Summary
Voltage-gated Ca2+ channels are multi-subunit membrane proteins that transduce depolarization into cellular functions such as excitation–contraction coupling in muscle or neurotransmitter release in neurons. The auxiliary β subunits function in membrane targeting of the channel and modulation of its gating properties. However, whether β subunits can reversibly interact with, and thus differentially modulate, channels in the membrane is still unresolved. In the present study we applied fluorescence recovery after photobleaching (FRAP) of GFP-tagged α1 and β subunits expressed in dysgenic myotubes to study the relative dynamics of these Ca2+ channel subunits for the first time in a native functional signaling complex. Identical fluorescence recovery rates of both subunits indicate stable interactions, distinct recovery rates indicate dynamic interactions. Whereas the skeletal muscle β1a isoform formed stable complexes with CaV1.1 and CaV1.2, the non-skeletal muscle β2a and β4b isoforms dynamically interacted with both α1 subunits. Neither replacing the I–II loop of CaV1.1 with that of CaV2.1, nor deletions in the proximal I–II loop, known to change the orientation of β relative to the α1 subunit, altered the specific dynamic properties of the β subunits. In contrast, a single residue substitution in the α interaction pocket of β1aM293A increased the FRAP rate threefold. Taken together, these findings indicate that in skeletal muscle triads the homologous β1a subunit forms a stable complex, whereas the heterologous β2a and β4b subunits form dynamic complexes with the Ca2+ channel. The distinct binding properties are not determined by differences in the I–II loop sequences of the α1 subunits, but are intrinsic properties of the β subunit isoforms.
Keywords: β subunit, Ca2+ channels, CaV1.1, FRAP, Skeletal muscle
Introduction
Voltage-gated Ca2+ channels are expressed in all excitable tissues where, in response to membrane depolarization, they control a variety of cell functions like contraction of muscles, secretion in endocrine cells and neurons, or gene regulation. Functional Ca2+ channels consist of one α1 subunit and at least one extracellular α2δ and a cytoplasmic β subunit. The α1 subunit forms the voltage-sensor and the channel pore, whereas the auxiliary α2δ and β subunits function in membrane targeting and modulation of gating and current properties. Multiple genes and splice variants of each subunit give rise to a considerable number of possible subunit combinations with distinct expression and distribution patterns, biophysical and pharmacological properties. A given α1 subunit can combine with different α2δ and β subunits in different cell types and at different developmental stages. However, it is still a matter of debate whether the auxiliary subunits can also dynamically exchange in native Ca2+ channel complexes and thus differentially modulate pre-existing channels in the membrane (Buraei and Yang, 2010).
In skeletal muscle the CaV 1.1 voltage-gated Ca2+ channel forms a signaling complex with the Ca2+ release channel (type 1 ryanodine receptor, RyR1) in the triad junctions between the transverse (T−) tubules and the sarcoplasmic reticulum (SR). Upon depolarization CaV1.1 activates the opening of the RyR1 and the resulting Ca2+ release from the SR then triggers excitation–contraction (EC−) coupling. This interaction of CaV1.1 and RyR1 depends on their physical interaction by the cytoplasmic loop between repeats II and III of the α1S subunit (Grabner et al., 1999) and probably also by the β1a subunit (Cheng et al., 2005). A highly regular spatial organization of groups of four CaV1.1s (termed tetrads) opposite the RyR1 is the structural correlate of this direct mode of EC coupling in skeletal muscle (Franzini-Armstrong et al., 1998). Whether the putative physical interactions between the CaV1.1 α1S and β1a subunits and the RyR1, which are essential for tetrad formation and direct EC coupling, also result in an increased stability of the Ca2+ channel signaling complex in skeletal muscle is hitherto unknown.
Here we applied fluorescence recovery after photobleaching (FRAP) analysis in dysgenic myotubes reconstituted with GFP-tagged CaV1 α1 and β subunits to study the dynamics or stability of Ca2+ channel subunits in the native environment of the triad junction. The skeletal muscle β1a subunit was stably associated with the α1S subunit. In contrast, higher fluorescence recovery rates of non-skeletal muscle β subunits compared with those of the skeletal muscle α1S and β1a subunits, for the first time demonstrate in a differentiated mammalian cell system that the auxiliary β subunits of the voltage-gated Ca2+ channel can dynamically exchange with the channel complex on a minute time scale. An affinity-reducing mutation in the β1a subunit increased the dynamic exchange of the β subunit within the channel clusters, whereas changing the sequence or orientation of the CaV1.1 I–II loop did not affect the stability of the Ca2+ channel complex. Thus, intrinsic properties of the β subunits determine whether they form stable (β1a) or dynamic (β2a, β4b) complexes with α1 subunits.
Results
CaV1.1 and CaV1.2 α1 subunits are both stably incorporated in triad junctions of dysgenic myotubes
In order to determine the dynamics of CaV1.1 α1S subunits in skeletal muscle triads and to establish a baseline for subsequent comparison with the dynamics of β subunits, we applied FRAP recordings in dysgenic myotubes reconstituted with GFP-tagged α1S subunits (GFP-α1S). Imaging of living myotubes using a laser scanning microscope (Fig. 1A) showed that, consistent with our previous immunofluorescence labeling experiments (Flucher et al., 2000a), GFP-α1S is localized in discrete clusters in the plane of the plasma membrane. These clusters colocalized with the RyR1 (supplementary material Fig. S1A) and thus resemble developing triad junctions between the plasma membrane and the SR. Moreover, extensive previous and ongoing functional studies demonstrated that these junctions are physiologically equivalent to Ca2+ release units, i.e. triad junctions, in mature skeletal muscle fibers (Kasielke et al., 2003; Obermair et al., 2005).
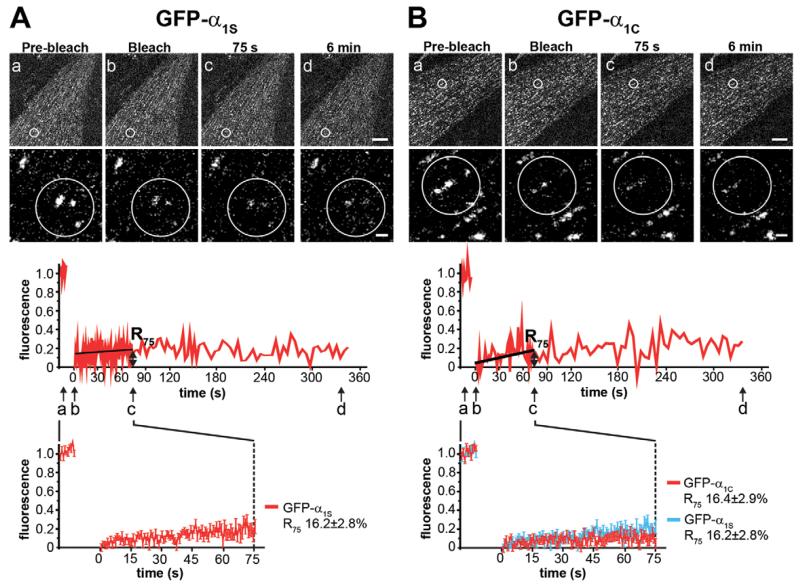
Fig. 1. FRAP analysis of GFP-α1S and GFP-α1C in skeletal muscle triad junctions.
Clusters of GFP-α1S (A) or GFP-α1C (B) in live dysgenic myotubes were photo-bleached (within circles) and imaged for up to 6 min. Representative high-magnification images and the corresponding normalized FRAP recordings show little fluorescence recovery of the Ca2+ channel α1 subunits (a–d, time points of example images). Average recovery curves [lower panels; mean±s.e., N=3, n(GFP-α1S)=16, n(GFP-α1C)=18] reveal a similarly low recovery of GFP-α1S and GFP-α1C and indistinguishable recovery rates 75 s after bleaching (R75). Upper scale bar: 10 μm. Lower scale bar: 1 μm.
For the FRAP analysis we bleached the fluorescence of the GFP-tagged channel subunit by applying high intensity laser power to a circular region of interest (ROI) containing several fluorescent clusters. Then the recovery of fluorescence in the clusters was observed at high sampling rate for 90 s followed by recording at reduced sampling rate to limit photobleaching for up to 6 min. Fluorescence outside the clusters in the bleached ROI was subtracted from the signal originating from clusters to specifically analyze the CaV1 channel dynamics within the junctional signaling complex. The magnified images of a representative experiment (Fig. 1A) show the degree of bleaching and recovery immediately after, 75 s and 6 min after bleaching. The trace below shows the corresponding example recording of the normalized and bleaching-corrected fluorescence intensity in the bleached clusters. As expected for a channel tightly incorporated into a signaling complex, the fluorescence of GFP-α1S showed little to no recovery within the 6-minute observation time. During the initial recording phase the sample was stable enough to allow fitting and calculation of mean recovery curves (Fig. 1A). The value of the fitted curve at 75 s after bleaching was chosen to calculate the fractional fluorescence recovery (R75) used for descriptive and comparative statistics. R75 of GFP-α1S was 16.2±2.8% of the pre-bleaching intensity.
The cardiac channel CaV1.2 also clusters in triad junctions (supplementary material Fig. S1B) but does not physically interact with the RyR1, as evidenced by the lack of tetrad formation and Ca2+ current-independent EC coupling (Takekura et al., 2004; Tuluc et al., 2007). Nevertheless, FRAP analysis of GFP-α1C revealed that this channel was just as stably incorporated in the triads as the skeletal muscle GFP-α1S (Fig. 1B). The mean recovery curves of the two α1 subunits were virtually indistinguishable and R75 for GFP-α1C was 16.4±2.9%, which was not significantly different from that of GFP-α1S. Together these results indicate that both CaV1 Ca2+ channels are stably incorporated into the EC coupling signaling apparatus of skeletal myotubes, and that the distinct coupling mechanisms of CaV1.1 and CaV1.2 to the RyR1 are not reflected by differences in their stability of incorporation.
Skeletal muscle β1a subunits form stable complexes with CaV1.1 in the triad junctions
Next we studied the dynamics of the CaV β subunit by coexpressing untagged α1S (CaV1.1) with GFP-tagged skeletal muscle β1a subunit (β1a-GFP). We hypothesized that β1a-GFP would show the same degree of fluorescence recovery as GFP-α1S, if both subunits form a stable channel complex. On the other hand, higher FRAP rates of β in the clusters compared with that of the α1 subunit would indicate a dynamic exchange of the β subunits with the channel.
When expressed without an α1 subunit in dysgenic myotubes, β1a-GFP revealed a diffuse cytoplasmic distribution pattern (Fig. 2A), consistent with previous immunofluorescence studies (Neuhuber et al., 1998a). After photobleaching the fluorescence in the ROI recovered almost instantaneously and R75 was 100.8±0.8% (Fig. 2A). This high recovery rate was similar to that of soluble eGFP expressed in dysgenic myotubes (supplementary material Fig. S2A), suggesting that in the absence of an α1 subunit, β1a-GFP is freely diffusible within the cytoplasm and has no relevant binding sites in the triads. In contrast, when coexpressed with α1S, β1a-GFP showed a clustered distribution pattern (supplementary material Fig. S3A). This demonstrates that recombinant β1a-GFP can readily compete with endogenous β1a for its binding sites in the junctional Ca2+ channel complex. After photobleaching β1a-GFP coexpressed with α1S showed little to no recovery within 6 min (Fig. 2B). The mean recovery curve during the first 75 s was practically identical to that of GFP-α1S and the R75 of 16.2±2.8% was not significantly different from that of GFP-α1S (Fig. 2B′). The observation that in triads the fluorescence of GFP-tagged β1a and GFP-α1S subunits recover at the same rates indicates that the two skeletal muscle Ca2+ channel subunits form a stable complex with one another and move or turn over together. But is this also the case for heterologous β subunits?
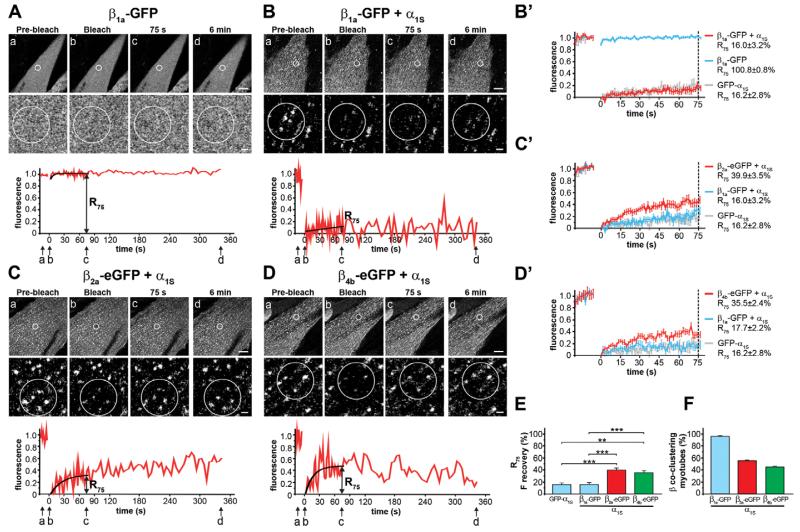
Fig. 2. FRAP analysis of β1a-GFP, β2a-eGFP, and β4b-eGFP with and without CaV1.1 α1S.
(A) β1a-GFP expressed without an α1 subunit in dysgenic myotubes is diffusely distributed and its fluorescence recovers instantaneously after photobleaching (mean±s.e., N=3, n=3). (B) Coexpressed with α1S, β1a-GFP is localized in clusters and does not recover within 6 min after bleaching. (B′) Average recovery curves (mean±s.e., N=5, n=19) and R75 of β1a-GFP reveal a high mobility when expressed alone (blue), but low mobility when coexpressed with α1S (red) similar to that of GFP-α1S (gray; from Fig. 1A). In contrast, β2a-eGFP (C) and β4b-eGFP (D) coexpressed with α1S show substantial fluorescence recovery. When coexpressed with α1S, mean recovery of β2a-eGFP (C′, red) and β4b-eGFP (D′, red) is approximately twofold higher than that of β1a-GFP+α1S (blue; from Fig. 2B for β2a, in parallel for β4b) or GFP-α1S (gray; from Fig. 1A). (E) R75 (mean±s.e.) of β2a-GFP (N=7, n=23) and β4b-eGFP (N=3, n=17) are significantly higher compared with β1a-GFP+α1S or GFP-α1S. Anova F(11,264)=15,6; P<0.001 (P values in the figure are for post-hoc analysis; **P<0.01, ***P<0.001). (F) Co-localization with α1S is seen in 96.6±1.9% of myotubes expressing β1a-GFP, but only in 56.6±1.9% expressing β2a-eGFP and 44.4±2.9% expressing β4b-eGFP (N=3, n=90). Upper scale bar: 10 μm. Lower scale bar: 1 μm.
Heterologous β subunits dynamically exchange with the CaV1.1 channel complex in the triad on a minute time scale
The β2a subunit is distinct from all other β subunits in that it is palmitoylated and thus associates with the plasma membrane even in the absence of an α1 subunit (Chien et al., 1996). Accordingly, β2a-eGFP expressed without an α1 subunit in dysgenic myotubes showed strong membrane localization (see below, Fig. 3A). When photobleached, its fluorescence recovered quickly (R75 79.9±4.1%), but not at the same rapid rate as the cytoplasmic β1a subunits. The recovery rate of β2a-eGFP was similar to that of GAP-GFP, another palmitoylated GFP probe (supplementary material Fig. S2C). When coexpressed with α1S, β2a-eGFP redistributed into clusters (supplementary material Fig. S3B), indicating that it too could successfully compete with endogenous β1a subunits for binding sites in the Ca2+ channel complex. However, different from β1a-GFP its fluorescent clusters substantially recovered within the first minutes after bleaching. Its R75 was 39.9±3.5% and thus 2.5×higher than that of GFP-α1S or β1a-GFP (Fig. 2C,C′,E).
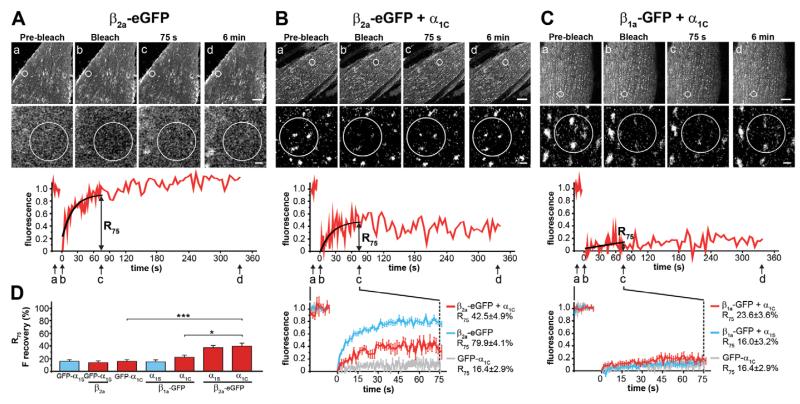
Fig. 3. FRAP analysis of β2a-eGFP and β1a-GFP with and without CaV1.2 α1C.
(A) The palmitoylated β2a-eGFP expressed without an α1 subunit in dysgenic myotubes is localized in the plasma membrane and its fluorescence fully recovers within 6 min after bleaching (mean recovery trace and R75 shown in B, blue, N=2 n=12). (B) When coexpressed with α1C, β2a-eGFP is recruited into junctional clusters and its average fluorescence recovery is significantly reduced (red), but not to the low rate of GFP-α1C (gray, from Fig. 1B, N=4, n=11). (C) In contrast, when β1a-GFP is coexpressed with α1C, it shows little recovery after bleaching (red), very similar to when β1a-GFP was coexpressed with α1S (in parallel N=3, n=18). (D) Comparison of R75 values shows that β2a-eGFP has approximately twofold higher recovery rates than β1a-GFP when coexpressed with α1S or α1C, but coexpression of β2a does not affect the recovery rate of GFP-α1S (mean±s.e.). Anova F(11,264)=15,6; P<0.001 (P values in the figure are for post-hoc analysis; *P<0.05, ***P<0.001). Upper scale bar: 10 μm. Lower scale bar: 1 μm.
This increased mobility could either reflect an increased exchange of β2a with CaV1.1 channels or an increased mobility of the entire channel complex due to the association of a heterologous β subunit. To distinguish between these two possibilities we analyzed the recovery of fluorescence of GFP-α1S when coexpressed with the heterologous β2a subunit. Interestingly, also under these conditions GFP-α1S clusters did not recover (supplementary material Fig. S4) and the R75 of GFP-α1S coexpressed with β2a (13.3±3.7%) was not significantly different from that of GFP-α1S coexpressed with β1a (R75 16.2±2.8%) (Fig. 3D). Thus, the substantial mobility of the β2a subunit in clusters of stable CaV1.1 α1S subunits clearly indicates that β2a-eGFP can dynamically exchange with the Ca2+ channel complex in skeletal muscle triads.
To clarify whether this reduced stability of β2a-eGFP in Ca2+ channel complexes is a general property of heterologous β subunits or is related to the fact that β2a is a palmitoylated membrane protein, we repeated the experiment with a non-palmitoylated heterologous β subunit, β4b-eGFP. Its diffuse distribution when expressed without an α1 subunit, and its rapid recovery in FRAP experiments similar to that of soluble eGFP verified that β4b-eGFP is cytoplasmic like β1a-GFP (supplementary material Fig. S2B). Similar to the other β isoforms and consistent with previous findings (Subramanyam et al., 2009), β4b also partitioned in the triadic Ca2+ channel complex when coexpressed with α1S (supplementary material Fig. S3C). However, different from β1a-GFP, β4b-eGFP showed an elevated recovery rate after photobleaching (Fig. 2D; Fig. 2D′). Its R75 of 35.5±2.4% was about twice as high and significantly different from that of GFP-α1S or that of the homologous GFP-tagged β1a subunits (Fig. 2E). This result indicates that, like the heterologous β2a-eGFP, also the heterologous β4b subunit dynamically exchanges with the Ca2+ channel complex in the triad.
In order to examine whether the difference in the stability/dynamics of the homologous β1a compared with the heterologous β2a-eGFP and β4b-eGFP subunits is also reflected in their ability to compete with the endogenous β1a for incorporation in the Ca2+ channel complex, we quantified the degree of co-clustering of the three β subunits with α1S. Myotubes co-transfected with α1S plus either β1a-GFP, β2a-eGFP, or β4b-eGFP were immunolabeled and analyzed for colocalization of the β subunits with α1S clusters. Whereas clusters of β1a-GFP and α1S were colocalized in practically all myotubes expressing α1S clusters (96.6±1.9%), co-clustering of β2a-eGFP and β4b-eGFP with α1S was only observed in about half of the myotubes (56.6±1.9% and 44.4±2.9%, respectively) (Fig. 2F; supplementary material Fig. S3A–C). Thus, increased dynamic exchange of the heterologous β2a and β4b subunits in the junctional Ca2+ channel complex correlates with their decreased ability to form identifiable complexes with α1S subunits in the developing triad junctions.
The stability of the β1a subunits in the triad Ca2+ channel complex is independent of the CaV1 α1 subunit isoform
Since the homologous β1a-GFP formed a stable complex with the skeletal muscle α1S subunit, whereas the heterologous β2a-eGFP and β4b-eGFP subunits formed dynamic complexes, we reasoned that these association characteristics might be altered or even reversed when the β subunits are coexpressed with the non-skeletal muscle CaV1.2 α1C subunit. On coexpression with α1C, β2a-eGFP also became redistributed into triad clusters and its fluorescence recovery rate was dramatically reduced compared with that of β2a-eGFP expressed alone (Fig. 3A,B). However, the mean R75 of 42.5±4.9% of β2a-eGFP combined with its homologous α1C subunit partner was still significantly higher than that of the GFP-α1C subunit itself and was not significantly different from β2a-eGFP’s recovery rate when combined with α1S (Fig. 3D). Thus, also when coexpressed with its native channel partner α1C, the non-skeletal muscle β2a-eGFP subunit formed a dynamic complex with the Ca2+ channel in the skeletal muscle triad. Therefore, the dynamic association of β2a with CaV1 channels is an intrinsic property of the β subunit that does not depend on differences between the CaV1.1 and CaV1.2 α1 subunits.
By itself this finding does, however, not exclude the possibility that the higher stability of the β1a-GFP subunit observed when coexpressed with CaV1.1 α1S may result from its specific association with its homologous skeletal muscle channel partner. Alternatively, the high stability might result from additional specific binding sites of this β isoform in the triad, including those suggested specifically between β1a and the RyR1. If so, its fluorescence recovery rate after photobleaching would be expected to increase when coexpressed with the heterologous CaV1.2 α1C subunit, which does not directly interact with RyR1. However this was not the case. When expressed together with α1C, β1a-GFP clusters showed little recovery within 6 min and the R75 of 23.6±3.6% was only slightly higher but not significantly different from those of GFP-α1C or of β1a-GFP coexpressed with GFP-α1S (Fig. 3C,D). Together these results suggest that the high stability of β1a in the triad Ca2+ channel complex does neither depend on its homologous association with the skeletal muscle CaV1.1 α1S subunit nor on its isoform-specific interactions with the RyR1 (Cheng et al., 2005; Grabner et al., 1999). Instead it seems to reflect an intrinsically strong binding of β1a to CaV1 channels either by a higher affinity to the AID site or by additional secondary binding sites.
Mutations of the CaV1.1 I–II loop and the β1a subunit differentially affect triad targeting and the stability of the β1a subunit in the Ca2+ channel complex
One possible mechanism explaining the differences in the stability/dynamics of distinct α1–β subunit pairs could be sequence differences within the primary protein–protein interaction site, the α1 subunit I–II loop containing the AID and the corresponding α binding pocket in the beta subunit. To examine the importance of the specific I–II loop sequence of L-type (CaV1) Ca2+ channels for the high stability of complexes with β1a we generated an CaV1.1 chimera containing the I–II loop of the CaV2.1 α1A subunit (α1SI–IIA) (Fig. 4A). The chimeric approach was necessary because α1A heterologously expressed in dysgenic myotubes is not targeted into triads (Flucher et al., 2000b). In contrast, the α1SI–IIA chimera was targeted into triads, albeit at a substantially reduced rate. Whereas 89±2.1% of myotubes expressing wild type α1S showed a clustered distribution pattern, clustering was achieved in only 32.6±3.0% of α1SI–IIA expressing myotubes (Fig. 4B; supplementary material Fig. S1C,D). This was not accompanied by a reduction of the whole-cell Ca2+ currents density (α1S −2.8±0.8 pA/pF; α1SI–IIA −4.4±1.0 pA/pF) indicating that replacing the I–II loop of α1S with that of α1A specifically perturbed triad targeting but not functional membrane expression of this chimera.
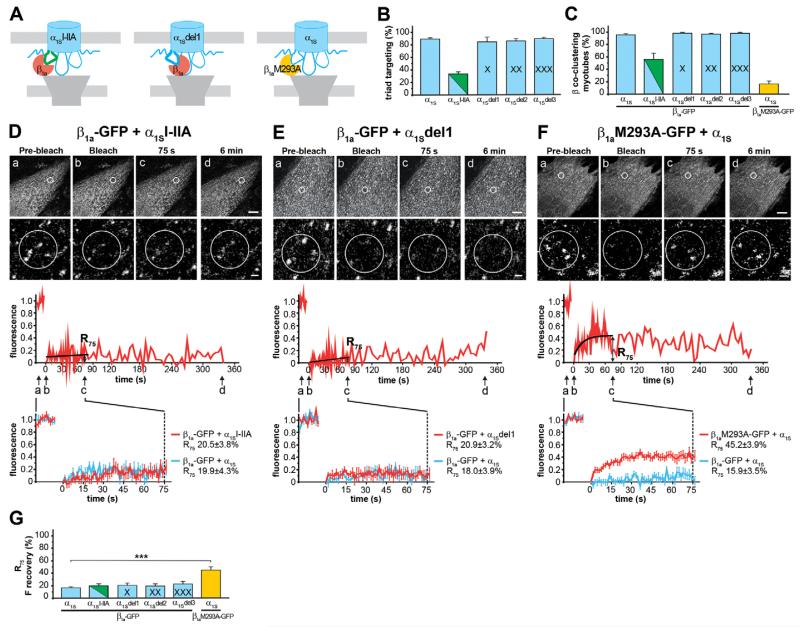
Fig. 4. Effects of mutations of the α1SI–II loop and the β1a subunit on co-clustering and the mobility of β1a-GFP.
(A) In α1SI–IIA, the I–II loop was replaced by that of α1A (CaV2.1); in α1Sdel1, one amino acid in the proximal I–II loop was deleted to alter the orientation of the β subunit relative to the channel; in β1aM293A-GFP, a single methionine was mutated to alanine. (B) Triad targeting was normal for the α1Sdel1 construct, but diminished to 32.6±3.0% for α1SI–IIA (N=3, n=300). (C) β1a-GFP co-clustered efficiently with the α1Sdel1 construct, but only in 50.6±11.4% of the myotubes expressing α1SI–IIA. β1aM293A-GFP co-clustered only in 17.8±4.8% of the myotubes expressing α1S (N=3, n=90). When coexpressed with α1SI–IIA (D) or with α1Sdel1 (E), β1a-GFP fluorescence did not recover within 6 min after bleaching. With both constructs, the mean recovery curves and R75 (G) (mean±s.e.; α1SI–IIA, N=6, n=7; α1Sdel1, N=3, n=13) were similar to that of β1a-GFP and the wild type α1S. When coexpressed with α1S (F), β1aM293A-GFP showed substantial fluorescence recovery: an approximately threefold higher recovery rate than β1a-GFP (N=7, n=25). Anova F(11,264)=15,6; P<0.001 (P values in the figure are for post-hoc analysis; ***P<0.001). Upper scale bar: 10 μm. Lower scale bar: 1 μm.
Analysis of β association with this construct using double immunofluorescence labeling demonstrated that only 50.6±11.4% of the myotubes forming α1SI–IIA clusters showed colocalized β1a-GFP clusters. By comparison, β1a-GFP was co-clustered in almost all (96.6±1.9%) myotubes expressing wild type α1S (Fig. 4C; supplementary material Fig. S3A,D). Surprisingly, even though the total number of myotubes with α1SI–IIA/β1a-GFP co-clusters was greatly reduced compared with that of wild type α1S/β1a-GFP, fluorescence recovery after photobleaching was not increased (Fig. 4D). For α1SI–IIA/β1a-GFP, R75 was 20.5±3.8%, which is not significantly different from that of β1a-GFP coexpressed with α1S (19.9±4.3%) (Fig. 4G). These similar recovery rates are consistent with the published results of an isothermal titration calorimetry study showing that CaV1.1 and CaV2.1 AID peptides bind β subunits with similar affinities in the low nanomolar range (Van Petegem et al., 2008). Apparently, replacing the I–II loop with that of α1A compromises triad targeting and the formation of stable Ca2+ channel complexes, but not their stability once they have been formed.
If sequence differences in the primary interaction domain, the I–II loop, do not explain the differential stability/dynamics of distinct α1–β subunit pairs, isoform-specific secondary interactions within the signaling complex may be involved. In order to displace β from such putative secondary interaction sites without affecting the primary interaction with the AID, we deleted one, two, or three amino acids from the proximal I–II loop of CaV1.1. This sequence forms a rigid connection between the IS6 transmembrane helix and the AID (Van Petegem et al., 2004). Therefore the three deletions are expected to rotate or tilt the I–II loop relative to the channel. Analogous deletions in CaV2.2 have previously been shown to displace secondary α1–β interactions and thus alter β-dependent modulation of N-type (CaV2.2) Ca2+ currents without changing the integrity of the AID (Mitra-Ganguli et al., 2009; Vitko et al., 2008). Immunofluorescence labeling showed that expression and clustering of the three deletion constructs were not significantly different from wild type α1S (α1Sdel1 85±8.2%, α1Sdel2 84.7±4.8%, α1Sdel3 91.3±2.3%, compared with α1S 89±2.1%) (Fig. 4B; supplementary material Fig. S1E–G). More importantly, also co-clustering of the β1a subunit with the three deletion constructs was not altered (α1Sdel1 98.9±1.1%, α1Sdel2 95±1.4%, α1Sdel3 98.3±1.4%, compared with α1S 96.6±1.9%) (Fig. 4C; supplementary material Fig. S3E–G), indicating that changing the orientation of the I–II loop and the β subunit relative to the channel does not affect the formation of channel complexes. Finally, FRAP analysis revealed that deletion of one or more amino acids did not reduce the stability of the complex with β1a-GFP (Fig. 4E; supplementary material Fig. S5). R75 was 20.9±3.2% for α1Sdel1, 19.9±3.8% for α1Sdel2 and 22.8±4.6% for α1Sdel3 and thus in no case significantly different from that of β1a-GFP coexpressed with wild type α1S (Fig. 4G). Together these experiments show that neither changing the I–II loop sequence nor the orientation of the I–II loop relative to the channel reduced the stability of the β1a-GFP/α1S complex in skeletal muscle triads.
Therefore we turned our attention to the β subunit and examined the importance of the α binding pocket by introducing a single residue exchange in β1a (M293A). In previous biochemical and functional studies the equivalent mutation in β2a has been shown to reduce the affinity of binding to AID peptides, but still allowed functional modulation of the channel, when coexpressed in oocytes at sufficiently high local concentrations (Maltez et al., 2005; Opatowsky et al., 2004; Van Petegem et al., 2008). Therefore we expected that on coexpression with α1S in dysgenic myotubes β1aM293A-GFP might still co-assemble with the channel in triads, and thus permit FRAP analysis. Indeed β1aM293A-GFP co-clustered with α1S but at a substantially reduced proportion of only 17.7±4.8% of myotubes with α1S clusters (Fig. 4C; supplementary material Fig. S3H). As expected the affinity-reducing mutation M293A diminish the ability of this β subunit to compete with endogenous β1a for association with the channel complex. Conversely, within the clusters β1aM293A-GFP had a dramatically increased fluorescence recovery. The fractional recovery of β1aM293A-GFP was 3-fold higher (R75, 45.2±3.9%) than that of wild type β1a-GFP (Fig. 4F,G). This indicates that a mutation in the α binding pocket known to reduce the affinity of β1a–α1S binding decreases the stability of the α1–β complex and increases the dynamic exchange of the mutated skeletal muscle β subunit to values similar to those of the non-skeletal muscle β isoforms.
Discussion
Here we used FRAP analysis of Ca2+ channel subunits expressed in dysgenic myotubes to study for the first time the dynamics of CaV α1 and β subunits in the native environment of a functional Ca2+ signaling complex. First, the relative dynamics of α1 and β subunits revealed that β1a forms a stable complex with CaV1 α1 subunits, whereas β2a, β4b and a β1a mutant (M293A) form dynamic complexes with these L-type Ca2+ channels. Secondly, our data suggest that the specific strengths of β association with the Ca2+ channel complex are intrinsic properties of the β subunits, regardless to whether they form homologous or heterologous pairs with the α1 subunit and likely independent of skeletal muscle-specific interactions with the RyR1.
Different β isoforms can form either stable or dynamic complexes with the α1 subunits
The question as to whether auxiliary β subunits can dynamically exchange with functional Ca2+ channels in the membrane has been highly controversial. High affinity binding of all β isoforms with the AID in the I–II loop of high-voltage-activated Ca2+ channels (De Waard et al., 1995; Van Petegem et al., 2008) indicates that α1 and β subunit form essentially irreversible complexes. However, emerging experimental evidence from heterologous expression systems suggests that in cells the α1–β interaction might be reversible (Buraei and Yang, 2010). Injection of β subunits into Xenopus oocytes expressing α1 subunits alone or in combination with another β isoform rapidly altered the gating properties of the Ca2+ currents (Hidalgo et al., 2006; Yamaguchi et al., 1998). Perfusion of skeletal muscle membrane vesicles with purified β1a doubled current densities but not ON gating charges within 15 minutes (García et al., 2002). Injection of competing AID peptide into HEK cells transfected with CaV1.2 and β2a inhibited β modulation of the single channel properties within a few minutes (Hohaus et al., 2000); and HEK cells cotransfected with CaV1.2 plus different ratios of β1a and β2b showed mode shifting in single channel recordings, consistent with the sequential association of distinct β subunits with the channel on a minute time scale (Jangsangthong et al., 2011). Whereas these and similar studies reviewed in (Buraei and Yang, 2010) indicate that in Xenopus oocytes and mammalian cells the α1–β interaction indeed can be reversed, the question as to whether this occurs in native Ca2+ channel signaling complexes remained hitherto unanswered.
Our FRAP analysis addresses this problem in one of the best characterized Ca2+ channel signaling complexes, the skeletal muscle triad. Unexpectedly, the results give a differentiated answer to this question. On the one hand, the homologous skeletal muscle β1a isoform forms stable complexes with CaV1 channels. Both the CaV1.1 α1S subunit and the β1a subunit have similarly low recovery rates, indicating that the two subunits remain stably associated to each other for the entire life time of the channel in the signaling complex. Although it has never before been demonstrated, the fact that homologous Ca2+ channel subunit pairs form stable complexes in its native environment may not appear surprising. But note that the skeletal muscle β1a subunit formed similarly stable complexes with the non-skeletal muscle CaV1.2 α1C subunit. On the other hand, the non-skeletal muscle β2a and β4b isoforms formed dynamic complexes with CaV1 channels in the junctions. Two to three times higher FRAP rates of β2a-eGFP and β4b-eGFP compared with the α1 subunit unambiguously demonstrate that these β isoforms can dynamically exchange with the α1 subunits in the triadic signaling complex on a minute time scale. Interestingly, dynamic interactions were not limited to heterologous α1–β pairs, but were also observed for β2a with its native partner CaV1.2. While such a differential ability to form stable or dynamic subunit complexes would not have been predicted from previous biochemical analysis of α1–β interactions, functionally it appears reasonable. Skeletal muscle expresses only one set of Ca2+ channel subunits and β1a serves primarily structural functions like the organization of tetrads (Schredelseker et al., 2005). Consequently there is no need for dynamic exchange. In contrast, neurons express multiple α1 and β isoforms including β2a and β4b, which confer distinct gating properties to the channels. Consequently, dynamic exchange of β subunits with α1 subunits expressed in the membrane provides a mechanism for current modulation. Recently we found very similar low FRAP recovery rates of α1C Ca2+ channels in somatodendritic Ca2+ channel clusters in hippocampal neurons (Di Biase et al., 2011). Apparently, voltage-gated Ca2+ channels are stably incorporated in signaling complexes of muscle and nerve cells. Whether β2a and β4b subunits also show dynamic exchange in these neuronal Ca2+ channel complexes remains to be shown.
The differential stability of β subunits in Ca2+ channel complexes is an intrinsic property of the β subunits
The observed differences in FRAP rates of β subunits could result from different affinity binding of the AID to the α binding pocket, by secondary binding sites between the two channel subunits, or by interactions with other binding proteins in the triad, foremost the RyR1. The molecular organization of the CaV1.1 channel in skeletal muscle triads and peripheral couplings is unique. It is arranged in tetrad arrays corresponding in size and orientation to the underlying RyR1s with which CaV1.1 physically interacts in the process of skeletal muscle EC-coupling (Franzini-Armstrong et al., 1998). The β1a subunit is essential for the organization of this functional assembly (Schredelseker et al., 2005). Therefore it is reasonable to assume that the same protein–protein interactions contribute to the stable anchoring of the Ca2+ channel subunits in the junctions. However, the stability of β1a-GFP did not decrease when it was coexpressed with the cardiac/neuronal CaV1.2, which does not form tetrads opposite the RyR1. Furthermore, introducing mutations into CaV1.1 expected to rotate the β1a subunit relative to the α1 subunit (Mitra-Ganguli et al., 2009; Vitko et al., 2008) and probably also in relation to the RyR1 did not reduce the stability of β1a association with the complex. Together these observations indicate that the stability of β1a in the triads and its role in tetrad formation are independent of its putative direct interactions with the RyR1, unless such interactions would be highly conformationally flexible. The conclusion that binding to the RyR1 does not substantially contribute to the immobilization of β1a in the triad is consistent with our previous observation that β1a-GFP expressed without an α1 subunit is not targeted into the junctional clusters (Neuhuber et al., 1998a), and is further substantiated by our present FRAP data, showing that β1a-GFP expressed alone recovered at the rate of free eGFP, indicating that it is freely diffusible in the cytoplasm. Thus, its stable anchoring in the triad junctions entirely depends on the coexpression of an α1 subunit and the strength of α1–β interactions in the context of skeletal muscle Ca2+ release units is the same for the homologous CaV1.1 and the heterologous CaV1.2 isoform.
The latter also indicates that the different strengths of α1–β complexes are independent of isoform-specific differences in the α1 subunit I–II loop sequences. The FRAP rates of β1a were equally low when expressed with CaV1.1, CaV1.2 and even α1SI–IIA carrying the I–II loop of CaV2.1. Conversely, the FRAP rates of β2a and β4b were always high regardless of the coexpressed α1 construct. This is consistent with biochemical studies in which similar affinities of β2a to the AID of CaV1.1 and CaV1.2 were measured (Van Petegem et al., 2008). Apparently, differences in the non-conserved residues of the AID and in the flanking sequences of the I–II loop do not explain the different strength of association of β1a versus β2a and β4b. Consequently, the differences appear to be intrinsic properties of the β subunits. This interpretation is substantiated by our experiment in which we mutated the α binding pocket of β1a subunit in position M293. Analogous mutations in β2a have previously been shown to reduce the affinity of binding to AID and expressed channels (Maltez et al., 2005; Opatowsky et al., 2004; Van Petegem et al., 2008). In our study the M293A substitution caused a threefold increase of the fluorescence recovery rate of β1a. This result provides a proof of principle for the suitability of our FRAP analysis to detect differences in α1–β affinity and it demonstrates that the α binding pocket, and thus the interaction with the AID, are crucial for the immobilization of β1a to the triadic Ca2+ channel complex. Nevertheless, it is important to note that the mutated methionine and other key residues of the α binding pocket are conserved between β1a, β2a and β4b, and therefore the intrinsic differences in their ability to form stable and dynamic complexes, respectively, must be determined by non-conserved residues affecting directly or indirectly the affinity of the α binding pocket or secondary interactions with the α1 subunit. As the modulatory functions of β subunits are highly sensitive to mutations in all domains of β (for a review, see Buraei and Yang, 2010), also the molecular mechanism resulting in more or less stable associations of β with the channel complex may arise from allosteric effects on the tertiary structure of β by non-conserved sequences anywhere in the protein.
In conclusion, determining the relative dynamics of Ca2+ channel α1 and β subunits using FRAP analysis represents a new approach to study protein–protein interactions of macromolecular signaling complexes live and in situ, and here it provided the first direct evidence for the dynamic exchange of β subunits within a functional Ca2+ channel complex.
Materials and Methods
Cell culture and transfection
Myotubes of the homozygous dysgenic (mdg/mdg) cell line GLT were cultured as previously described (Powell et al., 1996). At the onset of myoblast fusion, GLT cell cultures were transfected with plasmids coding for the Ca2+ channel subunits using FuGeneHD transfection reagent (Roche Diagnostics) according to the manufacturer’s instructions. A total of 2 μg of plasmid DNA was used per 60 mm culture dish.
Plasmids and cloning procedures
For the expression plasmids, see Table 1. pβA-β2a-eGFP. Rat β2a (GenBank number M80545) was isolated from pβA-β2a-V5 (Obermair et al., 2010) by HindIII/BglII digest and cloned in the respective sites of pβA-β4b-eGFP. pc-a1SI–IIa. Part of the α1S channel with the I–II loop of α1A was isolated from GFP-α1SSk-I–IIa (Flucher et al., 2000b) by SfiI/Bsu36I digest and cloned into the respective sites of pc-α1S. pc-α1Sdel1(Δ344), pc-α1Sdel2(Δ344–345), pc-α1Sdel3(Δ344–346). The deletions of amino acid 344, 344–345, and 344–345–346 of α1S were introduced by SOE-PCR. Briefly for each construct, the I–II loop cDNA sequence of α1S was PCR amplified with overlapping mutagenesis primers in separate PCR reactions using pc-α1S as template. The two separate PCR products were then used as templates for a final PCR reaction with flanking primers to connect the nucleotide sequences. This fragment was then SfiI/Bsu36I digested and cloned into the respective sites of pc-α1S. pcDNA3-β1aM293A-GFP. The mutation in position 293 was introduced by SOE-PCR. Briefly, the cDNA sequence of β1a was PCR amplified with overlapping mutagenesis primers in separate PCR reactions using pcDNA3-β1a-GFP as template. The two separate PCR products were then used as templates for a final PCR reaction with flanking primers to connect the nucleotide sequences. This fragment was then SacI/BamHI digested and cloned into the respective sites of pcDNA3-β1a-GFP.
Table 1. Expression plasmids.
| Plasmid | GenBank number | Promoter | Reference |
|---|---|---|---|
| GFP-α1S | NM_001101720 | CMV | Grabner et al., 1998 |
| GFP-α1C | X15539 | CMV | Grabner et al., 1998 |
| pc-α1S | NM_001101720 | CMV | Neuhuber et al., 1998b |
| pβA-α1C | M67515 | pβA | Di Biase et al., 2011 |
| pcDNA3-β1a-GFP | M25514 | CMV | Neuhuber et al., 1998b |
| pβA-β2a-eGFP | M80545 | pβA | The present study |
| pβA-β2a-V5 | M80545 | pβA | Obermair et al., 2010 |
| pβA-β4b-eGFP | LO2315 | pβA | Subramanyam et al., 2009 |
| pc-α1SI–IIA | CMV | Subramanyam et al., 2009 | |
| pc-α1Sdel1 | CMV | The present study | |
| pc-α1Sdel2 | CMV | The present study | |
| pc-α1Sdel3 | CMV | The present study | |
| pcDNA3-β1aM293A-GFP | CMV | The present study | |
| pβA-eGFP | pβA | Obermair et al., 2004 | |
| GAP-GFP | pAdV | Moriyoshi et al., 1996 |
FRAP experiments and data analysis
FRAP was performed on 9 days old transfected GLT myotubes using a SP-5 confocal microscope (Leica Microsystems) equipped with a 63×, 1.4 NA water-immersion lens at 37°C in an incubation chamber (EMBLEM). Cells growing on coverslips were mounted in a Ludin chamber in Tyrode’s physiological solution containing (in mM): 130 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 30 glucose. For all recordings myotubes with low to medium GFP fluorescence were selected to exclude overexpressing cells. Fluorescence was excited using the 488 nm line of the argon laser and recorded at a bandwidth of 500–550 nm. For GFP-α1S and GFP-α1C, images were acquired at 1.33 Hz in the pre-bleach, bleach and post-bleach phase (respectively 10, 6 and 100 frames) and for extended observation, an additional 30 and 40 frames were acquired at a 3 and 5 s interval, respectively. For all other experiments, images were acquired at 0.67 Hz in the pre-bleach, bleach and post-bleach phase (respectively 10, 3 and 50 frames). For extended observation, an additional 54 frames were acquired at a 5 s interval. For imaging in the pre-bleach and post-bleach phases the laser was set to 15–20% of the initially adjusted laser power (70%). A circular 6 μm diameter ROI was photobleached by scanning with the 488 nm line of argon laser at 100% intensity. Inside the bleached region, three 1.4 μm diameter ROIs were placed over clusters and three in the cluster-free regions in between. The average fluorescence of the cluster-free regions was set as background. The average fluorescence of the three ROIs on the clusters was background subtracted and corrected for the overall bleaching in each time frame. Then the average fluorescence of the clusters was normalized so that the pre-bleach intensity was set to 1 and the first frame after photobleaching to 0 and plotted as function of time (except for cytosolic β1a-GFP, β4b-eGFP and eGFP, where only the pre-bleach intensity was set to 1). The analysis of fluorescence was performed using LAS AF software (Leica Microsystems). Recovery curves were fitted with a straight line or a monoexponential fit with pClamp software (version 8.0, Molecular Devices) and the value of the fitted curve at 75 s after bleaching was chosen to calculate the mean rate of fluorescence recovery (R75). Results are expressed as mean±s.e. All data were organized in MS Excel and analyzed using ANOVA with Tukey post-hoc analysis in SPSS statistical software (SPSS Inc., Chicago IL, USA). Correlation analysis of the average fluorescence intensity of myotubes, as well as the average size and fluorescence intensity of the clusters with the corresponding FRAP (R75) values recorded in the same cell did not reveal any correlation between any of these parameters (supplementary material Fig. S6). This indicated that the variability of expression levels or differences in the subcellular distribution of the constructs cannot account for the observed differences of FRAP values.
Triad targeting and β co-clustering quantification
Paraformaldehyde-fixed cultures were double-immunolabeled [as previously described in (Flucher et al., 2000b)] with the monoclonal α1S antibody mAb 1A (1:4000) (Kugler et al., 2004) and the rabbit anti-GFP (serum, 1:10,000; Molecular Probes, Eugene, OR) and fluorescence-labeled with Alexa-594- and Alexa-488-conjugated secondary antibody, respectively. Thus, the anti-GFP label and the intrinsic GFP signal were both recorded in the green channel. Triad targeting of the α1S chimera and mutants was quantified by systematically screening the coverslips for transfected myotubes using a 63×, 1.4 NA objective Axioimager microscope (Carl Zeiss, Inc.). The labeling patterns of transfected myotubes with more than four nuclei were classified as either ‘clustered’ or ‘not clustered’. Quantitative analysis of β co-clustering was performed by systematically screening for clustered myotubes in the red channel (same criteria described for the triad targeting) and classifying them as β co-clustered or not in the green channel. The counts were obtained from samples of three separate experiments. For RyR staining, in GFP-α1S and GFP-α1C transfected cells, samples were double-immunolabeled with the rabbit anti-GFP (serum, 1:10,000) and mouse monoclonal anti RyR (34-C, 1:1000, Alexis Biochemicals, Lausen, Switzerland), and fluorescence-labeled with Alexa-594- and Alexa-488-conjugated secondary antibody, respectively. In untagged α1S expressing cells, samples were double-immunolabeled with the monoclonal α1S antibody mAb 1A (1:4000) and rabbit anti RyR1 [1:2000; (Flucher et al., 1999)] and fluorescence-labeled with Alexa-594- and Alexa-488-conjugated secondary antibody, respectively. 14-bit images were recorded with cooled CCD cameras (SPOT; Diagnostic Instruments, Stirling Heights, MI, USA) and Metaview image processing software (Universal Imaging, Corp., West Chester, PA, USA).
Image processing
Image composites were arranged in Adobe Photoshop CS3 (Adobe Systems Inc.) and, where necessary, linear adjustments were performed to correct black level and contrast.
Supplementary Material
Acknowledgements
We thank Ariane Benedetti and Roman Egger for excellent technical assistance, Bruno Benedetti for electrophysiology, Gerald Obermair for help with statistical analysis, Martin Offterdinger of the Biooptics Facility for assistance at the confocal microscope and Benedikt Nimmervoll for software assistance.
Funding: This study was supported by the Austrian Science Fund (FWF) [grant numbers P23479-B19 and W01101 to B.E.F. and T443-B18 to V.D.B.].
References
- Buraei Z, Yang J. The β subunit of voltage-gated Ca2+ channels. Physiol. Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Altafaj X, Ronjat M, Coronado R. Interaction between the dihydropyridine receptor Ca2+ channel beta-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 2005;102:19225–19230. doi: 10.1073/pnas.0504334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel beta2a subunit and effects on channel function. J. Biol. Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- De Waard M, Witcher DR, Pragnell M, Liu H, Campbell KP. Properties of the alpha 1-beta anchoring site in voltage-dependent Ca2+ channels. J. Biol. Chem. 1995;270:12056–12064. doi: 10.1074/jbc.270.20.12056. [DOI] [PubMed] [Google Scholar]
- Di Biase V, Tuluc P, Campiglio M, Obermair GJ, Heine M, Flucher BE. Surface traffic of dendritic CaV1.2 calcium channels in hippocampal neurons. J. Neurosci. 2011;31:13682–13694. doi: 10.1523/JNEUROSCI.2300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Conti A, Takeshima H, Sorrentino V. Type 3 and type 1 ryanodine receptors are localized in triads of the same mammalian skeletal muscle fibers. J. Cell Biol. 1999;146:621–630. doi: 10.1083/jcb.146.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Kasielke N, Gerster U, Neuhuber B, Grabner M. Insertion of the full-length calcium channel alpha(1S) subunit into triads of skeletal muscle in vitro. FEBS Lett. 2000a;474:93–98. doi: 10.1016/s0014-5793(00)01583-0. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Kasielke N, Grabner M. The triad targeting signal of the skeletal muscle calcium channel is localized in the COOH terminus of the alpha(1S) subunit. J. Cell Biol. 2000b;151:467–478. doi: 10.1083/jcb.151.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Ramesh V. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. N. Y. Acad. Sci. 1998;853:20–30. doi: 10.1111/j.1749-6632.1998.tb08253.x. [DOI] [PubMed] [Google Scholar]
- García R, Carrillo E, Rebolledo S, García MC, Sánchez JA. The beta1a subunit regulates the functional properties of adult frog and mouse L-type Ca2+ channels of skeletal muscle. J. Physiol. 2002;545:407–419. doi: 10.1113/jphysiol.2002.027433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Beam KG. Tagging with green fluorescent protein reveals a distinct subcellular distribution of L-type and non-L-type Ca2+ channels expressed in dysgenic myotubes. Proc. Natl. Acad. Sci. USA. 1998;95:1903–1908. doi: 10.1073/pnas.95.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner M, Dirksen RT, Suda N, Beam KG. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 1999;274:21913–21919. doi: 10.1074/jbc.274.31.21913. [DOI] [PubMed] [Google Scholar]
- Hidalgo P, Gonzalez-Gutierrez G, Garcia-Olivares J, Neely A. The alpha1-beta-subunit interaction that modulates calcium channel activity is reversible and requires a competent alpha-interaction domain. J. Biol. Chem. 2006;281:24104–24110. doi: 10.1074/jbc.M605930200. [DOI] [PubMed] [Google Scholar]
- Hohaus A, Poteser M, Romanin C, Klugbauer N, Hofmann F, Morano I, Haase H, Groschner K. Modulation of the smooth-muscle L-type Ca2+ channel alpha1 subunit (alpha1C-b) by the beta2a subunit: a peptide which inhibits binding of beta to the I-II linker of alpha1 induces functional uncoupling. Biochem. J. 2000;348:657–665. [PMC free article] [PubMed] [Google Scholar]
- Jangsangthong W, Kuzmenkina E, Böhnke AK, Herzig S. Single-channel monitoring of reversible L-type Ca(2+) channel Ca(V)α(1)-Ca(V)β subunit interaction. Biophys. J. 2011;101:2661–2670. doi: 10.1016/j.bpj.2011.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasielke N, Obermair GJ, Kugler G, Grabner M, Flucher BE. Cardiac-type EC-coupling in dysgenic myotubes restored with Ca2+ channel subunit isoforms alpha1C and alpha1D does not correlate with current density. Biophys. J. 2003;84:3816–3828. doi: 10.1016/S0006-3495(03)75109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler G, Grabner M, Platzer J, Striessnig J, Flucher BE. The monoclonal antibody mAB 1A binds to the excitation--contraction coupling domain in the II-III loop of the skeletal muscle calcium channel alpha(1S) subunit. Arch. Biochem. Biophys. 2004;427:91–100. doi: 10.1016/j.abb.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca(V)beta modulatory properties are AID-independent. Nat. Struct. Mol. Biol. 2005;12:372–377. doi: 10.1038/nsmb909. [DOI] [PubMed] [Google Scholar]
- Mitra-Ganguli T, Vitko I, Perez-Reyes E, Rittenhouse AR. Orientation of palmitoylated CaVbeta2a relative to CaV2.2 is critical for slow pathway modulation of N-type Ca2+ current by tachykinin receptor activation. J. Gen. Physiol. 2009;134:385–396. doi: 10.1085/jgp.200910204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi K, Richards LJ, Akazawa C, O’Leary DD, Nakanishi S. Labeling neural cells using adenoviral gene transfer of membrane-targeted GFP. Neuron. 1996;16:255–260. doi: 10.1016/s0896-6273(00)80044-6. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Gerster U, Döring F, Glossmann H, Tanabe T, Flucher BE. Association of calcium channel alpha1S and beta1a subunits is required for the targeting of beta1a but not of alpha1S into skeletal muscle triads. Proc. Natl. Acad. Sci. USA. 1998a;95:5015–5020. doi: 10.1073/pnas.95.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhuber B, Gerster U, Mitterdorfer J, Glossmann H, Flucher BE. Differential effects of Ca2+ channel beta1a and beta2a subunits on complex formation with alpha1S and on current expression in tsA201 cells. J. Biol. Chem. 1998b;273:9110–9118. doi: 10.1074/jbc.273.15.9110. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Szabo Z, Bourinet E, Flucher BE. Differential targeting of the L-type Ca2+ channel alpha 1C (CaV1.2) to synaptic and extrasynaptic compartments in hippocampal neurons. Eur. J. Neurosci. 2004;19:2109–2122. doi: 10.1111/j.0953-816X.2004.03272.x. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Kugler G, Baumgartner S, Tuluc P, Grabner M, Flucher BE. The Ca2+ channel alpha2delta-1 subunit determines Ca2+ current kinetics in skeletal muscle but not targeting of alpha1S or excitation-contraction coupling. J. Biol. Chem. 2005;280:2229–2237. doi: 10.1074/jbc.M411501200. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Schlick B, Di Biase V, Subramanyam P, Gebhart M, Baumgartner S, Flucher BE. Reciprocal interactions regulate targeting of calcium channel beta subunits and membrane expression of alpha1 subunits in cultured hippocampal neurons. J. Biol. Chem. 2010;285:5776–5791. doi: 10.1074/jbc.M109.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- Powell JA, Petherbridge L, Flucher BE. Formation of triads without the dihydropyridine receptor alpha subunits in cell lines from dysgenic skeletal muscle. J. Cell Biol. 1996;134:375–387. doi: 10.1083/jcb.134.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredelseker J, Di Biase V, Obermair GJ, Felder ET, Flucher BE, Franzini-Armstrong C, Grabner M. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. USA. 2005;102:17219–17224. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam P, Obermair GJ, Baumgartner S, Gebhart M, Striessnig J, Kaufmann WA, Geley S, Flucher BE. Activity and calcium regulate nuclear targeting of the calcium channel beta4b subunit in nerve and muscle cells. Channels. 2009;3:343–355. doi: 10.4161/chan.3.5.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekura H, Paolini C, Franzini-Armstrong C, Kugler G, Grabner M, Flucher BE. Differential contribution of skeletal and cardiac II-III loop sequences to the assembly of dihydropyridine-receptor arrays in skeletal muscle. Mol. Biol. Cell. 2004;15:5408–5419. doi: 10.1091/mbc.E04-05-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc. Natl. Acad. Sci. USA. 2007;104:11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F, Duderstadt KE, Clark KA, Wang M, Minor DL., Jr Alanine-scanning mutagenesis defines a conserved energetic hotspot in the CaValpha1 AID-CaVbeta interaction site that is critical for channel modulation. Structure. 2008;16:280–294. doi: 10.1016/j.str.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitko I, Shcheglovitov A, Baumgart JP, Arias-Olguín II, Murbartián J, Arias JM, Perez-Reyes E. Orientation of the calcium channel beta relative to the alpha(1)2.2 subunit is critical for its regulation of channel activity. PLoS ONE. 2008;3:e3560. doi: 10.1371/journal.pone.0003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Hara M, Strobeck M, Fukasawa K, Schwartz A, Varadi G. Multiple modulation pathways of calcium channel activity by a beta subunit. Direct evidence of beta subunit participation in membrane trafficking of the alpha1C subunit. J. Biol. Chem. 1998;273:19348–19356. doi: 10.1074/jbc.273.30.19348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.