], effects on reproduction [46, 47], stress response [48], and obesity [39, 43, 49]. Therefore, increased incidence of a wide variety of health abnormalities has been reported to occur transgenerationally after toxicant exposure. A number of these disease states were shown to occur in rats at high rates after ancestral exposure to any of several different environmental toxicants [40]. For example, certain ovarian diseases, including polycystic ovaries and reduction of the primordial follicle pool size, were found at high rates transgenerationally in females from all the toxicant exposure groups examined [28]. The speculation is that some physiological processes like ovarian follicle development are more susceptible to alterations in gene expression than are others. Exposure-induced germ cell epimutations lead to changes in gene expression, in all cells and tissues such that those tissues most susceptible will develop disease states more often.
Interestingly, some of the environmentally induced epigenetic transgenerational inherited disease susceptibilities appear to be exposure specific. For example, gestating rats exposed to jet fuel hydrocarbons transmit transgenerationally to the F3 generation females an increased rate of luteal ovarian cyst formation [39, 40]. This was not seen after exposure to plastics compounds, dioxin, or other environmental toxicants [25, 27, 38]. Similarly, increased risk of obesity is inherited transgenerationally after ancestral exposure to DDT and plastic compounds, but not vinclozolin [38, 49]. The role of environmentally induced epigenetic transgenerational inheritance in the etiology of disease requires further investigation.
In addition to disease etiology, epigenetic transgenerational inheritance likely plays a role in generating the phenotypic variation that is necessary for natural selection to act upon during evolution. Environmental factors that induce epigenetic changes that are passed in the germ line can result in inherited changes in gene expression, in all tissues [50]. This can lead to alterations in an animal’s phenotype that can then be acted upon by natural selection. This is a mechanism by which environmental exposures, as well as the induction of classical gene sequence mutations, can lead to increased phenotypic variation in future generations. A study in rats found that ancestral vinclozolin exposure caused marked changes in mate preference transgenerationally [51]. Since sexual selection is a major determinant in evolutionary biology, the epigenetic transgenerational inheritance of this altered mate preference behavior phenotype suggests that transgenerational epigenetics may have an important role in evolution [52, 53]. For example, the ability of environmental exposures to promote phenotypic variation (e.g. mate preference) in a population through epigenetic transgenerational inheritance will influence the natural selection process in that population [19, 51].
Etiology of Transgenerational Disease
The environmentally induced epigenetic transgenerational inheritance of disease susceptibility requires epimutations to be transmitted through the germ cells from generation to generation. Disease states such as cancer, obesity or prostate disease involve abnormal regulation of gene expression in the relevant somatic cells. The hypothesis is that epimutations in germ cells lead to epimutations in the somatic cells that develop from those germ cells and that the epimutations cause aberrant gene expression in somatic cells to increase disease susceptibility [19, 54]. There is ample evidence that ancestral toxicant exposure can cause a change in the transcriptome of a tissue (i.e. differences in gene expression) transgenerationally [1, 19, 35, 45, 55-57]. However, it does not appear to be the case that exactly the same changes in gene expression are seen in different somatic cells of the same animal [58]. What is observed is cell and tissue specific transgenerational transcriptomes. The epigenetic profile (epigenome) of each cell type in the body is very different from that of every other type. In a study of rats exposed ancestrally to vinclozolin, examination of the transcriptomes of eleven different adult tissues demonstrated that each tissue had genes differentially expressed between control and vinclozolin lineages with negligible overlap in the differentially expressed genes between tissues [58]. Consideration of how a relatively small number of sperm epimutations can promote such a large number of specific transcriptome changes led to an evaluation of the genomic locations of the epimutations and differentially expressed genes involved. Gene clusters were identified and termed “epigenetic control regions”. These regions are 2–5 megabases in size with statistically significant over-representation of regulated genes within the vicinity of both epimutations and long non-coding RNA. The long non-coding RNA is proposed to mediate the regional gene regulation, affecting those genes in that region that would normally be expressed in that particular cell type [58]. Further research is needed to uncover the unique molecular mechanisms that may be involved in epigenetic regulation of these regions and gene expression.
Several studies have helped clarify how an environmentally induced transgenerational inheritance of disease susceptibility within a specific tissue may develop. The ovarian diseases Polycystic Ovarian Disease (PCO) and Primary Ovarian Insufficiency (POI), premature reduction of the primordial follicle pool, were both induced transgenerationally by several environmental toxicants [28, 40]. In one study granulosa cells from ovarian follicles were isolated from younger animals prior to disease onset, and their epigenomes and transcriptomes were characterized [28]. The granulosa cells from vinclozolin lineage rats were found to have an altered epigenome and transcriptome that suggested specific signaling pathways were affected. A number of the differentially expressed genes identified were previously shown to be involved in the development of PCO and POI [59]. Similarly, studies have examined the molecular etiology of male infertility associated with testis disease that can be induced by ancestral exposure to vinclozolin. The somatic Sertoli cells in the testis were also found to have transgenerational alterations in their epigenomes and transcriptomes [60]. A number of the cellular processes and differentially regulated genes identified also have been previously shown to be involved in male infertility [57]. Therefore changes in the transcriptome of somatic cells that were induced transgenerationally by ancestral exposure to toxicants appear to be linked to increased susceptibility to specific diseases.
Conclusions
Research in this field has shown that a non-genetic (i.e. epigenetic) form of transgenerational inheritance of disease susceptibility exists that complements the well-known genetic inheritance of gene variants that pre-dispose disease [1, 19, 30]. Epigenetic transgenerational inheritance of disease susceptibility can occur following exposure to a number of environmental toxicants and nutritional abnormalities. The epigenetic changes induced in the germ line after ancestral exposure to environmental toxicants appear to be specific to the toxicant. The possibility that epigenetic testing could inform people of potential risks of disease susceptibility needs to be further investigated. Increased incidences of many different disease states have been linked to transgenerational epigenetic inheritance after exposure to toxicants. One mechanism for transgenerational epigenetic inheritance is for an environmental exposure to induce alterations in DNA methylation in the developing germ cells that become fixed or imprinted-like which are then transmitted transgenerationally. These epigenetic changes in germ cells will produce epigenetic changes in the resultant somatic cells that will lead to aberrant gene expression and the development of disease. Further studies clearly are needed to clarify the role of epigenetics and transgenerational inheritance in disease etiology, evolutionary biology, and other areas of cell and developmental biology. Increased knowledge in these areas will have a significant impact on our understanding of normal biology and disease etiology.
ACKNOWLEDGEMENTS
We thank Ms. Heather Johnson for assistance in preparation of the manuscript. Both authors have read and participated in the writing of the manuscript and have read the journal’s authorship agreement. No external editorial support was involved in the preparation of the manuscript. Neither author has any potential conflicts of interest or financial conflicts. This research was supported by an NIH grant to MKS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pembrey ME. Male-line transgenerational responses in humans. Hum Fertil (Camb) 2010;13:268–271. doi: 10.3109/14647273.2010.524721. [DOI] [PubMed] [Google Scholar]
- 4.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drake AJ, Walker BR, Seckl JR. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 6.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147:S11–17. doi: 10.1210/en.2005-1164. [DOI] [PubMed] [Google Scholar]
- 7.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 8.Kaati G, Bygren LO, Pembrey M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet. 2007;15:784–790. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 9.Ruden DM, Xiao L, Garfinkel MD, Lu X. Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstibesterol on uterine development and cancer. Hum Mol Genet. 2005;14(Spec No 1):R149–155. doi: 10.1093/hmg/ddi103. [DOI] [PubMed] [Google Scholar]
- 10.Walker AK, Hawkins G, Sominsky L, Hodgson DM. Transgenerational transmission of anxiety induced by neonatal exposure to lipopolysaccharide: implications for male and female germ lines. Psychoneuroendocrinology. 2012;37:1320–1335. doi: 10.1016/j.psyneuen.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 12.Zambrano E, Martinez-Samayoa PM, Bautista CJ, Deas M, Guillen L, Rodriguez-Gonzalez GL, Guzman C, Larrea F, Nathanielsz PW. Sex differences in transgenerational alterations of growth and metabolism in progeny (F2) of female offspring (F1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-de-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci (Lond) 2008;114:381–392. doi: 10.1042/CS20070302. [DOI] [PubMed] [Google Scholar]
- 14.Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218:200–205. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambrot R, Xu C, Saint-Phar S, Chountalos G, Cohen T, Paquet M, Suderman M, Hallett M, Kimmins S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat Commun. 2013;4:2889. doi: 10.1038/ncomms3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blewitt ME, Vickaryous NK, Paldi A, Koseki H, Whitelaw E. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006;2:e49. doi: 10.1371/journal.pgen.0020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB J. 2007;21:3380–3385. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 19.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Hamilton KJ, Lai AY, Burns KA, Li L, Wade PA, Korach KS. Diethylstilbestrol (DES)-Stimulated Hormonal Toxicity is Mediated by ERalpha Alteration of Target Gene Methylation Patterns and Epigenetic Modifiers (,, and) in the Mouse Seminal Vesicle. Environ Health Perspect. doi: 10.1289/ehp.1307351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris RM, Waring RH. Diethylstilboestrol--a long-term legacy. Maturitas. 72:108–112. doi: 10.1016/j.maturitas.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online. 2008;16:23–25. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS ONE. 2012;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol. 2012;34:694–707. doi: 10.1016/j.reprotox.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Pesticide and insect repellent mixture (permethrin and DEET) induces epigenetic transgenerational inheritance of disease and sperm epimutations. Reprod Toxicol. 2012;34:708–719. doi: 10.1016/j.reprotox.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS ONE. 2012;7:e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jodar M, Selvaraju S, Sendler E, Diamond MP, Krawetz SA. The presence, role and clinical use of spermatozoal RNAs. Hum Reprod Update. 2013;19:604–624. doi: 10.1093/humupd/dmt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27:868–879. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anway MD, Skinner MK. Transgenerational effects of the endocrine disruptor vinclozolin on the prostate transcriptome and adult onset disease. Prostate. 2008;68:517–529. doi: 10.1002/pros.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruner-Tran KL, Resuehr D, Ding T, Lucas JA, Osteen KG. The Role of Endocrine Disruptors in the Epigenetics of Reproductive Disease and Dysfunction: Potential Relevance to Humans. In: Stratton P, editor. Curr Obstet Gynecol Rep. Springer; 2012. pp. 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS ONE. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner MK. Hydrocarbons (jet fuel JP-8) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. Reprod Toxicol. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS ONE. 2012;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waterland RA. Is epigenetics an important link between early life events and adult disease? Horm Res. 2009;71(Suppl 1):13–16. doi: 10.1159/000178030. [DOI] [PubMed] [Google Scholar]
- 42.Jensen P. Transgenerational epigenetic effects on animal behaviour. Prog Biophys Mol Biol. 2013;113:447–454. doi: 10.1016/j.pbiomolbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bollati V, Baccarelli A. Environmental epigenetics. Heredity (Edinb) 2010;105:105–112. doi: 10.1038/hdy.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson EE, Anway MD, Stanfield J, Skinner MK. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction. 2008;135:713–721. doi: 10.1530/REP-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88:112. doi: 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A. 2012;109:9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skinner MK, Manikkam M, Tracey R, Guerrero-Bosagna C, Haque M, Nilsson EE. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11:228. doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerrero-Bosagna C, Sabat P, Valladares L. Environmental signaling and evolutionary change: can exposure of pregnant mammals to environmental estrogens lead to epigenetically induced evolutionary changes in embryos? Evol Dev. 2005;7:341–350. doi: 10.1111/j.1525-142X.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- 53.Guerrero-Bosagna C, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of phenotype and disease. Mol Cell Endocrinol. 2012;354:3–8. doi: 10.1016/j.mce.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker CL. Epigenomic reprogramming of the developing reproductive tract and disease susceptibility in adulthood. Birth Defects Res A Clin Mol Teratol. 2011;91:666–671. doi: 10.1002/bdra.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skinner MK, Haque CG, Nilsson E, Bhandari R, McCarrey JR. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE. 2013;8:e66318. doi: 10.1371/journal.pone.0066318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics. 2008;91:30–40. doi: 10.1016/j.ygeno.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guerrero-Bosagna C, Savenkova M, Haque MM, Nilsson E, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility. PLoS ONE. 2013;8:e59922. doi: 10.1371/journal.pone.0059922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skinner MK, Mohan M, Haque MM, Zhang B, Savenkova MI. Epigenetic transgenerational inheritance of somatic transcriptomes and epigenetic control regions. Genome Biol. 2012;13:R91. doi: 10.1186/gb-2012-13-10-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova M, Skinner M. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE. 2012;7:e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guerrero-Bosagna C, Savenkova M, Haque MM, Sadler-Riggleman I, Skinner MK. Environmentally Induced Epigenetic Transgenerational Inheritance of Altered Sertoli Cell Transcriptome and Epigenome: Molecular Etiology of Male Infertility. PLoS ONE. 2013;8:e59922. doi: 10.1371/journal.pone.0059922. [DOI] [PMC free article] [PubMed] [Google Scholar]