Abstract
A severely atrophied maxilla presents serious limitations for conventional implant placement. This presents challenge to the surgeon for implant placement in harmony with the planned prosthesis. Survey of various literatures using internet sources, manual searches, and common textbooks on dental implants shows, that a thorough knowledge of conventional augmentation procedures such as bone augmentation techniques, guided bone regeneration, alveolar distraction, maxillary sinus elevation techniques with or without grafting and contemporary techniques of implant placement provide effective long-term solutions in the management of the atrophic maxilla.
Keywords: Bone graft, Sinus lift, Zygoma implant, Pterygoid implant, Tilted implant, Mini-implant
Introduction
Implant rehabilitation has shown higher success rates of 84–92 %, when sufficient bone is available in maxilla. But, atrophy in maxilla is not an uncommon finding and conventional implant placement gets complicated in such situations. In maxilla, centripetal pattern of alveolar resorption, pneumatization of maxillary sinuses, presence of nasal fossae and nasopalatal duct, poor bone quality complicate implant placement [1].
The purpose of this review article is to describe various techniques available for rehabilitation of atrophic maxilla with dental implants. Among the techniques proposed for such anatomical limitations, mention has been made on the following: bone augmentation using grafts, guided bone regeneration (GBR), alveolar distraction osteogenesis, elevation of the sinus floor, implant placement in alternative anatomical regions, tilted placement of implants and the use of mini-implants.
Search Strategy
A survey of the literatures, without limitation regarding the year of publication, was conducted using three internet sources such as national library of medicine computerized bibliographic databases (MEDLINE-PubMed), Google Scholar and The Science Direct, all with links to related articles. The search was complemented by manual searches of the reference lists of all full-text articles selected. In addition, some common textbooks on dental implants were scrutinized for additional documentation.
Discussion
The treatment options for implant rehabilitation of atrophic maxilla can be broadly classified into two categories:
1. Augmentation of the bony defect.
2. Modified implant designs for specific conditions.
Augmentation of Bony Defect
The goal of hard tissue augmentation is to provide a foundation for ideal implant placement and also to support soft tissue for optimal esthetics. Through the years, multiple procedures and augmentation materials have emerged to augment deficient bony ridges. The augmentation of bony defects is done either in conjunction with the implant placement or during a surgical intervention prior to implant placement (staged approach). The staged approach is primarily the treatment of choice in situations with large bone defects, where the primary stability of the implants in the prosthetically desired position is questionable. The bone augmentation procedures used in implant dentistry includes, graft reconstruction, GBR, maxillary sinus floor elevation, and alveolar distraction osteogenesis.
Graft Reconstruction
The reconstruction of the resorbed ridges using bone grafts is considered as the gold standard procedure to which all other rehabilitation techniques were compared. Various types of grafts were available for ridge augmentation and are described in Table 1 [2, 3].
Table 1.
Graft materials for bone augmentation
| Description | Types | Advantage | Disadvantage | |
|---|---|---|---|---|
| Autografts (autogenous grafts) | Bone grafts transferred from one site to another site within the same individual | Block Grafts (inlays, onlays, veneers, or saddle), Particulate Grafts and Membranes. | No immunogenic graft rejection | Additional surgical procedure. |
| Treatment delay | ||||
| Provide the only source of transfer of osteocompetent cells. | ||||
| It can be cortical or cancellous or a combination of both | ||||
| They are obtained from the mandible, maxilla, tibia, illac crest, and cranium. | Morbidity at donor sites | |||
| Allografts (allogenic, homologous, homografts) | Bone grafts taken from different individual of the same species. | Fresh or fresh frozen bone | Closely matches the recipient in constitutional elements and architecture | Antigenic |
| Demineralized freeze-dried bone allografts (DFDBA) | Potential for transmission of disease. | |||
| Mineralized freeze-dried bone allografts (FDBA) | ||||
| Predominately space-occupying osteo-conductive lattices or frameworks. | ||||
| Osteo-inductive capability is minimal because of the low concentration of bone growth proteins due to the rigorous processes involved in the removal of potential antigenicity and pathogenicity. | ||||
| Xenografts (heterografts, xenogenic grafts) | Materials taken from different species. | Bovine bone, coral derivatives | Compared to allografts, xenografts show less resorption of graft substrate and form less new bone during the first few months. | Though negligible, antigenicity and infectious disease transmission are present. |
| Reduced operative time No morbidity at the donor site. |
Compared with allografts, xenografts form less new bone during the first few months | |||
| Alloplasts (alloplastic grafts, synthetic grafts) | Derived from inert synthetic materials | Hydroxyapatite (HA), cal-cium phosphate, β-tricalcium phosphate Calcium sulfate (gypsum), Bioactive glasses, polymethylmethacrylate | No cellular or protein material within these grafts | Increased resorption time and decreased new bone formation when compared with allografts or xenografts |
| Growth factors | Produced using recombinant | Platelet-rich plasma (PRP), Platelet-derived growth factors (PDGF), Transforming growth factor (TGF-β), Bone morphogenetic protein (BMP) | Can be added to all the above graft materials. | Localized swelling and increased costs as compared with other bone grafting alternatives. |
| DNA technology | ||||
| No risk of disease transmission. | ||||
| Reduce the healing time and enhance bone formation. |
The physiology of bone graft healing is analogous to primary/secondary healing. Principally grafts heal through a combination of 3 processes: osteogenesis, osteoinduction, and osteoconduction. Osteogenesis is the formation of new bone from osteocompetent cells, and is the only process where the graft itself can forms new bone; Osteoinduction induces bone formation from the differentiation and stimulation of mesenchymal cells by the bone-inductive proteins. Osteoconduction is the formation of bone along a scaffold from osteocompetent cells of the recipient site [3]. Table 2 shows the healing capability of each type of graft materials.
Table 2.
Healing capability Of each type Of graft materials
| Osteoconductive | Osteoinductive | Osteogenesis | |
|---|---|---|---|
| Alloplast | + | − | − |
| Xenograft | + | − | − |
| Allograft | + | ± | − |
| Autograft | + | + | + |
Grafting Protocol
According to Misch [4], the successful incorporation of bone grafts relies on following factors: surgical asepsis, soft-tissue coverage, graft space maintenance, graft immobilization, regional acceleratory phenomenon, host bone blood vessel and optimization of growth factors.
Surgical asepsis refers to the absence of acute infection. But, in general, the oral cavity is considered as a contaminated environment, and so, sterile positioning of any graft is practically impossible. On the other hand, grafts dissolve in pH of 5.5 or less. Infection within bone often results in a pH of 2 and increases the risk of insufficient bone formation.
Tension-free soft-tissue closure maintains the graft by encouraging osteo-competent cell proliferation and healing by primary intention. So, proper technique for grafts includes flap design with adequate releasing incisions, and scoring of the periosteum. Silk or Vicryl sutures provide better strength and adaptability than the chromic suture.
Without stability, graft may be jeopardized resulting in fibrous encapsulation and nonunion to the host bone. Excessive movement disturbs the blood supply and can create a sequestrum of the graft. So, it is important to ensure that no contact occurs between any existing dental prosthesis and the soft tissue overlying the membrane or graft.
Misch [4] elaborates on the process of the regional acceleratory phenomenon. According to him the tissue heals faster in response to noxious stimuli than during the normal regeneration process. To initiate this phenomenon, he recommends drilling holes into the host cortical bone at low speeds under copious irrigation. This aids the transfer of osteo-competent cells. Also, placing a resorbable/non-resorbable membrane promotes the bone regeneration by delaying the invasion from surface fibroblasts that may inhibit osteogenesis. Also, providing local growth factors can enhance formation and mineralization of bone.
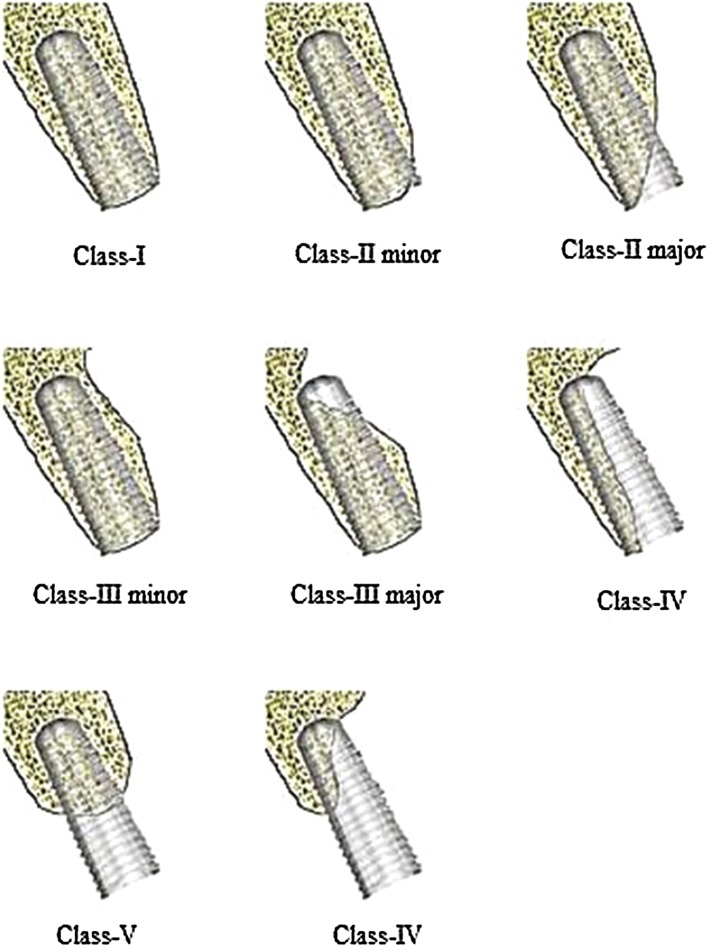
Table 3 describes graft selection based on UCLA classification [5] to deal with specific deficiencies (Fig. 1), either at the time of implant placement or as a separate procedure before implant placement.
Table 3.
Graft selection based on UCLA classification of ridge defects
| Description | Types | Advantage | Disadvantage | |
|---|---|---|---|---|
| Autografts (autogenous grafts) | Bone grafts transferred from one site to another site within the same individual | Block Grafts (inlays, onlays, veneers, or saddle), Particulate Grafts and Membranes. | No immunogenic graft rejection | Additional surgical procedure. |
| Provide the only source of transfer of osteocompetent cells. | ||||
| Treatment delay | ||||
| Morbidity at donor sites. | ||||
| It can be cortical or cancellous or a combination of both | ||||
| They are obtained from the mandible, maxilla, tibia, illac crest, and cranium. | ||||
| Allografts (allogenic, homologous, homografts) | Bone grafts taken from different individual of the same species. Predominately space-occupying osteo-conductive lattices or frameworks |
Fresh or fresh frozen bone | Closely matches the recipient in constitutional elements and architecture | Antigenic |
| Demineralized freeze-dried bone allografts (DFDBA) | ||||
| Mineralized freeze-dried bone allografts (FDBA) | ||||
| Osteo-inductive capability is minimal because of the low concentration of bone growth proteins due to the rigorous processes involved in the removal of potential antigenicity and pathogenicity. | Potential for transmission of disease. | |||
| Xenografts (heterografts, xenogenic grafts) | Materials taken from different species. | Bovine bone, coral derivatives | Compared to allografts, xenografts show less resorption of graft substrate and form less new bone during the first few months. | Though negligible, antigenicity and infectious disease transmission are present. |
| Reduced operative time | Compared with allografts, xenografts form less new bone during the first few months | |||
| No morbidity at the donor site. | ||||
| Alloplasts (alloplastic grafts, synthetic grafts) | Derived from inert synthetic materials | Hydroxyapatite (HA), cal-cium phosphate, β-tricalcium phosphate Calcium sulfate (gypsum), Bioactive glasses, polymethylmethacrylate | No cellular or protein material within these grafts | Increased resorption time and decreased new bone formation when compared with allografts or xenografts |
| Growth factors | Produced using recombinant | Platelet-rich plasma (PRP), Platelet-derived growth factors (PDGF), Transforming growth factor (TGF-β), Bone morphogenetic protein (BMP) | Can be added to all the above graft materials. | Localized swelling and increased costs as compared with other bone grafting alternatives. |
| DNA technology | ||||
| No risk of disease transmission. | ||||
| Reduce the healing time and enhance bone formation. |
Fig. 1.
UCLA classification for ridge deficiency
Through review of literatures [6], it is evident that survival rates of implants placed in reconstructed jaws are, lower than implants placed in native bone. The overall survival rate of implants in reconstructed maxillae after follow-up periods of 6–240 months was 79.5 %. Among the graft materials, onlay grafts (with or without associated sinus grafts) are one of the few options that permit the recreation of a more favorable environment for implant placement.
Regarding the timing of implant placement, whether to place it in conjunction with graft (immediate placement) or after consolidation of grafts (staged approach), the controversy still exists. The mean survival rate of implants placed in conjunction with bone graft placement was 81.8 % and with a staged approach was 89.9 % [6].
Advocates of immediate placement states that, resorption of an onlay graft are not a linear process and is most pronounced immediately after transplantation. Also, shortening of waiting time before rehabilitation potentially reduces the risk of bone resorption. On the other hand, those who advocate staged approach states that, immediate placement will increase the risk of wound dehiscence, infection/necrosis of the bone graft, leading to partial or total resorption of graft; also they states that during immediate placement, implants are placed in avascular bone, increasing the risk of non-integration. On the other hand, during staged approach implants are placed in a revascularized (albeit partly) graft permitting better osseo integration and better stability of implants [6].
Regarding the donor site, majority of implant failures occurred in patients reconstructed with iliac grafts (17.5 %) followed by calvarial grafts (6 %) and intraoral grafts (5.5 %). When considering the implant surface, machined-surface showed a lower survival rate (81.6 %) than rough-surfaced implants (94.2 %) [6].
Regarding the vertical bone resorption of grafts, resorption is greater in the first year following the reconstruction and after loading of implants, with a significant reduction in the consecutive years. Cortical thickness and density of donor bone influences the resorption pattern. To overcome these, oversized grafts should be harvested to maintain enough graft volume. In case of autogenous bone grafts, use of corticocancellous bone blocks is highly recommended. Cancellous bone, if used alone or particulated bones, if not used with barrier membranes, provide insufficient rigidity to withstand overlying soft tissue tension or the compression by provisional removable dentures, leading to complete resorption [6].
Guided Bone Regeneration (GBR)
Glossary of oral and maxillofacial implants (GOMI) [7] defines GBR for alveolar ridge augmentation as “principle of GBR using barrier membranes, either resorbable, to exclude certain cell types such as rapidly proliferating epithelium and connective tissue, thus promoting the growth of slower-growing cells capable of forming bone. GBR is often combined with bone grafting procedures”.
The first commercial membrane used for GBR was made from polytetrafluoroethylene (PTFE) and is considered as the gold standard for GBR treatments. Since conventional ePTFE membranes do not maintain adequate space unless supported by graft materials, PTFE was reinforced with fluorinated ethylene propylene (ePTFE) for rigidity and by titanium in situations like large defects or in supracrestal areas to increase the stability of the membrane. But, ePTFE membranes have high surface roughness, facilitating the bacterial adhesion. So, primary closure over the membrane is mandatory to avoid exposure. In addition, the removal of ePTFE membranes requires a second surgical procedure. To avoid these disadvantages, a high-density polytetrafluoroethylene (dPTFE), which does not require primary closure, was introduced by Barry Bartee. In addition, the comparatively smooth surface of dPTFE membranes facilitates the removal of the membrane without any additional surgical procedure [8].
The requirement of second surgical procedure for the removal of PTFE membranes led to the introduction of bio-resorbable membranes. The advantages of bio-resorbable membranes over non-resorbable membranes are, improved soft tissue healing, incorporation of the membranes by the host tissues (depending on material properties), and quick resorption in case of exposure, eliminating bacterial contamination. The resorbable membranes can be categorized into two groups, they are, aliphatic polyesters (polyglycolide and/or polylactide or copolymers) and collagen. At present, a wide range of resorbable membrane materials are available including collagen, freeze-dried fascia lata, freeze-dried dura mater allografts, polyglactin-910, polylactic acid, polyglycolic acid, polyorthoester, polyurethane, polyhydroxybutyrate etc.
However, the relative amount of bone formation using resorbable membranes was less favorable than ePTFE. Bio-resorbable membranes are not capable of maintaining adequate space unless the defect morphology is minimal and favorable, because they lose their mechanical strength soon after implantation into the tissues and need to be supported. Even though, bioresorbable membranes provide more bone regeneration, ePTFE membranes should be the material of choice for GBR, provided if soft tissue dehiscence are avoided.
In principle, bone regeneration using GBR is performed either in conjunction with implant placement (combined approach) or prior to implant placement (staged approach). The staged approach is the treatment of choice in situations with large bone defects, because the primary stability of implants in a prosthetically desired position is not possible.
Presently available data shows that GBR is a predictable and successful option for horizontal bone defects under standard conditions. Studies reported with 5-year data provides survival rates of 79.4 % for 37 implants with dehiscence/fenestration defects treated with ePTFE membranes [8].
In case of vertical defects, Simion et al. reported that vertical bone regeneration was possible to an extent of 4 mm in height with 42 % of implant-bone contact. In another study, twelve months following membrane placement, an average gain of 5 mm of vertical bone height was measured, reaching up to a maximum of 7 mm. Recent studies show that 3–20 mm of vertical bone gain is possible by using autogenous bone grafts or bone substitute materials in conjugation with titanium reinforced ePTFE membranes [8].
Maxillary Sinus Floor Elevation
Maxillary Sinus Floor Elevation procedures are indicated during free end situations in maxilla, where insufficient bone height is available for implant placement. Although Tatum was first credited with augmentation of the maxillary sinus for implant placement, Boyne’s landmark paper described the sinus augmentation using autogenous bone marrow graft with long-term follow-up [9]. From those initial investigations, many techniques have be-come available to the implant surgeon.
Surgical Techniques
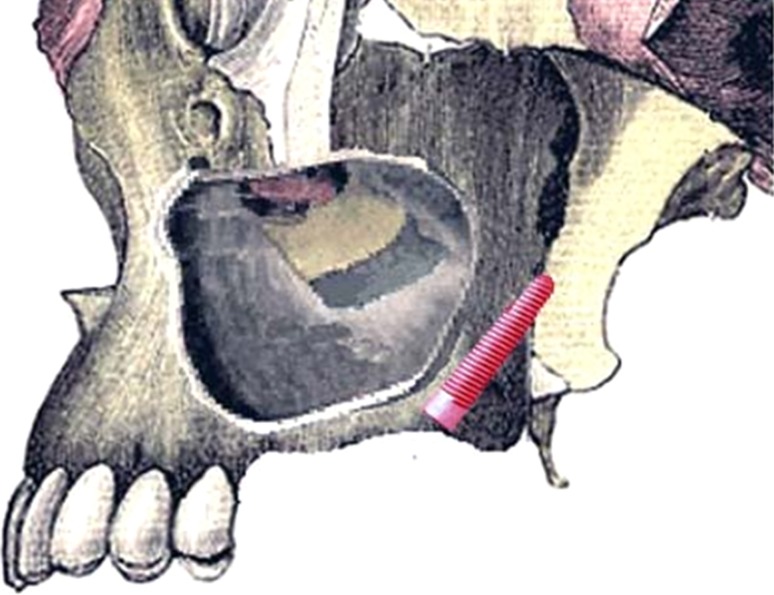
There are currently two techniques widely used for sinus augmentation: lateral window technique and sinus intrusion osteotomy technique [10]. These two methods are considered as the most stable techniques for vertical augmentation of posterior maxilla (Fig. 2).
Fig. 2.
Sinus lift procedure: lateral window (left side) and sinus intrusion osteotomy (right side)
Lateral Window (or) Direct Technique [10]
This was first demonstrated by Tatum using a modified Caldwell-Luc approach. It requires 4 linear osteotomies (2 horizontal and 2 vertical) to form a bony window without tearing the Schneiderian membrane. The inferior horizontal osteotomy runs from the area of first or second molar; superior horizontal osteotomy is performed at the level of the planned augmentation height. The vertical osteotomies are made parallel to the lateral nasal wall and the anterior border of the maxillary tuberosity (or the maxillary buttress) respectively. Then, the schneiderian membrane is exposed and the bone that is adherent is either removed or rotated in medially. The Schneiderian membrane is then elevated to a level higher than the superior osteotomy, using broad-based freers or curettes. This prevents excessive pressure on the bone graft material. Once the membrane is elevated, the graft is placed in the cavity loosely and should not be over-packed, in an anterior-inferior direction. Implants can be placed 6 months after the sinus lift procedure is performed. If there is adequate alveolar bone to stabilize the implants, the implant sites are prepared and the implants are placed before the bone graft, with the bone graft material being packed around the implants.
Sinus Intrusion Osteotomy/Indirect Technique [10]
The technique is indicated when minimal bone height is needed and there is sufficient bone for stabilization of an implant. This technique was developed in 1994 by Summers. To perform this technique, implant drills are used initially to create implant bed, leaving 1 mm of bone between the site and the sinus membrane. After preparing the site with the implant drills, sequential osteotomes with progressively increasing diameter are used to the depth of desired implant length; this compacts bone lateral and apical, and elevates the sinus membrane. Once at the desired length and diameter, bone graft material is placed in the apical portion of the prepared site. Finally implant is placed in the implant bed.
Other Techniques
Ferdinando Cosci [11] has described the one-stage crestal approach technique. The cortical bone of the sinus floor was perforated (not fractured) by the use of lifting drills with a small cutting angle of 30 degrees and a built-in water flow system. The set of drills included 8 pieces with the same diameter (3.10 mm) and sequentially increasing lengths (5–12 mm). Using this specific sequence of drills, the clinician slowly approached the Schneiderian membrane. The shape of the drill tip prevented perforation of the membrane. This system allowed the sinus floor to be elevated in a less traumatic approach with simultaneous bone grafting and implant placement.
Hydraulic Pressure Technique by Emmanouil [12] follows the Summers method to reach the sinus floor by using osteotomes in a specific sequence and fractures it. Then normal sa-line solution is injected under hydraulic pressure beneath the schneiderian membrane, using a suitably fitted syringe. This creates simultaneous detachment and elevation of the membrane.
Endo-Scopically Controlled Technique by Engelke et al. [13] involves transalveolar mobilization of the sinus membrane controlled by sinuscopy. This technique is indicated for moderately reduced alveolar sites. Later they modified the technique as, Subantroscopic Laterobasal Sinus Floor Augmentation (SALSA) which allowed augmentation of multiple maxillary sites via one small laterobasal trephination. Through this approach, a “tenting” of the complete sinus membrane from the premolar to the second molar site could be performed, thus allowing for large augmentations in case of primary and secondary implantation.
Francoise Tilotta [14] described a minimally invasive and safe technique to elevate sinus membrane using trephines and the osteotomes with stops. The guard prevents the instruments from invading the sinus. The repeated impaction movement, with or without grafting material, causes a greenstick fracture of the sinus floor, resulting in membrane elevation. The implant can then be placed.
Antral Membrane Balloon Elevation (AMBE) Technique by Muna et al. [15] is particularly useful in areas that are difficult to reach and is beneficial when teeth are adjacent to the edentulous area. The technique is accomplished with a minor incision, slight mucoperiosteal flap reflection, and a small window. Then the membrane is elevated to the medial wall of the sinus cavity avoiding sharp dissection around the roots of adjacent teeth. At this juncture, a balloon (Osseous Technologies of America, Huntington Beach, Calif) made of latex material is placed against the sinus floor midway between the lateral and the medial walls. The balloon is gently inflated with 2–4 mL of sterile saline. As it expands, the membrane is elevated. This technique offers optimal assurance that the fragile epithelium will be subjected to minimal trauma.
The Dentium Advanced Sinus Kit (DASK) introduced by Dentium, is the first and only sinus kit that can be used for both crestal and lateral approach. The kit includes six drills, four screw-on stoppers, four sinus elevation instruments, and five osteotome inserts. The drills are compatible to standard implant hand-pieces with diamond impregnated cutting edge and an optimal irrigation function. The diamond-coated burs were designed to prevent sinus perforation, while the internal irrigation holes placed inside the drill as “T” type, provides a pleasant cooling effect and adds hydraulic pressure to lifts the sinus membrane gently during the procedure.
Among the six drills provided in the kit, 3 drills (DASK #1, 2 and 3) were used for crestal approach procedure while the remaining 3 drills (DASK #4, 5 and 6) were used for lateral approach. The crestal approach was performed through grind-out technique with or without osteotome and the lateral approach was performed either by grind-out technique using DASK4, 5 or by wall-off technique using DASK 6 drill.
Grind-out technique—For crestal approach, the procedure site is prepared with twist drills in sequence up to 1 mm short of the sinus floor followed by drills #1/# 2 or, partial preparations with DASK Drill #1/#2 and up-fracture with osteotomes. Whereas for lateral approach, the drills #4 and #5 held at an angle of 45° against bone wall, were used for initial preparation with a pivoting action. Then the sinus floor is carefully approached by turning around a dome-shape sinus curette gently to lift the sinus membrane under light apical pressure. Finally drill #3 is used for broader detachment from the sinus floor which is facilitated with hydraulic pressure provided by the ‘T’ type irrigation system to make room for graft material.
Wall-off technique—In this technique, drill #6 is used with pivoting action to make a lateral window, until the sinus membrane shows through on the other side. DASK Drill #6 cut and detaches a bony island like a trephine bur from the lateral wall. Then the sinus floor is carefully approached using dome-shape sinus curette initially and drill #3 is used for broader detachment, to make room for graft material. The major drawback with this technique is uncontrolled over-drilling leading to sinus perforation and external irrigation is necessary when drilling.
Survival rates of implants placed in sinus augmented sites are consistent (60–100 %) with implants placed in non-grafted maxillae and the success rates, according to well-defined criteria ranges from 74.7 to 100 %. Regarding the type of implant surface, machined-surface implants have lower survival rates (88.7 %) when compared to rough-surfaced implants (97.1 %) [6].
Safety of Sinus Grafting Procedures [6]
The reduction in volume of the maxillary sinus following elevation does not affect the functions of sinus. However, maxillary sinus grafting is accompanied by a very low complication rate with most frequent intraoperative complication as sinus membrane perforation (4.8 to 58 %) and postoperative complications (3 %) as infection and/or postoperative maxillary sinusitis. Sinus mucosa perforations are usually well tolerated and regenerate over the bone graft postoperatively. These perforations can be corrected either by closing them with resorbable barriers, such as collagen sponge, fibrin adhesive, resorbable membranes or by simply folding the sinus mucosa after a more extended elevation. Post-operative complications such as sinusitis occur in previously unhealthy sinuses; therefore a thorough preoperative screening of maxillary sinus status is mandatory.
Choice of Grafting Material [6]
The use of different filling materials has no significant influence over the survival rates of implants placed in maxillary sinus floor elevation site. However, non-autogenous grafting materials show reliable result for sinus floor elevation, with no significant differences in clinical outcomes and implant survival. Autogenous bone also presents similar result, but holds the risk of higher morbidity rate when compared to non-autogenous materials. But, autogenous bone is the material of choice when sinus floor elevation is associated with onlay grafting of the maxilla as in the situations of severe atrophy. In the cases of delayed implant placement, sinuses grafted with autogenous grafts may receive implants earlier than with non-autogenous bone substitutes. The grafted sinuses may undergo re-expansion particularly in the first 2–3 years following the grafting procedures. This can be prevented by using the use of non-resorbable or slowly resorbable grafting materials in conjunction with autogenous grafts. For example, particulated autogenous bone used along with a mixture of xenografts or alloplastic materials should reduce the risk of bone resorption and sinus re-pneumatization.
Timing of Implant Placement [6]
Majority of authors suggests immediate implant placement when the residual alveolar bone has adequate quality and quantity to allow primary stability of implants and is contraindicated when the ridge height is <4–5 mm, and in cases of poor bone quality. The survival rate of immediately placed implants ranges from 61.2 to 100 % and from 72.7 to 100 % in case of a staged approach. The staged approach was indicated when the residual bone height might be insufficient (<4 mm) to achieve primary stability of implants, whereas immediate placement was suggested when bone height is >5 mm from alveolar crest to floor of the sinus. Implants placed in grafted sinuses were loaded 2 weeks to 13 months afterwards (on average 5 to 6 months after).
Alveolar Distraction Osteogenesis
Distraction osteogenesis (DO) is the process of bone generation between two bone segments in response to tensile stress [16]. The technique was first described by Codivilla in 1905 and was developed by Dr Gavriel A. IIlizarov in 1950s. He provided the basic principles for DO which consists of three distinctive phases such as,
Latency phase—initial post-surgical healing period of osteotomy site
Distraction phase—gradual and incremental bone separation at the rate of 1 mm/day
Consolidation phase—bone regeneration at distracted site
The main indication for alveolar distraction in implant rehabilitation is vertical augmentation of the ridge with a minimum of 6–7 mm remaining bone height above the vital anatomic structures and at least a 4 mm vertical defect when measured from adjacent bony walls [16]. The length of edentulous zone should include three or more missing teeth. In extremely atrophic areas, an onlay bone graft was performed initially, and vertically distracted after 4 months of healing period. In moderate horizontal atrophy situations, the distraction is performed first, followed by an onlay bone graft. If secondary grafting is not necessary in case of mild atrophy, implants are usually placed at the time of distractor removal, minimizing the resorption.
The alveolar distractors can be categorized as either extra-osseous or intra-osseous [16]. The following are some of the commonly used distractor systems,
ACE surgical distractor—The intra-osseous ACE distractor, made of titanium alloy has three main components. The external threads of the distractor body are of the same pattern as that of a conventional oral implant with a 5 or a 3 mm thread length. The axial distraction screw seated inside the base plug is threaded through the body to activate the dis-traction (2.5 turns/1 mm). During activation, the distractor body with the bony transport segment advances in coronal direction away from the intact bony bed with the stationary base plug. The simple removal procedure does not require flap reflection and was done by threading the base plug removal tool onto the internal threads of the base plug. With this system, one distractor is used for every three teeth with maximum of three distractors.
The Leibinger Endosseous Alveolar Distraction (LEAD) system—The intra-osseous LEAD system consists of a threaded transport plate, a stabilizing unthreaded base plate, and a 2 mm diameter threaded rod in 17, 22 and 32 mm lengths advanced by 0.4 mm per turn. The angle of the osteotomy for the threaded rod should be consistent with the proposed vector of distraction. Because of the narrow threaded rod, too much horizontal force will result in bone resorption and the rod may get displaced from the transport segment.
KLS Martin distractor—The extra-osseous Track distractors made of titanium consists of micro plates welded to the sliding mechanism of the actual distraction screw. In this system, various sizes are available depending on the regenerative needs. The extra-osseous distractor for ultimate implant reconstruction requires a 5-7 mm of bone width. So, autogenous bone grafting or a split ridge approach may be necessary prior to distraction.
Distractor and oral implant combination devices—The prosthetically restorable distractor concept was introduced by SIS Trade Systems. According to its concept, this approach eliminates the need of the secondary surgeries for distractor removal and implant placement. However, several major complications arise specific to this approach which includes, a lack of device osseointegration, crestal bone loss during distraction exposing the coronal threads, and inability to initially place the devices in ideal prosthetic position due to the interference of the vertical osteotomies leading to unfavorable angulation for restoration.
The order of distractor placement, fixation and final osteotomy preparation is specific to each system being used. Once the distractor is placed, distraction is initiated at a rate of up to 1 mm/day. The vertical bone generation at the end of distraction ranges from 3 to 20 mm. Since crestal resorption is often found during consolidation phase, it may be beneficial to over distract by 2–3 mm. However, overcorrection may result in non-union or incomplete distraction gap ossification. The minimum period of consolidation for long bones was 5 days/mm of distraction. Also, histologic results have demonstrated that the bone formed by DO is with adequate quality and quantity to provide primary stability of implants and to withstand the biomechanical demands of it [16].
Survival and success rates of implants placed in distracted areas are consistent with implants in native bone. Despite the greater predictability and success rate (98.9 %) of alveolar DO, complication rate of 75.7 % including soft tissue, tilting of the segments, change of the distraction vector, occlusal interferences and 21.6 % including fracture of basal bone or the transport segment, breakage of the distractor, and severe mechanical problems, leading to treatment discontinuation were reported [6]. In addition, Lingual/palatal inclination of the distracted segment is the another common problem encountered due to local muscle pull, inappropriate device positioning, and/or poor device trajectory, with an incidence varying from 13 to 35.4 %. To overcome this problem, fixed or removable prosthodontic and orthodontic devices can be used to guide the distracted segment to its proper final position. To correct the complications due to change in vector, a multidirectional alveolar distraction device can be used which allow the vector to be modified and guided in several planes of space [6].
Modified Implant Designs For Specific Conditions:
Zygomatic Implants
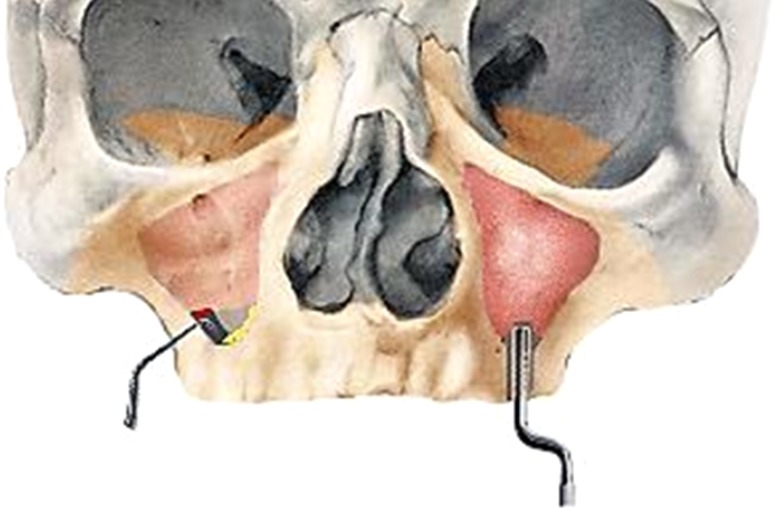
The availability of the zygoma implant has provided a viable alternative for treatment of extremely atrophied maxilla. The zygomatic implant is a self-tapping titanium implant with a machined surface, available in 8 different lengths of 30–52.5 mm (Fig. 3). The threaded apical part has a diameter of 4 mm and a crestal part of 4.5 mm. The implant head has an angulation of 45° and an inner thread for connection of abutments in order to compensate for the inclination of implant insertion with respect to zygoma. The implant has an oxidized rough surface with a smooth body and wide crestal neck.
Fig. 3.
Zygomatic implants: intra-sinus (left side) and extra-sinus approach (right side)
Surgical Techniques [17]
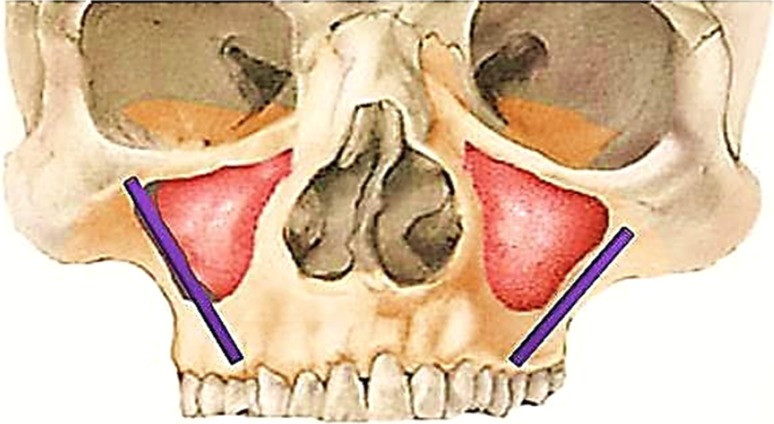
In 1993, Aparicio et al. mentioned the possibility of inserting dental implants in the zygomatic bone. The original technique proposed by Branemark in 1998, consisted of the insertion of a 35–55 mm long implant anchored in the zygomatic bone following an intra-sinusal trajectory (Fig. 4). Following this, many authors have varied the technique slightly.
Fig. 4.
Pterygoid implants
In 2000, Stella and Wagner described sinus slot technique in which the implant is positioned through the sinus via a narrow slot, following the contour of the malar bone and introducing the implant in the zygomatic process. Hence, fenestration of the maxillary sinus is avoided, and the implant emerges over the alveolar crest at first molar level, with a more vertical angulation. But, Boyes-Varley et al. disagree with the sinus slot technique, since perforation of the posterior antral wall is possible due to lack of visibility.
A new technique is currently being developed that involves placing extra-sinus zygomatic implants by fixing them to the lateral sinus and the zygomatic bone (Fig. 4). Aparicio et al. observed superior primary stability with this technique, than conventional technique since the implant is anchored to a larger amount of cortical bone.
Candel-Marti et al. [17] has reviewed 1,082 zygoma implants placed in the maxilla of 552 patients. The weighted average success rate was reported as 97.05 %. With this they concluded the zygoma implant as a suitable alternative to treat severe posterior maxillary atrophy.
Pterygoid Implants [18]
The use of pterygoid implants was described by Tulsane JF in 1992 and was subsequently used by many other researchers. Pterygoid implants have the advantage of allowing anchorage in the posterior atrophied maxilla, eliminating the need of sinus lifts or bone grafts. In addition posterior cantilever can be eliminated and axial loading is improved.
These implants can be placed in two different locations such as pterygoid process or in a most anterior position, the pterygomaxillary process (Fig. 5). The findings in the literature show no clear differences between these two locations and no consensus exists regarding the nomenclature of these implants.
Fig. 5.
Tilted implants (left side) and mini-implants (right side)
However, the implant length and angulations vary between these two locations. Shorter implants are generally placed in the pterygomaxillary region with angulation of 10°–20° to simulate the proper angulation of the third molar. On the other hand, the longer implants are anchored to the pterygoid plate of sphenoid bone.
Surgical Technique
The classic technique described by Tulsane involves the anchorage of pterygoid plate of the sphenoid bone by means of a 22 mm long pterygoid implant. The pterygoid plate is anchored through maxilla and palate with distal angulation between 35° and 55°. The angulation depends on the location of sinus floor and the height of tuberosity.
The implant site is prepared by combining drills and straight osteotomes, according to the technique described by Valeron and Valeron. The entry point is determined with round bur. Preparation of the implant bed then starts with the smallest osteotome, followed by a pilot drill to establish the direction of the implant axis. Preparation continues with consecutive cylindrical osteotomes in combination with drills of increased diameter.
Implants in the pterygomaxillary region are placed within the maxillary tuberosity, parallel to the posterior wall of the sinus. The surgical procedure is similar to that of pterygoid implants. The only difference is the use of curved osteotomes rather than using straight osteotomes. The angle should be 10°–20° to simulate the angulation of third molar.
Valeron et al. recommended the use of osteotomes, to preserve maximum bone and to reduce surgical risks especially hemorrhages. Nocini et al. used anatomically modified osteotomes to facilitate access to maxillary tuberosity area. Penarrocha et al. combined burs and osteotomes to place the implants in pterygomaxillary region, thus joining the advantages of two techniques: osteotomes—to minimize surgical risk, preserve bone, and tactile control, whereas the drills—to facilitate the formation of implant bed, especially in the dense cortical bone area.
Candel et al. [18] has reviewed 1,053 implants placed in the maxilla of 676 patients. The weighted average success rate was reported as 90.7 %. They concluded that pterygoid implants have high success rate, similar bone loss level to those of conventional implants, therefore a viable alternative to rehabilitate posterior atrophic maxilla.
Tilted Implants [19]
As early as 1999, tilting of dental implants in the posterior region of the jaw was demonstrated as an alternative to bone grafting for atrophied jaws. If the distal implant was tilted, a longer dental implant and a more posterior implant position could be achieved. The theory behind this philosophy was that a greater anterior-posterior position of implant would distribute the occlusal forces; therefore, the transverse force placed on the tilted implants would not be detrimental to them. In the maxilla, the distal implants could also get benefitted from the cortical bone wall of the sinus and the nasal fossa.
Bellini et al. [20] investigated the stress patterns at the bone-implant interface of tilted implants using three-dimensional finite element analysis and found that the numerical values of compressive stress were lower in the tilted implant configurations. He also found that tilting of the implants reduces the cantilever length by increasing the inter-implant distance. This may have produced a better load distribution, thereby reducing the stress level of the splinted implants. As a result, a biomechanical advantage is gained by using the tilted implants. Within the limitations, this study supports the use of tilted implants to treat the edentulous maxilla.
Menini et al. [19] has systematically reviewed 1,623 implants placed in the maxilla of 324 patients. Of these, 778 implants were tilted. The overall weighted cumulative success rate was reported as 98.62 %. They have suggested the use of tilted implants for full-arch immediate loading rehabilitations of the maxilla with a favorable short term prognosis.
Mini-Implants [21]
Mini-implants were first introduced in the literature as the “Miniplant” by Barber and Seckinger, in 1994 with an external connection. This study was followed by Sendax, who considered the ultra-small single piece implant. The primary intention was to support an interim prosthesis, as it was expected these implants would be easily removed. However, it was noted that removal of these implants from the bone was difficult as they appeared to have osseointegrated. Histologic studies later confirmed that bone appeared to be integrated to the surface of the ultra-small implant at the light microscopic level, and the bone appeared to be mature and healthy.
The GOMI [7] have defined the term mini implant as an “implant fabricated of the same biocompatible materials as other implants but of smaller dimensions. Implants can be made as one piece to include an abutment designed for support and/or retention of a provisional or definitive prosthesis”. The diameter threshold is not specified by the GOMI for these implants.
Also, the literature is not clear regarding the terminology associated with reduced diameter implants. The terms mini implants, narrow diameter implants, and small diameter implants have been used interchangeably. Additionally, the use of terms such as provisional implants, transitional implants, and orthodontic implants has further added to the confusion. In spite of these multiple terminologies, no consensus on the definition of reduced diameter implants exists in the literature.
Though mini implants were first introduced over 15 years ago, no studies compared the mini implants with standard dental implants for rehabilitation of complete edentulism. But the evidence for short-term survival of mini implants used for partial edentulous situations is encouraging, with a first year interval survival rate of 94.7 %. However, the follow-up period of several implants was reported to be <12 months. Limited evidence for the medium-term survival and no evidence for the long-term survival of mini implants for definitive prosthodontic treatment are available in the literature.
The advantages of using mini implants for definitive prosthodontic treatment are: Low cost, ability to be placed in narrow or wide ridges, simplified treatment procedures, flapless surgical procedure reducing postsurgical discomfort and morbidity for patients. Also, majority of mini-implants are designed as a 1-piece implant with the ability to immediately load the prosthesis.
The disadvantages of mini-implants for definitive prosthodontics treatment are: the need for multiple implants because of the unpredictability and lack of current scientific guidelines and understanding; limited scientific evidence about long-term survival; potential for fracture of the implant during placement; lack of parallelism between implants is less forgiving because of the 1-piece design; reduction in resistance to occlusal loading.
However, Bertil [22] has recommended the use of mini-implants (preferably three implants) in clinical situations with vertical height of 5–8 mm and width of 4–5 mm, to support a posterior maxillary fixed prosthesis.
Conclusion
Maxilla is different in its function, physiology, and bone density from the mandible. These differences, along with its varied anatomy, challenge the implant placement in harmony with planned prosthetic restoration. However, a thorough knowledge of various augmentation procedures, materials and proper patient selection will result effective long-term solutions in the management of the atrophied maxilla. By analyzing these augmentation procedures it can be concluded that,
The atrophic posterior maxilla should be evaluated and classified not only in terms of residual bone height and width, but also vertical and horizontal inter-maxillary relationships to optimize implant placement from a functional and esthetic point of view.
Despite the limitations discussed, reconstruction of atrophic maxilla with bone grafts is an acceptable treatment modality to restore with implant-supported prostheses, taking into account of its higher morbidity rate.
So, in cases of mild-moderate atrophy, other surgical options, such as distraction osteogenesis, guided bone regeneration, and sagittal osteotomies, which may present less morbidity, should be considered.
On the other hand, distraction osteogenesis provides natural bone formation between the distracted segment and basal bone in a relatively short time span, avoiding the necessity of autogenous bone harvesting.
But, when compared to other augmentation procedures, such as GBR or bone grafting, DO does not allow simultaneous correction of narrow ridges, which is only possible with over-distraction of the segment and secondary height reduction until adequate bone width is obtained. Also, DO is not the treatment of choice in cases of moderate-severe atrophy due to difficulties in maintaining an adequate vector for distraction.
Screw-shaped implants with rough surfaces offer a better prognosis than implants with machined surfaces in the regions of ridge augmentation, regardless of technique used.
For posterior atrophic areas, where esthetics is not a prime concern, contemporary treatment options such as zygoma implant, pterygoid implant, tilted implant, mini-implants can be the treatment of choice taking into account that they may result in a prosthetic and functional compromise.
However, the use of mini-implants should not be considered in severely atrophied edentulous maxillae and should be limited to mild-moderate atrophic situations.
References
- 1.Sorni Marco, Guarinos Juan, et al. Implant rehabilitation of the atrophic upper jaw: a review of the literature since 1999. Med Oral Patol Oral Cir Bucal. 2005;10:E45–E56. [PubMed] [Google Scholar]
- 2.Dym Harry, Huang David, et al. Alveolar bone grafting and reconstruction procedures prior to implant placement. Dent Clin North Am. 2012;56:209–218. doi: 10.1016/j.cden.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Precheur HV. Bone graft materials. Dent Clin North Am. 2007;51:729–746. doi: 10.1016/j.cden.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Misch CE (2007) Contemporary implant dentistry, 3rd edn. Mosby, St. Louis
- 5.Moustafa FN, et al. The single tooth dental implant: practical guidelines for hard tissue augmentation. J Calif Dent Asso. 2008;36(11):869–884. [PubMed] [Google Scholar]
- 6.Chiapasco Matteo, Casentini Paolo, Zaniboni Marco. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implant. 2009;24(Suppl):237–259. [PubMed] [Google Scholar]
- 7.Laney WR (2007) Glossary of oral and maxillofacial implants. Quintessence Publishing Co, Ltd., Berlin, Germany
- 8.Hammerle CHF, Jung RE. Bone augmentation by means of barrier membranes. Periodontology. 2000;2003(33):36–53. doi: 10.1046/j.0906-6713.2003.03304.x. [DOI] [PubMed] [Google Scholar]
- 9.Smiler DG, Johnson WP, et al. Sinus lift grafts and endosseous implants. Dent Clin North Am. 1992;36:151–186. [PubMed] [Google Scholar]
- 10.Stern Avichai, Green James. Sinus lift procedures: an overview of current techniques. Dent Clin North Am. 2012;56:219–233. doi: 10.1016/j.cden.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Cosci Ferdinando, et al. A new sinus lift technique in conjunction with placement of 265 implants: a 6-yearretrospective study. Implant Dent. 2000;9:363–368. doi: 10.1097/00008505-200009040-00014. [DOI] [PubMed] [Google Scholar]
- 12.Emmanouil G, et al. Elevation of the maxillary sinus floor with hydraulic pressure. J Oral Implantol. 2005;31(4):197–203. doi: 10.1563/1548-1336(2005)31[197:EOTMSF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Engelke, et al. Subantroscopic laterobasal sinus floor augmentation (SALSA): an up-to-5-year clinical study. Int J Oral Maxillofac Implant. 2003;18(1):135–143. [PubMed] [Google Scholar]
- 14.Francoise, et al. Gradual and safe technique for sinus floor elevation using trephines and osteotomes with stops: a cadaveric anatomic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:210–216. doi: 10.1016/j.tripleo.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 15.Muna sultan et al Antral membrane balloon elevation. J Oral Implantol. 2005;31(2):85–90. doi: 10.1563/0-773.1. [DOI] [PubMed] [Google Scholar]
- 16.Mcalister Bradley S, Gaffaney Thompson E. Distraction osteogenesis for vertical bone augmentation prior to oral implant reconstruction. Periodontology. 2000;2003(33):54–66. doi: 10.1046/j.0906-6713.2002.03305.x. [DOI] [PubMed] [Google Scholar]
- 17.Candel-Marti E, et al. Rehabilitation of atrophic posterior maxilla with zygomatic implants: review. J Oral Implantol. 2012;38(5):653–657. doi: 10.1563/AAID-JOI-D-10-00126. [DOI] [PubMed] [Google Scholar]
- 18.Candel E, Penarrocha D, et al. Rehabilitation of the atrophic posterior maxilla with pterygoid implants: a review. J Oral Implantol. 2012;38(Spl-1):461–466. doi: 10.1563/AAID-JOI-D-10-00200. [DOI] [PubMed] [Google Scholar]
- 19.Menini.M, Signori.A, et al. tilted implants in the immediate loading rehabilitation of the maxilla: a systematic review . J Dent Res. 2012;91(9):821–827. doi: 10.1177/0022034512455802. [DOI] [PubMed] [Google Scholar]
- 20.Bellini CM, Romeo D, et al. A finite element analysis of tilted versus non-tilted implant configurations in the edentulous maxilla. Int J Prosthodont. 2009;22:155–157. [PubMed] [Google Scholar]
- 21.Bidra Avinash S, Almas Khalid. Mini implants for definitive prosthodontic treatment: a systematic review. J Prosthet Dent. 2013;109:156–164. doi: 10.1016/S0022-3913(13)60035-9. [DOI] [PubMed] [Google Scholar]
- 22.Friberg Bertil. The posterior maxilla: clinical considerations and current concepts using Branemark system implants. Periodontology. 2000;2008(47):67–78. doi: 10.1111/j.1600-0757.2007.00238.x. [DOI] [PubMed] [Google Scholar]