Abstract
Donor-specific alloantibodies (DSA) mediate hyperacute and acute antibody-mediated rejection (AMR), which can lead to early graft damage and loss, and are also associated with chronic AMR and reduced long-term graft survival. Such alloantibodies can be generated by previous exposure to major histocompatibility (MHC) antigens (usually via blood transfusions, previous allografts or pregnancy) or can occur de novo after transplantation. Recent studies also suggest that non-MHC antibodies, including those recognising major histocompatibility complex class I-related chain A (MICA), MICB, vimentin, angiotensin II type I receptor may also have an adverse impact on allograft outcomes. In this review, we consider how the dose, route and context of antigen exposure influences DSA induction and describe factors which control the generation, maintenance and survival of alloantibody-producing plasma cells. Finally, we discuss the implications of these variables on therapeutic approaches to DSA.
Introduction
Donor-specific HLA alloantibodies (DSA) mediate hyperacute, acute and chronic antibody-mediated rejection (AMR) [1,2] and remain a major problem in the field of transplantation. Their presence is associated not only with humoral rejection which can lead to early graft damage and loss, but also with reduced long-term graft survival. Such alloantibodies can be generated by previous exposure to major histocompatibility (MHC) antigens (usually via blood transfusions, previous allografts or pregnancy) or can occur de novo after transplantation. New, sensitive assays for the detection of DSA in the serum of solid organ transplant recipients have implicated antibodies in adverse graft outcomes in many more patients than was previously thought. In particular, chronic microvascular injury mediated by DSA is emerging as a leading cause of progressive loss of function and failure of kidney transplants [3,4•]. The ability to diagnose AMR has been significantly enhanced by the use of anti-C4d staining of allograft biopsies [5], which identifies antibody-associated complement deposition on endothelium, though recent evidence suggests that C4d negative humoral rejection may also occur [6].
An understanding of the variables which alter the likelihood of the development and maintenance of serum DSA is needed in order to develop a logical approach to preventing alloantibody generation, transplanting sensitised patients and treating humoral rejection. In this review we will consider these variables in detail, after first providing an overview of the B cell response to protein antigen. We will consider how the dose, route and context of antigen exposure influences DSA induction. We will then describe factors which control the generation, maintenance and survival of alloantibody-producing plasma cells, and then factors that control the rate of production and half-life of such antibodies in the serum. Finally, we will discuss the implications of these variables on therapeutic approaches directed against DSA.
Overview of the B cell response to protein antigen
B cell activation is a multistep process which starts with B cell recognition of its cognate antigen, and leads to formation of antibody secreting cells or plasma cells. This process relies on the coordinated interaction and migration of different cell types in the secondary lymphoid organs (SLOs).
Antigen transport and recognition
The first step of B cell activation depends on the interaction between the B cell receptor (BCR) and its cognate antigen. The structure of SLOs plays a crucial role in this encounter, providing a platform in which tissue-derived antigens and cells in lymphatic fluid may interact with blood-derived cells, including naïve B cells, from the circulation.
The route of antigen delivery to SLOs and, more precisely, to the B cell follicle is function of the size and nature of the antigen. Direct diffusion of small soluble antigens through the pores of the subcapsular sinus (SCS) of lymph nodes (LNs) has been previously reported [7]. Alternatively, small antigens can reach the B cell follicle via the conduit system, which emerges from the SCS and extends throughout the LN. Afferent lymph circulates through the conduits, which are formed from a network of collagen fibres covered with stromal cells. [8,9••]. B cells can sample antigen from conduits and be directly activated. Alternatively, dendritic cells (DCs) and follicular dendritic cells (FDCs) can take up antigen and present it to B cells.
Large particulate antigens like immune complexes cannot cross the SCS floor nor pass through the conduits and thus need capturing and presenting by accessory cells. FDCs are restricted to the B cell follicle and are closely associated with conduits. They retain antigen on their surface for long periods of time via interaction with complement receptors [10,11] and the inhibitory receptor FcγRIIB [12]. Large antigens are shuttled from the blood to the FDCs in a cell-mediated manner. Marginal zone (MZ) B cells are involved in this process in the spleen. They bind blood-derived antigen in the MZ and then shuttle between the MZ and the follicle. Moreover, blockade of this shuttling leads to a defective antigen loading of FDCs, supporting the importance of MZ B cells in antigen delivery to the FDCs [13•]. In the spleen and the lymph nodes, transport of lymph-derived antigen from the SCS to the follicle relies on the sampling of the SCS and uptake of antigen by specialised macrophages. Cognate B cells can be directly activated by these SCS macrophages and migrate to the T/B border whereas non-cognate B cells uptake antigens from the SCS macrophages and transfer them to FDCs [14-16].
B cells can also interact with antigen presented by resident or newly emigrant DCs in the paracortex following their entrance in the LNs via the high endothelial venules (HEV) [17]. Indeed, immune complex binding to FcγRIIB expressed on DCs leads to recycling in non-degradative compartments and thus presentation of native antigen to B cells [18].
B cell migration to the T/B border
Once activated, B cells need to migrate to the interface between the B follicle and the T-cell zone to receive T cell help (Figure 1). SLOs are highly organized structures characterised by the segregation of T cells and B cells in distinct zones. This segregation is maintained by chemokine gradients and specific stromal cells [19]. B cell compartmentalization is dependent on the interaction between the chemokine receptor CXCR5 and its ligand, CXCL13, which is mainly secreted by FDCs. In the T-cell zone fibroblastic reticular cells (FRCs) are wrapped around the conduits and bind CCL19 and CCL21 promoting the homing and migration of CCR7-expressing T cells. Upon antigen stimulation B cells up-regulate CCR7 whilst maintaining CXCR5 expression, which leads to their migration to the T/B border where they present antigen in the context of MHC class II molecules, stimulating cognate CD4+ T cells. Subsequently, CD4 T cells provide help to B cells by direct cell-cell interaction via costimulatory molecules such as CD40/CD40-L and by cytokine secretion inducing clonal expansion of antigen-experienced B cells and further differentiation (Figures 1).
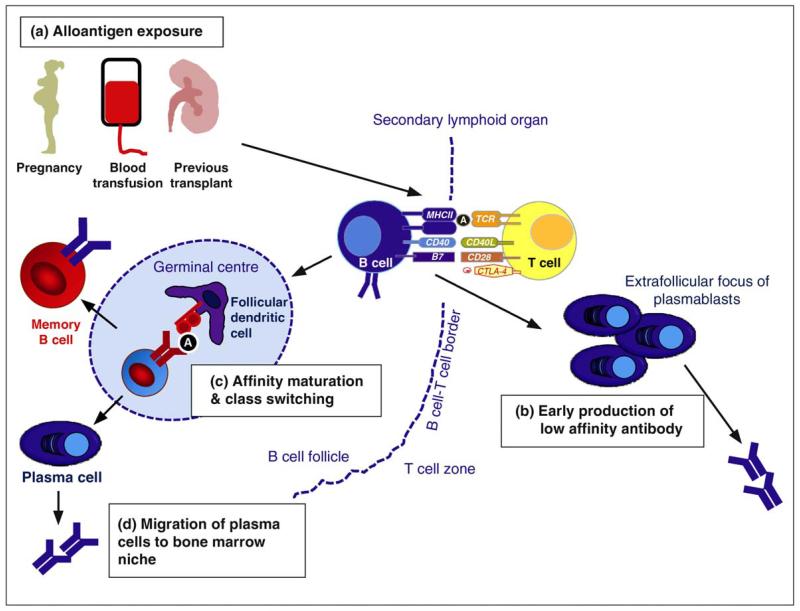
Figure 1.
B cell activation and alloantibody production. (a) Alloantigen exposure occurs via pregnancy, blood transfusions and previous transplants. B cell activation occurs within secondary lymphoid organs (spleen and lymph nodes) and also possibly within tertiary lymphoid organs within allografts. The first step of B cell activation depends on the interaction between the B cell receptor (BCR) and its cognate antigen (A). Once activated, B cells migrate to the interface between the B follicle and the T-cell zone where they present antigen in the context of MHC class II molecules to cognate CD4+ T cells. Activated CD4 T cells provide help to B cells via co-stimulatory molecules such as CD40/CD40-L and by cytokine secretion. After T-dependent activation B cells can undergo two different fates; either they can migrate out of the B cell follicle and form extrafollicular plasmablasts responsible for the early production of low affinity antibody (b), or they enter the germinal centre where they undergo somatic hypermutation and class switch recombination (c). Mutated clones with higher affinity for antigen are positively selected and differentiate into memory B cells or plasma cells. (d) A small proportion of plasma cells arising from the germinal centre migrate to the bone marrow where they become long-lived plasma cells responsible for maintenance of antibody titres.
B cell differentiation
Following T-dependent activation, B cells can undergo two different fates; they may differentiate into extrafollicular plasmablasts which are still dividing, and maintain the ability to migrate or they may enter germinal centres where they undergo somatic hyper mutation and class switch recombination (Figure 1c). Mutated clones with higher affinity for antigen are positively selected and differentiate into either memory B cells (which can be reactivated quickly following a second encounter with antigen) or plasma cells.
Most plasma cells are short-lived and die by apoptosis in a matter of days. These short-lived plasma cells are usually generated after a primary immune response and secrete class switched, low-affinity antibodies. They are generated quickly after antigenic challenge and form a first line of defence against pathogens.
A small proportion of plasma cells arising from the germinal centre and enriched for high affinity variants migrate to the bone marrow where they become fully differentiated long-lived, plasma cells responsible for maintaining antibody titres [20]. The survival of these long-lived plasma cells depends entirely upon their microenvironment as detailed below. Plasma cells have also been described in inflamed tissues for example, in the joints of patients with rheumatoid arthritis, the thymi of those with myasthenia gravis and the kidneys of those with SLE, suggesting that inflammatory lesions may provide supplementary survival niches. Similarly, plasma cells have also been described in renal and cardiac allograft [21-23].
In the context of transplantation, pre-existing donor-specific memory B cells or antibodies could lead to acute rejection. DSA can also be generated following transplantation. Graft-specific antigens may be transported via lymphatics to draining LNs, inducing B cell activation. Moreover, host DCs may infiltrate the graft following transplantation and transport graft antigen to the draining lymph nodes inducing B cell activation. Furthermore, lymphoid neogenesis and formation of tertiary lymphoid organs (TLOs) have been observed in several animal allograft models [21,24] and in human renal and cardiac allograft [21-23] suggesting that B cell activation could occur directly in the graft.
Antigen and alloantibody formation in transplantation
Antigen specificity
Antigens against which antibodies are commonly raised in solid organ transplantation include highly polymorphic molecules which may significantly differ between donor and recipient (for example, human leucocyte antigens (HLA)) as well as non-polymorphic molecules (for example, vimentin). The best-studied targets of alloantibodies are MHC class I and II antigens. Class I HLAs (A, B and C) are expressed on all cells (including graft endothelium) whilst class II (DP, DQ, DR) expression is limited to antigen presenting cells (such as dendritic cells, macrophages and B cells), and may also be variably found on renal endothelial cells. HLA molecules are not equally immunogenic, and some are more likely to initiate a humoral rather than a cellular immune response [25]. The relative immunogenicity is not an intrinsic property of a particular donor HLA molecule, but must be considered in the context of recipient HLA [26,27]. HLA epitopes recognised by alloantibodies are composed of three amino acids (triplets). The likelihood of developing antibodies to HLA class I molecules (either during pregnancy or following transplantation) is directly related to the number of non-self triplets present in antibody accessible sites of the Class I molecule [28,29]. The immunogenicity of an HLA alloantigen depends not only on the number of non-self triplets but also on their physiochemical characteristics. Differences in hydrophobicity and electrostatic charge between mismatched HLA class I molecules strongly influence the likelihood of class I-specific alloantibody formation post-transplantation [30].
The demonstration that higher panel reactive antibody was associated with reduced allograft survival in HLA identical sibling transplants emphasised the potential importance of non-HLA antibodies [31]. Endothelial cells express a number of antigens not found on leucocytes, some of which are polymorphic, for example, major histocompatibility complex class I-related chain A (MICA) and major histocompatibility complex class I-related chain B (MICB). Around 60 MICA alleles have been described and alloantibodies binding MICA have been identified in 11% of transplant recipients [32,33•], can be eluted from kidneys undergoing rejection [34], and their presence before transplantation is associated with an increase in allograft failure and with early graft loss [33•]. MICB is less polymorphic and antibodies directed against MICB have also been identified in the serum of patients awaiting transplantation and in rejecting kidneys [35].
Other non-HLA alloantibody targets include vimentin [36] and angiotensin II type I receptor [37•]. These molecules are not highly polymorphic, hence antibodies binding to them may be considered to be autoantibodies, rather than alloantibodies. In addition, anti-endothelial cell antibodies (of unknown precise specificity) have also been identified in transplant recipients and appear to be associated with worse outcomes [38]. More recently, protein microarrays have been used to identify novel antibody targets in paediatric transplant recipients, including protein kinase Ctζ [39] and a variety of autoantibodies in adult renal transplant recipients with chronic humoral rejection [40•]. In the latter study, there was minimal overlap in antigenic targets between patients, suggesting that each patient had developed autoantibodies to a unique set of antigens. These data imply that patient with chronic AMR may have widespread B cell dysregulation [40•].
Thus, alloantobodies against donor HLA, particularly MHC class I molecules, are well known, widely measured and correlate with rejection and with graft outcome. An increasing number of alloantibodies and autoantibodies are being described in transplantation, and more will no doubt follow. Anti-MHC antibodies will remain the major DSA of concern in transplantation until the functional and pathological importance of antibodies against these more recently described antigenic targets is determined.
Route of antigen exposure
There are three main stimuli which are thought to induce alloantibody production; pregnancy (through exposure to paternal, non-self antigens in the foetus), blood transfusions, and previous transplants (occasionally, following skin grafts, but more usually following solid organ transplantation). Of note, some patients develop HLA antibodies in the absence of any sensitising event [41,42], most likely in response to cross reactive epitopes on microorganisms, ingested proteins, and allergens [43].
Pregnancy
Previous pregnancy is one of the major causes of sensitisation due to exposure to foeto-paternal antigens at the matero-placental interface [44]. In addition, foetal cells enter the maternal circulation [45]. Studies suggest that 30% of single or multiparous women develop antibodies against foeto-paternal HLA during pregnancy [46,47]. Antibodies usually transiently appear in the third trimester [47] and at the time of delivery [48]. In most women, these antibodies disappear soon after delivery but can persist for many years [48]. The mechanisms underlying this variability in initial antibody response and subsequent persistence are unclear. Studies do not show any relationship between number of pregnancies and antibody appearance or persistence [47,48]. One factor which may play a role in maintaining antibody production is the ongoing presence of foeto-paternal antigens due to the persistence of foetal cells in the mother (microchimaerism). Foetal progenitor cells have been found in maternal peripheral blood decades after childbirth, and a third of parous women were found to have some peripheral blood mononuclear cells expressing foetopaternal HLA antigens long after pregnancy [49]. In a study of patients awaiting transplantation, there was evidence of microchimaerism in 66% of sensitised patients versus 25% of non-sensitised patients, suggesting that the presence of non-recipient HLA may drive antibody production in this group [50]. Other possible mechanisms for the persistence of antibodies to foeto-paternal antigens include the sequestration of such antigens by follicular dendritic cells within germinal centres, as has been demonstrated for model antigens [51].
The Class II genotype of the mother has been shown to play a role in determining whether antibodies will be formed against a foeto-paternal HLA class I molecules [52]. Presumably this effect occurs because these class II molecules are involved in the presentation of class I antigens to CD4 T cells, which subsequently provide help to B cells.
In conclusion, MHC class I genotype is the most important factor in determining sensitisation in association with pregnancy. Factors influencing the risk of foeto-maternal sensitisation have not been well defined, and this will be necessary before strategies to reduce risk can be out in place for those likely to subsequently need a transplant.
Blood transfusion
Patients who have received blood transfusions before transplantation have long been known to have worse graft survival [53]. By contrast, peri-operative transfusions (within 10 days of transplantation) do not adversely affect allograft survival, and indeed, may be associated with improved outcomes [54]. Blood transfusions given in the first few months after transplantation also do not appear to effect alloantibody formation [55]. Thus, the timing of antigen exposure appears important in this context. In terms of pre-transplant transfusions, sensitisation is infrequent and usually transient, occurring in around 10% of males who received up to 20 transfusions [56]. However, one or a few blood transfusions can induce broad and persistent HLA sensitisation in patients who have been previously exposed to HLA antigens through pregnancy, prior transplants, or massive transfusions [56,57]. It is therefore prudent to minimise blood transfusion in patients awaiting transplantation.
Previous transplants
Following renal transplantation, a proportion of non-sensitised patients develop HLA alloantibodies for the first time. The reported frequency is very variable, ranging from 1.6 to 60% [58,59]. Non-donor-specific antibodies appear earlier (1–5 years post-transplant) compared with donor-specific antibodies (5–10 years post-transplant) [59]. In larger studies, the development of HLA antibodies was associated with HLA-DR mismatch, pre-transplantation immunization, and acute rejection. In addition, the recipient HLA class II genotype plays a role in determining whether they will develop antibodies against certain donor class I molecules; for example, antibody formation against the HLABw4 epitope occurs more frequently in HLA-DR1 and DR3 positive recipients than in those of other DR genotypes [60]. This suggests that indirect recognition of allopeptides in the context of self-Class II molecules is important in the induction of alloantibody post-transplant.
Following transplant nephrectomy there is an increase in both alloantibody and autoantibody titres [61,62]. It has been suggested that this may be in part due to alloantibody absorption by the failing allograft and in part due to the reduction in immunosuppression which usually accompanies transplant nephrectomy. Whatever the mechanism, the rise in alloantibodies appears more pronounced in patients who have been minimally sensitised before nephrectomy (PRA <20%), and in cases in which nephrectomy was performed within six months of transplantation. In a small study, patients who were highly sensitised (PRA >80%) before nephrectomy had a statistically significant but modest reduction in PRA post-nephrectomy [62].
The adjuvant effect of danger signals
Infections may potentially contribute to the development of alloimmune response through the stimulation of pattern recognition receptors, such as Toll-like receptors (TLRs) on B cells or other antigen presenting cells, such as DCs, leading to the upregulation of co-stimulatory molecules and increased antigen presentation to T cells. CMV is one of the major infections encountered posttransplant, increases the risk of acute cellular rejection, and has been associated with an increase in IgM alloantibody levels [63]. Aielo et al. compared histological changes in transplant biopsies in patients with acute rejection, with and without a history of CMV or EBV infection. They noted an increase in plasma cell infiltrates and C4d staining in those with viral infections [64]. CMV infection has also been temporally associated with the development of anti-endothelial cell antibodies in cardiac and renal allograft recipients, with biopsy-proven humoral rejection [65] and with the appearance of anti-islet cell antibodies in pancreas transplant recipients [66]. In addition to the effects of CMV and EBV, Hepatitis C infection has also been associated with an increase in PRA and hence the risk of antibody-mediated rejection posttransplant [67].
More recently, Locke et al. observed an increase in both breadth and strength of HLA antibodies following systemic inflammatory events, such as bacteraemia and myocardial infarction, in sensitised renal transplant recipients [68•]. Given that the majority of renal transplant recipients develop an infection in the first year post-transplant, this may be a significant factor in enhancing alloantibody production in sensitised patients. It is currently unclear whether this would also apply to non-sensitised patients.
It has been appreciated for more than a decade that the ischaemia-reperfusion injury (IRI) observed following organ transplantation can initiate an alloimmune response [69,70]. The mechanism of this appears to require tissue resident dendritic cells and appears to involve TLR signalling [71,72]. There is little data examining the specific effect of IRI on B cell activation and antibody production; in a rat model of cardiac allograft vasculopathy (a process thought to involve antibody), ischaemia appeared to accelerate progression of vasculopathy, although alloantibody titres were not assessed [73].
To summarise, it is likely that both pathogen-associated and self-associated danger signals can augment an alloantibody response.
Recipient factors affecting alloantibody production
In autoimmunity both environmental and genetically determined host factors confer risk of autoantibody production, and similar factors may influence alloantibody production, though they have not been extensively investigated in the context of transplantation. Some risk factors will be transplant-specific (such as the effect of recipient class II genotype on alloantibody production in pregnancy and post-transplant, as described above) but many are likely to overlap with those driving B cell activation in antibody-mediated autoimmune diseases, for example systemic lupus erythematosus (SLE). Recent genome-wide association studies have implicated more than 40 genetic variants in the development of SLE [74] and many of these are clearly directly implicated in humoral immunity.
B cells can be activated by ligation of surface IgM (the BCR) and the threshold of BCR activation can be modulated by other receptors, both activatory and inhibitory. B cell inhibitory receptors include FcγRIIB, CD22, CD72, programmed cell death gene-1 (PDCD1) (or PD-1 as it is known in mice), PIR-B, CD5, CD66a, LAIR-1, and ILT-2 [75]. Overall, mice deficient in these molecules have hyperactive B cells and produce autoantibodies. Polymorphisms in these receptors may well effect alloantibody production in humans, although this has not yet been demonstrated. FcγRIIB is of particular interest in the context of alloantibody production, because it controls BCR activation via IgG immune complexes, cross-linking of FcγRIIB on plasma cells leads to plasma cell apoptosis [76•], and polymorphisms that reduce its function are associated with SLE in both mice and humans [77,78]. Genome-wide association studies in SLE have also implicated other genes involved in signalling downstream of the BCR, including the kinases B-lymphoid tyrosine kinase (BLK) and Lyn, and B cell scaffold protein with ankyrin repeats (BANK1) [74]. Genetic variants in these molecules might also alter alloantibody production.
B cell activity factor (BAFF, also known as BLyS, TALL-1 and THANK) is a member of the TNF ligand super-family and an important co-stimulator of B cell survival and expansion. Transgenic animals over-expressing BAFF in lymphoid cells develop autoantibodies and high levels of BAFF have been identified in patients with lupus and appear to be associated with autoantibody production [79,80]. There is also some data linking BAFF with both acute rejection and chronic decline in human renal allograft function. Xu et al. demonstrated the presence of BAFF by immunohistochemistry in renal transplant biopsies taken from ten patients with acute rejection. BAFF staining appeared to correlate with C4d staining [81]. Furthermore, in a small study of patients who were five or more years post-transplantation, surface expression of BAFF on CD4 T cells was associated with abnormal renal function. Elevated serum BAFF levels have also been noted in transplant patients following alemtuzumab treatment, and may partially explain the increased incidence of humoral rejection observed in these patients [82]. The human BAFF gene contains a number of single nucleotide polymorphisms (SNPs) which affect gene expression and might well alter susceptibility to alloantibody production.
Interleukin (IL-6) is a cytokine which can promote TH2 cell differentiation and the development of a humoral immune response. A SNP (rs1800795) at position 174 within the promoter region of the IL-6 gene has been associated with autoimmunity and altered plasma levels of IL-6. Martin et al. observed a significant association between this SNP and the development of donor-specific HLA antibodies post-transplant [83]. The same group also showed that an SNP in the gene encoding Class II transactivator, a master regulator of class II expression is also associated with alloantibody production post-transplant [83].
Many other host genetic factors are likely to drive risk of alloantibody production — for example those promoting increased T cell impact on the B cell response such as abnormalities of the CD40–CD40L pathway or increased T follicular helper cell function, both of which have been associated with SLE. Determination of these factors, which may be one benefit to come from the GWAS studies now under way in transplantation, may eventually allow us to more accurately define individual risk, allowing targeting preventative measures and immunosuppression strategies, and perhaps discovering novel pathways for therapeutic intervention.
Maintenance of alloantibody titres
Most serum antibody is made by long-lived bone marrow plasma cells, thus factors influencing the rate of plasma cell generation and plasma cell survival will be important in determining IgG titre [20]. Plasma cell survival relies on a combination of soluble survival factors including IL-6, CXCL12, and the TNF superfamily members TNFα, BAFF and APRIL [20,84] (Figure 2). Cross-linking of the inhibitory receptor FcγRIIB induces plasma cell apoptosis [76], while adhesion molecules CD44 and VLA-4 promote plasma cell survival [20], suggesting a role for cell-cell interaction in this process. Several aspects of the ’cellular niche’ in which plasma cells are thought to reside have been described. In the spleen and lymph nodes plasma cells are associated with different sub-populations of myeloid cells [85,86], with APRIL-secreting neutrophils in the mucosa [87], and some with megakaryocytes in the bone marrow although other cells, such as CXCL12-expressing stromal cells, are also likely to be involved [88,89].
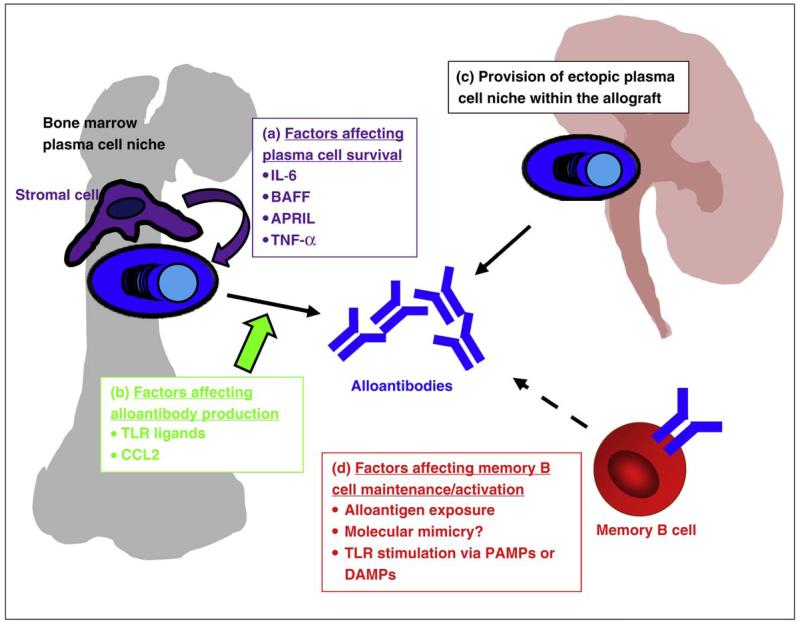
Figure 2.
Factors affecting alloantibody production - Alloantibodies are produced by plasma cells. Alloantibody titres will therefore by determined by: (a) The provision of plasma cell survival factors by stromal cells within bone marrow niches, for example cytokines such as IL-6 and BAFF. (b) The rate of alloantibody production by plasma cells, which may be increased by TLR stimulation and blocked by CCL2. (c) The provision of additional, ectopic plasma cell niches within rejecting, inflamed allograft. (d) Factors affecting memory B cell activation.
Plasma cells have also been identified in cardiac [23] and renal allografts where they are associated with poor prognosis [90,91•,92]. Formation of tertiary lymphoid organs (TLOs) may support the formation of HLA specific plasma cells directly in the graft (Figure 2). In an aortic allograft model in the rat, there was some evidence that more alloantibodies were generated by the graft than by draining nodes [21]. While specificity of bone marrow-derived plasma cells correlated with that of serum alloantibody [93], this may not be the case for graft-derived alloantibodies [91•,93], and analysis of their kinetics, longevity, antigen specificity and ’niche’ of the latter is required.
The maintenance of alloantibody titre will also depend on the production rate per plasma cell, and on antibody half-life once in the circulation. Very little is known about the control of the rate of antibody production per cell over time, though TLR ligands have been reported to activate antibody production by human plasma cells [94], and CCL2 produced by mesenchymal stromal cells can block antibody production [95] (Figure 2).
Alloreactive B cell memory — a requirement for antigen?
Germinal centres produce both long-lived plasma cells and memory B cells. The latter are often isotype-switched, and have undergone somatic mutation and selection resulting in affinity maturation. They are thought to comprise between 40 and 50% of circulating peripheral B cells in humans, and it is likely that as yet undescribed sub-populations of memory B cells may be present in secondary lymphoid organs and other tissues. It would be expected that in any patient with detectable DSA there would be alloantigen-specific memory B cells. This supposition has recently been confirmed by the directed detection of alloantigen-specific memory B cells in transplant patients, using HLA tetramers [96,97].
The maintenance of memory B cells in the circulation has been elegantly shown not to require continual antigen exposure [98], though ongoing contact with antigen may determine the relative size of different antigen-specific memory cell populations [99,100]. Reports that memory B cell reactivation and differentiation to plasma cells might be caused by TLR-driven ’bystander activation’, rather than by specific antigen [94], have not been confirmed [76•] and specific memory cell reactivation upon transplantation is likely to be a major source of increased DSA. This likelihood requires careful consideration when designing therapies for the transplantation of sensitised patients (for example, as a proportion of memory B cells may not express CD20, Rituximab may not be an effective agent in this context).
Although B cells can induce antibody formation and support T-cell immune responses, a sub-population may, in certain circumstances, act as inhibitory cells. This was first demonstrated in the mouse [101], and it has also been suggested that these cells may be found in humans and be important in controlling autoimmune disease [102]. Whether these cells represent a distinct lineage analogous to FoxP3 positive regulatory T-cells, and whether or not they are of relevance to transplantation, remains to be determined.
Therapeutic implications
Current standard of care in renal transplantation includes HLA antibody monitoring by solid phase assay both before and after transplantation, and for cytotoxic and flow cytometric cross-match testing before transplantation [103,104]. Patients with graft dysfunction undergo allograft biopsy, now routinely assessed for C4d deposition [5]. Such intensive DSA monitoring and the emergence of DSA as important mediators of acute and chronic graft damage [3,4•,105] have highlighted the need for effective therapies, particularly in three clinical situations:
Prophylactic therapy against AMR in patients with DSA against a potential live donor (desensitisation) [106]
Treatment of acute AMR [107]
Treatment of chronic antibody-mediated graft damage
Currently available therapies are targeted at DSA removal and prevention of DSA synthesis.
Antibody removal
Plasma exchange (PEX) has been widely used to remove DSA before transplantation [108] and to treat AMR [109,110]. PEX removes DSA from the circulation, but does not prevent ongoing DSA synthesis by plasma cells. Consequently there is a high incidence of AMR (40%) once PEX is discontinued, and emerging evidence of chronic DSA-mediated graft damage leading to reduced graft survival in many patients [111], emphasising the need for therapies designed to prevent ongoing DSA synthesis.
Intravenously immunoglubulin (IVIG) has been used as an adjunct to PEX, or in place of PEX. Low dose IVIG (100 mg/kg) is often given at the end of each PEX treatment in the hope of preventing DSA re-synthesis [108]. High dose IVIG (2 g/kg), administered without PEX, effectively reduces DSA titre when administered before transplantation [112], and has been used as treatment for AMR [113,114]. The mechanisms by which IVIG reduces DSA titre are unclear, but may include plasma cell apoptosis induced by FcγRIIB ligation and cross-linking [76•] (reviewed in [115]). The efficacy of IVIG is enhanced if co-administered with Rituximab [112].
Prevention of DSA re-synthesis
Until recently, and with the possible exception of IVIG, there have been no therapies targeted against plasma cells. Rituximab effectively depletes B cells and thus may block the development of new DSA-secreting plasma cells. Rituximab has been used for the treatment of AMR [116], in patients with steroid resistant rejection [117], and in conjunction with IVIG for the prevention of AMR [112]. It has also been used as part of the conditioning regimens before ABO blood group incompatible kidney transplantation [118,119], although recent reports suggest no additional benefit of Rituximab when added to PEX-based protocols [120]. Anti-thymocyte globulin (ATG) contains antibodies which can bind syndecan (CD138), a plasma cell-specific molecule [121], although in vivo ATG treatment is not associated with a reduction in either splenic or bone marrow plasma cells [93,122] Clinical studies suggest that ATG may reduce the risk of AMR in patients with preformed DSA [123].
Future therapies (Figure 3)
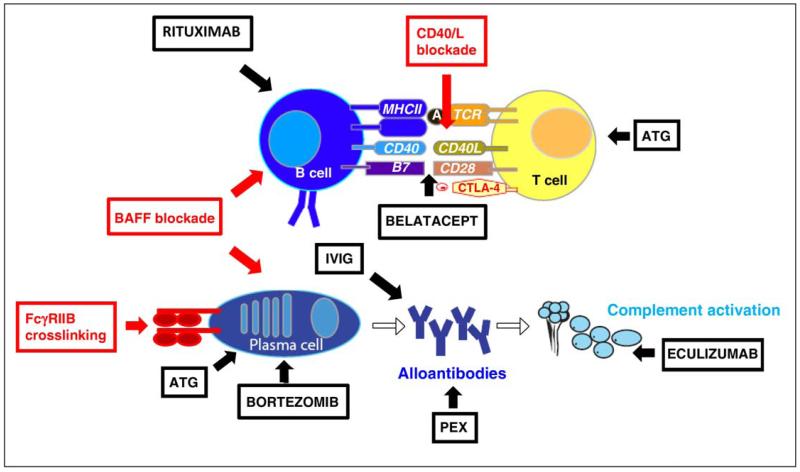
Figure 3.
Current (shown in black) and potential (shown in red) alloantibody-targeted therapies in transplantation. Current management strategies include: (a) antibody removal, using plasma exchange (PEX) or intravenous immunoglobulin (IVIG). (b) the prevention of DSA re-synthesis by depleting B cells using rituximab, depriving B cells of T cell help through T cell depletion using ATG, or depleting plasma cells with bortezomib. (c). Prevention of antibody mediated complement activation (eculizumab). Potential future therapeutic targets include blockade of survival factors for plasma cells and B cells, particularly those provided via BAFF or APRIL, blockade of T cell costimulation using belatacept or CD40L antagonist, or cross-linking of FcyRIIB on plasma cells to induce apoptosis.
A promising new therapy is the proteasome inhibitor Bortezomib, which kills alloantibody-producing plasma cells in vitro [124•], may reduce anti-HLA antibodies in sensitised patients and has been used in a small number of cases to treat antibody-mediated rejection. An alternative to antibody elimination is to block antibody-mediated graft injury. Eculizumab is an antibody directed against the complement component C5 and is effective in preventing complement-mediated red cell lysis in patients with paroxysmal nocturnal haemoglobinuria. Unpublished reports suggest that Eculizumab is also effective in preventing AMR in renal transplant recipients transplanted despite a positive XM.
Whether or not Bortezomib or Eculizumab proves effective, there is no question that new therapies for acute and chronic DSA-mediated graft damage are required. PEX or IVIG-based protocols effectively reduce DSA titre to permit transplantation despite preformed DSA, but with an unacceptable 40% incidence of AMR, a predictor poor graft survival. As yet no therapy has proven effective in treating chronic DSA-mediated graft injury. A greater understanding of the factors controlling alloantibody production will be required to achieve this goal.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 2.Gloor J, Cosio F, Lager DJ, Stegall MD. The spectrum of antibody-mediated renal allograft injury: implications for treatment. Am J Transplant. 2008;8:1367–1373. doi: 10.1111/j.1600-6143.2008.02262.x. [DOI] [PubMed] [Google Scholar]
- 3.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520–2531. doi: 10.1111/j.1600-6143.2009.02799.x. [DOI] [PubMed] [Google Scholar]
- 4•.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68–74. doi: 10.1097/TP.0b013e3181e065de. [These two papers (Refs. [3,4 •]) provide the first robust evidence that alloantibodies are responsible for long-term allograft damage.] [DOI] [PubMed] [Google Scholar]
- 5.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 6.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 7.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Bajenoff M, Germain RN. B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells. Blood. 2009;114:4989–4997. doi: 10.1182/blood-2009-06-229567. [(Roozendaal et al. [8] + Bajénoff et al. [9••]): Together these two studies describe the nature and origin of the conduit system in B cell follicles. They also elegantly demonstrate the role of the conduit system in small Ag delivery to FDCs and cognate B cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 11.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcgammaRIIB receptors. J Exp Med. 2002;196:1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin D, Wu J, Vora KA, Ravetch JV, Szakal AK, Manser T, Tew JG. Fc gamma receptor IIB on follicular dendritic cells regulates the B cell recall response. J Immunol. 2000;164:6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 13•.Cinamon G, Zachariah MA, Lam OM, Foss FW, Jr, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [This paper demonstrates that MZ B cells constantly shuttle between the MZ and the follicle depending on their expression of S1P1 and S1P3 and of CXCR5 respectively. This shuttling is necessary for normal antigen capture in the MZ and antigen delivery to the FDCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 16.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 17.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 18.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 21.Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF, Mandet C, Belair MF, Bruneval P, Meilhac O, et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA. 2005;102:14723–14728. doi: 10.1073/pnas.0507223102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerjaschki D, Regele HM, Moosberger I, Nagy-Bojarski K, Watschinger B, Soleiman A, Birner P, Krieger S, Hovorka A, Silberhumer G, et al. Lymphatic neoangiogenesis in human kidney transplants is associated with immunologically active lymphocytic infiltrates. J Am Soc Nephrol. 2004;15:603–612. doi: 10.1097/01.asn.0000113316.52371.2e. [DOI] [PubMed] [Google Scholar]
- 23.Wehner JR, Fox-Talbot K, Halushka MK, Ellis C, Zachary AA, Baldwin WM., III B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89:1141–1148. doi: 10.1097/TP.0b013e3181d3f271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baddoura FK, Nasr IW, Wrobel B, Li Q, Ruddle NH, Lakkis FG. Lymphoid neogenesis in murine cardiac allografts undergoing chronic rejection. Am J Transplant. 2005;5:510–516. doi: 10.1111/j.1600-6143.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 25.Claas FH, Roelen DL, Mulder A, Doxiadis II, Oudshoorn M, Heemskerk M. Differential immunogenicity of HLA class I alloantigens for the humoral versus the cellular immune response: “towards tailor-made HLA mismatching”. Hum Immunol. 2006;67:424–429. doi: 10.1016/j.humimm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Doxiadis II, Smits JM, Schreuder GM, Persijn GG, van Houwelingen HC, van Rood JJ, Claas FH. Association between specific HLA combinations and probability of kidney allograft loss: the taboo concept. Lancet. 1996;348:850–853. doi: 10.1016/s0140-6736(96)02296-9. [DOI] [PubMed] [Google Scholar]
- 27.Dankers MK, Roelen DL, Van Der Meer-Prins EM, De Lange P, Korfage N, Smits JM, Persijn GG, Welsh KI, Doxiadis II, Claas FH. Differential immunogenicity of HLA mismatches: HLA-A2 versus HLA-A28. Transplantation. 2003;75:418–420. doi: 10.1097/01.TP.0000044456.51462.E2. [DOI] [PubMed] [Google Scholar]
- 28.Dankers MK, Witvliet MD, Roelen DL, de Lange P, Korfage N, Persijn GG, Duquesnoy R, Doxiadis II, Claas FH. The number of amino acid triplet differences between patient and donor is predictive for the antibody reactivity against mismatched human leukocyte antigens. Transplantation. 2004;77:1236–1239. doi: 10.1097/01.tp.0000120385.03278.28. [DOI] [PubMed] [Google Scholar]
- 29.Kosmoliaptsis V, Bradley JA, Sharples LD, Chaudhry A, Key T, Goodman RS, Taylor CJ. Predicting the immunogenicity of human leukocyte antigen class I alloantigens using structural epitope analysis determined by HLAMatchmaker. Transplantation. 2008;85:1817–1825. doi: 10.1097/TP.0b013e31817441d6. [DOI] [PubMed] [Google Scholar]
- 30.Kosmoliaptsis V, Chaudhry AN, Sharples LD, Halsall DJ, Dafforn TR, Bradley JA, Taylor CJ. Predicting HLA class I alloantigen immunogenicity from the number and physiochemical properties of amino acid polymorphisms. Transplantation. 2009;88:791–798. doi: 10.1097/TP.0b013e3181b4a9ff. [DOI] [PubMed] [Google Scholar]
- 31.Opelz G. Non-HLA transplantation immunity revealed by lymphocytotoxic antibodies. Lancet. 2005;365:1570–1576. doi: 10.1016/S0140-6736(05)66458-6. [DOI] [PubMed] [Google Scholar]
- 32.Zwirner NW, Marcos CY, Mirbaha F, Zou Y, Stastny P. Identification of MICA as a new polymorphic alloantigen recognized by antibodies in sera of organ transplant recipients. Hum Immunol. 2000;61:917–924. doi: 10.1016/s0198-8859(00)00162-2. [DOI] [PubMed] [Google Scholar]
- 33•.Zou Y, Stastny P, Susal C, Dohler B, Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293–1300. doi: 10.1056/NEJMoa067160. [This paper, together with Dragun et al. [37•] was the first to demonstrate in large patient cohort robust associations between non-HLA antibodies and adverse outcomes in transplant recipients.] [DOI] [PubMed] [Google Scholar]
- 34.Zou Y, Heinemann FM, Grosse-Wilde H, Sireci G, Wang Z, Lavingia B, Stastny P. Detection of anti-MICA antibodies in patients awaiting kidney transplantation, during the post-transplant course, and in eluates from rejected kidney allografts by Luminex flow cytometry. Hum Immunol. 2006;67:230–237. doi: 10.1016/j.humimm.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Quiroga I, Salio M, Koo DD, Cerundolo L, Shepherd D, Cerundolo V, Fuggle SV. Expression of MHC class I-related Chain B (MICB) molecules on renal transplant biopsies. Transplantation. 2006;81:1196–1203. doi: 10.1097/01.tp.0000205788.05322.42. [DOI] [PubMed] [Google Scholar]
- 36.Carter V, Shenton BK, Jaques B, Turner D, Talbot D, Gupta A, Chapman CE, Matthews CJ, Cavanagh G. Vimentin antibodies: a non-HLA antibody as a potential risk factor in renal transplantation. Transplant Proc. 2005;37:654–657. doi: 10.1016/j.transproceed.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 37•.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [This paper, together with Zou et al. [33•] was the first to demonstrate in large patient cohort robust associations between non-HLA antibodies and adverse outcomes in transplant recipients.] [DOI] [PubMed] [Google Scholar]
- 38.Sun Q, Liu Z, Chen J, Chen H, Wen J, Cheng D, Li L. Circulating anti-endothelial cell antibodies are associated with poor outcome in renal allograft recipients with acute rejection. Clin J Am Soc Nephrol. 2008;3:1479–1486. doi: 10.2215/CJN.04451007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutherland SM, Li L, Sigdel TK, Wadia PP, Miklos DB, Butte AJ, Sarwal MM. Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int. 2009;76:1277–1283. doi: 10.1038/ki.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Porcheray F, Devito J, Yeap BY, Xue L, Dargon I, Paine R, Girouard TC, Saidman SL, Colvin RB, Wong W, et al. Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation. 2010;89:1239–1246. doi: 10.1097/TP.0b013e3181d72091. [This paper uses protein microarray technology to extensively characterise the antigen specificity of alloantibodies in chronic rejectors and surprisingly showed few common antigenic targets pointing to broad B cell dysfunction in this group.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins ZV, Arnold PF, Peetoom F, Smith GS, Walford RL. A naturally occurring monospecific anti-HL-A8 isoantibody. Tissue Antigens. 1973;3:358–363. doi: 10.1111/j.1399-0039.1973.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 42.Morales-Buenrostro LE, Terasaki PI, Marino-Vazquez LA, Lee JH, El-Awar N, Alberu J. “Natural” human leukocyte antigen antibodies found in nonalloimmunized healthy males. Transplantation. 2008;86:1111–1115. doi: 10.1097/TP.0b013e318186d87b. [DOI] [PubMed] [Google Scholar]
- 43.Hirata AA, Terasaki PI. Cross-reactions between streptococcal M proteins and human transplantation antigens. Science. 1970;168:1095–1096. doi: 10.1126/science.168.3935.1095. [DOI] [PubMed] [Google Scholar]
- 44.Sanfilippo F, Vaughn WK, Bollinger RR, Spees EK. Comparative effects of pregnancy, transfusion, and prior graft rejection on sensitization and renal transplant results. Transplantation. 1982;34:360–366. doi: 10.1097/00007890-198212000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Lo YM, Lo ES, Watson N, Noakes L, Sargent IL, Thilaganathan B, Wainscoat JS. Two-way cell traffic between mother and fetus: biologic and clinical implications. Blood. 1996;88:4390–4395. [PubMed] [Google Scholar]
- 46.Suciu-Foca N, Reed E, Rohowsky C, Kung P, King DW. Anti-idiotypic antibodies to anti-HLA receptors induced by pregnancy. Proc Natl Acad Sci USA. 1983;80:830–834. doi: 10.1073/pnas.80.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regan L, Braude PR, Hill DP. A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Hum Reprod. 1991;6:294–298. doi: 10.1093/oxfordjournals.humrep.a137325. [DOI] [PubMed] [Google Scholar]
- 48.van Kampen CA, Versteeg-vd Voort Maarschalk MF, Langerak-Langerak J, Roelen DL, Claas FH. Kinetics of the pregnancy-induced humoral and cellular immune response against the paternal HLA class I antigens of the child. Hum Immunol. 2002;63:452–458. doi: 10.1016/s0198-8859(02)00396-8. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–708. doi: 10.1073/pnas.93.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SivaSai KS, Jendrisak M, Duffy BF, Phelan D, Ravenscraft M, Howard T, Mohanakumar T. Chimerism in peripheral blood of sensitized patients waiting for renal transplantation: clinical implications. Transplantation. 2000;69:538–544. doi: 10.1097/00007890-200002270-00013. [DOI] [PubMed] [Google Scholar]
- 51.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dankers MK, Roelen DL, Nagelkerke NJ, de Lange P, Persijn GG, Doxiadis II, Claas FH. The HLA-DR phenotype of the responder is predictive of humoral response against HLA class I antigens. Hum Immunol. 2004;65:13–19. doi: 10.1016/j.humimm.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Sanfilippo F, Vaughn WK, Bollinger RR, Spees EK. The influence of pretransplant transfusions, using different blood products, on patient sensitization and renal allograft survival. Transplantation. 1984;37:350–356. doi: 10.1097/00007890-198404000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Sanfilippo F, Spees EK, Vaughn WK. The timing of pretransplant transfusions and renal allograft survival. Transplantation. 1984;37:344–350. doi: 10.1097/00007890-198404000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Scornik JC, Schold JD, Bucci M, Meier-Kriesche HU. Effects of blood transfusions given after renal transplantation. Transplantation. 2009;87:1381–1386. doi: 10.1097/TP.0b013e3181a24b96. [DOI] [PubMed] [Google Scholar]
- 56.Opelz G, Graver B, Mickey MR, Terasaki PI. Lymphocytotoxic antibody responses to transfusions in potential kidney transplant recipients. Transplantation. 1981;32:177–183. doi: 10.1097/00007890-198109000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Scornik JC, Brunson ME, Howard RJ, Pfaff WW. Alloimmunization, memory, and the interpretation of crossmatch results for renal transplantation. Transplantation. 1992;54:389–394. doi: 10.1097/00007890-199209000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trial. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 59.Hourmant M, Cesbron-Gautier A, Terasaki PI, Mizutani K, Moreau A, Meurette A, Dantal J, Giral M, Blancho G, Cantarovich D, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16:2804–2812. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- 60.Fuller TC, Fuller A. The humoral immune response against an HLA class I allodeterminant correlates with the HLA-DR phenotype of the responder. Transplantation. 1999;68:173–182. doi: 10.1097/00007890-199907270-00002. [DOI] [PubMed] [Google Scholar]
- 61.McCarty GA, King LB, Sanfilippo F. Autoantibodies to nuclear, cytoplasmic, and cytoskeletal antigens in renal allograft rejection. Transplantation. 1984;37:446–451. doi: 10.1097/00007890-198405000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Khakhar AK, Shahinian VB, House AA, Muirhead N, Hollomby DJ, Leckie SH, McAlister VC, Chin JL, Jevnikar AM, Luke PP. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35:862–863. doi: 10.1016/s0041-1345(02)04031-9. [DOI] [PubMed] [Google Scholar]
- 63.Matinlauri IH, Kyllonen LE, Eklund BH, Koskimies SA, Salmela KT. Weak humoral posttransplant alloresponse after a well-HLA-matched cadaveric kidney transplantation. Transplantation. 2004;78:198–204. doi: 10.1097/01.tp.0000128190.08238.a1. [DOI] [PubMed] [Google Scholar]
- 64.Aiello FB, Calabrese F, Rigotti P, Furian L, Marino S, Cusinato R, Valente M. Acute rejection and graft survival in renal transplanted patients with viral diseases. Mod Pathol. 2004;17:189–196. doi: 10.1038/modpathol.3800033. [DOI] [PubMed] [Google Scholar]
- 65.Toyoda M, Galfayan K, Galera OA, Petrosian A, Czer LS, Jordan SC. Cytomegalovirus infection induces anti-endothelial cell antibodies in cardiac and renal allograft recipients. Transpl Immunol. 1997;5:104–111. doi: 10.1016/s0966-3274(97)80050-0. [DOI] [PubMed] [Google Scholar]
- 66.Zanone MM, Favaro E, Quadri R, Miceli I, Giaretta F, Romagnoli R, David E, Perin PC, Salizzoni M, Camussi G. Association of cytomegalovirus infections with recurrence of humoral and cellular autoimmunity to islet autoantigens and of type 1 diabetes in a pancreas transplanted patient. Transpl Int. 2010;23:333–337. doi: 10.1111/j.1432-2277.2009.00994.x. [DOI] [PubMed] [Google Scholar]
- 67.Forman JP, Tolkoff-Rubin N, Pascual M, Lin J, Hepatitis C. acute humoral rejection, and renal allograft survival. J Am Soc Nephrol. 2004;15:3249–3255. doi: 10.1097/01.ASN.0000145896.16153.43. [DOI] [PubMed] [Google Scholar]
- 68•.Locke JE, Zachary AA, Warren DS, Segev DL, Houp JA, Montgomery RA, Leffell MS. Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant. 2009;9:2136–2139. doi: 10.1111/j.1600-6143.2009.02764.x. [This paper is the first to convincingly demonstrate that non-viral infectious stimuli and non-infectious stimuli may influence alloantibody production.] [DOI] [PubMed] [Google Scholar]
- 69.Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation. 2005;79:505–514. doi: 10.1097/01.tp.0000153160.82975.86. [DOI] [PubMed] [Google Scholar]
- 70.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation. 2005;79:1370–1377. doi: 10.1097/01.tp.0000158355.83327.62. [DOI] [PubMed] [Google Scholar]
- 71.Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, Tobita K, Zuckerbraun BS, McCurry KR, Murase N, et al. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 73.Knight RJ, Dikman S, Liu H, Martinelli GP. Cold ischemic injury accelerates the progression to chronic rejection in a rat cardiac allograft model. Transplantation. 1997;64:1102–1107. doi: 10.1097/00007890-199710270-00003. [DOI] [PubMed] [Google Scholar]
- 74.Harley IT, Kaufman KM, Langefeld CD, Harley JB, Kelly JA. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat Rev Genet. 2009;10:285–290. doi: 10.1038/nrg2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pritchard NR, Smith KG. B cell inhibitory receptors and autoimmunity. Immunology. 2003;108:263–273. doi: 10.1046/j.1365-2567.2003.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KGC. FcγRIIB controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [This paper demonstrates that FcγRIIB cross-linking induces bone marrow plasma cell apoptosis in a Bim-dependent manner. Moreover, FcγRIIB expression is strikingly reduced on plasma cells from autoimmune mice strains. These results suggest that FcγRIIB expression on long-lived plasma cells may constitute a late peripheral tolerance check-point.] [DOI] [PubMed] [Google Scholar]
- 77.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, et al. Loss of function of a lupus-associated FcγRIIB polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 78.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pers JO, Daridon C, Devauchelle V, Jousse S, Saraux A, Jamin C, Youinou P. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–39. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 81.Xu H, He X, Sun J, Shi D, Zhu Y, Zhang X. The expression of B-cell activating factor belonging to tumor necrosis factor superfamily (BAFF) significantly correlated with C4D in kidney allograft rejection. Transplant Proc. 2009;41:112–116. doi: 10.1016/j.transproceed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 82.Bloom D, Chang Z, Pauly K, Kwun J, Fechner J, Hayes C, Samaniego M, Knechtle S. BAFF is increased in renal transplant patients following treatment with alemtuzumab. Am J Transplant. 2009;9:1835–1845. doi: 10.1111/j.1600-6143.2009.02710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin J, Worthington J, Harris S, Martin S. The influence of class II transactivator and interleukin-6 polymorphisms on the production of antibodies to donor human leucocyte antigen mismatches in renal allograft recipients. Int J Immunogenet. 2009;36:235–239. doi: 10.1111/j.1744-313X.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 84.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, Maquelin A, Belnoue E, Siegrist CA, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 85.Garcia De Vinuesa C, Gulbranson-Judge A, Khan M, O’Leary P, Cascalho M, Wabl M, Klaus GG, Owen MJ, MacLennan ICM. Dendritic cells associated with plasmablast survival. Eur J Immunol. 1999;29:3712–3721. doi: 10.1002/(SICI)1521-4141(199911)29:11<3712::AID-IMMU3712>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 86.Mohr E, Serre K, Manz RA, Cunningham AF, Khan M, Hardie DL, Bird R, MacLennan IC. Dendritic cells and monocyte/macrophages that create the IL-6/APRIL-rich lymph node microenvironments where plasmablasts mature. J Immunol. 2009;182:2113–2123. doi: 10.4049/jimmunol.0802771. [DOI] [PubMed] [Google Scholar]
- 87.Huard B, McKee T, Bosshard C, Durual S, Matthes T, Myit S, Donze O, Frossard C, Chizzolini C, Favre C, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest. 2008;118:2887–2895. doi: 10.1172/JCI33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, Roth K, Wong DM, Dame C, Tarlinton DM, et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood. 2010;116:1867–1875. doi: 10.1182/blood-2009-12-259457. [DOI] [PubMed] [Google Scholar]
- 90.Xu X, Shi B, Cai M, Han Y, Wang Q, Xu L, Xiao L, Zhou W. A retrospective study of plasma cell infiltrates in explanted renal allografts. Transplant Proc. 2008;40:1366–1370. doi: 10.1016/j.transproceed.2008.03.097. [DOI] [PubMed] [Google Scholar]
- 91•.Thaunat O, Patey N, Caligiuri G, Gautreau C, Mamani-Matsuda M, Mekki Y, Dieu-Nosjean MC, Eberl G, Ecochard R, Michel JB, et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J Immunol. 2010;185:717–728. doi: 10.4049/jimmunol.0903589. [This paper suggests that ectopic formation of germinal centre in the renal allograft involves a transcriptional program similar to that involved in embryonic lymph node formation. Moreover, comparison of alloantibody specificities in the graft and in the serum suggests that local and systemic alloimmune responses may be uncoupled.] [DOI] [PubMed] [Google Scholar]
- 92.Martin L, Charon-Barra C, Bocrie O, Guignier F, D’Athis P, Dautin G, de la Vega MF, Justrabo E, Rifle G, Mousson C. Detection of plasma cells, C4d deposits and donor-specific antibodies on sequential graft biopsies of renal transplant recipients with chronic dysfunction. Transpl Immunol. 2010;22:110–114. doi: 10.1016/j.trim.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Perry DK, Pollinger HS, Burns JM, Rea D, Ramos E, Platt JL, Gloor JM, Stegall MD. Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant. 2008;8:133–143. doi: 10.1111/j.1600-6143.2007.02039.x. [DOI] [PubMed] [Google Scholar]
- 94.Dorner M, Brandt S, Tinguely M, Zucol F, Bourquin JP, Zauner L, Berger C, Bernasconi M, Speck RF, Nadal D. Plasma cell toll-like receptor (TLR) expression differs from that of B cells, and plasma cell TLR triggering enhances immunoglobulin production. Immunology. 2009;128:573–579. doi: 10.1111/j.1365-2567.2009.03143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, Forner K, Boivin MN, Doody K, Tremblay M, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112:4991–4998. doi: 10.1182/blood-2008-07-166892. [DOI] [PubMed] [Google Scholar]
- 96.Mulder A, Eijsink C, Kardol MJ, Franke-van Dijk ME, van der Burg SH, Kester M, Doxiadis II, Claas FH. Identification, isolation, and culture of HLA-A2-specific B lymphocytes using MHC class I tetramers. J Immunol. 2003;171:6599–6603. doi: 10.4049/jimmunol.171.12.6599. [DOI] [PubMed] [Google Scholar]
- 97.Zachary AA, Kopchaliiska D, Montgomery RA, Leffell MS. HLA-specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation. 2007;83:982–988. doi: 10.1097/01.tp.0000259017.32857.99. [DOI] [PubMed] [Google Scholar]
- 98.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636–642. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 99.Gray D, Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988;336:70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- 100.Gray D. A role for antigen in the maintenance of immunological memory. Nat Rev Immunol. 2002;2:60–65. doi: 10.1038/nri706. [DOI] [PubMed] [Google Scholar]
- 101.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 102.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 103.Bray RA, Nolen JD, Larsen C, Pearson T, Newell KA, Kokko K, Guasch A, Tso P, Mendel JB, Gebel HM. Transplanting the highly sensitized patient: the emory algorithm. Am J Transplant. 2006;6:2307–2315. doi: 10.1111/j.1600-6143.2006.01521.x. [DOI] [PubMed] [Google Scholar]
- 104.Cai J, Terasaki PI. Post-transplantation antibody monitoring and HLA antibody epitope identification. Curr Opin Immunol. 2008;20:602–606. doi: 10.1016/j.coi.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 105.Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, Sellares J, Reeve J, Halloran PF. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9:2532–2541. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 106.Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. Am J Transplant. 2010;10:449–457. doi: 10.1111/j.1600-6143.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- 107.Jordan SC, Vo AA, Tyan D, Nast CC, Toyoda M. Current approaches to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:408–415. doi: 10.1111/j.1399-3046.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 108.Warren DS, Montgomery RA. Incompatible kidney transplantation: lessons from a decade of desensitization and paired kidney exchange. Immunol Res. 2010;47:257–264. doi: 10.1007/s12026-009-8157-y. [DOI] [PubMed] [Google Scholar]
- 109.Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 110.Shah A, Nadasdy T, Arend L, Brennan J, Leong N, Coppage M, Orloff M, Demme R, Zand MS. Treatment of C4d-positive acute humoral rejection with plasmapheresis and rabbit polyclonal antithymocyte globulin. Transplantation. 2004;77:1399–1405. doi: 10.1097/01.tp.0000122187.76518.bc. [DOI] [PubMed] [Google Scholar]
- 111.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM, Cosio FG, Gandhi MJ, Kremers W, Stegall MD. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant. 2010;10:582–589. doi: 10.1111/j.1600-6143.2009.02985.x. [DOI] [PubMed] [Google Scholar]
- 112.Vo AA, Peng A, Toyoda M, Kahwaji J, Cao K, Lai CH, Reinsmoen NL, Villicana R, Jordan SC. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89:1095–1102. doi: 10.1097/TP.0b013e3181d21e7f. [DOI] [PubMed] [Google Scholar]
- 113.Luke PP, Scantlebury VP, Jordan ML, Vivas CA, Hakala TR, Jain A, Somani A, Fedorek S, Randhawa P, Shapiro R. Reversal of steroid- and anti-lymphocyte antibody-resistant rejection using intravenous immunoglobulin (IVIG) in renal transplant recipients. Transplantation. 2001;72:419–422. doi: 10.1097/00007890-200108150-00010. [DOI] [PubMed] [Google Scholar]
- 114.Jordan SC, Vo AA, Toyoda M, Tyan D, Nast CC. Post-transplant therapy with high-dose intravenous gammaglobulin: applications to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:155–161. doi: 10.1111/j.1399-3046.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 115.Jordan SC, Toyoda M, Vo AA. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation. 2009;88:1–6. doi: 10.1097/TP.0b013e3181a9e89a. [DOI] [PubMed] [Google Scholar]
- 116.Garrett HE, Jr, Groshart K, Duvall-Seaman D, Combs D, Suggs R. Treatment of humoral rejection with rituximab. Ann Thorac Surg. 2002;74:1240–1242. doi: 10.1016/s0003-4975(02)03824-9. [DOI] [PubMed] [Google Scholar]
- 117.Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996–1001. doi: 10.1111/j.1600-6143.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 118.Sawada T, Fuchinoue S, Teraoka S. Successful A1-to-O ABO-incompatible kidney transplantation after a preconditioning regimen consisting of anti-CD20 monoclonal antibody infusions, splenectomy, and double-filtration plasmapheresis. Transplantation. 2002;74:1207–1210. doi: 10.1097/00007890-200211150-00001. [DOI] [PubMed] [Google Scholar]
- 119.Sonnenday CJ, Warren DS, Cooper M, Samaniego M, Haas M, King KE, Shirey RS, Simpkins CE, Montgomery RA. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004;4:1315–1322. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 120.Segev DL, Simpkins CE, Warren DS, King KE, Shirey RS, Maley WR, Melancon JK, Cooper M, Kozlowski T, Montgomery RA. ABO incompatible high-titer renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5:2570–2575. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 121.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, Bozorgzadeh A, Sanz I, Briggs BJ. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79:1507–1515. doi: 10.1097/01.tp.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 122.Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7:402–407. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 123.Bachler K, Amico P, Honger G, Bielmann D, Hopfer H, Mihatsch MJ, Steiger J, Schaub S. Efficacy of induction therapy with ATG and intravenous immunoglobulins in patients with low-level donor-specific HLA-antibodies. Am J Transplant. 2010;10:1254–1262. doi: 10.1111/j.1600-6143.2010.03093.x. [DOI] [PubMed] [Google Scholar]
- 124•.Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201–209. doi: 10.1111/j.1600-6143.2008.02461.x. [This paper provides evidence that bortezomib may be useful in targeting alloantibody-producing plasma cells, in addition to its previously well-accepted role in treating myeloma.] [DOI] [PubMed] [Google Scholar]