Abstract
Identification of agents that target human leukemia stem cells (LSCs) is an important consideration for the development of new therapies. The present study demonstrates that rocaglamide and silvestrol, closely related natural products from the flavagline class of compounds, are able to preferentially kill functionally defined LSCs while sparing normal stem and progenitor cells. In addition to efficacy as single agents, flavaglines sensitize leukemia cells to several anti-cancer compounds, including front-line chemotherapeutic drugs used to treat leukemia patients. Mechanistic studies indicate that flavaglines strongly inhibit protein synthesis, leading to the reduction of short-lived anti-apoptotic proteins. Notably though, treatment with flavaglines alone or in combination with other drugs, yields a much stronger cytotoxic activity towards leukemia cells than the translational inhibitor temsirolimus. These results indicate that the underlying cell death mechanism of flavaglines is more complex than simply inhibiting general protein translation. Global gene expression profiling and cell biological assays identified Myc inhibition and the disruption of mitochondrial integrity to be features of flavaglines, which we propose contribute to their efficacy in targeting leukemia cells. Together, these findings indicate that rocaglamide and silvestrol are distinct from clinically available translational inhibitors and represent promising candidates for the treatment of leukemia.
Keywords: leukemia, stem cells, silvestrol, rocaglamide, flavaglines
Introduction
In the past decade, a relatively new focus in drug development has been the concept of a cancer stem cell (CSCs), where the diversity within tumors arises at least in part as a consequence of developmental programs that mirror normal tissue systems (1). Perhaps the best characterized CSCs are those found in the blood cancer acute myelogenous leukemia (AML). Several studies have identified malignant stem cells, termed leukemia stem cells (LSCs), which are central to the initiation, growth and relapse of AML (2, 3). The high rates of relapse in AML patients suggest that current chemotherapeutic strategies do not adequately target LSCs. Thus there is great interest in the development/identification of compounds that target LSCs yet still maintain an optimal therapeutic index.
Nearly half of the agents used in cancer therapy today are either natural products or derivatives of natural products (4). A group of flavagline compounds from the plant genus Aglaia has attracted attention due to their insecticidal activities and inhibition of tumor growth (5). Two members of this family, rocaglamide and silvestrol have shown toxicity towards leukemia cells (6–9). The Li-Weber group has shown that rocaglamide induces apoptosis in malignant but not normal proliferating lymphocytes, possibly attributed to its ability to selectively suppress MAPK/ERK survival activity in the cancer(6, 8). Silvestrol has shown efficacy in vitro and in mouse models of the B-cell malignancies CLL, ALL and MCL at doses that caused no discernable toxicity. In these studies the activity of silvestrol was due at least in part to loss of the anti-apoptotic protein Mcl-1, with subsequent mitochondrial depolarization and caspase-dependent apoptosis (7, 10). In addition to leukemia silvestrol has shown activity towards lung, breast and prostate cancer cells and thus the utility of these compounds may extend beyond hematologic malignancies (11, 12). Studies have shown that silvestrol promotes an aberrant interaction between capped mRNA and eIF4A, thereby interfering with the assembly of the eIF4F translation complex and blocking translation initiation (13, 14). Consistent with these observations, recent work has identified eIF4A as one of the primary targets of rocaglamide and silvestrol (15). Hence, the activity of these compounds appear to be related to their ability to inhibit translation. Extensive in vitro evidence now points to the translational machinery as a powerful therapeutic target in cancer including hematologic malignancies (16, 17). The translation initiation complex constitutes a major node of convergence for numerous signaling pathways, however few agents impact this machinery directly, leaving this avenue largely unexplored in vivo. Thus, flavaglines are a unique set of compounds that represent the first direct inhibitor of translation initiation with clinical potential, as evidenced by their preclinical activity on an array of tumor types in the nanomolar range.
Here we show rocaglamide and silvestrol preferentially kill phenotypically and functionally defined LSCs, while sparing normal stem and progenitor cells. Importantly these compounds are significantly more toxic to leukemia cells as single agents or in combination with other anti-cancer drugs than clinically available translational inhibitors. This difference in cytotoxicity however is not attributable to the respective differences global protein synthesis inhibition; rather it appears that they more efficiently decrease levels of Myc protein and also alter mitochondrial integrity via p53 activation.
Materials and Methods
Primary AML and normal hematopoietic cells
Normal and leukemic human bone marrow samples were obtained after informed consent from volunteer donors at the University of Rochester Medical Center. Total bone marrow mononuclear cells were isolated by standard Ficoll procedures (GE Healthcare) and cryopreserved in freezing medium consisting of Cryostor CS10 (BioLife Solutions). The viability of leukemic cells after thawing was 50 – 90%. Normal bone marrow total mononuclear cells were further enriched for CD34 positive cells using MACS CD34 enrichment kit (Milltenyi Biotec).
Cell death assays
For in vitro cell death assays, normal and leukemic cells were cultured in serum-free media for 24 or 48 hours in the presence of drug and analyzed with AnnexinV/7AAD staining using the LSRII flow cytometer (BD, San Jose, CA). For ex vivo toxicity assays, cells were treated in vitro with Rocaglamide (ENZO life sciences) for 48hr, and then harvested and injected in irradiated NSG mice. For AML and NBM specimens, engraftment of human cells was evaluated after 6–8 weeks by flow cytometry.
Colony forming assay
5×104/ml of AML or normal cells were plated in Methocult GF H4534 as previously described (17). Colonies were scored after 21 days of culture.
Methionine-Incorporation assay
Methionine labeling experiments and subsequent click-it chemistry were performed using reagents and protocols provided by Invitrogen/Life Sciences. Briefly, cells were grown in media lacking Methionine for one hour, incubated with Click-IT AHA for 2hrs, then washed and lysed. Click-it chemistry was performed on lysates and which were subsequently run on SDS-PAGE gel, transferred to nitrocellulose and probed for the presence of labeled methionine with straptavidin HRP antibody.
Western Blot
Primary antibodies for Mcl-1, Bcl-Xl, Myc, phospho-p53, Bax and actin were purchased from Cell Signaling Technologies. Primary antibody for BCL-2 and GAPDH were purchased from Santa-Cruz. Western Blot was performed as previously described(18).
Combination index calculation
Combination index (CI) was calculated by Calcusyn software (Biosoft). The results were input into Calcusyn software that applies a Chou-Talalay method to calculate the CI value for each specific dose combo. Parthenolide (Biomol), Cytarabine (Sigma), Daunorubicin (pfizer), ABT-737 (Selleckchem), PU-H71 (Calbiochem), were used in assays.
Whole transcriptome analysis
Total RNA was isolated from AML 24hrs after rocaglamide treatment using the RNeasy Plus Kit (Qiagen, Valencia, CA) per manufacturer’s recommendations. The TruSeq RNA Sample Preparation Kit V2 (Illumina, San Diego, CA) was used for next generation sequencing library construction per manufacturer’s protocols and as previously described (19).
sh-RNA mediated p53 knock down and Myc overexpression
The coding sequences of p53 (NM_001126114.1) targeted by short hairpin RNAs are: sh-p53 A: 5′-GTCCAGATGAAGCTCCCAGAA-3′; sh-p53 E: 5′-GCATCTTATCCGAGTGGAAGG-3′; sh-P53 H 5′GGTCTTTGAACCCTTGCTTGC3′ The detailed methods for cloning shRNA, generating lentiviral particles, and infecting AML cells as previously described(20). MYC overexpression construct was created as follows: c-MYC gene was excised from MSCVh c-MYC IRES GFP (addgene plasmid 18119) with EcoRI restriction enzyme and ligated into pLVX-mcherry (Clontech).
Cell Cycle analysis
For evaluation of cell cycle profile, cells were fixed by the BD Fixation/Permeabilization kit (554714, BD Biosciences) according to manufacturer’s instructions, stained with anti-Ki67-Alexa Fluor 647 (BD Biosciences) for 45min at 4°C and re-suspended overnight at 4°C in 0.5ml of 5μg/ml 7AAD staining solution. Cells were analyzed using BD LSRII flow cytometer (BD Biosciences).
Mitochondrial Depolarization
Mitochondrial membrane depolarization was assessed using Tetramethylrhodamine, Ethyl Ester, Percholate (TMRE) purchase from Life Sciences. Following treatment with drugs, cells were incubated with TMRE for 30 minutes at 37 degrees, washed, stained with Annexin V/7AAD. All analyses show the percentage of viable cells with depolarized mitochondrial membranes.
Measurement of endogenous ROS levels
Endogenous ROS levels of M9-ENL cells were detected by labeling 1×106 cells for 15min at 37°C with the redox sensitive probes CM-H2DCFDA (5μ), or MitoSox (5μ) (Life Technologies). Labeled cells were re-suspended in 0.2ml of 10uM DAPI (Invitrogen) solution and analyzed using BD LSRII flow cytometer (BD Biosciences) and FlowJo software (Treestar).
In vivo treatment of xenografts with Silvestrol
Irradiated NSG mice were transplanted with human leukemia cells (3×106/mouse) via tail vein in a final volume of 0.2 mL of PBS with 0.5% FBS. After 3 weeks, animals were size matched to treatment and control groups (10 animals per group) and received either Silvestrol (0.75mg/kg) or vehicle. Drugs were injected IP for 3 weeks with a schedule of 5 days on, 2 days off (15 injections total). Silvestrol was formulated at of 0.1 mg/mL in 30% HPBCD. Following treatment, mice were sacrificed and human engrafted cells were evaluated by flow cytometry.
Results
Rocaglamide selectively ablates leukemia blast cells and stem cells
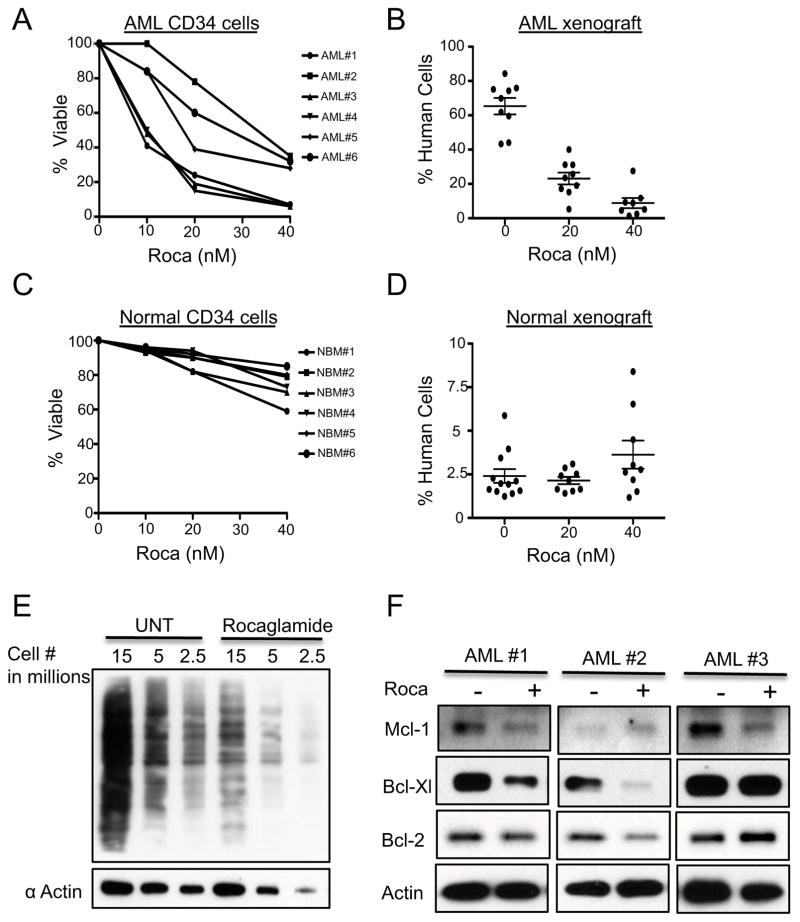
To establish the in vitro cytotoxicity of flavaglines, we initially treated cultures of primary human AML specimens or normal bone marrow controls with varying concentrations of rocaglamide. AML cells showed minimal cell death as measured by annexin and 7AAD at 24 hours of treatment, however toxicity increased significantly at 48 hours in both total and the more primitive CD34+ populations (Figure 1A, S1A). Parallel experiments on normal cells showed very modest decreases in viability (Figure 1C, S1B). To functionally assess the effects of rocaglamide on stem cells, primary AML and normal specimens were treated with rocaglamide for 48 hours and then transplanted into sublethally irradiated NSG (NOD/SCID/IL2Rg) mice. At 8 weeks post-transplantation, bone marrow cells were analyzed to determine the engraftment of human cells. Rocaglamide treated cells were strongly impaired with respect to xenograft engraftment (Figure 1B, S1C), whereas normal controls show no significant reduction in engraftment potential (Figure 1D, S1D). In agreement with xenograft experiments, in vitro colony assays showed that rocaglamide treatment resulted in only a modest reduction in normal myeloid and erythroid colony forming units (CFUs) (Figure S1E). Taken together these results indicate that rocaglamide is able to induce death in bulk leukemia cells, as well the primitive LSCs, while sparing normal stem and progenitor cells.
Figure 1.
A) Percentage of CD34 viable hematopoietic cells in primary human AML cells (N=6) exposed to rocaglamide. Cells were stained with annexin-V and 7-AAD and viability measured by a flow cytometer, viability values are relative to untreated controls. B) Percentage engraftment achieved in NSG mice receiving AML cells after 48hrs culture with or without rocaglamide. Each symbol represents a single animal analyzed 8 weeks after transplantation. Mean engraftment is indicated by the horizontal bars. C)Percentage of viable CD34+ cells from healthy donors exposed to rocaglamide., D) Percentage engraftment achieved in NOG mice receiving hematopoietic cells from healthy donors after 48hrs culture with or without rocaglamide. E)Methionine metabolic labeling of untreated leukemia cells and leukemia cells treated with rocaglamide for 24hrs. Cells were labeled with an epitope tagged version of methionine to quantify the extent of inhibition of new protein synthesis. F) Whole cell lysates were generated from primary AML cells treated with Rocaglamide for 24hrs for western blot analysis
Rocaglamide inhibits translation, resulting in reduced levels of anti-apoptotic proteins
Flavaglines have previously been shown to inhibit the translation of nascent proteins (13, 22). To verify this activity for rocaglamide, we used an epitope tagged form of methionine and pulse-chase studies to determine and quantify inhibition of new protein synthesis. Rocaglamide dramatically reduced the amount of nascent translation after 24 hours treatment (Figure 1E). Next, we performed western blot analysis on primary AML cells treated with rocaglamide and observed reduced levels of phosphorylated 70S6K and 4EBP1, proteins critical for the initiation/regulation of cap-dependent translation (Figure S1G). It should be noted that the loss of phosphorylation does not appear to be the result of rocaglamide affecting mTOR machinery directly; rather it is a response to the decrease in translation caused by rocaglamide (data not shown). Taken together these results clearly indicate that rocaglamide affects the translational status of treated cells.
Next, we examined levels of the Bcl-2 family of anti-apoptotic proteins Mcl-1, Bcl-2 and Bcl-XL, some of which have been reported to decrease in other systems upon inhibition of translation (7, 9). We observed decreases in the levels of these proteins in primary AML cells 24 hours after treatment with rocaglamide (Figure 1F). The changes in expression levels of these proteins following rocaglamide treatment are not consistent between samples. There are clear differences in the identity of the proteins that decrease as well as the degree of change, suggesting that the apoptosis induced by rocaglamide cannot be attributed to a decrease of one particular protein of this family, consistent with recent genetic experiments (23).
Despite the inconsistency in Bcl-2 family member reduction caused by rocaglamide treatment, in all AML specimens tested the level of at least one of the mitochondrial-associated protein decreased leading us to further evaluate mitochondrial function. Using TMRE, a fluorescent dye indicative of mitochondrial membrane integrity, we observed that rocaglamide increased the number of cells with depolarized mitochondria (Figure S1F). This observation is consistent with previous studies using the closely related compound silvestrol, where LNCaP prostate cancer cells and CLL cells also demonstrated a loss of membrane potential (7, 24). Overall our results show that rocaglamide inhibits translation in AML cells, decreases the levels of anti-apoptotic proteins, and disrupts mitochondrial integrity.
Rocaglamide is more cytotoxic to leukemia cells than other translational inhibitors
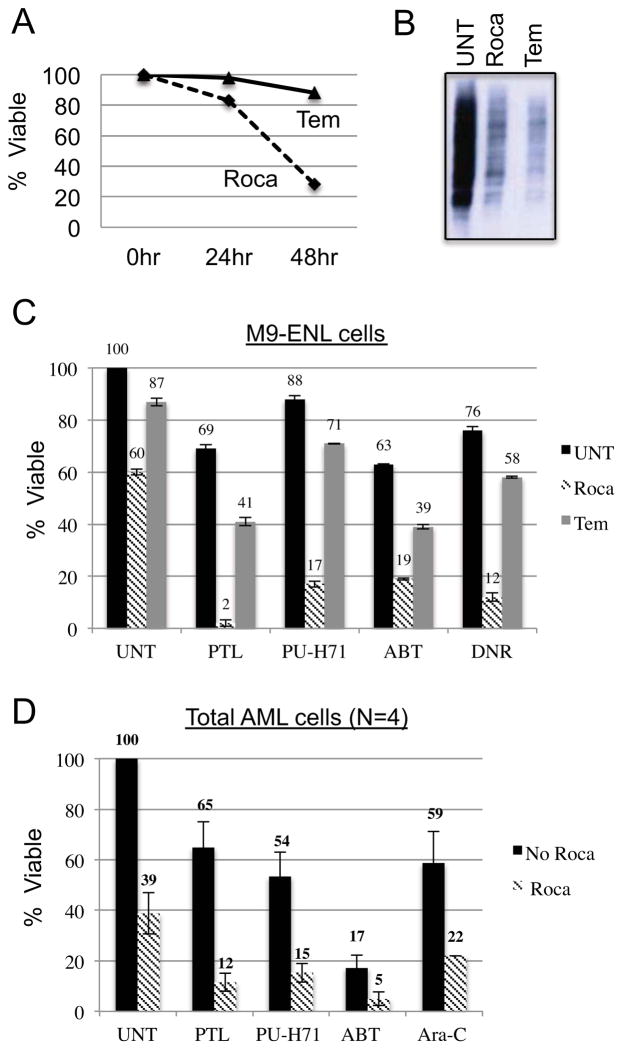
The deregulation of translation has been implicated in cancer initiation and progression and consequently there is significant interest in targeting this process with therapeutic agents (25). To assess how flavaglines compare to other agents, we examined Temsirolimus, a rapamycin derivative that targets the mTOR pathway, and has been investigated in numerous treatment regimens for AML (26, 27) and other cancers (28, 29). We treated the M9-ENL cell line with temsirolimus or rocaglamide for 48hrs and found that rocaglamide was markedly more cytotoxic towards leukemia cells (Figure 2A). In fact, rocaglamide was more toxic to leukemia cells than the combination of temsirolimus and ribavirin, another translational inhibitor that blocks the binding of eIF4E to the mRNA cap structure (Figure S2A) (27, 30). We performed methionine labeling in parallel with the cytotoxicity assays to evaluate the effect rocaglamide and temsirolimus had on the rate of translation. Strikingly, temsirolimus, which is minimally toxic to leukemia cells, inhibits global translation to a degree that is comparable to that of rocaglamide (Figure 2B). Thus, translational inhibition alone cannot explain the relative efficacy of rocaglamide.
Figure 2.
A) Percent survival of leukemia cells treated with different translation inhibitors (40nM Rocaglamide, 2.5ug/mL Temsirolimus). B) Met-label experiment comparing translation after treatment with indicated compounds (40nM Rocaglamide, 2.5ug/mL Temsirolimus). Graph represents percent survival of C) M9-ENL leukemia cells D) CD34 primary leukemia cells (N=4) treated with indicated drugs: , PTL = 2.5uM Parthenolide, , PU-H71 = 2uM PU-H71, , ABT737 = 25nM ABT-737, DNR = 25nM Daunorubicin. Solid bars represent percent survival of compounds given individually. Striped and gray bars represent the percent survival when compounds were given following 24hr pretreatment with rocaglamide or temsirolimus, respectively.
Next, we surveyed a broad range of drugs in combination with rocaglamide to determine it’s potential in combination regimens. M9-ENL cells pretreated with rocagamide for 24 hours prior to additiona of a second drug showed strong cytotoxicity for several agents (Figure 2C). Temsirolimus also enhanced the activity of compounds tested however the degree was much less than that observed with rocaglamide (Figure 2C). We calculated combination indices (CI) according to the Chou-Talalay method, which indicated that all combinations using rocaglamide were synergistic (Figure S2B–E). We also observed increased toxicity when rocaglamide was given simultaneously with the second drug, however increases were not as large as those achieved using the pretreatment strategy (Figure S2G). It should be noted that not all drugs synergized with rocaglamide, in fact the proteasome inhibitor bortezomib was antagonistic with rocaglamide, presumably because it slowed the turnover of short-lived anti-apoptotic proteins (Figure S2F).
To further explore drug synergies, we examined several combinations in primary human AML CD34+ cell populations in vitro (Figure 2D). Importantly, rocaglamide enhanced the cytotoxicity of Ara-C, a mainstay of AML therapeutic regimens. In addition rocaglamide enhanced the activity of several experimental agents: 1) ABT-737, a Bcl-2 inhibitor (31), 2) PU-H71 is a new class of HSP90 inhibitor (32) and 3) parthenolide, a potent inducer of reactive oxygen species (33). Importantly, ABT-737 and parthenolide, like rocaglamide show selectivity towards the LSC population as single agents indicating that their combination holds promise for targeting more primitive leukemia cells (18, 20). Taken together the data indicate that rocaglamide has the potential to increase the efficacy of multiple agents, a degree of versatility that could be useful in building novel treatment regimens.
Rocaglamide modulates the activity of key transcriptional factors
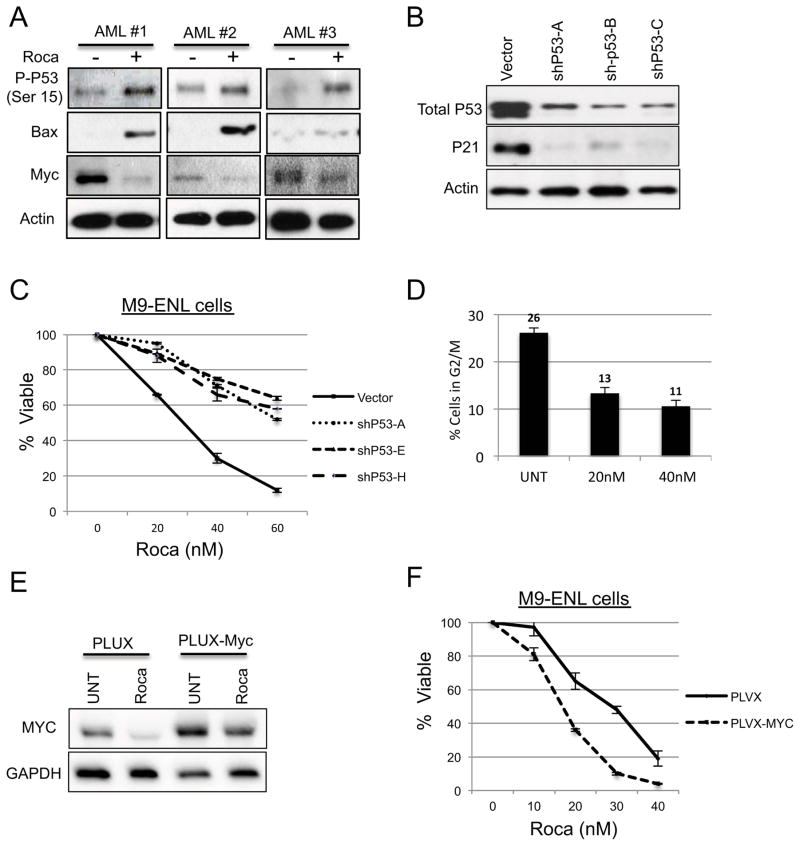
To better understand the mechanism of rocaglamide-mediated cell death, we performed RNA-seq based studies to generate a comprehensive profile of gene expression changes that occur as a consequence of drug treatment. Using four primary AML specimens and the M9-ENL cell line, cells treated with or without 40nM rocaglamide for 24hrs were assayed. A total of 794 genes were differentially expressed using a P <.05 statistical cutoff (Table S1). Bioinformatic analyses of the data demonstrated that the transcription factors p53 and MYC were strongly influenced by rocaglamide treatment (Figure S3B).
The tumor-suppressor p53 is mutated in less than 10% of AML patients and thus its activity may be relevant to the biology of most AML cells. We observed increased phosphorylation of p53 on serine 15 following rocaglamide treatment, as well as increased levels of the pro-apoptotic and p53 regulated protein Bax (Figure 3A). We speculate that increased Bax is due to enhanced protein stability, since protein synthesis is strongly inhibited by rocaglamide. To test whether p53 activation is required for rocaglamide mediated cell death, we performed shRNA-mediated knock down of P53 mRNA expression in M9-ENL cells and tested their sensitivity to rocaglamide. We generated three independent clones all of which had reduced levels of p53 protein (Figure 3B). All three clones showed resistance to rocaglamide treatment, indicating that p53 activation is a component of rocaglamide mediated cell-death (Figure 3C). It should be noted that there is not a complete rescue of cell death in our p53 deficient cells, indicating that p53 may not be the sole driver of rocaglamide-induced apoptosis. The potential for a p53-independent mechanism is consistent with experiments performed in CLL leukemia and LNCaP cells treated with silvestrol (7, 24).
Fig. 3.
A)Whole cell lysates were generated from primary AML cells treated with 40nM rocaglamide for 24hrs for western blot analysis. B) Protein expression of M9-ENL cells infected with empty vector and shRNA targeting P53. C) Graph represents percent survival of cells treated with indicated concentrations of rocaglamide. D) Cell cycle analysis of M9 cells treated with indicated amounts of rocaglamide. E) Protein expression of M9 cells infected with empty vector and MYC expressing vector. F) Graph represents percent survival of M9 cells treated with indicated amounts of rocagalmide.
The Myc oncogene contributes to the genesis of many human cancers and appears to play a role in LSC self-renewal (34). We observed a strong decrease in Myc protein levels following rocaglamide treatment (Figure 3A), consistent with our RNA-seq analysis where >85% (33 out of 38) of Myc regulated genes were inhibited (Figure S3A). Importantly, the RNA-seq data and subsequent qPCR analyses (data not shown) showed no significant decreases in Myc mRNA following rocaglamide treatment, indicating that the decreases in the level of Myc protein is the result of post-transcriptional events. Many of these down-regulated genes controlled by Myc are implicated in cell cycle progression, thus we co-labeled M9 cells with anti-Ki67 and 7AAD to evaluate cell cycle status after rocaglamide treatment. Rocaglamide decreased the percentage of actively dividing cells, while increasing the percentage of cells in G0 (Figure 3D, S3B). To test the importance of Myc inhibition for rocaglamide mediated cell death we generated M9-ENL cells in which Myc cDNA was ectopically expressed from a lentiviral promoter, which resulted in a significant increase in Myc protein levels (Figure 3E). Despite the elevated Myc levels rocaglamide was able to reduce Myc protein and induce apoptosis to an even greater extent than cells transduced with an empty vector (Figure 3E, F). The increased sensitivity of the Myc expressing cells to rocaglamide is consistent with the synthetic lethal relationship between Myc and the eIF4F complex (35). We also tested the sensitivity of the transduced M9-ENL cells to the Myc inhibitor JQ1 and observed that, like rocaglamide, the Myc expressing cells were more sensitive to JQ1. The similar toxicity profiles of JQ1 and rocaglamide, as well as the ability of rocaglamide to reduce the forced expression of the Myc protein indicate that Myc inhibition is a component of the rocaglamide mechanism.
Rocaglamide has a greater affect on mitochondrial integrity and MYC levels than Temsirolimus
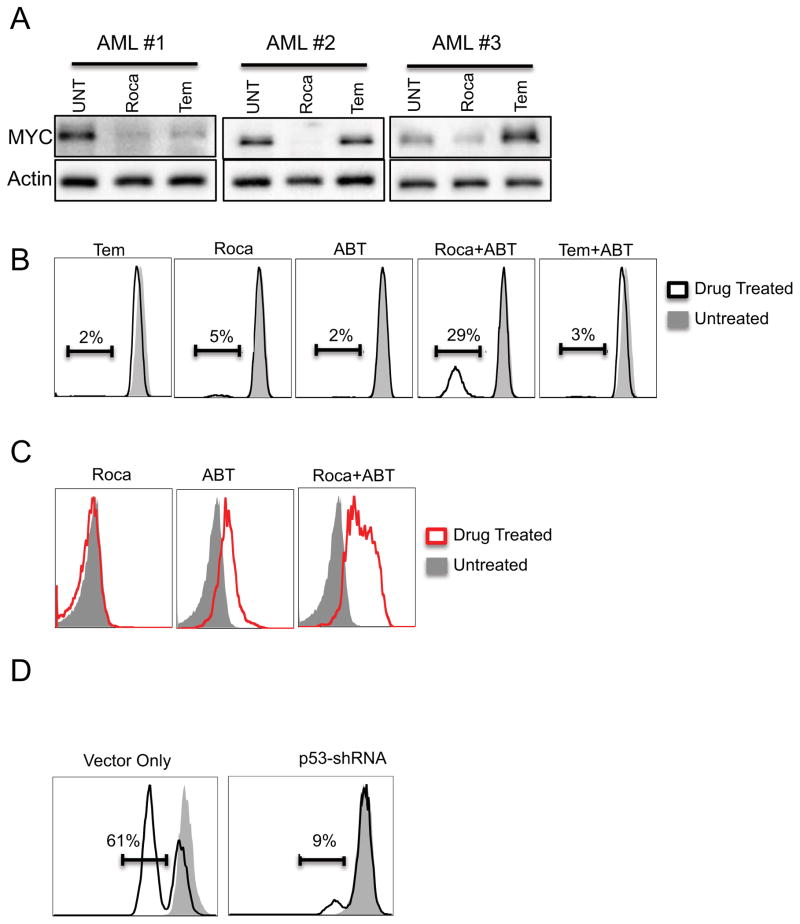
To further examine the relevance of Myc reduction, we compared protein levels between rocaglamide vs. temsirolimus treated cells. Rocaglamide more effectively reduced Myc, consistent with a role for this protein in cell death (Figure 4A).
Figure 4.
A) Whole cell lysates were generated from primary AML cells treated with 40nM rocaglamide for 24hrs for western blot analysis. Blots were probed with antibodies against either cMYC or GAPDH B) M9-ENL Cells were treated with indicated drugs, depolarized mitochondria was determined by flow cytometry following staining with TMRE dye. C) M9-ENL cells were incubated with indicated drugs followed by labeling with the fluorescent dye Mitosox which detects superoxide, and analysis by flow cytometry. D) M9-ENL cells infected with empty vector or shRNA targeting P53 were treated with Rocaglamide and ABT-737 and stained with TMRE.
We next investigated the possibility that mechanism of rocaglamide may extend beyond translation inhibition and that these additional activities are not shared by temsirolimus. Rocaglamide affects mitochondrial integrity (Figure S1F), and synergizes with the Bcl-2 inhibitor ABT-737 to a much greater degree than temsirolimus (Figure 2C). Thus, we compared the extent of mitochondrial depolarization induced by rocaglamide and temsirolimus as single agents as well as in combination with ABT-737. The amount of depolarization induced by rocaglamide is more than that of temsirolimus (Figure 4B, S4B). Strikingly, the combination of rocaglamide and ABT-737 shows a significant increase in depolarization compared to ABT-737 alone or in combination with temsirolimus, In fact, the degree of depolarization is similar to levels induced by the well-characterized mitochondrial depolarizer, CCCP (Figure 4B, S4B, C). These results led us to further evaluate the effects rocaglamide drug combinations have on mitochondria events. Using the fluorescent dye Mitosox, which detects mitochondrial specific superoxide we observed significant increases in superoxide levels in cells treated with rocaglamide and ABT (Figure 4C), but not for cells treated with either agent individually (Figure 4C). Interestingly, the combination had no effect on reactive oxygen species as measured by the fluorescent dye CM-H2DCFDA (DCF) (Figure S4D). DCF provides a more broad measure of intracellular redox levels than Mitosox, These results indicate that the disruption of mitochondrial integrity/function is critical to the mechanism of rocaglamide. Having already established a role for p53 activation in the rocaglamide induced cell death (Figure A and C) we asked if the impaired mitochondrial function could be explained by p53 activation. Employing our p53 deficient M9-ENL cells we observed a significant reduction in mitochondrial depolarization (and increased viability, data not shown) induced by the combination of rocaglamide and ABT-737 compared to an empty vector control (Figure 4D) This result indicates that p53 activation is critical to the mitochondrial defects induced by rocaglamide and thus its ultimate mechanism of action and represent properties distinct from other translational inhibitors such as temsirolimus.
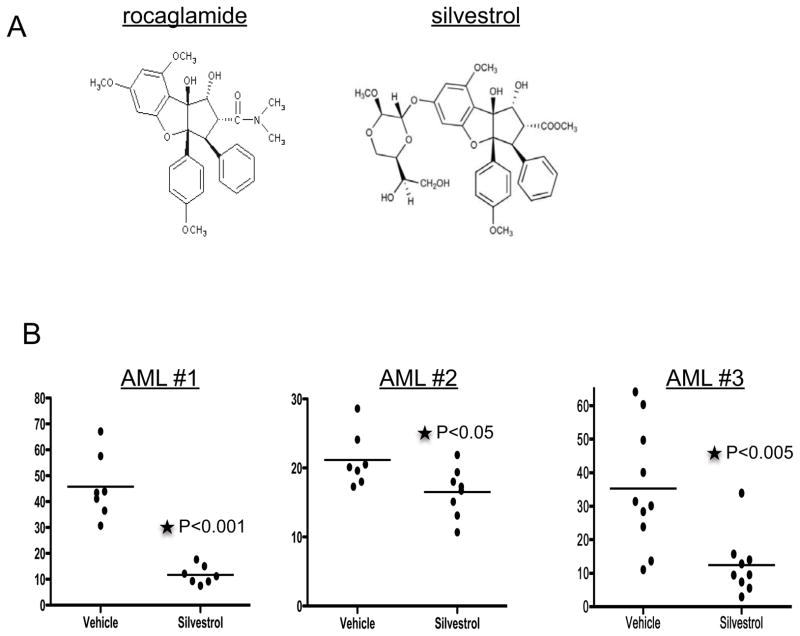
The flavagline compound silvestrol reduces leukemia burden in vivo
Although several labs have successfully synthesized rocaglamide (36–41), it is still difficult to obtain rocaglamide in quantities necessary for in vivo studies. Thus, we chose to evaluate the in vivo activity of silvestrol, a closely related member of the flavagline family (Figure 5A) which we have successfully isolated in gram quantities from the plant source (4, 11, 42, 43). Silvestrol has previously demonstrated in vivo toxicity towards chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL) and mantle cell lymphoma cells (7, 10). We first compared the in vitro toxicity of silvestrol to rocaglamide using several primary AML samples and found that both compounds produced similar dose curves, with silvestrol showing a slight increased potency (~2-fold) (Figure S5A). Further, as observed for rocaglamide, silvestrol showed minimal toxicity towards normal hematopoietic cells, including primitive populations (Figure S5B, C). Next we compared the ability of silvestrol to synergize with the collection of anti-cancer drugs that we previously tested with rocaglamide. Similar to rocaglamide, silvestrol significantly enhanced the toxicity of all the drugs tested (Figure S5D). In addition to analogous cytotoxicity profiles, the molecular response induced by both compounds, including protein synthesis inhibition and a subsequent reduction in Myc protein levels, is also similar (Figure S5E,F). Thus, from a mechanistic standpoint, silvestrol appears to be extremely similar to rocaglamide, inducing the same molecular events and changes in cell physiology.
Figure 5.
A) Chemical structure of rocaglamide and silvestrol. B) Primary AML cells were injected into sublethally irradiated mice. Three weeks after transplant, mice were either treated with silvestrol or vehicle for 3 weeks. For AML #1 and AML#2 silvestrol was administered at 0.75mg/kg daily, 5X a week for 3 weeks. Silvestrol was administered 3X a week for 3 weeks at a dose of 1.5mg/kg for mouse engrafted with AML#3. Mice were euthanatized and bone marrow was analyzed for percentage of human cells using anti-human CD45 antibody
Next we sought to determine the efficacy of silvestrol for reducing tumor burden in an established human-mouse xenograft. NSG mice were sub-lethally irradiated and injected with primary human AML cells from three independent specimens. Beginning at 3 weeks after transplant, mice were treated with 0.75mg/kg silvestrol 5 days a week for 3 weeks, after which tumor burden, as a function of percent bone marrow engraftment, was determined (Figure 5B). Silvestrol significantly reduced engraftment for all three specimens (P<0.05), with minimal deleterious effects on the mice as measured by viability of the marrow and weight loss during treatment (Figure S5G). In addition, we were able to more specifically evaluate in vivo activity of silvestrol for specimen #1, which had a distinct CD33+, CD3+ leukemic population. As shown in Figure S5H, analysis of the CD33+/CD3+ cells following treatment showed a preferential reduction in this aberrant clone in comparison to other cells in the graft which may represent a mixture of normal and leukemic cells. Thus the overall decrease of CD45+ cells may under-represent the degree of tumor reduction, which may be upwards of 10-fold (Figure S5I). Taken together, the data clearly indicate in vivo activity of silvestrol towards primary human AML populations.
Discussion
Numerous lines of investigation have demonstrated that cellular heterogeneity for leukemia is evident at multiple levels, including immunophenotype, morphology, cell cycle status, growth potential, and response to chemotherapy (44, 45). Thus, in order for a drug to provide durable remissions it must be capable of targeting multiple cell types, each having distinct properties. Furthermore, drugs that target LSC, while sparing normal HSC are of particular interest. Results presented here indicate that drugs of the flavagline class (rocaglamide and silvestrol) represent promising candidates for the development of improved AML regimens. They are toxic to total AML cells as well as AML stem cells as assayed by engraftment of NSG mice; while normal hematopoietic cells, including HSCs, are largely unaffected under the same conditions. Further, in vivo experiments show that silvestrol is able to reduce the tumor burden of mice engrafted with primary AML cells, with minimal deleterious effects on the mice.
In comparison to Temsirolimus, rocaglamide and silvestrol are significantly more toxic to leukemia cells when given as single agents or in combination with other drugs. Interestingly, this increased cytotoxicity does not directly correlate with the ability of the compounds to inhibit translation as temsirolimus inhibits translation at levels equal to or greater than rocaglamide This observation led us to further investigate the molecular mechanism of cell death induced by rocaglamide. Using several assays, we showed that rocaglamide leads to p53 activation, Myc reduction, cell cycle inhibition as well as defects in mitochondrial integrity. Each of these activities appears to contribute to the overall activity of rocaglamide..
The observation that rocaglamide and temsirolimus inhibit global protein synthesis to similar extents does not eliminate the possibility that the compounds differentially affect the translation rates of a subset of proteins critical for the survival of leukemia cells. We looked at Myc levels following drug treatment and observed a striking difference in the level of reduction following treatment with rocaglamide as compared to temsirolimus. An inability to reduce Myc levels has been reported for other mTOR inhibitors in AML cells and may represent a limitation to this class of compounds for treating AML (17). The importance of Myc activity in the biogenesis and progression of cancer has long been appreciated however up until recently the ability to target Myc remained elusive (46–48). Silvestrol, JQ1 and other BET bromodomain inhibitors currently in development present viable therapeutic options for targeting this nexus.
In addition to their potency as single agents we also show that rocaglamide and silvestrol increase the cytotoxicity of several types of drugs towards AML cells, including daunorubicin and Ara-C, two chemotherapeutic drugs currently used to treat AML patients. The broad range of drug classes that rocaglamide and silvestrol are able to enhance is quite striking. It is possible that these compounds are blocking translation-based survival mechanisms induced by the second drug, however one would expect all translational inhibitors to synergize with these compounds, which is not the case (Figure 2C). We propose that the ability of rocaglamide to synergize is related, at least in part, to the affect it has on mitochondrial integrity/function (Figure 4B). The mitochondrial defect induced by rocaglamide treatment in essence “primes” cells for death when the second drug hits. Employing M9-ENL cells harboring shRNA constructs targeting p53 we were able link the mitochondrial defects with p53 activation (Figure 4D), indicating a critical role for this node in the mechanism of action of flavaglines.
The deregulation of translation is a critical feature of cancer initiation and progression and thus there is great interest in targeting protein synthesis therapeutically (49, 50). Data presented here and in other labs has shown that targeting the translational initiation complex with flavaglines can be preferentially toxic to transformed cells (51, 52). Silvestrol is the first direct inhibitor of translation initiation with clinical potential. It is now under development at the National Cancer Institute’s NExT Program, with current efforts dedicated to toxicology and scale-up using supercritical fluid extraction. Adequate raw materials for preclinical toxicology studies have already been collected by the source country and Ohio State University has received an exclusive license to develop silvestrol for the treatment of cancer. We believe that the therapeutic flexibility, in vivo efficacy, and ability to target LSCs, pose intriguing possibilities for these compounds to serve as adjuvants to current therapeutic regimes.
Supplementary Material
Acknowledgments
This work was supported by the Leukemia and Lymphoma Society (CTJ, 6230-11), Department of Defense (CTJ, W81XWH-07-1-0601), NY State Stem Cell Foundation (CTJ, C024964), National Institutes of Health (AF, RO1 GM079364), (ADK, PO1 CA125066) and a Wilmot-Roswell Park Collaborative Seed Grant. KPC was supported by American Cancer Society (PF-10-054-01-LIB) and Wilmot Cancer Center post-doctoral fellowships.
Footnotes
Authorship Contributions
K.P.C designed research, performed experiments, wrote the paper; C.T.J. designed research, wrote the paper; M.M, C.C., E.D.L., R.M.R., M.B.B., L.P, S.J., A.F., M.R.G., A.D.K., J.L.L., M.W.B. performed experiments and analyzed data.
Disclosure of Conflicts of Interest: The authors declare no competing financial interests.
Supplementary information is available at Leukemia’s website.
References
- 1.Jordan CT, Guzman ML, Noble M. Cancer stem cells. The New England journal of medicine. 2006;355(12):1253–61. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 2.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15(9):494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Becker MW, Jordan CT. Leukemia stem cells in 2010: Current understanding and future directions. Blood Rev. 2011 doi: 10.1016/j.blre.2010.11.001. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 4.Lucas DM, Still PC, Perez LB, Grever MR, Kinghorn AD. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Current drug targets. 2010;11(7):812–22. doi: 10.2174/138945010791320809. Epub 2010/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Salim AA, Swanson SM, Kinghorn AD. Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anti-cancer agents in medicinal chemistry. 2006;6(4):319–45. doi: 10.2174/187152006777698123. Epub 2006/07/18. [DOI] [PubMed] [Google Scholar]
- 6.Zhu JY, Lavrik IN, Mahlknecht U, Giaisi M, Proksch P, Krammer PH, et al. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. International journal of cancer Journal international du cancer. 2007;121(8):1839–46. doi: 10.1002/ijc.22883. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 7.Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA, et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood. 2009;113(19):4656–66. doi: 10.1182/blood-2008-09-175430. Epub 2009/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JY, Giaisi M, Kohler R, Muller WW, Muhleisen A, Proksch P, et al. Rocaglamide sensitizes leukemic T cells to activation-induced cell death by differential regulation of CD95L and c-FLIP expression. Cell death and differentiation. 2009;16(9):1289–99. doi: 10.1038/cdd.2009.42. Epub 2009/04/18. [DOI] [PubMed] [Google Scholar]
- 9.Bleumink M, Kohler R, Giaisi M, Proksch P, Krammer PH, Li-Weber M. Rocaglamide breaks TRAIL resistance in HTLV-1-associated adult T-cell leukemia/lymphoma by translational suppression of c-FLIP expression. Cell death and differentiation. 2011;18(2):362–70. doi: 10.1038/cdd.2010.99. Epub 2010/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alinari L, Prince CJ, Edwards RB, Towns WH, Mani R, Lehman A, et al. Dual targeting of the cyclin/Rb/E2F and mitochondrial pathways in mantle cell lymphoma with the translation inhibitor silvestrol. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(17):4600–11. doi: 10.1158/1078-0432.CCR-12-0839. Epub 2012/07/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang BY, Su BN, Chai H, Mi Q, Kardono LB, Afriastini JJ, et al. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. The Journal of organic chemistry. 2004;69(10):3350–8. doi: 10.1021/jo040120f. Epub 2004/05/11. [DOI] [PubMed] [Google Scholar]
- 12.Malina A, Cencic R, Pelletier J. Targeting translation dependence in cancer. Oncotarget. 2011;2(1–2):76–88. doi: 10.18632/oncotarget.218. Epub 2011/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cencic R, Carrier M, Galicia-Vazquez G, Bordeleau ME, Sukarieh R, Bourdeau A, et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PloS one. 2009;4(4):e5223. doi: 10.1371/journal.pone.0005223. Epub 2009/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers JM, Lindqvist LM, Webb A, Huang DC, Savage GP, Rizzacasa MA. Synthesis of biotinylated episilvestrol: highly selective targeting of the translation factors eIF4AI/II. Organic letters. 2013;15(6):1406–9. doi: 10.1021/ol400401d. Epub 2013/03/07. [DOI] [PubMed] [Google Scholar]
- 15.Sadlish H, Galicia-Vazquez G, Paris CG, Aust T, Bhullar B, Chang L, et al. Evidence for a Functionally Relevant Rocaglamide Binding Site on the eIF4A-RNA Complex. ACS chemical biology. 2013 doi: 10.1021/cb400158t. Epub 2013/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grzmil M, Hemmings BA. Translation regulation as a therapeutic target in cancer. Cancer Res. 2012;72(16):3891–900. doi: 10.1158/0008-5472.CAN-12-0026. Epub 2012/08/02. [DOI] [PubMed] [Google Scholar]
- 17.Tamburini J, Green AS, Bardet V, Chapuis N, Park S, Willems L, et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood. 2009;114(8):1618–27. doi: 10.1182/blood-2008-10-184515. Epub 2009/05/22. [DOI] [PubMed] [Google Scholar]
- 18.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005 doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagadinou ED, Ziros PG, Tsopra OA, Dimas K, Kokkinou D, Thanopoulou E, et al. c-Jun N-terminal kinase activation failure is a new mechanism of anthracycline resistance in acute myeloid leukemia. Leukemia. 2008;22(10):1899–908. doi: 10.1038/leu.2008.192. Epub 2008/07/25. [DOI] [PubMed] [Google Scholar]
- 20.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell stem cell. 2013;12(3):329–41. doi: 10.1016/j.stem.2012.12.013. Epub 2013/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashton JM, Balys M, Neering SJ, Hassane DC, Cowley G, Root DE, et al. Gene sets identified with oncogene cooperativity analysis regulate in vivo growth and survival of leukemia stem cells. Cell stem cell. 2012;11(3):359–72. doi: 10.1016/j.stem.2012.05.024. Epub 2012/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SM, Wendel HG, et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. The Journal of clinical investigation. 2008;118(7):2651–60. doi: 10.1172/JCI34753. Epub 2008/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindqvist LM, Vikstrom I, Chambers JM, McArthur K, Ann Anderson M, Henley KJ, et al. Translation inhibitors induce cell death by multiple mechanisms and Mcl-1 reduction is only a minor contributor. Cell death & disease. 2012;3:e409. doi: 10.1038/cddis.2012.149. Epub 2012/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S, Hwang BY, Su BN, Chai H, Mi Q, Kinghorn AD, et al. Silvestrol, a potential anticancer rocaglate derivative from Aglaia foveolata, induces apoptosis in LNCaP cells through the mitochondrial/apoptosome pathway without activation of executioner caspase-3 or -7. Anticancer research. 2007;27(4B):2175–83. Epub 2007/08/19. [PMC free article] [PubMed] [Google Scholar]
- 25.Lindqvist L, Pelletier J. Inhibitors of translation initiation as cancer therapeutics. Future medicinal chemistry. 2009;1(9):1709–22. doi: 10.4155/fmc.09.122. Epub 2009/12/01. [DOI] [PubMed] [Google Scholar]
- 26.Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM, et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood. 2005;105(6):2527–34. doi: 10.1182/blood-2004-06-2494. [DOI] [PubMed] [Google Scholar]
- 27.Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A, et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood. 2009;114(2):257–60. doi: 10.1182/blood-2009-02-205153. Epub 2009/05/13. [DOI] [PubMed] [Google Scholar]
- 28.Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(23):5314–22. doi: 10.1200/JCO.2005.66.130. Epub 2005/06/16. [DOI] [PubMed] [Google Scholar]
- 29.Staehler M, Haseke N, Khoder W, Stief CG. Profile of temsirolimus in the treatment of advanced renal cell carcinoma. OncoTargets and therapy. 2010;3:191–6. doi: 10.2147/ott.s7657. Epub 2010/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(52):18105–10. doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–81. doi: 10.1038/nature03579. Epub 2005/05/20. [DOI] [PubMed] [Google Scholar]
- 32.Chiosis G, Kang Y, Sun W. Discovery and development of purine-scaffold Hsp90 inhibitors. Expert opinion on drug discovery. 2008;3(1):99–114. doi: 10.1517/17460441.3.1.99. Epub 2008/01/01. [DOI] [PubMed] [Google Scholar]
- 33.Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. The Journal of biological chemistry. 2002;277(41):38954–64. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG, et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143(2):313–24. doi: 10.1016/j.cell.2010.09.010. Epub 2010/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CJ, Nasr Z, Premsrirut PK, Porco JA, Jr, Hippo Y, Lowe SW, et al. Targeting synthetic lethal interactions between Myc and the eIF4F complex impedes tumorigenesis. Cell reports. 2012;1(4):325–33. doi: 10.1016/j.celrep.2012.02.010. Epub 2012/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malona JA, Cariou K, Frontier AJ. Nazarov cyclization initiated by peracid oxidation: the total synthesis of (+/−)-rocaglamide. Journal of the American Chemical Society. 2009;131(22):7560–1. doi: 10.1021/ja9029736. Epub 2009/05/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerard B, Sangji S, O’Leary DJ, Porco JA., Jr Enantioselective photocycloaddition mediated by chiral Bronsted acids: asymmetric synthesis of the rocaglamides. Journal of the American Chemical Society. 2006;128(24):7754–5. doi: 10.1021/ja062621j. Epub 2006/06/15. [DOI] [PubMed] [Google Scholar]
- 38.Davey AE, Schaeffer MJ, Taylor RJK. Synthesis of the Novel Antileukemic Tetrahydrocyclopenta[b]benzofuran, Rocaglamide. Journal of the Chemical Society, Chemical Communications. 1991:1137–9. [Google Scholar]
- 39.Dobler MR, Bruce I, Cederbaum F, Cooke NG, Diorazio LJ, Hall RG, et al. Total synthesis of (+/−)-rocaglamide and some aryl analogues. Tetrahedron Letters. 2001;42(47):8281–4. [Google Scholar]
- 40.Hongsen L, Fu B, Wang MA, Li N, Liu WJ, Xie ZQ, et al. Total synthesis and biological activity of (+/−)-Rocaglamide and its 2,3-di-epi analogue. European Journal of Organic Chemistry. 2008:1753–8. [Google Scholar]
- 41.Trost BM, Greenspan PD, Yang BV, Saulnier MG. An Unusual Oxidative Cyclization - a Synthesis and Absolute Stereochemical Assignment of (−)-Rocaglamide. Journal of the American Chemical Society. 1990;112(24):9022–4. [Google Scholar]
- 42.Saradhi UV, Gupta SV, Chiu M, Wang J, Ling Y, Liu Z, et al. Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry. The AAPS journal. 2011;13(3):347–56. doi: 10.1208/s12248-011-9273-x. Epub 2011/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan L, Kardono LB, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM, et al. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale re-collection of Aglaia foveolata. Journal of natural products. 2010;73(11):1873–8. doi: 10.1021/np100503q. Epub 2010/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan CT. Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia. 2002;16(4):559–62. doi: 10.1038/sj.leu.2402446. Epub 2002/04/18. [DOI] [PubMed] [Google Scholar]
- 45.Dick JE. Acute myeloid leukemia stem cells. Annals of the New York Academy of Sciences. 2005;1044:1–5. doi: 10.1196/annals.1349.001. Epub 2005/06/17. [DOI] [PubMed] [Google Scholar]
- 46.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–17. doi: 10.1016/j.cell.2011.08.017. Epub 2011/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(40):16669–74. doi: 10.1073/pnas.1108190108. Epub 2011/09/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–8. doi: 10.1038/nature10334. Epub 2011/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10(4):254–66. doi: 10.1038/nrc2824. Epub 2010/03/25. [DOI] [PubMed] [Google Scholar]
- 50.Stumpf CR, Ruggero D. The cancerous translation apparatus. Curr Opin Genet Dev. 2011;21(4):474–83. doi: 10.1016/j.gde.2011.03.007. Epub 2011/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. The Journal of clinical investigation. 2007;117(9):2638–48. doi: 10.1172/JCI32044. Epub 2007/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128(2):257–67. doi: 10.1016/j.cell.2006.11.046. Epub 2007/01/27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.