Abstract
Allergen-specific immunotherapy is the only allergen-specific and disease-modifying treatment for allergy. The construction and characterization of a vaccine for birch pollen allergy is reported. Two nonallergenic peptides, PA and PB, derived from the IgE-reactive areas of the major birch pollen allergen Bet v 1 were fused to the hepatitis B surface protein, PreS, in four recombinant fusion proteins containing different numbers and combinations of the peptides. Fusion proteins expressed in Escherichia coli and purified to homogeneity showed a lack of IgE reactivity and allergenic activity when tested with sera and basophils from patients allergic to birch pollen. Compared to Bet v 1 allergen, peptides PA and PB showed reduced T cell activation in PBMCs from allergic patients, whereas PreS fusion proteins induced less IL-5 and more IL-10 and IFN-γ. Immunization of rabbits with the fusion proteins, in particular with a PreS fusion protein 2PAPB-PreS, containing two copies of each peptide, induced high levels of IgG Abs against the major IgE-reactive site on Bet v 1 and related allergens. These IgG Abs inhibited allergic patients’ IgE binding to Bet v 1 better than did IgG induced by immunization with complete Bet v 1. Furthermore, 2PAPB-PreS–induced IgG inhibited Bet v 1–induced basophil activation in allergic patients and CD23-facilitated allergen presentation. Our study exemplifies novel beneficial features for a PreS carrier–based peptide vaccine for birch pollen, which, in addition to the established reduction in allergenic activity, include the enhanced focusing of blocking Ab responses toward IgE epitopes, immunomodulatory activity, and reduction of CD23-facilitated allergen presentation.
Immunoglobulin E Ab–mediated allergy affects >25% of the population (1). Since the first clinical trial conducted by Leonard Noon in patients allergic to grass pollen >100 y ago, allergen-specific immunotherapy (SIT) is the only Ag-specific and disease-modifying treatment for allergy (2, 3). SIT is based on the administration of the disease-causing allergens with the goal to reduce allergic inflammation upon allergen exposure. Furthermore, it was shown that SIT can prevent the progression from mild to severe forms of allergy and that it has long-lasting effects, even after its discontinuation (4, 5). Major immunological mechanisms underlying SIT are the induction of allergen-specific IgG Abs that inhibit IgE-mediated allergic inflammation and alterations in cellular responses, including the induction of IL-10–producing regulatory T cells, shifts toward Th1 responses, and effects on APCs and effector cells (e.g., mast cells, basophils) (3).
However, several disadvantages, which are mainly due to the poor quality of allergen preparations, limit the broad applicability of SIT. They include varying allergen compositions, cumbersome forms of treatment requiring multiple injections, and severe anaphylactic side effects due to varying potency of vaccines (6–11).
With the identification of allergen structures by cDNA cloning it has become possible to produce recombinant allergens to study allergen-specific immune responses, as well as to develop new forms of diagnosis and new approaches of SIT that target different immunological mechanisms (12, 13). Several of these molecular approaches were tested in clinical trials in allergic patients. As exemplified for the major cat allergen, Fel d 1, induction of allergen-specific T cell tolerance in allergic patients by administration of synthetic allergen-derived, non-IgE–reactive peptides was attempted (14, 15). Other approaches were based on vaccination with recombinant hypoallergenic allergen derivatives, highly purified recombinant allergens, or purified allergens conjugated to immunomodulatory substances (16–18).
It was demonstrated for birch pollen allergy that SIT with the recombinant major allergen of birch, Bet v 1, is equally effective as SIT with birch pollen extract or natural purified Bet v 1 (19).
Birch pollen is one of the most important inhalant allergen sources in northern and central Europe, North America, and certain parts of Asia and Australia. The cDNA coding for Bet v 1 was isolated (20), rBet v 1 equaling natural Bet v 1 (21) was produced, and the major T cell epitopes of Bet v 1 were mapped (22). The three-dimensional structure of Bet v 1 was solved using x-ray and nuclear magnetic resonance technology (23). Bet v 1 contains mainly conformational IgE epitopes that are lost when its structure and fold are destroyed (24). Using site-directed mutagenesis and epitope mapping based on peptide-specific Abs, it was shown that a surface patch defined by peptides comprising aa 49–58, 73–88, and 88–103 of Bet v 1 represents a major IgE binding site (25, 26).
In this article, we report the construction and characterization of a nonallergenic vaccine for birch pollen allergy that is based on recombinant fusion proteins consisting of nonallergenic peptides derived from the major IgE-binding area of Bet v 1 and the viral carrier molecule PreS derived from hepatitis B. In addition to a reduction in IgE reactivity, T cell reactivity, and allergenic activity, which together should decrease IgE- and T cell–mediated side effects during SIT, our study reveals important novel and unique features of this vaccine. In contrast to Bet v 1, which induced Th2 responses in patients’ PBMCs, the fusion proteins led to production of the tolerogenic cytokine IL-10 and to Th1-biased immune responses. Moreover, immunization with the fusion proteins focused blocking IgG Abs better toward the major IgE epitopes than did immunization with the complete Bet v 1 allergen.
Materials and Methods
Recombinant allergens, synthetic peptides, and allergic patients
rBet v 1, rAln g 1, rCor a 1, and rMal d 1 were purchased from BIOMAY (Vienna, Austria). Bet v 1–derived peptides PA, PB, P1′, P2′, P3′, P4′, P5′, and P6′ (Fig. 1A) were synthesized and purified as described (25). Serum and heparinized blood samples were obtained from patients allergic to birch pollen (n = 54) and, for control purposes, from nonallergic individuals (n = 3) after informed consent and approval of the local ethics committee were obtained. Allergic patients were characterized by case history, determination of allergen-specific IgE, and/or skin testing, as described (27).
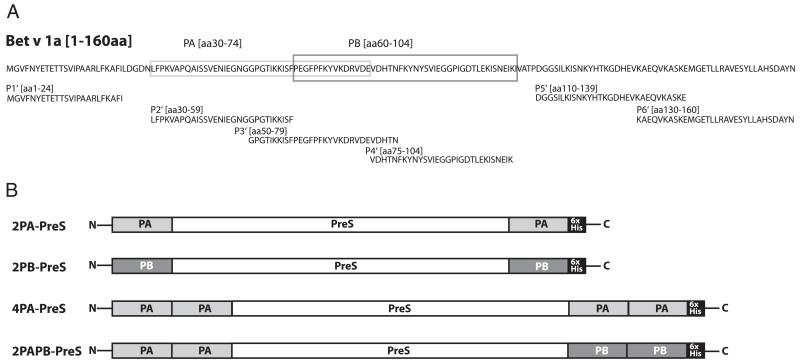
FIGURE 1. Scheme of the construction of rPreS fusion proteins for vaccination against birch pollen allergy.
(A) Amino acid sequence of Bet v 1a. Peptide PA (light gray box) and peptide PB (dark gray box), which are part of the recombinant fusion proteins, as well as Bet v 1–derived peptides P1′– P6′ used for epitope-mapping experiments, are indicated. (B) Illustration of the four rPreS fusion proteins (2PA-PreS, 2PB-PreS, 4PA-PreS, 2PAPB-PreS). Peptide A (PA: light gray), peptide B (PB: dark gray), the carrier protein PreS (white), and the C-terminal hexahistidine tags (6xHis: black) are indicated.
Expression and purification of rPreS and rPreS fusion proteins
DNAs coding for fusion proteins consisting of PreS fused with Bet v 1–derived peptides (Fig. 1B), which were codon optimized for expression in Escherichia coli, were synthesized (2PA-PreS, 2PB-PreS, 4PA-PreS: ATG: biosynthetics, Merzhausen, Germany; 2PAPB-PreS: GenScript, Piscataway, NJ) and inserted into the NdeI/XhoI sites of plasmid pET-27b (Novagen, Darmstadt, Germany). The DNA sequences were confirmed by DNA sequencing of both strands (Microsynth, Balgach, Switzerland).
rPreS and rPreS fusion proteins were expressed in E. coli strain BL21 (DE3) (Stratagene, La Jolla, CA) by growing transformed cells in liquid Luria–Bertani medium containing 50 μg/ml kanamycin to an OD of 0.6. Protein expression was then induced by adding isopropyl-β-d-thio-galactopyranoside to a final concentration of 1 mmol/l overnight at 37°C. Cells were harvested by centrifugation. For PreS, 2PA-PreS, and 2PB-PreS inclusion bodies were prepared, whereas for 4PA-PreS and 2PAPB-PreS the bacterial cell pellets were dissolved directly in 6 M GuHCl, 100 mM NaH2PO4, and 10 mM Tris [pH 8]. Recombinant proteins were purified by nickel-affinity chromatography, as described (28). Fusion proteins were eluted with 8 mol/l urea, 100 mmol/l NaH2PO4, and 10 mmol/l Tris-HCl (pH 3.5); after dialysis either against water (2PA-PreS, 2PB-PreS, 4PA-PreS) or against 10 mM NaH2PO4 (pH 4.8) (PreS, 2PAPB-PreS) they remained soluble at a concentration of 1 mg/ml.
Characterization of rPreS and rPreS fusion proteins
The purity of recombinant proteins was analyzed by SDS-PAGE (12.5%) and subsequent Coomassie staining (29).
Molecular weights of PreS and PreS fusion proteins were compared with those calculated according to their sequence using Expasy (http://www.expasy.org).
Aliquots of 1 μg PreS fusion proteins, PreS, and human serum albumin (HSA; control) were dotted onto nitrocellulose and incubated with mAbs specific for peptide P2′ (mAb2) or P4′ (mAb12), as well as with rabbit Ig specific for PreS or the corresponding preimmune Ig diluted 1:1000 at 4°C (26). Bound mouse and rabbit Abs were detected with 125I-labeled rabbit anti-mouse and 125I-labeled goat anti-rabbit IgG, respectively (Perkin-Elmer, Waltham, MA), diluted 1:500 for 2 h and visualized by autoradiography (30).
IgE reactivity of PreS fusion proteins determined by radioallergosorbent test–based dot blot and ELISA
Purified rBet v 1, recombinant fusion proteins, and PreS were tested for IgE reactivity by radioallergosorbent test-based, nondenaturing dot blot assays. Two micrograms of the purified proteins and, for control purposes, HSA were dotted onto nitrocellulose membrane strips (Schleicher & Schuell, Dassel, Germany), which were blocked in buffer A (31) and then incubated with 1:10 diluted sera from patients allergic to birch pollen (n = 47), sera from nonallergic persons (n = 3), or buffer alone. Bound IgE Abs were detected with 125I-labeled anti-human IgE Abs (Demeditec, Kiel-Wellsee, Germany). The presence of rBet v 1 and PreS fusion proteins on the nitrocellulose was demonstrated using a 1:1000 diluted rabbit anti-rBet v 1 antiserum, followed by 125I-labeled goat anti-rabbit Abs (Perkin-Elmer) and visualization by autoradiography (30).
In additional IgE-binding experiments, ELISA plates were coated with rBet v 1 or with the purified PreS fusion proteins (5 μg/ml) diluted in 0.1 mol/l carbonate buffer (pH 9.6) overnight at 4°C. Plates were washed three times with PBS containing 0.05% (v/v) Tween 20 (PBST), blocked for 2 h with 1% (w/v) BSA in PBST, and subsequently incubated with sera from patients allergic to birch pollen (n = 21) and three nonallergic individuals diluted 1:5. Bound IgE was detected with purified mouse anti-human IgE (BD Pharmingen, Franklin Lakes, NJ) diluted 1:1000 overnight and visualized with HRP-labeled sheep anti-mouse IgG (GE Healthcare, Buckinghamshire, U.K.) diluted 1:2000 (25). Results (i.e., OD values corresponding to bound IgE) are means of duplicate determinations with a variation <10%. The blank control (i.e., OD from empty wells developed with the color substrate) was subtracted.
Allergen-induced upregulation of CD203c on basophils of allergic patients
Heparinized blood samples were obtained from patients allergic to birch pollen (n = 6) after informed consent was given; they were incubated with increasing concentrations of rBet v 1, 2PA-PreS, 2PB-PreS, 4PA-PreS, or 2PAPB-PreS (0.00001–0.1 μg/ml), with a monoclonal anti-IgE Ab (1 μg/ml; Immunotech, Marseille, France) or PBS alone (negative control) for 15 min (37°C). Upregulation of CD203c expression was determined as described (32), and results (stimulation index [SI]) are displayed as means of triplicate determinations ± SD.
Lymphocyte proliferation and cytokine responses of PBMCs from patients allergic to birch pollen
PBMCs from patients allergic to birch pollen (n = 6) were isolated by Ficoll (Amersham Biosciences, Uppsala, Sweden) density-gradient centrifugation, resuspended in AIM V medium (Life Technologies, Grand Island, NY; 2 × 105 cells/well), and stimulated with 2.5 μg/well rBet v 1, with equimolar concentrations of PA, PB, PreS, 2PA-PreS, 2PB-PreS, 4PA-PreS, or 2PAPB-PreS, medium alone (negative control), or IL-2 (4 IE/well; positive control). Endotoxin contents of proteins were determined by Endosafe Technology (Charles River, Kiselegg, Germany) and were found to range at low levels (PreS: 0.16 EU/l; 2PA-PreS: 2.00 EU/l; 2PB-PreS: 0.44 EU/l; 4PA-PreS: 6.28 EU/l; 2PAPB-PreS: 0.78 EU/l; Bet v 1: 0.05 EU/l; PA: 0.01 EU/l; PB: 0.01 EU/well). After 6 d of culture, proliferative responses were measured by [3H]thymidine incorporation and expressed as SIs (33). The concentrations of 17 cytokines (i.e., IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, IFN-γ, TNF-α, G-CSF, GM-CSF, MIP-1β, MCP-1) were measured in identically prepared PBMC cultures using the Bio-Plex Pro Human Cytokine 17-Plex Panel (Bio-Rad, Hercules, CA), according to the manufacturer’s instructions. Briefly, aliquots of 50 μl of each supernatant were mixed with anti-cytokine/chemokine mouse mAbs coupled to different beads as capture Abs (Bio-Rad). An eight-point standard curve was used to achieve low-end sensitivity. After washing, anti-cytokine biotinylated detection Abs were added, and the reactions were visualized by adding streptavidin-labeled PE and assay buffer. Samples were analyzed on a Luminex 100 instrument (Biosource, Nivelles, Belgium), and the data were acquired using Bio-Plex Manager 6.0 software. All samples were analyzed in one run.
The Mann–Whitney U test was used to determine differences in SI. To calculate the variations in cytokine measurements, data were transformed into a logarithmic scale to achieve a normal distribution, and one-way ANOVA was used to visualize intersubject variability. A p value < 0.05 was considered statistically significant. SPSS Version 17 was used to calculate all statistical analyses.
Rabbit Abs specific for PreS fusion proteins
Specific rabbit anti-sera were obtained by immunization of New Zealand white rabbits three times in monthly intervals with 200 μg purified rAgs (i.e., 2PA-PreS, 2PB-PreS, 4PA-PreS, 2PAPB-PreS, rBet v 1) adsorbed either to aluminum hydroxide or to Freund’s adjuvant (once with CFA, twice with IFA) (Charles River Breeding Laboratories, Kissleg, Germany). For determination of Bet v 1–specific IgG Ab responses, ELISA plates (MaxiSorp; Nunc, Roskilde, Denmark) were coated with rBet v 1 (2 μg/ml) diluted in 0.1 mol/l carbonate buffer (pH 9.6). Plates were washed three times with PBST, blocked for 2 h with 1% (w/v) BSA-PBST, and subsequently incubated with serial 1:2 dilutions of rabbit Abs ranging from 1:500 to 1:1,280,000 overnight at 4°C. After washing the plates five times, bound rabbit IgG Abs were detected with HRP-labeled donkey anti-rabbit Abs (GE Healthcare), and the color reaction was developed as described (25, 34). Results (OD values corresponding to bound IgG Abs) are means of duplicate determinations with variations <5%.
Cross-reactivity and epitope specificity of rabbit Abs were also analyzed by ELISA using rBet v 1 (birch), rAln g 1 (alder), rCor a 1 (hazel), rMal d 1 (apple), and synthetic peptides P1′–P6′, which had been coated at a concentration of 1 μg/ml. After washing and blocking, 1:1000 diluted anti-sera were added, and bound rabbit IgG was detected with HRP-labeled donkey anti-rabbit Abs (GE Healthcare).
Inhibition of allergic patients’ IgE binding to rBet v 1
The inhibition of the binding of IgE from patients allergic to birch pollen to rBet v 1 was determined by an IgE-inhibition ELISA (26, 35). ELISA plates were coated with rBet v 1 at a concentration of 1 μg/ml at 4°C overnight. After washing and blocking, plates were preincubated with protein G–purified (Immunopure [G] IgG Purification Kit, Pierce, Rockford, IL) rabbit anti–2PAPB-PreS, rabbit anti-PreS, or anti-Bet v 1 IgG obtained by immunization with alum-adsorbed Ags overnight at 4°C. IgG Ab preparations were diluted according to titration experiments to contain the same concentrations of Bet v 1–specific IgG Abs (~300 μg/ml). For control purposes, plates were incubated with the corresponding rabbit preimmune IgG. After an additional washing step, 1:5 diluted sera from patients allergic to birch pollen were added overnight at 4°C, and bound human IgE Abs were detected with a 1:1000 diluted alkaline phosphatase–conjugated mouse monoclonal anti-human IgE Ab (BD Pharmingen). The percentages of inhibition of IgE binding to rBet v 1 after preincubation with anti–2PAPB-PreS rabbit IgG, anti–Bet v 1 rabbit IgG, and anti-PreS rabbit IgG were calculated for each patient serum as follows: percentage inhibition = 100 − (ODi × 100/ODp). ODp and ODi represent the extinctions after preincubation with specific rabbit IgG (ODi) or preimmune sera (ODp), respectively. A Student t test (SPSS Version 17) was used to compare patients’ IgE binding to Bet v 1 after preincubation with anti–2PAPB-PreS or anti–Bet v 1 Abs. A p value < 0.05 was considered statistically significant.
Inhibition of allergen-induced upregulation of CD203c
Whether anti–2PAPB-PreS or anti–Bet v 1 rabbit IgG can inhibit Bet v 1–induced upregulation of CD203c on basophils of patients allergic to birch pollen was studied by exposing heparinized blood samples from the patients with increasing concentrations of rBet v 1 (0.0000132–0.01 μg/ml), which were preincubated at 37°C for 30 min with 9% rabbit anti–2PAPB-PreS or anti–Bet v 1 Abs, obtained by immunization with CFA-adsorbed Ags. For control purposes, allergen dilutions were preincubated with 9% of a serum from a rabbit without Bet v 1–specific IgG (i.e., nonimmunized rabbit). Rabbit anti-sera were heat-inactivated at 56°C for 30 min for these experiments. CD203c upregulation was analyzed as described in Ref. 32.
IgE-facilitated allergen-binding assay
The inhibition of IgE-facilitated binding of Bet v 1 to CD23-expressing B cells by anti–2PAPB-PreS, anti-PreS, or anti–Bet v 1 IgG was determined as described (36). Aliquots consisting of 5 μl rBet v 1 (0.0025 μg/ml) and 20 μl serum of a patient allergic to birch pollen (indicator serum) were incubated with 20 μl of each of the heat-inactivated rabbit sera for 1 h at 37°C to allow allergen–IgE complex formation in triplicates. Five microliters of EBV-transformed B cells (i.e., 100,000 cells/sample) were added to each of these aliquots and incubated for 30 min at room temperature. After washing with facilitated allergen-binding (FAB) assay buffer, cells were incubated with 20 μl PE-labeled anti-IgE (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) in a 1:30 dilution for 30 min at room temperature. IgE binding was analyzed by flow cytometry after washing with FAB buffer, as described (36–38). Results are displayed as mean ± SD of triplicate measurements.
Results
Construction, purification, and biochemical characterization of rPreS fusion proteins containing Bet v 1–derived peptides
Peptides PA and PB (Fig. 1A) from the IgE epitope–containing area of the major birch pollen allergen Bet v 1 (26) were incorporated in the birch pollen allergy vaccine. PA and PB comprise aa 30–74 and aa 60–104 of Bet v 1, respectively, and, thus, include three non–IgE-reactive peptides (i.e., P2′, P3′, P4′, Fig. 1A), which, after coupling to keyhole limpet hemocyanin, induced Bet v 1–specific blocking IgG Abs after immunization of animals (25). PA and PB are outside of the major Bet v 1–specific T cell epitope-containing areas, which have been mapped to the C-terminal portion of Bet v 1 (39). Following the hapten-carrier principle described by Benacerraf and colleagues (40), which we also used for the construction of a hypoallergenic vaccine for cat allergy (28), we produced fusion proteins consisting of different numbers and combinations of the Bet v 1–derived peptides PA and PB and the PreS surface Ag of hepatitis B (Fig. 1B). These fusion proteins were thought to induce IgG Abs specific for the peptides and, thus, for Bet v 1, as well as for PreS, mainly with T cell help derived from epitopes of PreS. In total, we constructed, expressed, and purified four rPreS fusion proteins in E. coli: two had either one copy of PA or PB fused to the N and C terminus of PreS (i.e., 2PA-PreS, 2PB-PreS, Fig. 1B), one had two copies of PA fused to the N and C terminus of PreS (i.e., 4PA-PreS, Fig. 1B), and one contained two copies of PA and two copies of PB fused to the N and C terminus, respectively (i.e., 2PAPB-PreS, Fig. 1B).
The four recombinant fusion proteins were expressed with good yields in E. coli (i.e., ~10 mg/l culture) mainly in the inclusion body fraction and could be purified to homogeneity by nickel affinity chromatography as proteins that, after reconstitution in physiological buffers, were soluble up to concentrations of 1 mg/ml. Fig. 2A shows an SDS-PAGE containing the purified PreS fusion proteins and PreS, which migrated at apparent molecular masses corresponding to the predicted values (Table I). Gel-filtration experiments (i.e., size-exclusion chromatography) indicated that neither PreS nor the PreS fusion proteins formed aggregates in solution, although circular dichroism analysis showed that the proteins remained unfolded in physiological buffers (data not shown).
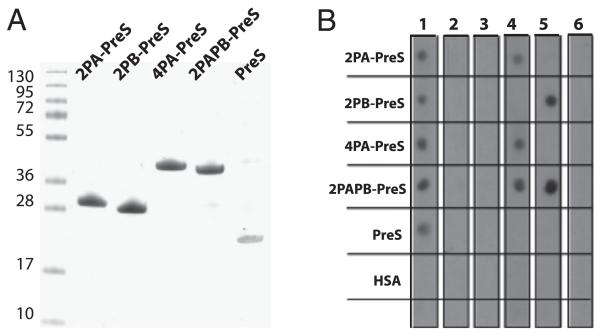
FIGURE 2. Characterization of recombinant fusion proteins.
(A) Coomassie-stained SDS-PAGE showing purified rPreS fusion proteins and rPreS. (B) Nitrocellulose-dotted PreS fusion proteins and PreS probed with rabbit anti-PreS Ig (lane 1), rabbit preimmune Ig (lane 2), or buffer alone (lane 3) were reacted with 125I-labeled goat anti-rabbit Ig. In addition, dotted proteins were probed with mAbs specific for Bet v 1–derived peptide P2′ (mAb2) (lane 4), Bet v 1–derived peptide P4′ (mAb12) (lane 5), or buffer alone (lane 6) and reacted with 125I-labeled rabbit anti-mouse Ig.
Table I. Characteristics of rPreS and PreS fusion proteins.
| Construct | Calculated Molecular Mass (kDa) | Amino Acids | Isoelectric Point |
|---|---|---|---|
| 2PA-PreS | 28.87 | 270 | 8.84 |
| 2PB-PreS | 29.44 | 270 | 6.38 |
| 4PA-PreS | 38.65 | 360 | 8.59 |
| 2PAPB-PreS | 39.21 | 360 | 6.39 |
| PreS | 19.2 | 173 | 8.3 |
Using specific Ab probes for the peptides and PreS, it was shown in a nondenaturing dot blot assay that each of the proteins contained the PreS backbone and that the peptides PA and PB were accessible in the respective fusion proteins (Fig. 2B). When dot-blotted proteins were incubated only with buffer or rabbit preimmune Igs, no signals were observed (Fig. 2B). Dot blot results were confirmed by ELISA experiments (data not shown).
rPreS fusion proteins containing Bet v 1 peptides lack IgE reactivity and allergenic activity
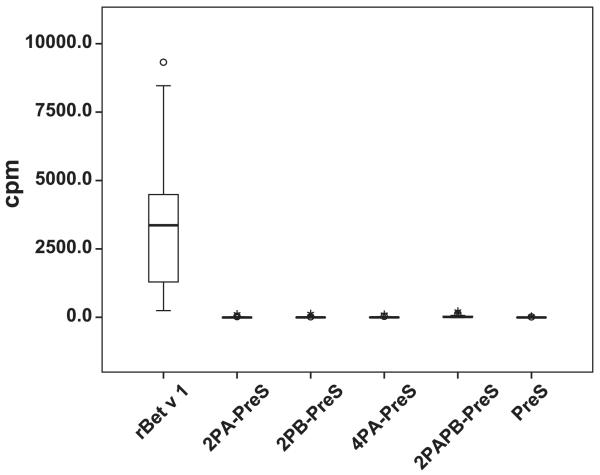
In a first set of experiments, we tested 47 patients with allergy to birch pollen for IgE reactivity to rBet v 1, the four PreS fusion proteins, and PreS in a nondenaturing, radioallergosorbent test-based dot blot assay (Fig. 3). Each of the patients showed IgE reactivity of varying intensities to rBet v 1, whereas no IgE reactivity to PreS or the four PreS fusion proteins was observed (Fig. 3). For control purposes, we also tested sera from three nonallergic individuals and buffer alone. Furthermore, HSA (negative control) was included on the membranes. Under these conditions, no binding was found (data not shown).
FIGURE 3. IgE reactivity of rBet v 1 and of recombinant fusion proteins.
Box plots showing cpm values (y-axis) corresponding to bound IgE Abs (x-axis) for sera from 47 patients allergic to birch pollen.
In a second set of experiments, 17 of the 47 sera used in the dot blot experiments (i.e., A1–A17) and sera from four additional patients allergic to birch pollen (A18–A21) were tested in an ELISA for their IgE reactivity to Bet v 1, PreS, and the four PreS fusion proteins (Table II). None of the allergic patients showed any IgE reactivity to the four PreS fusion proteins or to PreS. Similar results were obtained with sera from the nonallergic individuals. Sera from patients allergic to birch pollen but not from the nonallergic individuals showed IgE reactivity to rBet v 1 (allergic patients A1–A21: OD 0.110–0.994; mean OD, 0.340; nonallergic individuals NA1–NA3: OD 0.014–0.031; mean OD, 0.021) (Table II).
Table II. IgE reactivity of rBet v 1, recombinant fusion proteins, and PreS.
| Subject ID | 2PA-PreS | 2PB-PreS | 4PA-PreS | 2PAPB-PreS | PreS | rBet v 1 |
|---|---|---|---|---|---|---|
| NA1 | 0.048 | 0.036 | 0.037 | 0.024 | 0.017 | 0.018 |
| NA2 | 0.072 | 0.063 | 0.055 | 0.055 | 0.039 | 0.031 |
| NA3 | 0.039 | 0.027 | 0.027 | 0.024 | 0.013 | 0.014 |
| Mean OD nonallergics | 0.053 | 0.042 | 0.039 | 0.034 | 0.023 | 0.021 |
| A1 | 0.046 | 0.013 | 0.032 | 0.011 | 0.004 | 0.309 |
| A2 | 0.038 | 0.014 | 0.021 | 0.015 | 0.004 | 0.380 |
| A3 | 0.038 | 0.024 | 0.030 | 0.029 | 0.012 | 0.417 |
| A4 | 0.035 | 0.024 | 0.029 | 0.023 | 0.012 | 0.344 |
| A5 | 0.067 | 0.063 | 0.045 | 0.040 | 0.022 | 0.494 |
| A6 | 0.044 | 0.023 | 0.029 | 0.021 | 0.012 | 0.296 |
| A7 | 0.049 | 0.041 | 0.043 | 0.035 | 0.030 | 0.299 |
| A8 | 0.026 | 0.004 | 0.012 | −0.004 | −0.005 | 0.194 |
| A9 | 0.043 | 0.027 | 0.038 | 0.025 | 0.021 | 0.665 |
| A10 | 0.025 | 0.011 | 0.019 | 0.010 | 0.005 | 0.130 |
| A11 | 0.037 | 0.014 | 0.029 | 0.012 | 0.009 | 0.994 |
| A12 | 0.029 | 0.023 | 0.031 | 0.018 | 0.015 | 0.681 |
| A13 | 0.020 | 0.007 | 0.012 | 0.005 | 0.000 | 0.124 |
| A14 | 0.033 | 0.023 | 0.030 | 0.020 | 0.010 | 0.116 |
| A15 | 0.029 | 0.021 | 0.022 | 0.023 | 0.008 | 0.231 |
| A16 | 0.032 | 0.018 | 0.022 | 0.015 | 0.008 | 0.383 |
| A17 | 0.039 | 0.030 | 0.038 | 0.033 | 0.018 | 0.110 |
| A18 | 0.048 | 0.026 | 0.039 | 0.022 | 0.011 | 0.411 |
| A19 | 0.031 | 0.019 | 0.024 | 0.010 | 0.003 | 0.122 |
| A20 | 0.019 | 0.013 | 0.015 | 0.009 | −0.001 | 0.279 |
| A21 | 0.028 | 0.022 | 0.029 | 0.016 | 0.011 | 0.166 |
| Mean OD allergics | 0.036 | 0.022 | 0.028 | 0.018 | 0.010 | 0.340 |
OD values (means of duplicate determinations) corresponding to IgE levels specific for the fusion proteins (2PA-PreS, 2PB-PreS, 4PA-PreS, 2PAPB-PreS), PreS and for rBet v 1 are shown for sera from 21 patients allergic to birch pollen (A1–A21) and three nonallergic individuals (NA1–NA3).
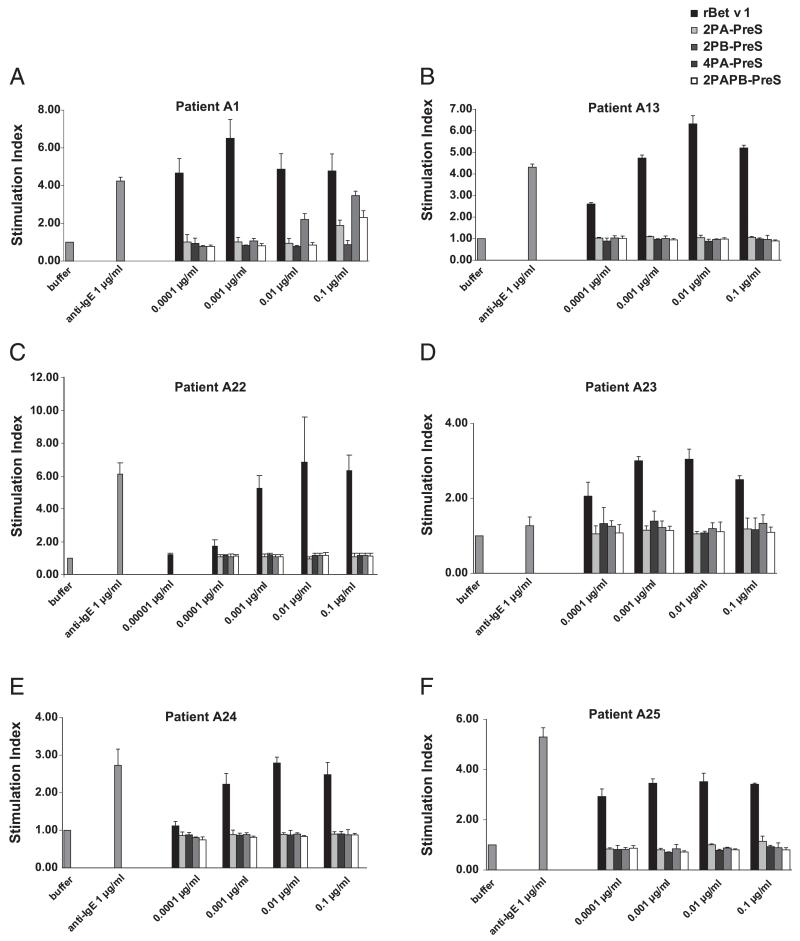
Next, we compared the allergenic activity of PreS fusion proteins with rBet v 1 by measuring the upregulation of CD203c on basophils from six patients allergic to birch pollen (Fig. 4). Incubation of blood samples with increasing concentrations (i.e., 0.00001–0.1 μg/ml) of rBet v 1 strongly upregulated CD203c expression on patients’ basophils, whereas no relevant upregulation was observed with the same concentrations of the PreS fusion proteins. Anti-human IgE induced an upregulation of CD203c in each of the samples, whereas buffer alone (negative control) showed no effect (Fig. 4).
FIGURE 4. Reduction in allergenic activity of PreS fusion proteins, as shown in basophil-activation tests.
Blood samples from six patients allergic to birch pollen [A1 (A), A13 (B), A22 (C), A23 (D), A24 (E), and A25 (F)] were exposed to increasing concentrations (0.00001–0.1 μg/ml) of Ags, anti-IgE, or buffer alone. Upregulation of CD203c expression is displayed as SI. Means of triplicate measurements are shown, and SD are indicated. The mean fluorescence intensities of unstimulated cells were as follows: A1: 119; A13: 141.7; A22: 112.1; A23: 242; A24: 135; and A25: 183.
rPreS fusion proteins show reduced activation of Bet v 1–specific T cells but induced tolerogenic and Th1-like immune responses in cultured PBMCs from patients allergic to birch pollen
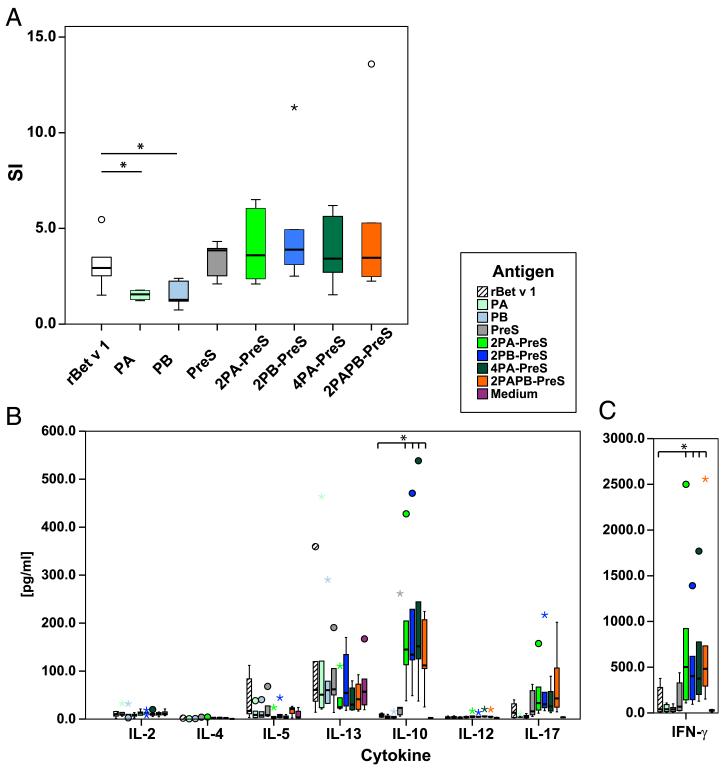
Cultured PBMCs from patients allergic to birch pollen were exposed to rBet v 1, PA, PB, PreS, and the four PreS fusion proteins (Fig. 5A). PA (p = 0.025) and PB (p = 0.010) induced significantly lower proliferative responses compared with rBet v 1 (Fig. 5A). PreS and PreS fusion proteins induced comparable proliferations of lymphocytes that, therefore, were mainly specific for PreS.
FIGURE 5. Lymphocyte proliferation and cytokine responses of PBMCs from patients allergic to birch pollen.
PBMCs from six patients allergic to birch pollen were stimulated with equimolar amounts of rBet v 1, PA, PB, PreS fusion proteins, or PreS. Box plots of SI (A) and cytokines (B, C), for which 50% of the values are within the boxes; extremes and outliers are indicated by asterisks and circles, respectively. Horizontal lines represent median values. The mean cpm of unstimulated cells for the patients analyzed were A1: 1027.2; A20: 4674.3; A21: 2779.0; A25: 2066.8; A26: 9165.5; and A27: 748.0. *p < 0.05.
We also investigated the production of 17 cytokines in the PBMC cultures (Fig. 5B, 5C). Interestingly, we found that the PreS fusion proteins induced significantly higher levels of the tolerogenic cytokine IL-10 and the Th1 cytokine IFN-γ than did Bet v 1, Bet v 1–derived peptides, or PreS (Fig. 5B, 5C). We also noted a decreased production of the Th2 cytokine IL-5 in peptide, PreS, and PreS fusion protein–stimulated cultures compared with Bet v 1 (Fig. 5B). Furthermore, we found that PreS fusion proteins, but not PreS, Bet v 1, or the isolated peptides, led to an increased production of certain proinflammatory cytokines linked to innate immunity (i.e., IL-1β, IL-6, TNF-α, IL-17, G-CSF) (data not shown). No relevant differences with regard to IL-8, IL-13, MIP-1β, or MCP-1 were observed, and levels of IL-2, IL-4, IL-7, IL-12, and GM-CSF were low for each of the tested Ags (Fig. 5B, data not shown).
Enhancement of allergen-specific and cross-reactive IgG responses of PreS fusion proteins through duplication and combination of fused Bet v 1 peptides
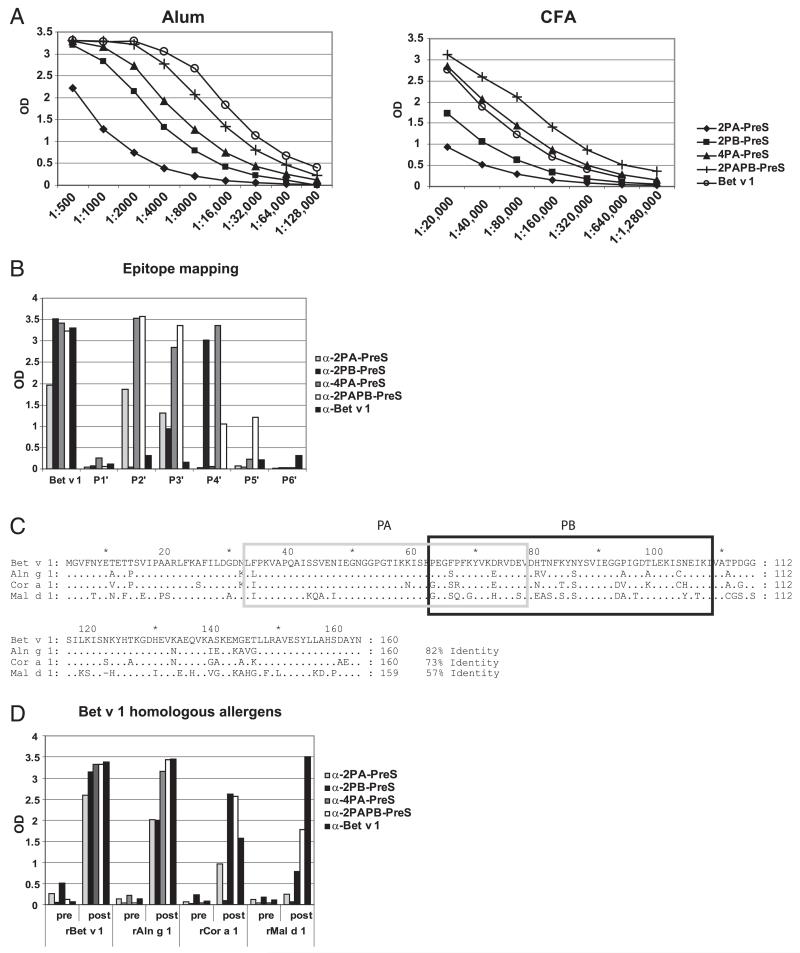
Rabbits immunized with PreS fusion proteins using different adjuvants (i.e., aluminum hydroxide, CFA) showed a strong and robust Bet v 1–specific IgG response over a wide range of serum dilutions (Fig. 6A). IgG levels of CFA-immunized rabbits were approximately 10-fold higher than were those of alum-immunized rabbits. Interestingly, when the number of Bet v 1–derived peptides was doubled in the PreS fusion proteins (e.g., 2PA-PreS versus 4PA-PreS), a much stronger induction of Bet v 1–specific IgG was obtained (Fig. 6A). The highest Bet v 1–specific IgG titers, which were comparable to those obtained with the complete Bet v 1 allergen, were achieved with 2PAPB-PreS, consisting of two copies of each of the peptides PA and PB fused to PreS (Fig. 6A).
FIGURE 6. Titers and specificities of Abs raised by immunization of rabbits with rBet v 1 and PreS fusion proteins.
(A) Bet v 1–specific IgG levels (y-axes: OD values) for different serum dilutions of rabbits that were immunized with Ags (2PA-PreS, 2PB-PreS, 4PA-PreS, 2PAPB-PreS, rBet v 1) adsorbed to aluminum hydroxide (Alum) (left panel) or CFA (right panel). (B) IgG levels (y-axis: mean OD values) of the anti-sera shown in (A) for Bet v 1 and for six Bet v 1–derived peptides (P1′–P6′). (C) Sequence alignment of Bet v 1 and Bet v 1–homologous allergens from alder (Aln g 1), hazel (Cor a 1), and apple (Mal d 1). Identical amino acids are indicated by dots, and gaps are represented by dashes. Numbers of amino acids and percentage of sequence identity with Bet v 1 are shown to the right. Peptides A (PA: light gray box) and B (PB: dark gray box) are indicated. (D) IgG levels (y-axis: mean OD values) of the preimmune and immune sera shown in (A) for Bet v 1, Aln g 1, Cor a 1, and Mal d 1 (x-axis).
A detailed epitope mapping performed with Bet v 1–derived synthetic peptides P1′–P6′ (Fig. 1A) showed that immunization with alum-adsorbed complete rBet v 1 allergen induced IgG Abs recognizing each of the peptides with low intensity, thus yielding a broad immune response. In contrast, immunization with alum-adsorbed PreS fusion proteins focused the IgG responses mainly toward those peptides that are derived from the major IgE-binding area of Bet v 1 (i.e., peptides P2′, P3′, and P4′; Fig. 6B) (25, 26). Many patients who are allergic to birch pollen also exhibit symptoms caused by Bet v 1–homologous allergens present in tree pollen and plant-derived foods (27, 41, 42). Therefore, we tested whether Abs induced by immunization with PreS fusion proteins also cross-react with Bet v 1–homologous allergens. Immunization with PreS fusion proteins induced IgG Abs specific for Bet v 1–homologous allergens in alder (rAln g 1), hazel (rCor a 1), and apple (rMal d 1). The broadest cross-reactivity was observed with PreS fusion proteins containing peptide PA, which exhibited the highest sequence homology among Bet v 1–related pollen and food allergens (Fig. 6C, 6D).
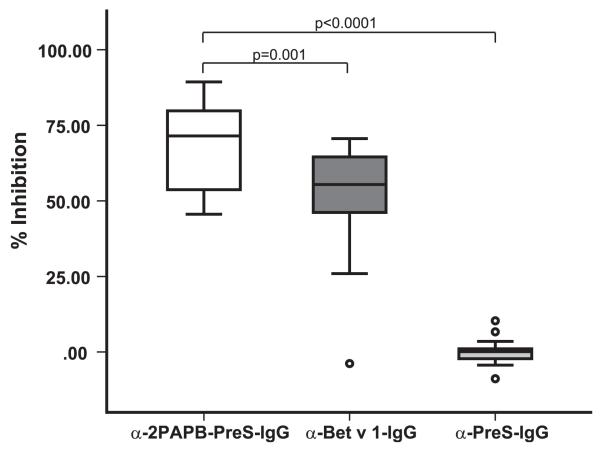
Focusing of blocking IgG responses to Bet v 1 IgE epitopes by the 2PAPB-PreS–based vaccine
In the next series of experiments, we studied whether IgG Abs obtained by immunization of rabbits with alum-adsorbed 2PAPB-PreS, which had induced the highest Bet v 1–specific IgG response, could inhibit the binding of allergic patients’ IgE to Bet v 1. Fig. 7 shows that anti–2PAPB-PreS IgG Abs caused a mean inhibition of allergic patients’ (n = 20) IgE binding to Bet v 1 of 69.7%, which was significantly higher (p = 0.001) than that obtained with anti–rBet v 1 IgG Abs (i.e., 51.1%). Anti–PreS-IgG showed no inhibition (Fig. 7).
FIGURE 7. Inhibition of allergic patients’ IgE binding to Bet v 1 by anti–2PAPB-PreS and anti–rBet v 1 Abs.
The percentage inhibition of IgE binding to rBet v 1 obtained with anti–2PAPB-PreS, anti–rBet v 1, or anti–PreS rabbit IgG Abs was determined for sera from 20 patients allergic to birch pollen. Data are displayed as box plots, for which 50% of the values are within the boxes; horizontal lines indicate the median values. Significant differences in inhibition, calculated using a Student t test, are indicated.
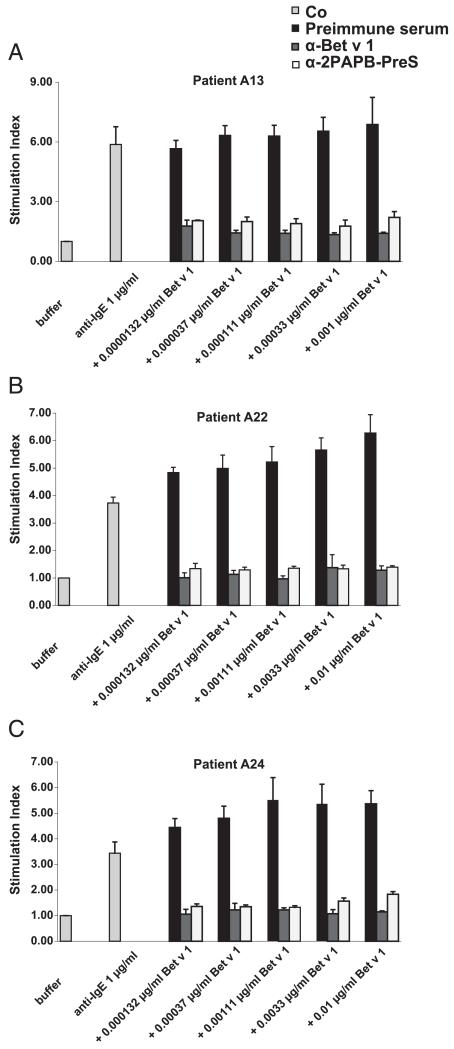
IgG Abs induced by immunization with 2PAPB-PreS inhibit Bet v 1–specific basophil activation and IgE-facilitated allergen presentation
Furthermore, we investigated whether anti–2PAPB-PreS IgG Abs could inhibit Bet v 1–induced activation of the basophils of patients who are allergic to birch pollen. As shown in Fig. 8, a 1:10 diluted anti–2PAPB-PreS and anti–Bet v 1 rabbit anti-serum, but not serum from a nonimmunized rabbit or a rabbit immunized with PreS (data not shown), inhibited activation of basophils from three patients who are allergic to birch pollen. Reductions in basophil activation were also observed with a 1:40 dilution of the anti–2PAPB-PreS antiserum and with a 1:80 dilution of the anti–rBet v 1 antiserum (data not shown).
FIGURE 8. Inhibition of basophil activation in allergic patients by anti–2PAPB-PreS and anti–rBet v 1 Abs.
Increasing concentrations (0.0000132–0.01 μg/ml) of rBet v 1 in the presence of anti–2PAPB-PreS, anti–rBet v 1, or rabbit preimmune Ig were exposed to blood samples of three patients allergic to birch pollen [A13 (A), A22 (B), A24 (C)]. Anti-IgE Abs or buffer alone were used as controls (Co). Upregulation of CD203c expression is displayed as SI. Means of triplicate measurements are shown, and SD are indicated. The mean fluorescence intensity of unstimulated cells were as follows: A13: 126.3; A22: 127.7; A24: 99.3.
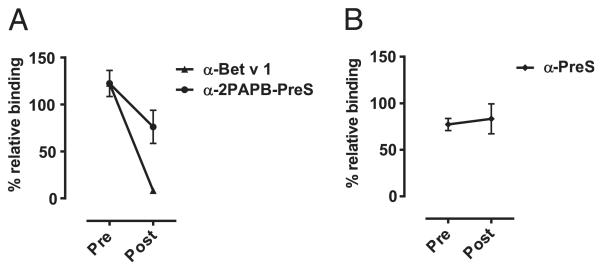
We also studied whether anti–2PAPB-PreS Abs could inhibit IgE-facilitated allergen presentation via CD23 using the FAB assay (36). Anti–2PAPB-PreS Abs caused a reduction >37% of IgE-facilitated allergen binding to CD23-expressing B cells, although the reduction was less than that observed with anti–Bet v 1 Abs (Fig. 9A). Anti-PreS Abs had no effect (Fig. 9B).
FIGURE 9. Reduction in binding of Bet v 1–IgE complexes to CD23-expressing B cells by anti–2PAPB-PreS and anti–Bet v 1 Ig.
Percentage of relative binding of IgE to B cells after the addition of pre- or postimmune Ig (A) or after the addition of anti-preS Ig (B). The percentage reduction achieved with post-Ig are indicated. SD of triplicate measurements are shown.
Discussion
In this study, we developed and characterized a therapeutic vaccine for the treatment of birch pollen allergy, which affects >100 million patients worldwide. The vaccine is based on the major birch pollen allergen, Bet v 1, which harbors the majority of immunologically relevant epitopes responsible for birch pollen and related pollen and food allergies (21, 22, 39). Furthermore, rBet v 1 has been used successfully for birch pollen–specific immunotherapy and shown to replace total birch pollen extract (19). Based on previous work identifying the major IgE-reactive sites of Bet v 1 (26), we constructed four fusion proteins consisting of a nonallergenic carrier protein, the PreS protein from hepatitis B, a component of hepatitis B vaccines (28, 43), and different numbers and combinations of nonallergenic peptides derived from the major IgE-reactive area of Bet v 1 (Fig. 1). According to the hapten-carrier principle described by Benacerraf and colleagues (40, 44–46), upon immunization the fusion proteins should induce IgG Abs against the allergen-derived peptides, the corresponding allergen, and the allergen-unrelated carrier molecule with T cell help from the carrier.
Testing the four fusion proteins for IgE reactivity and allergenic activity with sera and basophils of patients allergic to birch pollen showed that the PreS fusion proteins lacked any detectable IgE reactivity or relevant allergenic activity and, thus, appeared to exhibit an even lower allergenic activity than all previously described hypoallergenic versions of Bet v 1 (47). In addition to the reduction in allergenic activity, the peptides used for construction of the fusion proteins showed a significant reduction with regard to the activation of Bet v 1–specific T cells. The latter feature may be another important advantage over previously characterized Bet v 1 hypoallergens, because the recombinant hypoallergens were made to preserve allergen-specific T cell epitopes and, hence, induced late-phase side effects during SIT and IgE-independent delayed-type inflammation in allergic patients by atopy patch testing (48, 49). An unexpected finding was that the fusion proteins shifted the T cell response in cultured PBMCs from patients allergic to birch pollen toward a tolerogenic and Th1 phenotype that has been described as beneficial for the modulation of allergy by SIT (3). This effect may be explained by the covalent linkage of allergen-derived peptides to the PreS protein.
Immunization of rabbits with the fusion proteins adsorbed to CFA, as well as to aluminum hydroxide, an adjuvant used in most current allergy vaccines, induced high levels of IgG Abs that were directed against the major IgE-reactive areas on the Bet v 1 allergen and cross-reacted with Bet v 1–related pollen and food allergens (27, 42). Aluminum hydroxide is frequently added as adjuvant to enhance the immune response; furthermore, it has a depot effect that can reduce systemic side effects (8).
Fusion proteins containing four Bet v 1–derived peptides (i.e., 2PAPB-PreS, 4PA-PreS) induced consistently higher levels of Bet v 1–specific IgG Abs than did fusion proteins containing only two copies of Bet v 1–derived peptides. The induction of allergen-specific blocking IgG has been identified as an important mechanism of SIT (3, 12, 50). Therefore, we investigated whether Abs induced by immunization with the fusion proteins could inhibit allergic patients’ IgE binding to Bet v 1. Interestingly, we found that 2PAPB-PreS–induced IgG inhibited allergic patients IgE binding to Bet v 1 significantly better than did IgG induced by immunization with the complete Bet v 1 allergen. This finding may be explained by the fact that 2PAPB-PreS focuses IgG predominantly against the IgE-reactive areas of Bet v 1, whereas immunization with Bet v 1 also induces IgG against epitopes that are not recognized by IgE. In this context, it was reported that birch pollen–specific SIT induces IgG Abs that lack blocking effects; this SIT may even have harmful effects because the Abs were reported to enhance IgE binding to Bet v 1 and Bet v 1–induced skin reactions (51, 52). Therefore, it is tempting to speculate that a birch vaccine based on 2PAPB-PreS may have another advantage over vaccines based on Bet v 1: as a result of the focusing of IgG responses toward IgE epitopes, a 2PAPB-PreS–based vaccine may reduce the formation of unwanted and potentially harmful IgG responses during SIT. In fact, we found that 2PAPB-PreS–induced IgG had beneficial effects because these Abs inhibited Bet v 1–induced activation of allergic patients’ basophils, indicating that a 2PAPB-PreS–based vaccine likely will reduce Bet v 1–induced immediate allergic inflammation.
The beneficial effects of SITon allergen-specific T cell responses may be explained by at least two mechanisms. One possibility is the induction of allergen-specific T cell tolerance by allergen-derived T cell epitopes or linked immunological suppression (53). The second possibility is based primarily on the induction of blocking IgG Abs that inhibit IgE-facilitated allergen presentation to T cells and, thus, lead to a suppression of T cell and proinflammatory cytokine responses (54, 55). In fact, our study provides evidence that a 2PAPB-PreS–based vaccine may induce allergen-specific T cell tolerance by both mechanisms, because we found that the 2PAPB-PreS–based vaccine induced the tolerogenic cytokine IL-10 in PBMCs from allergic patients, and 2PAPB-PreS–induced IgG inhibited IgE-facilitated allergen binding to CD23-expressing B cells. An additional beneficial effect for allergen-specific IgG may be the induction of tolerance by the activation of the inhibitory pathway via binding on FcγRIIB (56).
Our study thus reports the characterization of a vaccine for the treatment of birch pollen allergy based on the peptide carrier concept (i.e., 2PAPB-PreS), which holds promise for reducing immediate, as well as delayed-type, side effects better than previously described Bet v 1 hypoallergens. In addition, our study reveals novel and unexpected features of the PreS carrier–based allergy vaccines that include the highly efficient focusing of blocking IgG toward IgE epitopes and their potential to modulate allergen-specific T cell responses toward a tolerogenic type.
Acknowledgments
This work was supported by research grants from the Christian Doppler Research Association, BIOMAY AG, and projects F4604, 4605, and 4611 of the Special Research Program of the Austrian Science Fund (Fonds zur Foerderung der wissenschaftlichen Forschung). K.M. was supported by a research fellowship from the European Academy of Allergy and Clinical Immunology.
Abbreviations used in this article
- FAB
facilitated allergen-binding
- HSA
human serum albumin
- PBST
PBS containing 0.05% (v/v) Tween 20
- SI
stimulation index
- SIT
allergen-specific immunotherapy
Footnotes
Disclosures: R.V. has received research grants from the Christian Doppler Research Association and from BIOMAY and serves as a consultant for BIOMAY. The other authors have no financial conflicts of interest.
References
- 1.Flöistrup H, Swartz J, Bergström A, Alm JS, Scheynius A, van Hage M, Waser M, Braun-Fahrländer C, Schram-Bijkerk D, Huber M, et al. Parsifal Study Group Allergic disease and sensitization in Steiner school children. J. Allergy Clin. Immunol. 2006;117:59–66. doi: 10.1016/j.jaci.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 2.Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572–1573. [Google Scholar]
- 3.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 4.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N. Engl. J. Med. 1999;341:468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 5.Möller C, Dreborg S, Ferdousi HA, Halken S, Høst A, Jacobsen L, Koivikko A, Koller DY, Niggemann B, Norberg LA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study) J. Allergy Clin. Immunol. 2002;109:251–256. doi: 10.1067/mai.2002.121317. [DOI] [PubMed] [Google Scholar]
- 6.Focke M, Marth K, Flicker S, Valenta R. Heterogeneity of commercial timothy grass pollen extracts. Clin. Exp. Allergy. 2008;38:1400–1408. doi: 10.1111/j.1365-2222.2008.03031.x. [DOI] [PubMed] [Google Scholar]
- 7.Calderón M, Cardona V, Demoly P, EAACI 100 Years of Immunotherapy Experts Panel One hundred years of allergen immunotherapy European Academy of Allergy and Clinical Immunology celebration: review of unanswered questions. Allergy. 2012;67:462–476. doi: 10.1111/j.1398-9995.2012.02785.x. [DOI] [PubMed] [Google Scholar]
- 8.Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin. Exp. Allergy. 2006;36:254–260. doi: 10.1111/j.1365-2222.2006.02340.x. [DOI] [PubMed] [Google Scholar]
- 9.van der Veen MJ, Mulder M, Witteman AM, van Ree R, Aalberse RC, Jansen HM, van der Zee JS. False-positive skin prick test responses to commercially available dog dander extracts caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J. Allergy Clin. Immunol. 1996;98:1028–1034. doi: 10.1016/s0091-6749(96)80187-4. [DOI] [PubMed] [Google Scholar]
- 10.Curin M, Reininger R, Swoboda I, Focke M, Valenta R, Spitzauer S. Skin prick test extracts for dog allergy diagnosis show considerable variations regarding the content of major and minor dog allergens. Int. Arch. Allergy Immunol. 2011;154:258–263. doi: 10.1159/000321113. [DOI] [PubMed] [Google Scholar]
- 11.Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen KW, de Blay F, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int. Arch. Allergy Immunol. 2012;159:253–262. doi: 10.1159/000337654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, Vrtala S. From allergen genes to allergy vaccines. Annu. Rev. Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 13.Thomas WR. The advent of recombinant allergens and allergen cloning. J. Allergy Clin. Immunol. 2011;127:855–859. doi: 10.1016/j.jaci.2010.12.1084. [DOI] [PubMed] [Google Scholar]
- 14.Larché M. T cell epitope-based allergy vaccines. Curr. Top. Microbiol. Immunol. 2011;352:107–119. doi: 10.1007/82_2011_131. [DOI] [PubMed] [Google Scholar]
- 15.Worm M, Lee HH, Kleine-Tebbe J, Hafner RP, Laidler P, Healey D, Buhot C, Verhoef A, Maillere B, Kay AB, Larche M. Development and preliminary clinical evaluation of a peptide immunotherapy vaccine for cat allergy. J. Allergy Clin. Immunol. 2011;127:89–97. 97.e1–14. doi: 10.1016/j.jaci.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, Reisinger J, Pelzmann M, Hayek B, Kronqvist M, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. USA. 2004;101(Suppl. 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jutel M, Jaeger L, Suck R, Meyer H, Fiebig H, Cromwell O. Allergen-specific immunotherapy with recombinant grass pollen allergens. J. Allergy Clin. Immunol. 2005;116:608–613. doi: 10.1016/j.jaci.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Creticos PS, Schroeder JT, Hamilton RG, Balcer-Whaley SL, Khattignavong AP, Lindblad R, Li H, Coffman R, Seyfert V, Eiden JJ, Broide D, Immune Tolerance Network Group Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N. Engl. J. Med. 2006;355:1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 19.Pauli G, Larsen TH, Rak S.z, Horak F, Pastorello E, Valenta R, Purohit A, Arvidsson M, Kavina A, Schroeder JW, et al. Efficacy of recombinant birch pollen vaccine for the treatment of birch-allergic rhinoconjunctivitis. [Published erratum appears in 2009 J. Allergy Clin. Immunol. 123: 166.] J. Allergy Clin. Immunol. 2008;122:951–960. doi: 10.1016/j.jaci.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, Scheiner O, Breitenbach M. The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J. 1989;8:1935–1938. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira FD, Hoffmann-Sommergruber K, Breiteneder H, Pettenburger K, Ebner C, Sommergruber W, Steiner R, Bohle B, Sperr WR, Valent P, et al. Purification and characterization of recombinant Bet v I, the major birch pollen allergen. Immunological equivalence to natural Bet v I. J. Biol. Chem. 1993;268:19574–19580. [PubMed] [Google Scholar]
- 22.Ebner C, Szépfalusi Z, Ferreira F, Jilek A, Valenta R, Parronchi P, Maggi E, Romagnani S, Scheiner O, Kraft D. Identification of multiple T cell epitopes on Bet v I, the major birch pollen allergen, using specific T cell clones and overlapping peptides. J. Immunol. 1993;150:1047–1054. [PubMed] [Google Scholar]
- 23.Gajhede M, Osmark P, Poulsen FM, Ipsen H, Larsen JN, Joost van Neerven RJ, Schou C, Løwenstein H, Spangfort MD. X-ray and NMR structure of Bet v 1, the origin of birch pollen allergy. Nat. Struct. Biol. 1996;3:1040–1045. doi: 10.1038/nsb1296-1040. [DOI] [PubMed] [Google Scholar]
- 24.Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, Valent P, Ebner C, Kraft D, Valenta R. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J. Clin. Invest. 1997;99:1673–1681. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, Thalhamer J, Kraft D, Valenta R. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin. Exp. Allergy. 2004;34:1525–1533. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 26.Gieras A, Cejka P, Blatt K, Focke-Tejkl M, Linhart B, Flicker S, Stoecklinger A, Marth K, Drescher A, Thalhamer J, et al. Mapping of conformational IgE epitopes with peptide-specific monoclonal antibodies reveals simultaneous binding of different IgE antibodies to a surface patch on the major birch pollen allergen, Bet v 1. J. Immunol. 2011;186:5333–5344. doi: 10.4049/jimmunol.1000804. [DOI] [PubMed] [Google Scholar]
- 27.Niederberger V, Pauli G, Grönlund H, Fröschl R, Rumpold H, Kraft D, Valenta R, Spitzauer S. Recombinant birch pollen allergens (rBet v 1 and rBet v 2) contain most of the IgE epitopes present in birch, alder, hornbeam, hazel, and oak pollen: a quantitative IgE inhibition study with sera from different populations. J. Allergy Clin. Immunol. 1998;102:579–591. doi: 10.1016/s0091-6749(98)70273-8. [DOI] [PubMed] [Google Scholar]
- 28.Niespodziana K, Focke-Tejkl M, Linhart B, Civaj V, Blatt K, Valent P, van Hage M, Gronlund H, Valenta R. A hypoallergenic cat vaccine based on Fel d 1-derived peptides fused to hepatitis B PreS. J. Allergy Clin. Immunol. 2011;127:1562–1570.e6. doi: 10.1016/j.jaci.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, Ferreira F, Tejkl M, Edelmann H, Kraft D, et al. Profilins constitute a novel family of functional plant pan-allergens. J. Exp. Med. 1992;175:377–385. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vrtala S, Akdis CA, Budak F, Akdis M, Blaser K, Kraft D, Valenta R. T cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J. Immunol. 2000;165:6653–6659. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- 32.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, Bühring HJ, Valenta R, Valent P. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J. Allergy Clin. Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 33.Jahn-Schmid B, Kelemen P, Himly M, Bohle B, Fischer G, Ferreira F, Ebner C. The T cell response to Art v 1, the major mugwort pollen allergen, is dominated by one epitope. J. Immunol. 2002;169:6005–6011. doi: 10.4049/jimmunol.169.10.6005. [DOI] [PubMed] [Google Scholar]
- 34.Marth K, Focke M, Flicker S, Valenta R. Human monoclonal antibody-based quantification of group 2 grass pollen allergens. J. Allergy Clin. Immunol. 2004;113:470–474. doi: 10.1016/j.jaci.2003.11.042. [DOI] [PubMed] [Google Scholar]
- 35.Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, Kraft D, Valenta R. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–2044. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 36.Shamji MH, Wilcock LK, Wachholz PA, Dearman RJ, Kimber I, Wurtzen PA, Larché M, Durham SR, Francis JN. The IgE-facilitated allergen binding (FAB) assay: validation of a novel flow-cytometric based method for the detection of inhibitory antibody responses. J. Immunol. Methods. 2006;317:71–79. doi: 10.1016/j.jim.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pree I, Shamji MH, Kimber I, Valenta R, Durham SR, Niederberger V. Inhibition of CD23-dependent facilitated allergen binding to B cells following vaccination with genetically modified hypoallergenic Bet v 1 molecules. Clin. Exp. Allergy. 2010;40:1346–1352. doi: 10.1111/j.1365-2222.2010.03548.x. [DOI] [PubMed] [Google Scholar]
- 38.Francis JN. The facilitated antigen binding (FAB) assay—a protocol to measure allergen-specific inhibitory antibody activity. Methods Mol. Med. 2008;138:255–261. doi: 10.1007/978-1-59745-366-0_21. [DOI] [PubMed] [Google Scholar]
- 39.Jahn-Schmid B, Radakovics A, Lüttkopf D, Scheurer S, Vieths S, Ebner C, Bohle B. Bet v 1142-156 is the dominant T-cell epitope of the major birch pollen allergen and important for cross-reactivity with Bet v 1-related food allergens. J. Allergy Clin. Immunol. 2005;116:213–219. doi: 10.1016/j.jaci.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Paul WE, Siskind GW, Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. II. Carrier specificity of anti-2,4-dinitrophenyl-poly-l-lysine antibodies. J. Exp. Med. 1966;123:689–705. doi: 10.1084/jem.123.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebner C, Hirschwehr R, Bauer L, Breiteneder H, Valenta R, Ebner H, Kraft D, Scheiner O. Identification of allergens in fruits and vegetables: IgE cross-reactivities with the important birch pollen allergens Bet v 1 and Bet v 2 (birch profilin) J. Allergy Clin. Immunol. 1995;95:962–969. doi: 10.1016/s0091-6749(95)70096-x. [DOI] [PubMed] [Google Scholar]
- 42.Kazemi-Shirazi L, Pauli G, Purohit A, Spitzauer S, Fröschl R, Hoffmann-Sommergruber K, Breiteneder H, Scheiner O, Kraft D, Valenta R. Quantitative IgE inhibition experiments with purified recombinant allergens indicate pollen-derived allergens as the sensitizing agents responsible for many forms of plant food allergy. J. Allergy Clin. Immunol. 2000;105:116–125. doi: 10.1016/s0091-6749(00)90186-6. [DOI] [PubMed] [Google Scholar]
- 43.Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, Wiesinger K, Kollaritsch H. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055–2060. doi: 10.1016/s0264-410x(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 44.Siskind GW, Paul WE, Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. I. Effect of carrier on the nature of the antibody synthesized. J. Exp. Med. 1966;123:673–688. doi: 10.1084/jem.123.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katz DH, Paul WE, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J. Exp. Med. 1970;132:261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paul WE, Katz DH, Goidl EA, Benacerraf B. Carrier function in anti-hapten immune responses. II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J. Exp. Med. 1970;132:283–299. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, Focke-Tejkl M, Blatt K, Gieras A, Zafred D, et al. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J. Allergy Clin. Immunol. 2010;126:1024–1031. 1031.e1–8. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Purohit A, Niederberger V, Kronqvist M, Horak F, Grönneberg R, Suck R, Weber B, Fiebig H, van Hage M, Pauli G, et al. Clinical effects of immunotherapy with genetically modified recombinant birch pollen Bet v 1 derivatives. Clin. Exp. Allergy. 2008;38:1514–1525. doi: 10.1111/j.1365-2222.2008.03042.x. [DOI] [PubMed] [Google Scholar]
- 49.Campana R, Mothes N, Rauter I, Vrtala S, Reininger R, Focke-Tejkl M, Lupinek C, Balic N, Spitzauer S, Valenta R. Non-IgE-mediated chronic allergic skin inflammation revealed with rBet v 1 fragments. J. Allergy Clin. Immunol. 2008;121:528–530.e1. doi: 10.1016/j.jaci.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 50.Linhart B, Valenta R. Mechanisms underlying allergy vaccination with recombinant hypoallergenic allergen derivatives. Vaccine. 2012;30:4328–4335. doi: 10.1016/j.vaccine.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denépoux S, Eibensteiner PB, Steinberger P, Vrtala S, Visco V, Weyer A, Kraft D, Banchereau J, Valenta R, Lebecque S. Molecular characterization of human IgG monoclonal antibodies specific for the major birch pollen allergen Bet v 1. Anti-allergen IgG can enhance the anaphylactic reaction. FEBS Lett. 2000;465:39–46. doi: 10.1016/s0014-5793(99)01703-2. [DOI] [PubMed] [Google Scholar]
- 52.Visco V, Dolecek C, Denépoux S, Le Mao J, Guret C, Rousset F, Guinnepain MT, Kraft D, Valenta R, Weyer A, et al. Human IgG monoclonal antibodies that modulate the binding of specific IgE to birch pollen Bet v 1. J. Immunol. 1996;157:956–962. [PubMed] [Google Scholar]
- 53.Campbell JD, Buckland KF, McMillan SJ, Kearley J, Oldfield WL, Stern LJ, Grönlund H, van Hage M, Reynolds CJ, Boyton RJ, et al. Peptide immunotherapy in allergic asthma generates IL-10-dependent immunological tolerance associated with linked epitope suppression. J. Exp. Med. 2009;206:1535–1547. doi: 10.1084/jem.20082901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wachholz PA, Soni NK, Till SJ, Durham SR. Inhibition of allergen-IgE binding to B cells by IgG antibodies after grass pollen immunotherapy. J. Allergy Clin. Immunol. 2003;112:915–922. doi: 10.1016/s0091-6749(03)02022-0. [DOI] [PubMed] [Google Scholar]
- 55.van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J. Immunol. 1999;163:2944–2952. [PubMed] [Google Scholar]
- 56.Cady CT, Powell MS, Harbeck RJ, Giclas PC, Murphy JR, Katial RK, Weber RW, Hogarth PM, Johnson S, Bonvini E, et al. IgG antibodies produced during subcutaneous allergen immunotherapy mediate inhibition of basophil activation via a mechanism involving both FcgammaRIIA and FcgammaRIIB. Immunol. Lett. 2010;130:57–65. doi: 10.1016/j.imlet.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]