Abstract
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are the most common cause of genital ulceration in humans worldwide. Typically, HSV-1 and 2 infections via mucosal route result in a lifelong latent infection after peripheral replication in mucosal tissues, thereby providing potential transmission to neighbor hosts in response to reactivation. To break the transmission cycle, immunoprophylactics and therapeutic strategies must be focused on prevention of infection or reduction of infectivity at mucosal sites. Currently, our understanding of the immune responses against mucosal infection of HSV remains intricate and involves a balance between innate signaling pathways and the adaptive immune responses. Numerous studies have demonstrated that HSV mucosal infection induces type I interferons (IFN) via recognition of Toll-like receptors (TLRs) and activates multiple immune cell populations, including NK cells, conventional dendritic cells (DCs), and plasmacytoid DCs. This innate immune response is required not only for the early control of viral replication at mucosal sites, but also for establishing adaptive immune responses against HSV antigens. Although the contribution of humoral immune response is controversial, CD4+ Th1 T cells producing IFN-γ are believed to play an important role in eradicating virus from the hosts. In addition, the recent experimental successes of immunoprophylactic and therapeutic compounds that enhance resistance and/or reduce viral burden at mucosal sites have accumulated. This review focuses on attempts to modulate innate and adaptive immunity against HSV mucosal infection for the development of prophylactic and therapeutic strategies. Notably, cells involved in innate immune regulations appear to shape adaptive immune responses. Thus, we summarized the current evidence of various immune mediators in response to mucosal HSV infection, focusing on the importance of innate immune responses.
Keywords: Herpes simplex virus, Mucosal infection, Innate immunity, Adaptive immunity, Toll-like receptors, Type I IFN receptors
INTRODUCTION
Herpes simplex viruses (HSV), the most prevalent and pestilent causes of human viral infections, belong to the genus simplexvirus of the family Herpesviridae including two members herpes simplex virus 1 and 2 (HSV-1 and HSV-2). Genome of HSV is linear double-stranded DNA being enclosed with enveloped icosahedral capsid. The envelope holds at least 10 different glycoproteins protruding from the outer side (gB, gC, gD, gE, gG, gH, gI, gK, gL, and gM), which have their primary mechanical functions in viral attachment and entry as well several immune regulatory effects. The immune response against HSV involves intricate and multifactorial; yet these viruses have various immune evasion and modulation mechanisms that are resulted in their evolutionary success. On that ground, the study of anti-HSV immune responses, including both innate and adaptive immune responses as well as the corresponding viral oppugn measures, is valuable to our understanding of HSV pathogenesis and anti-HSV immunity (1,2,3,4).
The innate antiviral response is account to play a vital role in determining the outcome of an HSV infection. The first line of defense against HSV1/2 infection is provided by innate humoral (complement, cytokines, chemokines) and cellular (interferon-producing cells, macrophages, neutrophils, NK cells and γδ T cell) responses. These orchestrate the lysis of virions and virus-infected cells and subsequently provide a link to effective adaptive immunity, too (3,4,5,6). The adaptive immune system, also called the acquired immune system, has been shown to play important roles in disease progression, latency and control of virus spread. Key components of the adaptive immune system are B-cells, T-cells, antibodies and the secondary lymphoid organs (7,8,9,10).
Developing novel strategies to eradicate HSV is a global public health priority. There are three ways to eradicate HSV infections in terms of anti-HSV drugs, microbicides and vaccines (Summarized in Table I). Prevention is better than cure as antiviral drugs do not destroy their target pathogen; instead they inhibit their development. There are three classes of drugs currently licensed for the treatment of HSV infection, all of which target viral DNA replication; these include guanosine analogues (acyclovir, famcilovir, and ganciclovir), acyclic nucleotide analogue, cidofovir and the pyrophosphate analogue, foscarnet. A microbicide is any biocidal compound or substance whose purpose is to reduce the infectivity of microbes, such as viruses or bacteria. A novel strategy for HSV prevention is the development of mucosally delivered microbicides. Historically, prevention acquired by vaccines is considered as the best way to control the HSV epidemic. The development of vaccines/biologicals that elicit sterilizing immunity to completely prevent the establishment of infection ultimately results in prevention and eradication of infection (11,12,13).
Table I.
Some experimental trails for prophylactic and therapeutic strategies focused on the enhancement of innate and adaptive immune response against HSV-1 and HSV-2
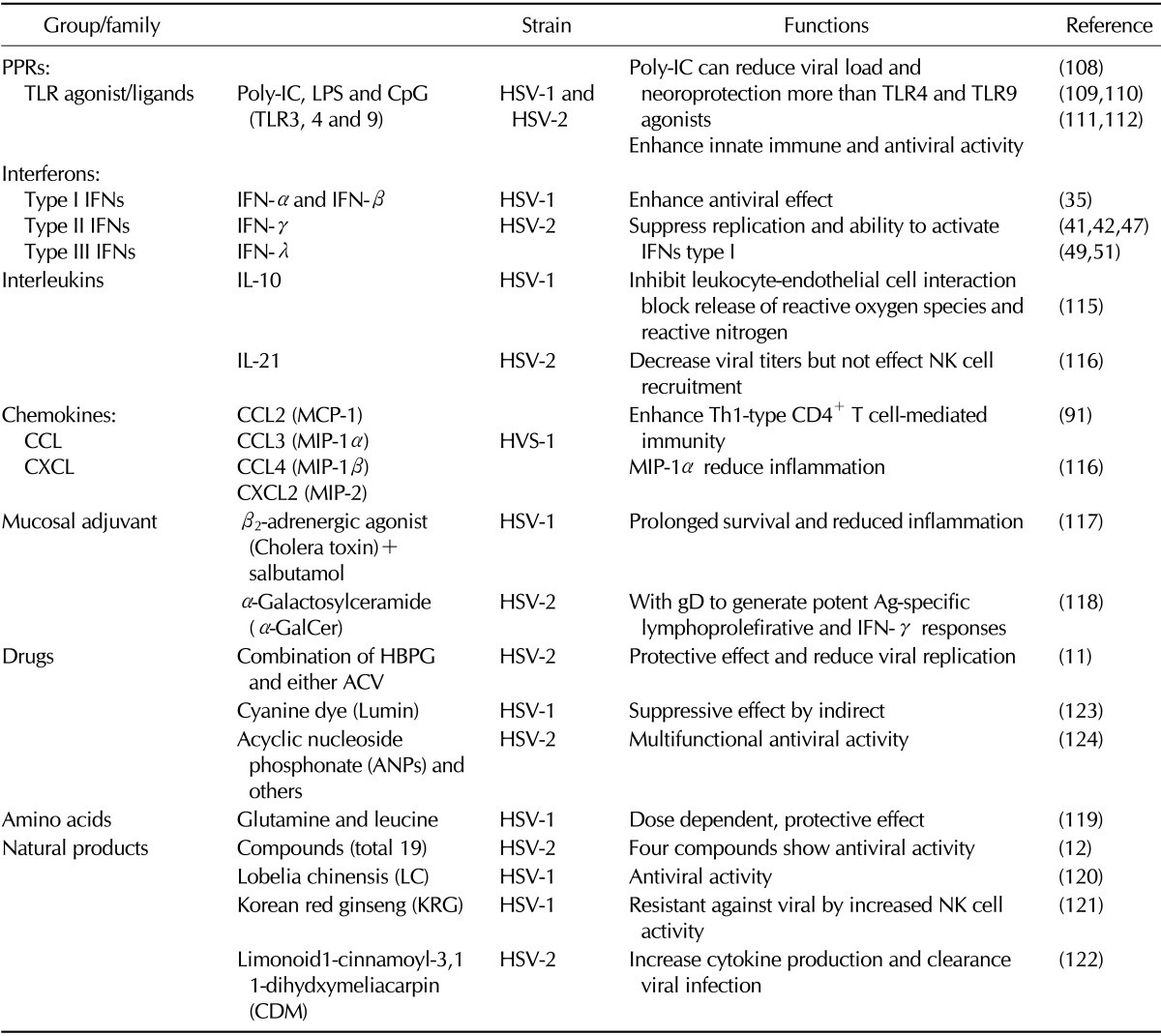
This review provides an update on researchers regarding the modulation on innate and adaptive immunity against HSV, which is focused on mucosal route of infection. In addition, current status of treatment and prophylactic measures for development against both HSV-1 and HSV-2 was discussed. Also, here focus on how immune system modulation help in developed novel strategies regarding drugs, microbicides and biologicals to eradicate HSV.
MODULATION OF INNATE IMMUNITY AGAINST MUCOSAL INFECTION OF HSV
The innate or non-specific immune system is one of the major subdivisions of immune system. The innate immune system is our first line of defense against invading organisms. The elements of the innate (non-specific) immune system include anatomical barriers, secretory molecules and cellular components.
Importance of TLR signaling against HSV mucosal infection
Toll-like receptors (TLRs) are important element from innate immunity. TLRs are membrane-bound pattern recognition receptor (PPR) proteins expressed by cells of the innate immune system to proclaim pathogen associated molecular patterns (PAMPs). TLRs are single transmembrane non-catalytic receptor protein which plays informational role in innate immunity network against microbial pathogens and successive induction of adaptive immunity. All TLRs possess amino-terminal leucine-rich repeats, which are responsible for the recognition of PAMPs, and also possess a carboxy-terminal Toll-interleukin-1 (IL-1) receptor (TIR) domain, which is required for initiating intracellular signaling (14,15). TLRs interact with their specific PAMP to induce NF-kB signaling and the MAP kinase pathway, and subsequently led to the secretion of proinflammatory cytokines and co-stimulatory molecules. Although engagement of each TLR activates a different specific molecular cascade, many induce the production of Th1-type cytokines, such as IL-12 and IFN-γ (16). These molecules act as activation signal to other cells of the immune system making TLRs as key link between innate immunity and adaptive immunity (17).
TLRs had been first discovered in Drosophila and subsequently have been found in many species. There are 10 human (TLRs 1 to 10) and 12 murine (TLR1 to 9 and TLR11 to 13) TLR family members. TLR3 and 7 to 9 recognize PAMPs in endosomes and all remaining TLRs in extracellular space (14). There are several TLRs monitors, especially TLR2, TLR3, and TLR9, involved in early recognition of HSV components (18). TLR2 recognizes an unidentified molecular structure on the virion. A number of studies revealed that TLR2 mediates the induction of inflammatory cytokines response to HSV-1, but that expression of TLR2 is not protective against lethal viral encephalitis on HSV-1 infection (19,20). In vitro study demonstrates the importance of TLR2 in microglial cell to induce oxidative stress through decreasing activation of p38 MAPK and p42/p44 ERK and neuronal damage in response to HSV-1 infection (21). TLR9 recognizes unmethylated CpG sequences in DNA molecules. One study result indicates that TLR9 mediates cellular response to unmethylated CpG dinucleotides in bacterial DNA and viral DNA to mount an innate immune response (22). Some studies showed that TLR9 plays an important role for dendritic cell (DC) response to HSV-1, but they did not find differences in viral replication or in susceptibility in TLR9- and MyD88-deficient mice infected with HSV-1 via the footpad or the cornea route (23,24,25). Herein, some studies have shown the secretion of type I IFN in response to HSV in vivo as well as in vitro mediated by TLR9/MyD88-dependent and independent pathway (23,25). However, others demonstrated that MyD88-deficient mice showed fully 100% lethal encephalitis when infected via mucosal route (i.e. intranasal and intravaginal) with HSV-1 and HSV-2, respectively (2,26).
TLR3 is efficient in the recognition of double stranded RNA set up during the virus replication. About TLR3 signaling, some study suggested that TLR3 provides early control of HSV-1 and 2 infections immediately after entry into the CNS by mediating type I IFN responses in central nervous system (CNS) (18,27,28).
The role of type I, II and III IFNs in mucosal infection of HSV
The interferon system is one of the major elements from the innate immune system, which is the first line of defense against various viral infections in mammals. This system is designed to block the spread of virus infection in the body, sometimes at the expense of accelerating the death of the infected cells. Interferons (INFs) are cytokines that cause cells to limit or prohibit viral replication, and they have a variety of functions in the innate immune response. They can control macrophage and NK cell activation, stimulate cytotoxic lymphocytes, induce cell surface costimulatory molecules, activate cytokine production, and stimulate local inflammation (3,4,29). IFNs were described and named in 1957 by Alick Isaacs and Jean Lindenmann (30). Seven IFNs have been described for humans out of ten distinct IFNs identified in mammals. They are typically divided among three IFN classes: type I IFN, type II IFN and type III IFN. All IFN classes are very important for fighting viral infections and tumors (31). The type I IFNs in humans are IFN-α, IFN-β and IFN-ω and bind to common receptor known as IFN-α receptor (IFNAR) that consists of IFNAR1 and IFNAR2 chains. In human type II IFNs is IFN-γ and signals intracellular transduction through IFN-γ receptor (IFNGR) complex that consists of IFNGR1 and IFNGR2 chains (32). Types I and III IFNs were produced by most cell types in particularly pDCs and cDCs (30,31,33,34) and INF-γ is produced by natural killer (NK) cells and CD4+ T cells, CD8+ T cells (3,4,6,7).
Many various studies are focused on the role of type I and II IFNs during the HSV-1 and HSV-2 infection, respectively. Initially, Gill et al. and others demonstrated that TLR-mediated protection is dependent on type I signaling (35,36). Importantly, recent data suggested that IRF-3 activation and subsequent IFN-α/β signaling are required for poly (I:C)-induced innate protection against intravaginal HSV-2 challenge. Collectively, local delivery of murine recombinant IFN-β alone was shown to protect C57BL/6 and IRF-3-/- mice against subsequent intravaginal HSV-2 challenge (35). Also, Conrady et al. clearly showed the importance of IFN production in innate immune response as well as evoking several chemokine's production necessitated to assist adaptive immune response in response to both HSV-1 and HSV-2 infection via ocular and intravaginal route (37,38,39). More recently, one study demonstrated that type I IFN induced the production of IL-15, which promotes NK cell survival and proliferation during the HSV-2 infection (40).
The antiviral effect of IFN-γ may be direct (intracellular, NO secretion) or indirect, involving activation of effector cells of the immune system. Several studies were focused on the type II IFN (IFN-γ). Initial some studies reported that IFN-γ is important for controlling in vivo-reactivated HSV-1 and thereby contributes to the maintenance of virological latency, meaning the absence of infectious HSV-1 in the ganglion as opposed to molecular latency, which is manifested as repression of viral gene expression at the cellular level (41,42). Additionally, one study found that both IFN-γ and T cell-mediated cytolytic mechanisms which are mediated by either perforin or Fas are required for complete clearance of HSV-2 from the genital epithelium (43). However, contrast one research team suggested that IFN-γ is not fully helpful for virus clearance but plays a key role in enhancing T cell immune response in HSV-1 reactivation (44).
Type III IFN, also known as interferon lambda (IFN-λ), is recently classified subfamily of interferon. IFN-λ1-3 or IL-28A/B and IL-29 are three structurally related members of this group. They signal through a receptor complex consisting CRF2-4 (IL-10Rβ) and CRF2-12 (IFNLR1) (33,34). A number of studies suggested the role of type III IFN during HSV infection. It has been shown to have antiviral activity in vitro as types III IFNs trigger type I IFN-like gene expression profile (45,46,47). Ank et al. shows that IFN-λ produced by DCs at mucosal sites elicited limited antiviral activity to HSV-2 infection with its stronger dependence on NF-κB through TLR9, compared to IFN-α (48,49). After that, Marie et al. reported that DCs are a key source of IFN-λ at epithelial surfaces in the vaginal tract and that expression of type III IFN show high level of dependent on the NF-kB pathway than type I IFNs (50). Also, Jieliang et al. showed that the replication of HSV-1 infection is suppressed in microglia and astrocytes by treatment of IFN-λ through TLR3 activation (51,52).
NK cells, macrophages, and dendritic cells
NK cells, monocytes, neutrophils, macrophages and DCs are innate immune cells. They play a crucial role during the early phase of a viral infection. NK cells are important cellular component of the innate immune response against tumor cells and virus-infected cells and to produce cytokines such as IFN-γ and granzyme B (3,4,5,53). Studies clearly demonstrated that cytokines, such as IL-2, IL-12, IL-15, and IL-18, involved in NK cell activation (40,54,55,56). For instance, Ashkar and Rosenthal clearly demonstrated that NK, NKT and IL-15-ablated mice were very high susceptible to low dose (100 PFU) challenge and showed 100% mortality, compared to B6 control mice during HSV-2 infection via vaginal route (55). Also, IL-18 plays a key role in the rapid activation of NK cells, thereby resulting in control of early HSV-1 replication in the lung in intranasal HSV-1 infection (56). Also others result suggested that type I IFNs signaling are key mediator for NK cell activation by IL-15-help during genital HSV-2 infection (40). Several studies have suggested that NK cell depletion via anti-NK1.1 or anti-asialo-GM1 antibodies increases the susceptibility of mice to ocular, genital, cutaneous and intravascular challenge with HSV (40,53,57,58). Importantly, recent one study also revealed that NK cells are stimulated by HSV Ag through TLR2 activation, and subsequently can contact with CD4+T cells in direct cell-to-cell manner (6).
DC is professional antigen-presenting cells and bridges between the innate and adaptive immune system (59). Although many studies focused on DC against HSV-1 and HSV-2, initially Zao et al. demonstrated that vaginal submucosal CD11b+ DCs, but not Langerhans cells (LCs), induce protective role of Th1 responses in the draining lymph nodes (60). Next others using mucosal viral infection, it is shown that viral recognition and induction of antiviral immunity by both the infected stromal cells and uninfected Ag-presenting DCs requires TLR signaling (61). HSV-1 entry glycoproteins act as a target for innate immune recognition that is going to activate DCs independenting on TLR2 signaling (62). Sadik et al. suggested that DCs are essential not only in the optimal activation of NK cells and CD4+ and CD8+ T cells but also need for resistance to HSV-1 infection (63). Following early HSV-1 corneal infection, resident DCs, but not polymorphnuclear neutrophils, had essential role for migration of NK cells and inflammatory monocytes into the central cornea (64). Interestingly herein, one research team suggested that PD-1: PD-L1 signaling produced by DC mediates T-cell exhaustion and latency during acute ocular HSV-1 infection (65). Additionally, several research teams tried to clearly describe the role of plasmacytoid DCs (pDC) during HSV mucosal infection. pDCs have been identified as a potent secretor of the type I IFNs in response to CpG as well as several viruses. Therefore, Lund et al. and other research teams demonstrated that pDC can recognize both HSV-1 and HSV-2 exclusively via TLR9 (66,67,68).
Macrophages are anti-herpetic actions during the first hours of the infection. Besides that, macrophages also play wide variety of immune functions which are including phagocytosis, tumor cytotoxicity and secretion of cytokine and antigen presentation (3). Mott et al. clearly suggested that STAT1 may have critically important role for allowing bone marrow-derived DCs and macrophages to blocking HSV-1 replication (69). Present study hypothesized that macrophage treated with IL-27 might induce the expression of novel micro RNAs that may be affecting the anti HSV function in IL-27 and M-CSF (I-Mac) (70). Also, recent study recognized that ablation of macrophages, but not DCs, NK cells, B cells, CD4+ T cells, or CD8+ T cells, induced CNS demyelination during the infected ocular with HSV-1. Finally, their results showed that macrophage IL-12p70 signaling could inhibit development autoaggressive CD4+ Treg cells, resulting in the prevention of HSV-1 induced CNS injury (71). More importantly, others demonstrate that macrophage-mediated immunity against infection occurs efficiently through iNOS in trigeminal ganglia and appears to be organized by TLR2 and TLR9, which contributes to HSV-1 infection control (72). Also, inflammatory monocytes were found to play main role in antiviral defense against HSV-2 genital mucosa infection through CCL2 molecule (73). Neutrophils also appear to have a key role in T and Bcell recruitment and control of viral replication during both HSV-1 and HSV-2 in corneal infection and vaginal mucosa (74,75). But constantly, recent one study suggested, using depletion model of Gr-1+ cells, that neutrophils may be do not play a major role in HSV-1 clearance in an intranasal model (76).
MODULATION OF ADAPTIVE IMMUNITY AGAINST MUCOSAL INFECTION OF HSV
The adaptive immune response against pathogen is primarily responsible for viral clearance and the generation of long-term memory. Current vaccine development strategies have focuses on understanding adaptive cellular-mediated immune responses. The effective adaptive responses predominantly require a balance between CD4+ and CD8+ T-cell activation. Acquired protective immunity can be mediated by both humoral and cell-mediated immune mechanisms.
Is humoral immunity indispensable for the clearance of HSV?
Many studies showed that B cells producing natural antibodies including IgA and IgG have been shown to have anti-viral and inflammatory effects and can control both HSV-1 and HSV-2 infection via vaginal route (7,77,78,79). Additionally, several research teams tried to determine the role of B cells using human and mouse model in ocular infection (80,81). Iijima et al. suggested that B cells together with DC contribute to restimulate memory CD4+T cells to secrete IFN-γ (82). In general, vaccine against HSV was focused on the production of neutralizing antibodies against HSV several glycoproteins (83,84,85). On the other hand, recent one researcher team reported that HSV-1 induced humoral response, especially induction of memory B cells, is dependent on complement system (9).
Although many studies have demonstrated the protective role of B cells against HSV infection, there is also contrasting reports that B cells are not required for HSV-1 and HSV-2 clearance (86,87,88). Therefore, it is still not clear about the specific contribution of humoral immunity to HSV control. While antibodies against HSV can mediate prophylatic protection in mice, B cells are not absolutely required for protection in the context of an acute infection; rather, they likely interact with other immune effectors such as T cells. Taken together, a better understanding of this area of HSV immunity is warranted.
T cell-mediated immunity to HSV mucosal infection
Prominent role of Th1-type CD4+ T cells to HSV mucosal infection
IFN-γ is signature cytokine produced by CD4+Th1 cells which are derived by IL-12 through T-bet transcription factor, and promote cell-mediated immunity against intracellular pathogens (89,90). Several studies showed that IFN-γ-secreting CD4+ T cells are crucial for development of protective immunity against to genital and ocular HSV-1 and HSV-2 infection, respectively (6,7,41,91). Also, Kuklin et al. clearly suggested that CD4+ T cells act as the principal mediators of vaginal immunity against HSV-1 (86). The local NK cells are likely to contribute to early control of HSV replication in infection and make some IFN-γ in the infected tissue (6). Importantly, Kumamoto et al. and other revealed that CD4+ T cells support and help cytotoxic T lymphocyte priming and generation (92).
Some research teams were interested and tried to determine the relative impact of both CD4+ T cells and CD8+ T cells during HSV infection. Herein, against ocular HVS-1, one study showed that both CD4-deficient and CD8+ deficient mice significantly developed corneal scarring compared than control C57BL/6 mice. Also, virus clearance from the eyes of the CD4-deficient mice was longer than CD8-deficient mice (93,94). Additionally, Koelle et al. suggested the clearance of HSV-2 from recurrent genital lesions correlates with the infiltration of both HSV-2-specific CD4+ and CD8+ cytotoxic T cells (95). More importantly, memory Th1 cells, but not CD8 T cells, are localized in the vaginal mucosa and are required for virus clearance after HSV-2 secondary challenge (41).
Paradoxical role of CD4+Foxp3+ Treg and IL-17+CD4+ Th17 cells to HSV mucosal infection
For determining the role of Treg in the control of HSV infections some publications focused on the suppressive role of Treg on CD4+ and CD8+ T cells (96,97). Notably, herein one study showed that adoptive transfer of in vitro-converted CD4+CD25+Foxp3+ Treg cells could decreased lesion severity in initial phase of three different models of herpetic stromal keratitis (HSK) (98). On the other hand, present study found in the female genital tract of mice that TCRγδ+CD4-CD8- T cells are the major population of IL-17A-secreting cells and TCRγδ+ T cells exhibit different expression profiles of cytokines and transcription factors compared to those from spleen (99).
Cytotoxic CD8+ T cells for viral clearance
Rapid induction of CD8+ cytotoxic T lymphocyte (CTL) responses is critical to combat acute infection with intracellular pathogens. Initial study suggested that cross presentation is main roles for induction of CTL to the Ag presentation capacity of CD8α+ DC (100). Also, one study tried to define about HSV epitopes that are recognized by CD8+ T cells in BL/6 mice, and found that nearly 50% of CD8+ T cells participate in recognizing HSV-1epitope (101). In summary, several studies data suggested that CD8+ T cells are require for complete clearance of HSV-1 and 2 (42,43,102,103), whereas CD8+ T cells are dispensable to control virus replication in the vaginal mucosa and other infected area (82,86). Additionally, in latent infection, augmenting the number of circulating HSV-specific CD8+ T cells is not sufficient to bolster the HSV-specific memory T cell population in sensory ganglia (104).
PROPHYLACTIC AND THERAPEUTIC STRATEGIES TO HSV MUCOSAL INFECTION
The development of effective prophylactic and therapeutic against herpes mucosal infection still has proven problematic. Difficulties are associated with the complexity of the virus life cycle (latency) and our relatively poor understanding of the mechanism of immune control of primary and recurrent disease (105). The morbidity and socioeconomic burden associated with genital herpes as well as the alarming relationship between genital herpes and the increased risk of acquiring a HIV infection emphasize the need for development of an effective vaccine and/or therapeutics. Primary HSV exposures first elicit innate immune responses and therefore, immunomodulatory approaches are being explored that boost innate immunity and engender increased resistance to HSV infection (91,106,107). Regarding the TLR expression profile in the female genital tract, immunoprophylactics and therapies targeting on TLR2, 3, 7 and 9 of the mucosa have been investigated for the utility to prevent or attenuate herpes virus disease. For prophylaxis, these synthetic agonists were designed to transiently activate the innate immune response to establish a more HSV-resistant environment, thereby increasing the threshold of infection or attenuating recurrent shedding events (108). Recent some works have also suggested that TLR3 agonist (poly: IC) induces more potent antiviral response than the agonists of TLR4 and TLR9 in genital HSV-2 and HSV-1 encephalitis, respectively (109,110). Additionally, some studies tried to determine role of CpG during mucosal HSV infection (111,112). For instance, one study has shown that CpG of HSV genome directly activates pDCs to induce the expression of IFN-α (113). Interestingly, herein one study suggested the mucosa delivery of CpG oligodeoxynucleotides expands functional DCs and macrophages in the vagina (114).
Cytokines may exert antiviral effects via either directly their receptors or indirectly immune modulatory effects. The efficacy of cytokine therapies have been demonstrated in several human and animal studies against HSV infection. It has been already suggested that IFN treatment could be effective to inhibit virus infection and spread in HSV-1 (42,51). Also, others results tried to determine role of some chemokines during HSV infection. Tumpey et al. indicate that IL-10-mediated suppression of MIP-1α synthesis was a significant factor reducing inflammation in the HSV-1 infected cornea (115).
Alteration of innate immunity to viral infectious using treatment with mIL-21 resulted in decreased vaginal viral titers, but did not affect NK cell recruitment and did not subsequently alter IFN-γ production or degranulation activity in vaginal NK cells in the absence of the IL- 21R after intra-vaginal HSV-2 infection (116). Others also examined effects of murine chemokine DNA which are including CC chemokines macrophage inflammatory protein 1β (MIP-1β) and monocyte chemotactic protein 1 (MCP-1), as genetic adjuvants given mucosally. Their results indicated that chemokines function may be displayed by affecting the interaction between innate and adaptive immunity during the HSV-1 infection (91). Moreover, co-administration of salbutamol with DNA vaccine could provide the effective and rapid responses to HSV-1 mucosal challenge, thereby conferring prolonged survival and reduced inflammation against viral infection (117). Another one team also tried to determine effect of α-GalCer, which is mucosal adjuvant and they found protective immunity by intravaginal immunization against vaginal HSV-2 challenge (118). Next, administration of amino acids such as Glutamine and Leucine, but not their combination, showed enhanced production of IFN-γ by NK and suggested that Th1 type CD4+ T cells may be critical to control the outcome of disease following HSV-1 mucosal infection (119).
Since natural products are considered powerful sources of novel drug discovery and development against pathogen. Therefore, many studies investigated the protective effect and immune modulatory of those natural products such as plant extracts against mucosal herpes infection (120,121). Importantly, initial results demonstrated that four compounds, especially eugenol, carrageenan lambda type IV, cineole and curcumin, provide the significant protection than others totally 19 such compounds both in-vitro and in-vivo (12). Interestingly, just few months ago, one research team tried to determine effect of one natural product which is isolated from leaf extract named by limonoid 1 - cinnamoyl - 3, 11 - dihydroxymeliacarpin (CDM) which can inhibit HSV-2 multiplication in epithelial cells and also increases cytokine production in macrophages, both important actions to the clearance of infecting virus in the mouse vagina (122).
There are a variety of nucleoside analog drugs used to treat herpes infections, though the person still harbors the virus for life. Some of these drugs are very specific and are only activated by specific viral enzymes, meaning that these drugs show few side effects such as acyclovir, famciclovir and valacyclovir, and these drugs act against the replicating virus and therefore they are ineffective against latent virus (11,12,122). One report suggested that thymidine kinase inhibitor (2-phenylamino-6-oxo-9-(4-hydroxybutyl)purine; HBPG) may have synergistic activity against HSV encephalitis (11), but others results showed, that cyanine dye (lumen) exhibits significantly suppressive effects on human amnionic FL cell line by suggesting the inhibition of virus invasion into the cell. Thus, that may potential new preventive anti-herpetic drug together with IFN (123).
The relationship between genital herpes and the increased risk of acquiring a HIV infection emphasize the need for development of an effective vaccine and/or therapeutics. Especially, important task is to develop a compound that is highly potent against both viruses to suppress their transmission and replication (91). Herein, Jan Balzarini et al. was focused on a distinct new subclass of acyclic nucleoside phosphonate (ANP), which are structurally and functionally different from previously used drugs such as tenofovir and adefovir that has significant relevance over the commonly used drugs. In their study they use representative drug PMEO-DAPym decisively suppresses HSV DNA polymerase and at the same time the drug also activate anti HIV CC chemokine. This combine results into dual anti-viral therapy (124).
Existing control measures against various infectious diseases includes the combined use of vaccines, antibiotics and chemicals. Vaccines can provide long term immunity and thereby confer specific protection against a particular pathogen following immunization. Studies on efficacy of various vaccines that prevent or reduce the primary and recurrent HSV infection have demonstrated the importance of cellular immunity for protection against the infection. Generally, against HSV vaccine strategies within the last few years have focused on the use of HSV viral epitopes such as gB, gC, gD and gG (77,78,83,84,85,125,126), some specific peptides (127,128), DNA-based vaccine or plasmid viral vectors (129) and attenuated/replication-detective versions of both HSV-1 and HSV-2 respectively (130,131). For example, in human, glycoprotein D-based subunit gD-2 vaccines can provide effective protections in HSV-1 but not HSV-2 seronegative women (132). Additionally, several studies suggested that subunit vaccines in combination with adjuvant appeared to be safe and effective against genital herpes in guinea pigs (133,134), but in clinical trials still failed to provide general protection (135). Importantly, present study demonstrated that vaccination with HSV-1 recombinant CJ9-gD elicits strong and protective immune responses against primary and recurrent HSV-2 genital disease and significantly reduces the extent of latent infection (136). Also, another team tested gB1 vaccine delivered by feline immunodeficiency virus (FIV) vector to elicit cross-neutralizing antibodies and cell-mediated responses that protected 100% and 75% animals from both HSV-1 and HSV-2 associated severe disease, respectively (137). Interestingly, herein one research team tried to determine role of DC for vaccine design and employed to assess their value in protection against live virus challenge in an experimental model using lethal and latent herpes simplex virus (HSV) infection in Balb/c mice (138).
CONCLUSIONS AND PERSPECTIVES
Generally, the immune response involves multiple mechanisms to effectively clear viral infection. The innate immune response is the host's first line of antiviral defense, and is mediated through the production of type I IFNs and TLR signaling. While NK cells, DC, especially pDC, as well as macrophages and other innate factors, are required to eradicate both HSV-1 and HSV-2, it is well established that adaptive immune responses, especially CD4+ Th1 cells except CD8+ T cells, are necessary to clear the infection. Although functional adaptive immunity is required to clear viral infection, these responses alone are not sufficient to protect against infection in the absence of innate immune mechanisms. Therefore, many studies investigate the protective effect and immune modulatory role of drugs, natural products such as plant extracts and prophylactics agents against mucosal herpes infection. Collectively, it is important to consider a functional innate defense and its role in assisting subsequent adaptive immune responses, when developing effective therapeutic and vaccine strategies against both HSV-1 and HSV-2.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MISP) (2013R1A4A1069486 and 2012R1A2A1A03670284).
Abbreviations
- CCL
chemokine (C-C motif) ligand
- CDM
limonoid 1-cinnamoyl-3,11-dihydromeliacarpin
- CNS
central nervous system
- CTL
cytotoxic T lymphocyte
- CXCL
chemokine (C-X-C motif) ligand
- DCs
dendritic cells
- gB
glycoprotein B
- HSV-1
herpes simplex virus type 1
- HSV-2
herpes simplex virus type 2
- HIV
human immunodeficiency virus
- LCs
langerhans cells
- LPS
lipopolysaccharide
- MAPKs
mitogen-activated protein kinases
- M-CSF
macrophage-colony stimulating factor
- MIP-1α
macrophage inflammatory protein-1 alpha (CCL3)
- MyD88
myeloid differentiation primary response gene 88
- Poly-IC
polyinocinic-polycytidylic acid
- PRRs
pattern recognition receptors
- PAMPs
pathogen associated molecular patterns
- TCR
T cell receptor
- TLRs
Toll-like receptors
Footnotes
The authors have no financial conflict of interest.
References
- 1.Mansur DS, Kroon EG, Nogueira ML, Arantes RM, Rodrigues SC, Akira S, Gazzinelli RT, Campos MA. Lethal encephalitis in myeloid differentiation factor 88-deficient mice infected with herpes simplex virus 1. Am J Pathol. 2005;166:1419–1426. doi: 10.1016/S0002-9440(10)62359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roizman B, Knipe DM. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edition. Lippincott Williams & Wilkins; 2001. pp. 2399–2459. [Google Scholar]
- 3.Ellermann-Eriksen S. Macrophages and cytokines in the early defence against herpes simplex virus. Virol J. 2005;2:59. doi: 10.1186/1743-422X-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan T, Barra NG, Lee AJ, Ashkar AA. Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa. J Reprod Immunol. 2011;88:210–218. doi: 10.1016/j.jri.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Osborne NR, Zeng W, Donaghy H, McKinnon K, Jackson DC, Cunningham AL. Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol. 2012;188:4158–4170. doi: 10.4049/jimmunol.1103450. [DOI] [PubMed] [Google Scholar]
- 7.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 8.Verschoor A, Brockman MA, Knipe DM, Carroll MC. Cutting edge: myeloid complement C3 enhances the humoral response to peripheral viral infection. J Immunol. 2001;167:2446–2451. doi: 10.4049/jimmunol.167.5.2446. [DOI] [PubMed] [Google Scholar]
- 9.Da Costa XJ, Brockman MA, Alicot E, Ma M, Fischer MB, Zhou X, Knipe DM, Carroll MC. Humoral response to herpes simplex virus is complement-dependent. Proc Natl Acad Sci U S A. 1999;96:12708–12712. doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwant-Mitchell A, Ashkar AA, Rosenthal KL. Mucosal innate and adaptive immune responses against herpes simplex virus type 2 in a humanized mouse model. J Virol. 2009;83:10664–10676. doi: 10.1128/JVI.02584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebhardt BM, Focher F, Eberle R, Manikowski A, Wright GE. Effect of combinations of antiviral drugs on herpes simplex encephalitis. Drug Des Devel Ther. 2009;3:289–294. doi: 10.2147/dddt.s8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourne KZ, Bourne N, Reising SF, Stanberry LR. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antiviral Res. 1999;42:219–226. doi: 10.1016/s0166-3542(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein DI, Stanberry LR. Herpes simplex virus vaccines. Vaccine. 1999;17:1681–1689. doi: 10.1016/s0264-410x(98)00434-4. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 17.Doyle SL, O'Neill LA. Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem Pharmacol. 2006;72:1102–1113. doi: 10.1016/j.bcp.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Menasria R, Boivin N, Lebel M, Piret J, Gosselin J, Boivin G. Both TRIF and IPS-1 adaptor proteins contribute to the cerebral innate immune response against herpes simplex virus 1 infection. J Virol. 2013;87:7301–7308. doi: 10.1128/JVI.00591-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JP, Bowen GN, Zhou S, Cerny A, Zacharia A, Knipe DM, Finberg RW, Kurt-Jones EA. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J Virol. 2012;86:2273–2281. doi: 10.1128/JVI.06010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-ediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 21.Schachtele SJ, Hu S, Little MR, Lokensgard JR. Herpes simplex virus induces neural oxidative damage via microglial cell Toll-like receptor-2. J Neuroinflammation. 2010;7:35. doi: 10.1186/1742-2094-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–459. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 23.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 25.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 26.Tengvall S, Harandi AM. Importance of myeloid differentiation factor 88 in innate and acquired immune protection against genital herpes infection in mice. J Reprod Immunol. 2008;78:49–57. doi: 10.1016/j.jri.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Perez de DR, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Valerie D, Jouanguy E, Chaussabel D, Geissmann F, Abel L, Casanova JL, Zhang SY. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, gnaes-Hansen F, Ulhoi BP, Holm TH, Mogensen TH, Owens T, Nyengaard JR, Thomsen AR, Paludan SR. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 30.Isaacs A, Lindenmann J, Valentine RC. Virus interference. II. Some properties of interferon. Proc R Soc Lond B Biol Sci. 1957;147:268–273. [PubMed] [Google Scholar]
- 31.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 32.Janeway CA, Travers P, Walport M, Shlomchik MJ. Immunobiology. The immune system in health and disease. 6th Edition. New York: Garland Science; 2005. pp. 461–516. [Google Scholar]
- 33.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 34.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 35.Gill N, Deacon PM, Lichty B, Mossman KL, Ashkar AA. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J Virol. 2006;80:9943–9950. doi: 10.1128/JVI.01036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol. 2007;81:13315–13324. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conrady CD, Halford WP, Carr DJ. Loss of the type I interferon pathway increases vulnerability of mice to genital herpes simplex virus 2 infection. J Virol. 2011;85:1625–1633. doi: 10.1128/JVI.01715-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conrady CD, Jones H, Zheng M, Carr DJ. A functional type I interferon pathway drives resistance to cornea herpes simplex virus type 1 infection by recruitment of leukocytes. J Biomed Res. 2011;25:111–119. doi: 10.1016/S1674-8301(11)60014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conrady CD, Zheng M, Mandal NA, van RN, Carr DJ. IFN-alpha-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013;6:45–55. doi: 10.1038/mi.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill N, Chenoweth MJ, Verdu EF, Ashkar AA. NK cells require type I IFN receptor for antiviral responses during genital HSV-2 infection. Cell Immunol. 2011;269:29–37. doi: 10.1016/j.cellimm.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 42.Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J Virol. 2001;75:11821–11826. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dobbs ME, Strasser JE, Chu CF, Chalk C, Milligan GN. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. J Virol. 2005;79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J Virol. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J Virol. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 48.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, gnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 50.Iversen MB, Ank N, Melchjorsen J, Paludan SR. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J Virol. 2010;84:4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J, Hu S, Zhou L, Ye L, Wang X, Ho J, Ho W. Interferon lambda inhibits herpes simplex virus type I infection of human astrocytes and neurons. Glia. 2011;59:58–67. doi: 10.1002/glia.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Ye L, Wang X, Hu S, Ho W. Induction of interferon-gamma contributes to Toll-like receptor 3-mediated herpes simplex virus type 1 inhibition in astrocytes. J Neurosci Res. 2012;90:399–406. doi: 10.1002/jnr.22758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 2000;45:33–45. doi: 10.1016/s0166-3542(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 54.Lehmann C, Zeis M, Uharek L. Activation of natural killer cells with interleukin 2 (IL-2) and IL-12 increases perforin binding and subsequent lysis of tumour cells. Br J Haematol. 2001;114:660–665. doi: 10.1046/j.1365-2141.2001.02995.x. [DOI] [PubMed] [Google Scholar]
- 55.Ashkar AA, Rosenthal KL. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J Virol. 2003;77:10168–10171. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reading PC, Whitney PG, Barr DP, Wojtasiak M, Mintern JD, Waithman J, Brooks AG. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J Immunol. 2007;179:3214–3221. doi: 10.4049/jimmunol.179.5.3214. [DOI] [PubMed] [Google Scholar]
- 57.Nandakumar S, Woolard SN, Yuan D, Rouse BT, Kumaraguru U. Natural killer cells as novel helpers in anti-herpes simplex virus immune response. J Virol. 2008;82:10820–10831. doi: 10.1128/JVI.00365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staats HF, Oakes JE, Lausch RN. Anti-glycoprotein D monoclonal antibody protects against herpes simplex virus type 1-induced diseases in mice functionally depleted of selected T-cell subsets or asialo GM1+ cells. J Virol. 1991;65:6008–6014. doi: 10.1128/jvi.65.11.6008-6014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J Virol. 2006;80:3985–3993. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci U S A. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reske A, Pollara G, Krummenacher C, Katz DR, Chain BM. Glycoprotein-dependent and TLR2-independent innate immune recognition of herpes simplex virus-1 by dendritic cells. J Immunol. 2008;180:7525–7536. doi: 10.4049/jimmunol.180.11.7525. [DOI] [PubMed] [Google Scholar]
- 63.Kassim SH, Rajasagi NK, Ritz BW, Pruett SB, Gardner EM, Chervenak R, Jennings SR. Dendritic cells are required for optimal activation of natural killer functions following primary infection with herpes simplex virus type 1. J Virol. 2009;83:3175–3186. doi: 10.1128/JVI.01907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frank GM, Buela KA, Maker DM, Harvey SA, Hendricks RL. Early responding dendritic cells direct the local NK response to control herpes simplex virus 1 infection within the cornea. J Immunol. 2012;188:1350–1359. doi: 10.4049/jimmunol.1101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryant-Hudson KM, Carr DJ. PD-L1-expressing dendritic cells contribute to viral resistance during acute HSV-1 infection. Clin Dev Immunol. 2012;2012:924619. doi: 10.1155/2012/924619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen H, Iwasaki A. A crucial role for plasmacytoid dendritic cells in antiviral protection by CpG ODN-based vaginal microbicide. J Clin Invest. 2006;116:2237–2243. doi: 10.1172/JCI28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 69.Mott KR, Underhill D, Wechsler SL, Town T, Ghiasi H. A role for the JAK-STAT1 pathway in blocking replication of HSV-1 in dendritic cells and macrophages. Virol J. 2009;6:56. doi: 10.1186/1743-422X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swaminathan S, Hu X, Zheng X, Kriga Y, Shetty J, Zhao Y, Stephens R, Tran B, Baseler MW, Yang J, Lempicki RA, Huang D, Lane HC, Imamichi T. Interleukin-27 treated human macrophages induce the expression of novel microRNAs which may mediate anti-viral properties. Biochem Biophys Res Commun. 2013;434:228–234. doi: 10.1016/j.bbrc.2013.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mott KR, Gate D, Zandian M, Allen SJ, Rajasagi NK, van RN, Chen S, Arditi M, Rouse BT, Flavell RA, Town T, Ghiasi H. Macrophage IL-12p70 signaling prevents HSV-1-induced CNS autoimmunity triggered by autoaggressive CD4+ Tregs. Invest Ophthalmol Vis Sci. 2011;52:2321–2333. doi: 10.1167/iovs.10-6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zolini GP, Lima GK, Lucinda N, Silva MA, Dias MF, Pessoa NL, Coura BP, Cartelle CT, Arantes RM, Kroon EG, Campos MA. Defense against HSV-1 in a murine model is mediated by iNOS and orchestrated by the activation of TLR2 and TLR9 in trigeminal ganglia. J Neuroinflammation. 2014;11:20. doi: 10.1186/1742-2094-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milligan GN. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J Virol. 1999;73:6380–6386. doi: 10.1128/jvi.73.8.6380-6386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molesworth-Kenyon SJ, Popham N, Milam A, Oakes JE, Lausch RN. Resident corneal cells communicate with neutrophils leading to the production of IP-10 during the primary inflammatory response to HSV-1 infection. Int J Inflam. 2012;2012:810359. doi: 10.1155/2012/810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol. 2010;91:2158–2166. doi: 10.1099/vir.0.021915-0. [DOI] [PubMed] [Google Scholar]
- 77.Parr MB, Parr EL. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology. 2000;101:126–131. doi: 10.1046/j.1365-2567.2000.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gorander S, Harandi AM, Lindqvist M, Bergstrom T, Liljeqvist JA. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J Virol. 2012;86:7544–7553. doi: 10.1128/JVI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu K, Jiang D, Zhang L, Yao Z, Chen Z, Yu S, Wang X. Identification of B- and T-cell epitopes from glycoprotein B of herpes simplex virus 2 and evaluation of their immunogenicity and protection efficacy. Vaccine. 2012;30:3034–3041. doi: 10.1016/j.vaccine.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Deshpande SP, Zheng M, Daheshia M, Rouse BT. Pathogenesis of herpes simplex virus-induced ocular immunoinflammatory lesions in B-cell-deficient mice. J Virol. 2000;74:3517–3524. doi: 10.1128/jvi.74.8.3517-3524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peek R, Verjans GM, Meek B. Herpes simplex virus infection of the human eye induces a compartmentalized virus-specific B cell response. J Infect Dis. 2002;186:1539–1546. doi: 10.1086/345555. [DOI] [PubMed] [Google Scholar]
- 82.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Campo J, Lindqvist M, Cuello M, Backstrom M, Cabrerra O, Persson J, Perez O, Harandi AM. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine. 2010;28:1193–1200. doi: 10.1016/j.vaccine.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 84.Cortesi R, Ravani L, Rinaldi F, Marconi P, Drechsler M, Manservigi M, Argnani R, Menegatti E, Esposito E, Manservigi R. Intranasal immunization in mice with non-ionic surfactants vesicles containing HSV immunogens: a preliminary study as possible vaccine against genital herpes. Int J Pharm. 2013;440:229–237. doi: 10.1016/j.ijpharm.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 85.Chiuppesi F, Vannucci L, De LA, Lai M, Matteoli B, Freer G, Manservigi R, Ceccherini-Nelli L, Maggi F, Bendinelli M, Pistello M. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J Virol. 2012;86:6563–6574. doi: 10.1128/JVI.00302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuklin NA, Daheshia M, Chun S, Rouse BT. Role of mucosal immunity in herpes simplex virus infection. J Immunol. 1998;160:5998–6003. [PubMed] [Google Scholar]
- 87.Dudley KL, Bourne N, Milligan GN. Immune protection against HSV-2 in B-cell-deficient mice. Virology. 2000;270:454–463. doi: 10.1006/viro.2000.0298. [DOI] [PubMed] [Google Scholar]
- 88.Morrison LA, Zhu L, hebeau LG. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J Virol. 2001;75:1195–1204. doi: 10.1128/JVI.75.3.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eo SK, Lee S, Chun S, Rouse BT. Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J Virol. 2001;75:569–578. doi: 10.1128/JVI.75.2.569-578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci U S A. 2011;108:8749–8754. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rajasagi NK. The role of CD4+ Helper T cells, IL-2 and IL-15 in the generation of an optimal CD8+ T cell response following infection with herpes simplex virus-1 (HSV-1) 2007. pp. 56–61. Louisiana State University Health Sciences Center-Shreveport, ProQuest, UMI Dissertations Publishing Number: 3311956. [Google Scholar]
- 94.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol. 2000;84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coleman CA, Muller-Trutwin MC, Apetrei C, Pandrea I. T regulatory cells: aid or hindrance in the clearance of disease? J Cell Mol Med. 2007;11:1291–1325. doi: 10.1111/j.1582-4934.2007.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dasgupta G, Chentoufi AA, You S, Falatoonzadeh P, Urbano LA, Akhtarmalik A, Nguyen K, Ablabutyan L, Nesburn AB, BenMohamed L. Engagement of TLR2 reverses the suppressor function of conjunctiva CD4+CD25+ regulatory T cells and promotes herpes simplex virus epitope-specific CD4+CD25- effector T cell responses. Invest Ophthalmol Vis Sci. 2011;52:3321–3333. doi: 10.1167/iovs.10-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol. 2008;82:6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim JO, Cha HR, Kim ED, Kweon MN. Pathological effect of IL-17A-producing TCRgammadelta(+) T cells in mouse genital mucosa against HSV-2 infection. Immunol Lett. 2012;147:34–40. doi: 10.1016/j.imlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Jirmo AC, Nagel CH, Bohnen C, Sodeik B, Behrens GM. Contribution of direct and cross-presentation to CTL immunity against herpes simplex virus 1. J Immunol. 2009;182:283–292. doi: 10.4049/jimmunol.182.1.283. [DOI] [PubMed] [Google Scholar]
- 101.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J Immunol. 2011;186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J Immunol. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 103.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Invest. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koelle DM, Corey L. Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med. 2008;59:381–395. doi: 10.1146/annurev.med.59.061606.095540. [DOI] [PubMed] [Google Scholar]
- 106.Wilson SS, Fakioglu E, Herold BC. Novel approaches in fighting herpes simplex virus infections. Expert Rev Anti Infect Ther. 2009;7:559–568. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petrera E, Coto CE. Effect of the potent antiviral 1-cinnamoyl-3,11-dihydroxymeliacarpin on cytokine production by murine macrophages stimulated with HSV-2. Phytother Res. 2014;28:104–109. doi: 10.1002/ptr.4974. [DOI] [PubMed] [Google Scholar]
- 108.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 109.Miller RL, Tomai MA, Harrison CJ, Bernstein DI. Immunomodulation as a treatment strategy for genital herpes: review of the evidence. Int Immunopharmacol. 2002;2:443–451. doi: 10.1016/s1567-5769(01)00184-9. [DOI] [PubMed] [Google Scholar]
- 110.Ashkar AA, Yao XD, Gill N, Sajic D, Patrick AJ, Rosenthal KL. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J Infect Dis. 2004;190:1841–1849. doi: 10.1086/425079. [DOI] [PubMed] [Google Scholar]
- 111.Boivin N, Sergerie Y, Rivest S, Boivin G. Effect of pretreatment with toll-like receptor agonists in a mouse model of herpes simplex virus type 1 encephalitis. J Infect Dis. 2008;198:664–672. doi: 10.1086/590671. [DOI] [PubMed] [Google Scholar]
- 112.Ashkar AA, Bauer S, Mitchell WJ, Vieira J, Rosenthal KL. Local delivery of CpG oligodeoxynucleotides induces rapid changes in the genital mucosa and inhibits replication, but not entry, of herpes simplex virus type 2. J Virol. 2003;77:8948–8956. doi: 10.1128/JVI.77.16.8948-8956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gill N, Davies EJ, Ashkar AA. The role of toll-like receptor ligands/agonists in protection against genital HSV-2 infection. Am J Reprod Immunol. 2008;59:35–43. doi: 10.1111/j.1600-0897.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 114.Sajic D, Patrick AJ, Rosenthal KL. Mucosal delivery of CpG oligodeoxynucleotides expands functional dendritic cells and macrophages in the vagina. Immunology. 2005;114:213–224. doi: 10.1111/j.1365-2567.2004.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tumpey TM, Cheng H, Yan XT, Oakes JE, Lausch RN. Chemokine synthesis in the HSV-1-infected cornea and its suppression by interleukin-10. J Leukoc Biol. 1998;63:486–492. doi: 10.1002/jlb.63.4.486. [DOI] [PubMed] [Google Scholar]
- 116.Kratholm SK, Iversen MB, Reinert L, Jensen SK, Hokland M, Andersen T, Rankin A, Young D, Frische S, Paludan SR, Holm CK. Interleukin-21 receptor signalling is important for innate immune protection against HSV-2 infections. PLoS One. 2013;8:e81790. doi: 10.1371/journal.pone.0081790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim SB, Han YW, Rahman MM, Kim SJ, Yoo DJ, Kang SH, Kim K, Eo SK. Modulation of protective immunity against herpes simplex virus via mucosal genetic co-transfer of DNA vaccine with beta2-adrenergic agonist. Exp Mol Med. 2009;41:812–823. doi: 10.3858/emm.2009.41.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lindqvist M, Persson J, Thorn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182:6435–6443. doi: 10.4049/jimmunol.0900136. [DOI] [PubMed] [Google Scholar]
- 119.Uyangaa E, Lee HK, Eo SK. Glutamine and leucine provide enhanced protective immunity against mucosal infection with herpes simplex virus type 1. Immune Netw. 2012;12:196–206. doi: 10.4110/in.2012.12.5.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuo YC, Lee YC, Leu YL, Tsai WJ, Chang SC. Efficacy of orally administered Lobelia chinensis extracts on herpes simplex virus type 1 infection in BALB/c mice. Antiviral Res. 2008;80:206–212. doi: 10.1016/j.antiviral.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 121.Cho A, Roh YS, Uyangaa E, Park S, Kim JW, Lim KH, Kwon J, Eo SK, Lim CW, Kim B. Protective effects of red ginseng extract against vaginal herpes simplex virus infection. J Ginseng Res. 2013;37:210–218. doi: 10.5142/jgr.2013.37.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petrera E, Coto CE. Effect of the potent antiviral 1-cinnamoyl-3,11-dihydroxymeliacarpin on cytokine production by murine macrophages stimulated with HSV-2. Phytother Res. 2014;28:104–109. doi: 10.1002/ptr.4974. [DOI] [PubMed] [Google Scholar]
- 123.Ushio C, Ariyasu H, Ariyasu T, Arai S, Ohta T, Fukuda S. Suppressive effects of a cyanine dye against herpes simplex virus (HSV)-1 infection. Biomed Res. 2009;30:365–368. doi: 10.2220/biomedres.30.365. [DOI] [PubMed] [Google Scholar]
- 124.Balzarini J, Andrei G, Balestra E, Huskens D, Vanpouille C, Introini A, Zicari S, Liekens S, Snoeck R, Holy A, Perno CF, Margolis L, Schols D. A multi-targeted drug candidate with dual anti-HIV and anti-HSV activity. PLoS Pathog. 2013;9:e1003456. doi: 10.1371/journal.ppat.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu K, He X, Yu F, Yuan X, Hu W, Liu C, Zhao F, Dou J. Immunization with DNA vaccine expressing herpes simplex virus type 1 gD and IL-21 protects against mouse herpes keratitis. Immunol Invest. 2011;40:265–278. doi: 10.3109/08820139.2010.534219. [DOI] [PubMed] [Google Scholar]
- 126.Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, Cai M, Brown M, Smith JF, Kowalski R, Swoyer R, Galli J, Copeland V, Rios S, Davidson RC, Salnikova M, Kingsley S, Bryan J, Casimiro DR, Friedman HM. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J Virol. 2014;88:2000–2010. doi: 10.1128/JVI.03163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brans R, Yao F. Immunization with a dominant-negative recombinant Herpes Simplex Virus (HSV) type 1 protects against HSV-2 genital disease in guinea pigs. BMC Microbiol. 2010;10:163. doi: 10.1186/1471-2180-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koelle DM, Magaret A, McClurkan CL, Remington ML, T Warren, Teofilovici F, Wald A. Phase I dose-escalation study of a monovalent heat shock protein 70-herpes simplex virus type 2 (HSV-2) peptide-based vaccine designed to prime or boost CD8 T-cell responses in HSV-naive and HSV-2-infected subjects. Clin Vaccine Immunol. 2008;15:773–782. doi: 10.1128/CVI.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009;2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jamali A, Roostaee MH, Soleimanjahi H, Ghaderi PF, Bamdad T. DNA vaccine-encoded glycoprotein B of HSV-1 fails to protect chronic morphine-treated mice against HSV-1 challenge. Comp Immunol Microbiol Infect Dis. 2007;30:71–80. doi: 10.1016/j.cimid.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 131.Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. J Clin Invest. 2011;121:4600–4609. doi: 10.1172/JCI57148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987;155:914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 134.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis. 2003;187:542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 135.Bourne N, Milligan GN, Stanberry LR, Stegall R, Pyles RB. Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis. 2005;192:2117–2123. doi: 10.1086/498247. [DOI] [PubMed] [Google Scholar]
- 136.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 137.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE Chiron HSV Vaccine Study Group. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA. 1999;282:331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 138.Ghasemi M, Erturk M, Buruk K, Sonmez M. Induction of potent protection against acute and latent herpes simplex virus infection in mice vaccinated with dendritic cells. Cytotherapy. 2013;15:352–361. doi: 10.1016/j.jcyt.2012.11.012. [DOI] [PubMed] [Google Scholar]


