Abstract
FcγRIIB is the only inhibitory Fc receptor. It controls many aspects of immune and inflammatory responses, and variation in the gene encoding this protein has long been associated with susceptibility to autoimmune disease, particularly systemic lupus erythematosus (SLE). FcγRIIB is also involved in the complex regulation of defence against infection. A loss-of-function polymorphism in FcγRIIB protects against severe malaria, the investigation of which is beginning to clarify the evolutionary pressures that drive ethnic variation in autoimmunity. Our increased understanding of the function of FcγRIIB also has potentially far-reaching therapeutic implications, being involved in the mechanism of action of intravenous immunoglobulin, controlling the efficacy of monoclonal antibody therapy and providing a direct therapeutic target.
The receptors for the Fc region of IgG (FcγRs) are expressed by many immune cells (FIG. 1) and are important in both promoting and regulating the immune and inflammatory response to immune complexes. Most FcγRs are activating receptors and include the high-affinity receptor FcγRI and a family of low affinity receptors, including FcγRIIA, FcγRIIC, FcγRIIIA and FcγRIIIB in humans, and FcγRIII and FcγRIV in mice1. FcγRIIB is the only FcγR that has an inhibitory function.
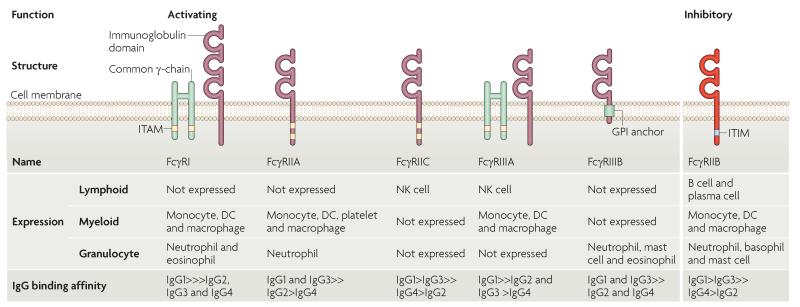
Figure 1. Structure, cellular distribution and IgG isotype-binding affinity of human activating and inhibitory FcγRs.
Human Fc receptors for IgG (FcγRs) differ in function, affinity for the Fc fragment of antibody and in cellular distribution. There are five activating FcγRs: the high-affinity receptor FcγRI, which can bind monomeric IgG, and four low-affinity receptors (FcγRIIA, FcγRIIC, FcγRIIIA and FcγRIIIB), which bind only immune-complexed IgG. Cross-linking of activating FcγRs by immune complexes results in the phosphorylation of immunoreceptor tyrosine-based activating motifs (ITAMs) that are present either in the cytoplasmic domain of the receptor (FcγRIIA and FcγRIIC), or in the associated FcR common γ-chain (FcγRI and FcγRIIIA), resulting in an activating signalling cascade. FcγRIIIB is a glycosylphosphatidylinositol (GPI)-linked receptor that has no cytoplasmic domain. FcγRIIB is the only inhibitory FcγR. It is a low affinity receptor that binds immune-complexed IgG and contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic domain. FcγRIIB cross-linking by immune complexes results in ITIM phosphorylation and inhibition of the activating signalling cascade. FcγRs differ in their cellular expression; myeloid cells express FcγRI, FcγRIIA and FcγRIIIA, whereas granulocytes express FcγRI, FcγRIIA and FcγRIIIB. In such cells, immune complex-mediated activation of these receptors is negatively regulated by FcγRIIB. FcγRIIB is the only FcγR expressed by B cells and negatively regulates B cell receptor activation by immune-complexed antigen. FcγRs bind different IgG subtypes with differing affinity. For example, in the case of FcγRIIB, binding affinity is highest for IgG1, followed by IgG3, which in turn has higher affinity than IgG4 followed by IgG2. The ratio of binding of an IgG subtype to activating FcγRs and inhibitory FcγRIIB is known as the A/I ratio, and it determines the activation threshold of the cell. DC, dendritic cell; NK, natural killer.
IgG has an important role in defence against pathogens, as shown by the increased susceptibility to infection of patients with hypogammaglobulinaemia. The interaction between pathogen-bound IgG and activating FcγRs directly mediates pathogen clearance by antibody-dependent cell-mediated cytotoxicity (ADCC), degranulation of cytotoxic cells and phagocytosis, and indirectly through the release of cytokines and other inflammatory mediators1. FcγRs are also important in mediating IgG-associated pathogen toxin neutralization2,3. In addition to binding to IgG, FcγRs can also bind to pentraxins and acute-phase proteins, including serum amyloid P and C-reactive protein4,5; however, the physiological importance of these interactions remains to be determined.
Cross-linking of activating FcγRs by immune complexes results in the phosphorylation of immunoreceptor tyrosine-based activating motifs (ITAMs) that are present either in the cytoplasmic domain of the receptor (FcγRIIA and FcγRIIC) or in the associated FcR common γ-chain (FcγRI and FcγRIIIA). This ITAM phosphorylation results in the activation of the signalling molecule SYK and the initiation of an activating signalling cascade1.
It has long been known that the Fc fragments of IgG could suppress humoral immunity6, an effect mediated by the specific interaction of these fragments with B cell-expressed FcγRIIB7,8. FcγRIIB is the only FcγR expressed by B cells9, and if it is cross-linked to the B cell receptor (BCR) the B cell activation threshold is increased and antibody production decreased. FcγRIIB is also expressed by other immune cells, including dendritic cells (DCs), macrophages, activated neutrophils, mast cells and basophils1,10,11. When expressed by these cells, FcγRIIB inhibits the functions of activating FcγRs, such as phagocytosis and pro-inflammatory cytokine release. When expressed by follicular DCs (FDCs), FcγRIIB is important for trapping the antigen-containing immune complexes that are thought to be crucial for driving the germinal centre response12,13. This diversity of its expression and function underlies the importance of FcγRIIB in regulating defence against infection and susceptibility to autoimmune disease.
In humans and mice, three FcγRIIB isoforms have been identified14-17. Two of these, FcγRIIB1 and FcγRIIB2, encode membrane proteins that have an immunoreceptor tyrosine-based inhibitory motif (ITIM) within the cytoplasmic domain. FcγRIIB1 is the main isoform and is expressed by B cells and, at lower levels, by monocytes18. It has inhibitory functions and is not thought to mediate immune complex internalization. FcγRIIB2 is the main isoform expressed by myeloid-derived cells and can mediate endocytosis19, a process dependent on a dileucine motif in its cytoplasmic domain20,21. Although FcγRIIB1 and FcγRIIB2 are encoded by the same gene, the FcγRIIB1 isoform is generated by alternative mRNA splicing that results in a 47-amino acid cytoplasmic insertion upstream of the ITIM, which inhibits endocytosis by preventing receptor accumulation in clathrin-coated pits22. FcγRIIB3 is a soluble isoform that lacks the transmembrane and first cytoplasmic domains23, and it can inhibit the presentation of IgG-complexed antigen24. Another soluble form of FcγRIIB can be generated by cleavage of the two extracellular domains25 and can suppress plasmablast production in vitro26. Thus, there are two soluble forms of FcγRIIB that can inhibit immune complexmediated immunity in vitro. However, remarkably little is known about their physiological role in vivo, and the remainder of this review will therefore focus on the membrane-bound isoforms. When studies do not differentiate between the isoforms (as is usually the case) they are referred to collectively as FcγRIIB.
In this Review, the regulation of FcγRIIB expression and function, as well as its role in controlling immune responses, is discussed. The signalling pathways downstream of FcγRIIB have been recently reviewed elswhere1, and therefore we focus on the influence of FcγRIIB on cellular immunity, autoimmunity and responses to infection. Although FcγRIIB has a conserved and important role in regulating immunity in mice and humans, common genetic variants have been reported that result in diverse receptor expression and function within populations of both species. The effect of these variants on the predisposition to both autoimmunity and infection, and the potential evolutionary effects of the interplay between them, is discussed. Finally, we consider the effect that our increasing knowledge of the biology and function of FcγRIIB might have on the optimal use of current biological therapies, such as intravenous immunoglobulin (IVIG), and on the development of new therapeutic approaches.
The functions of FcγRIIB
The main function of FcγRIIB is to inhibit activating signals, which is achieved through co-ligation of FcγRIIB with either activating FcγRs or with the BCR by immune complexes (FIG. 2). This leads to phosphorylation of the cytoplasmic domain ITIM of FcγRIIB27,28 by the Src-family kinase lYN29. This phosphorylation event is thought to require access of FcγRIIB to sphingolipid rafts in which activating FcγRs and the BCR reside following cross-linking30,31. Subsequent binding of SH2-domain-containing inositol phosphatases (SHIPs), in particular SHIP1, result in the dephosphorylation of downstream targets and inhibition of the activating signalling cascade32,33.
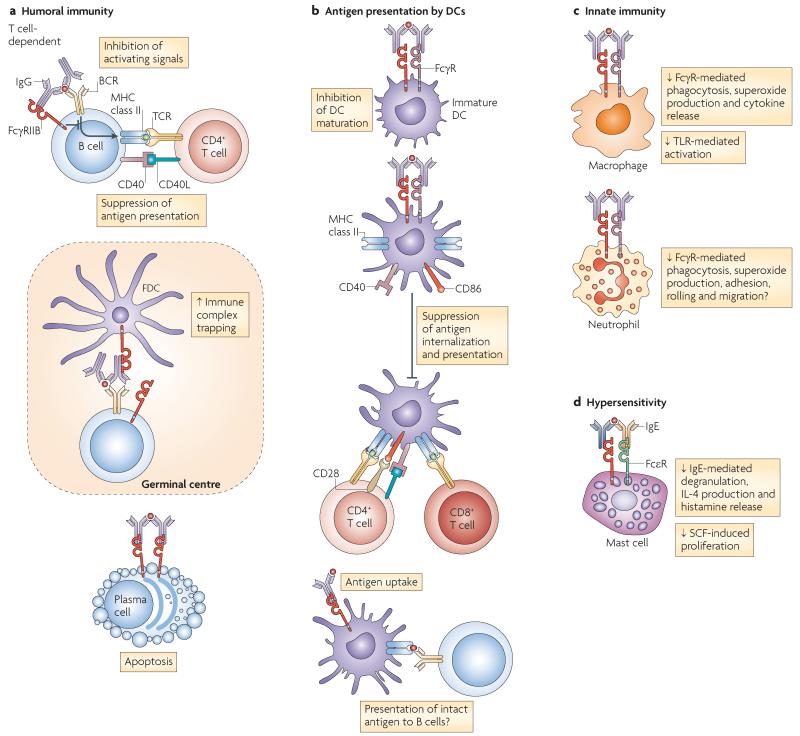
Figure 2. The functions of FcγRIIB.
a | Fc receptor IIB for IgG (FcγRIIB) has an important role in controlling humoral immunity by regulating B cell activation, localization of B cells in the germinal centres, as well as plasma cell survival. FcγRIIB regulates B cell activation by increasing the B cell receptor (BCR) activation threshold and suppressing B cell-mediated antigen presentation to T cells. Follicular dendritic cells (FDCs) express FcγRIIB, which is thought to be important for trapping immune-complexed antigen for presentation to germinal centre B cells. The absence of FcγRIIB on germinal centre FDCs results in impaired antibody and memory responses. Terminally differentiated plasma cells express little or no BCR but express high levels of FcγRIIB, and cross-linking FcγRIIB with immune complexes in vitro can induce apoptosis. b | FcγRIIB influences antigen presentation by inhibiting FcγR-dependent internalization of immune-complexed antigen by DCs, as well antigen presentation to both CD4+ and CD8+ T cells (cross-presentation). FcγRIIB is also thought to provide a basal level of inhibition to DC maturation, as blockade of immune complex binding to FcγRIIB results in DC maturation and type I interferon production. There is also a possibility that FcγRIIB may deliver intact antigen to a non-degradative compartment, allowing its recycling to the cell surface where it could interact with the BCR and activate B cells. c | FcγRIIB can also influence innate immunity: in macrophages, FcγRIIB cross-linking inhibits FcγR-mediated phagocytosis and cytokine release (including tumour necrosis factor, interleukin-6 (IL-6) and IL-1α), as well as Toll-like receptor 4 (TLR4)-mediated activation. In neutrophils, cross-linking of activating FcγRs results in phagocytosis, superoxide production and enhanced neutrophil adhesion, rolling and migration, all of which are probably inhibited by ligating FcγRIIB. d | FcγRIIB also inhibits IgE-induced mast cell and basophil degranulation, thus contributing to hypersensitivity responses. FcεR, Fc recpetor for IgE; SCF, stem cell factor.
FcγRIIB regulates B cell activation by increasing the BCR activation threshold and suppressing B cellmediated antigen presentation to T cells through the ITIM-dependent inhibitory mechanism described above (FIG. 2). FcγRIIB can interrupt immune synapse formation, which is important in early B cell activation164, at least in part by altering BCR–lipid interactions and preventing antigen-induced BCR oligomerization165. In addition, cross-linking FcγRIIB in the absence of BCR ligation can induce apoptosis of mature B cells, independently of ITIM- and SHIP-mediated signalling. This induction of apoptosis depends on phosphorylation of a membrane proximal tyrosine residue (probably at position 269) of FcγRIIB by the tyrosine protein kinase ABL34,35 and activation of the BCL-2 family members BH3-interacting-domain death agonist (BID) and BCL-2-antagonist of cell death (BAD)36. FcγRIIB is also expressed by pre-B cells, suggesting that it might contribute to central tolerance37,38, although this is not supported by data from FcγRIIB-deficient mice39.
Recent studies have shown that cross-linking FcγRIIB on plasma cells can also result in apoptosis. Terminally differentiated plasma cells express little or no BCR on the cell surface but express high levels of FcγRIIB; cross-linking FcγRIIB with immune complexes in vitro can induce apoptosis in a BCL-2-interacting mediator of cell death (BIM)-dependent manner40. A possible physiological role for this phenomenon is in regulating the long-lived bone marrow plasma cell population, as FcγRIIB-deficient mice have increased numbers of bone marrow plasma cells and no immune complex-mediated apoptosis; however, a causal link between these two observations has not been conclusively shown.
Inhibitory signalling has been shown in all cell types that express FcγRIIB with the exception of FDCs, in which only an immune complex-binding function for FcγRIIB has been observed. In contrast to FDCs in primary follicles, FDCs in germinal centres express high levels of FcγRIIB, and in fact FcγRIIB expression may be required for FDC activation166. The absence of FcγRIIB on germinal centre FDCs of knockout mice also impairs the trapping of immune complexes, resulting in impaired antibody and memory responses12,13.
FcγRIIB-deficient mice have elevated antibody levels in response to T cell-dependent antigens, and to a lesser extent T cell-independent antigens41. Transgenic overexpression of FcγRIIB by B cells leads to a decrease in T cell-dependent IgG responses, with little effect on IgM titres, whereas T cell-independent responses are unaffected42. The lack of effect of FcγRIIB on IgM titres in both FcγRIIB B cell transgenic42 and FcγRIIB-deficient40 mice could be because most of the short-lived extra-follicular plasmablasts responsible for the early production of IgM are generated before the IgG required to ligate FcγRIIB is produced (if antigen-specific IgG is indeed required for such feedback regulation). Another explanation might be that FcγRIIB-mediated suppression of IgG responses requires a level of cross-linking only achieved by FDC-associated immune complexes, thereby limiting the inhibitory effects of FcγRIIB to cells that develop in the germinal centre. Cross-linking of FcγRIIB may be decreased in T cell-independent responses dominated by IgG3, which has low affinity for FcγRIIB43,44 (FIG. 1).
Thus, FcγRIIB is an important regulator of humoral immunity, although many of the details of this effect on B cells remain to be determined, a process that would benefit from cell-type specific knockout mice. FcγRIIB is particularly important in maintaining B cell tolerance in the periphery (see later), implicating variations in its expression and function in the pathogenesis of autoimmunity.
In addition to its effect on B cells, FcγRIIB inhibits FcγR-dependent internalization and presentation of antigen, as well as subsequent T cell priming, by DCs45. FcγRIIB also provides a basal level of inhibition to DC maturation, as blockade of immune complex binding to FcγRIIB, or FcγRIIB deficiency in DCs, results in DC maturation and type I interferon (IFN) production46-48. Healthy individuals have immune complexes in their serum49 that induce DC maturation by binding activating FcγRs, unless this process was suppressed by FcγRIIB. In addition, a study has suggested that FcγRIIB expressed by DCs may deliver intact antigen to a non-degradative compartment, allowing its recycling to the cell surface where it could interact with the BCR and activate B cells50.
In macrophages, engagement of FcγRIIB can reduce FcγR-mediated phagocytosis and cytokine release (including tumour necrosis factor (TNF), interleukin-6 (IL-6), IL-1α and neutrophil chemotactants)51-53, as well as Toll-like receptor 4 (TLR4)-mediated activation54 (FIG. 2). FcγRIIB may also have a non-inhibitory role in macrophage function by mediating endocytosis and non-inflammatory clearance of immune complexes from arthritic joints by macrophages55.
FcγRIIB inhibits several aspects of mast cell and basophil function (FIG. 2), including degranulation, IL-4 production and histamine release induced through the Ige receptor56-58, as well as stem cell factor (also known as KIT ligand)-mediated cell proliferation59. Resting human neutrophils express FCGR2B2 mRNA60,61 but express low levels of FcγRIIB2 on the cell surface62. In mouse neutrophils, cross-linking of activating FcγRs results in phagocytosis, superoxide production and enhanced neutrophil adhesion, rolling and migration63-65, and it is likely that FcγRIIB inhibits all of these responses51,66,67. In humans, the precise role of FcγRIIB on neutrophils has not been defined.
Regulation of FcγRIIB expression and function
Given that FcγRIIB has a central role in regulating immune responses, its function must be carefully controlled. FcγRIIB is expressed together with its activating counterpart (or counterparts) (either an activating FcγR or the BCR), and the ratio of binding to activating FcγRs and inhibitory receptors on a given cell (known as the A/I ratio) determines the activation threshold of the cell. This threshold can be influenced by factors that control cell surface expression of the receptor itself, its signalling capacity or its interaction with IgG.
Regulation of expression
The regulation of FcγRIIB expression is complex and differs depending on the cell type. For example, stimulation of B cells with IL-4 decreases FCGR2B mRNA and cell surface expression (in a signal transducer and activator of transcription 6 (STAT6)-dependent manner)68,69, whereas in monocytes, IL-4 increases FcγRIIB expression60,70. This might be expected to increase antibody titres while reducing inflammatory cytokines production by macrophages in response to the resultant immune complexes. By contrast, IFNγ increases FCGR2B mRNA and cell surface expression by lipopolysaccharide-stimulated B cells68 but decreases it on monocytes60, which would polarize the immune response away from antibody production towards clearance of pathogens. It thus seems likely that the cytokine milieu is important in fine-tuning FcγRIIB expression in a cell- and context-specific manner.
Activation of complement may also affect FcγRIIB expression. In a mouse model of immune complex-induced alveolitis, the complement component C5a, an anaphylatoxin, was shown to directly upregulate FcγRIII and downregulate FcγRIIB expression by alveolar macrophages71. This regulation of FcγR expression would lower the activation threshold of macrophages and enhance the immune response to immune complexes, coordinating complement and phagocyte activation.
The transcription factors responsible for the regulation of FCGR2B expression are not well defined. The mouse Fcgr2b promoter contains a glucocorticoid response element, an AP4 binding site, an SP1 binding site, an e box (which is a binding site for helix–loop–helix factors) and an S box72. In addition, there are thought to be enhancer and repressor elements within the gene, particularly within demethylated regions in the third intron, which have an important role in control of Fcgr2b transcription17,73,74.
Ubiquitylation of surface receptors, and their subsequent degradation, provides an important mechanism to control their expression and to regulate immune responses. A recent study showed that FcγRIIB may be a substrate for MARCH9, a membrane-associated RING-containing ubiquitin E3 ligase expressed by B cells, T cells and DCs75.
Regulation of IgG–FcγRIIB interaction
All low-affinity FcγRs have two extracellular immunoglobulinlike domains termed D1 and D2; D2 interacts with the CH2 domains of the IgG Fc region76. The affinity of this interaction differs with variations in the protein backbone of IgG and therefore with differing isotypes and with variations in the levels of glycosylation of the IgG Fc region. The variation in the binding affinity of activating and inhibitory receptors for IgG isotypes, that is the A/I ratio, differs by several orders of magnitude between isotypes in mice. IgG1 has the lowest A/I ratio (0.1), suggesting the activity of this isotype is strongly influenced by FcγRIIB, whereas the activity of IgG2a is much less affected, with an A/I ratio of 70 (REFS 43,44). In humans, FcγRIIB binds IgG1 with the highest affinity, followed by IgG3, IgG4 and IgG2 (REF. 77; reviewed in REF. 78) (FIG. 1). The CH2 domains of IgG also contain an asparagine residue at position 297 that can be variably glycosylated. These sugar moieties vary in complexity, and this variation is associated with conformational changes in the Fc region79 that may substantially alter their binding to FcγRs80,81. enrichment of α2,6-sialylated forms of mouse IgG1 results in a tenfold reduction in its affinity for FcγRIIB with the net effect of sialylation increasing the A/I ratio for IgG1 (REF. 81). It is clear that a crucial component in the regulation of FcγR-mediated immune responses involves not only the FcγRs, but also the IgG subclass involved and the nature and degree of IgG glycosylation.
Genetic variation in FcγRIIB
The human 1q23.3 locus contains five FCGR genes (FCGR2A, FCGR2B, FCGR2C, FCGR3A and FCGR3B) encoding the FcγRII and FcγRIII receptor families. There is high sequence similarity between these genes, in part due to an ancestral segmental duplication82,83 that arose following the division of the great apes from new world monkeys84,85. The locus is complicated by the presence of at least three copy number variant (CNV) regions, two of which are common in Caucasians but do not seem to involve FCGR2B62,86. The homology in the region of the ancestral duplication is likely to facilitate CNV formation87. A similar complexity is seen in the mouse FcR locus, although the presence of CNVs has not been described.
In both species, genetic variation at the FcR locus exists within and between different populations. This combination of gene duplication, several common single nucleotide polymorphisms (SNPs) and CNVs is characteristic of regions involved in immune regulation. The generation and maintenance of such genetic diversity is thought to increase the probability of successful defence against varying pathogens. Such evolutionary plasticity has best been described at the MHC and killer cell immunoglobulin-like receptor loci88, and the FcR locus seems to provide another, less complex, example. This genetic variability has implications for susceptibility to both autoimmunity and infection.
Variations in mice
Several variants in the putative promoter region and the demethylated region of intron 3 of Fcgr2b have been described, which together form three haplotypes in inbred strains of mice (FIG. 3) and are also found in wild mice (M.R.C. and K.G.C.S., unpublished observations). They include a 13-base-pair deletion that disrupts a putative AP4 binding site and an S box close to the transcription start site of Fcgr2b, as well as two deletions in intron 3. These variants were first described in several autoimmune-prone inbred strains of mice and were found to be associated with decreased expression and function of FcγRIIB72,89,90. Studies in congenic mouse strains have provided evidence they these variants may influence the expression of FcγRIIB by B cells91,92, although the causative genetic variant and a direct link to autoimmune predisposition remain to be shown.
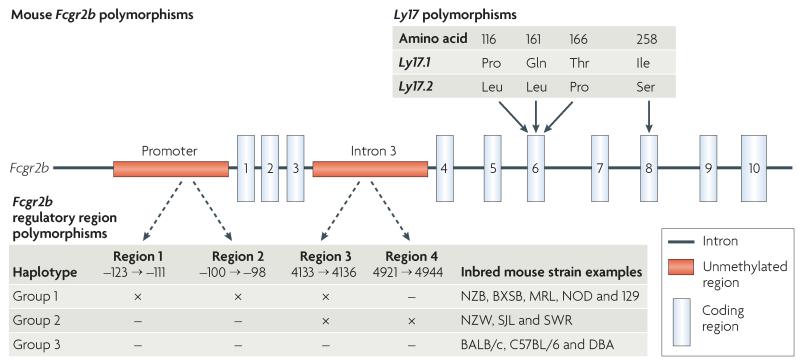
Figure 3. Mouse Fcgr2b polymorphisms.
Fcgr2b polymorphisms include the Ly17 polymorphisms, which are found in the coding region that comprises the Ly17 haplotype. These include three single nucleotide polymorphisms (SNPs) within exon 6 and one in exon 8 that appear to have no effect on receptor function. Regulatory region polymorphisms identified within the promoter region and intron 3 form 3 haplotypes and vary between different strains of inbred mice. In the promoter there is a 13 base pair deletion (region 1) and a 3 base pair deletion (region 2). In intron 3, there is a 4 base pair deletion at nucleotide 4133 to 4136 (region 3) and a 24 base pair deletion at nucleotide 4921 to 4944 (region 4). Inbred strains with the group 1 haplotype (NZB, BXSB, MRL, non-obese diabetic (NOD) and 129 mice) have region 1, region 2 and region 3 deletions (×), have decreased expression of FcγRIIB by macrophages and activated B cells and are prone to autoimmune disease. The precise contribution of deletions in the promoter and intron 3 to autoimmune susceptibility in these strains has yet to be determined. Inbred strains with the group 2 haplotype have a promoter with no deletions but have deletions in region 3 and 4 of intron 3 (which may contain enhancer and repressor elements). Inbred strains with the group 3 haplotype do not have deletions in either the promoter or intron 3 and include BALB/c and C57BL/6 mice.
In Fcgr2b, two coding variants have been identified, termed Ly17.1 and Ly17.2 (REF. 93) (FIG. 3). Despite resulting in amino acid exchanges in the extracellular D2 domain, there is no evidence of a detectable functional effect of these coding variants or of a consistent association with autoimmunity94.
Human variations
Two promoter haplotypes have been described; the common −386G:−120T variant and the less common −386C:−120A variant95 (FIG. 4). The −386C:−120A variant has been shown in one study to result in increased binding of the transcription factors GATA-binding protein 4 (GATA4) and Yin-Yang 1 (YY1) to the promoter95 and in increased expression of FcγRIIB by monocytes, neutrophils and myeloid DCs96, whereas, another study showed that −386C homozygosity decreased the transcription and surface expression levels of FcγRIIB in peripheral B cells compared with −386G homozygotes97, creating an apparent paradox that awaits resolution.
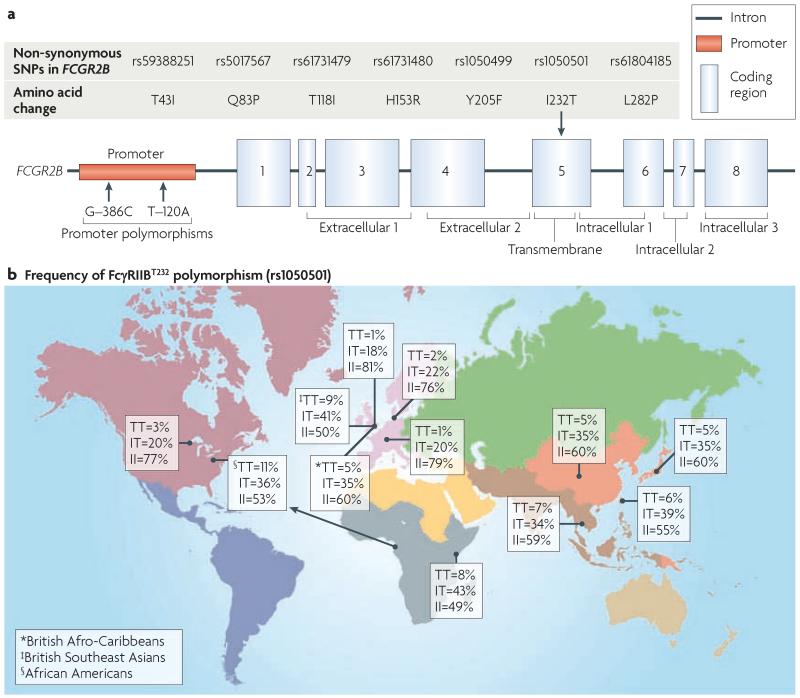
Figure 4. Human FCGR2B polymorphisms.
a | Several single nucleotide polymorphisms (SNPs) have been identified within the promoter and coding regions of FCGR2B. In the promoter, there are two SNPs at nucleotides −386 and −120. These SNPs form two haplotypes, a common −386G:−120T haplotype and a less common −386C:−120A haplotype. These haplotypes may alter transcription factor binding and expression. Seven non-synonymous SNPs have been identified in the human FCGR2B gene but only one has been studied in detail. This non-synonymous T-to-C transition (rs1050501) in exon 5 of the gene results in the substitution of a threonine for isoleucine at position 232 within the transmembrane domain of FcγRIIB (FcγRIIBT232). The FcγRIIBT232 variant is excluded from lipid rafts and has notably impaired inhibitory function. The polymorphism that encodes FcγRIIBT232 has been associated with increased susceptibility to systemic lupus erythematosus (SLE) in both Southeast Asian and Caucasian populations. b | The frequency of the FcγRIIBT232 variant in different populations varies substantially. It is uncommon in Caucasians (1% homozygosity) but more common in populations found in areas of malarial endemicity such as Africa and Southeast Asia (5–11% homozygosity) suggesting that decreased FcγRIIB function may provide a survival advantage against this disease. This might provide the first example of an immune polymorphism predisposing to polygenic autoimmunity, selected and retained by virtue of its protective effect in malaria infection, and thus it is beginning to provide an explanation for the ethnic differences seen in SLE susceptibility.
Seven non-synonymous SNPs have been identified in human FCGR2B98,99 (FIG. 4), but only one of these SNPs has been shown to occur at notable frequency. This non-synonymous T-to-C transition in exon 5 (rs1050501) results in the substitution of a threonine for isoleucine at position 232 within the transmembrane domain of FcγRIIB (referred to as FcγRIIBT232). The FcγRIIBT232 variant is excluded from lipid rafts, leading to reduced phosphorylation by LYN, abnormal SHIP recruitment100,101 and a decrease in FcγRIIB-mediated inhibition of both macrophage and B cell responses100. The frequency of the homozygous FcγRIIBT232 variant is subject to considerable ethnic variation and is lower in Caucasians (1%) than Africans (8–11%)102 or Southeast Asians (5–7%)98,103.
FcγRIIB and autoimmunity
Within the adaptive immune system, cells are generated with antigen receptors capable of recognizing selfantigen. During lymphocyte development mechanisms exist in the bone marrow to modify autoreactive B cells or to destroy or inactivate autoreactive cells (central tolerance); however, some autoreactive lymphocytes persist in the periphery. Thus, additional measures exist to limit autoreactivity in mature lymphocytes (peripheral tolerance)104. Failure of both central and peripheral tolerance leads to activation of the immune system against self-antigens, which is termed auto immunity. Autoimmune diseases may be tissue specific, such as type 1 diabetes, or may involve multiple organ systems, such as systemic lupus erythematosus (SLE) and they tend to be polygenic: there are many genes that contribute to disease susceptibility. FCGR2B is one of the genes thought to influence susceptibility to several autoimmune diseases in humans.
SLE
Abnormalities in B cell function and in the clearance and inflammatory response to immune complexes are characteristic features of SLE. As FcγRIIB is a key regulator of B cells, and low affinity FcγRs are central to the response to immune complexes, FcγRIIB might be expected to be involved in the pathogenesis of SLE and perhaps other autoimmune diseases.
The most direct evidence for the role of FcγRIIB in SLE comes from studies involving genetically modified mice1. FcγRIIB deficiency renders normally resistant strains of mice susceptible to several antibody- or immune complex-dependent models of autoimmunity, including immune complex-mediated alevolitis51 and glomerular basement membrane-specific antibody-mediated nephritis67,105.
FcγRIIB-deficient mice backcrossed to a C57BL/6 background spontaneously develop hypergamma-globulinaemia, autoantibodies and an immune complex-mediated disease resembling SLE106; this does not occur on mice backcrossed to a BAlB/c background, indicating the importance of strain-specific epistatic effects on autoimmune susceptibility. The restoration of FcγRIIB expression levels on bone marrow cells of (NZB × NZW) F1 mice (a mouse model of spontaneous SLE) using an FcγRIIB-expressing retrovirus can prevent autoimmunity107, and modest transgenic overexpression of FcγRIIB on B cells markedly reduces the development of SLE in MRL-lpr mice42. Thus, studies in genetically modified mice implicate FcγRIIB in the pathogenesis of auto immunity and the maintenance of B cell tolerance. Some uncertainty remains, however, owing to the possibility that neighbouring genes might be influencing the phenotype of FcγRIIB-deficient mice (see BOX 1).
Box 1. FcγRIIB and spontaneous SLE in mice.
Fcgr2b is found in a region of distal chromosome 1 that contains both the low affinity Fc receptors for IgG (FcγRs) and several other immunologically important genes, such as those encoding signalling lymphocytic activation molecule (SLAM) and complement receptor 2 (CR2). Therefore, studies on the association between naturally occurring FcγRIIB variants and spontaneous systemic lupus erythematosus (SLE), as well as the phenotype of FcγRIIB-deficient mice, have to be interpreted carefully. This region has been linked with SLE in mice, although initial fine-mapping studies suggested that the FcγR region did not contribute to disease susceptibility (however, this region was of NZW origin, and a role for the NZB haplotype was not excluded)157. Studies using congenic mice with genomic regions containing the NZW-derived Fcgr2b allele have shown evidence of abnormal FcγRIIB expression and immune function, but not spontaneous autoimmunity92 (although, in a polygenic disease, any single polymorphism would be expected to act in concert with other genetic variants before exerting a measurable effect on disease susceptibility). Consistent with this, a recent study has shown that the FcγR interval in NZB mice can combine with the SLAM locus to increase SLE susceptibility158. Thus, the importance of FcγRIIB in controlling SLE has been unequivocally shown by abrogation of SLE through partial restoration of FcγRIIB expression107 and transgenic overexpression of FcγRIIB on B cells42 and, in addition, genetic evidence demonstrates that the FcγR region contributes to the pathogenesis of SLE158. Nonetheless, careful studies of the immunological and pathological implications of naturally occurring FcγRIIB variants are still required, preferably using C57BL/6 embryonic stem cell ‘knock-in’ mice to exclude the possible influence of neighbouring genetic variation present in congenic regions.
The telomeric region of chromosome 1 derived from the 129 strain of mouse is similar to that in autoimmune-prone strains of mice157 including NZB mice72, and thus the phenotype of FcγRIIB-deficient mice made with 129 mouse-derived embryonic stem cells41 might be influenced by carry-over of adjacent 129 mouse-derived regions despite backcrossing106. C57BL/6 mice congenic for the 129 mouse-derived region develop autoimmunity159,160, which may explain why mice that are deficient in closely linked genes, such as SAP, CR2 and C1q, develop similar phenotypes161-163. Thus, although it is likely that Fcgr2b contributes to spontaneous SLE in mice (in concert with other variants) and to the autoimmune phenotype of FcγRIIB-deficient mice, the analysis of FcγRIIB-deficient mice made with C57BL/6 embryonic stem cells, and/or autoimmune-associated variant ‘knock-in’ C57BL/6 mice, will be necessary to formally prove this association.
FcγRIIB may also have an important role in determining susceptibility to spontaneous autoimmunity in several inbred strains of mice that develop a lupus-like disease; the major locus contributing to SLE susceptibility in the (NZB × NZW) F1 and BXSB mouse models of spontaneous SLE, excluding the MHC, is on distal chromosome 1 and contains the FcR region108. Furthermore, Fcgr2b promoter variants (FIG. 3) are present in polygenic models of SLE (MRL-lpr, (NZB × NZW) F1 and BXSB mice)72,89,90,109. Studies with congenic mice suggest, but do not prove, that variability in the Fcgr2b promoter can alter FcγRIIB expression by B cells and alter B cell and macrophage activation72,91,92, consistent with a role in SLE pathogenesis. All of these studies, however, need to be interpreted in the light of the potential for other polymorphic genes in the locus to influence autoimmune susceptibility (BOX 1).
Collectively, these studies show a role for FcγRIIB in the control of the B cell-mediated immune responses. FcγRIIB is also important in the maintenance of peripheral B cell tolerance, and although the mechanisms underlying this are as yet not fully defined, possibilities include the induction of apoptosis of autoreactive germinal centre B cells, the exclusion of autoreactive B cells from B cell follicles110 and the control of plasma cell development and apoptosis40.
The polymorphism encoding FcγRIIBT232 in humans is associated with susceptibility to SLE in Southeast Asian populations98,103,111 and in Caucasians99. This association is only seen when FcγRIIBT232 is homozygous; it is associated with an odds ratio of 1.73, making this one of the strongest genetic associations with SLE99. The increased frequency of FcγRIIBT232 in individuals of Southeast Asian and African descent may therefore contribute to the increased prevalence and/or severity of SLE, which has long been noted in these ethnic groups112. The effect of the FCGR2B promoter variant −386C:−120A on its expression is debated, but an association of the −386C:−120A haplotype with SLE in Caucasians has been shown by two studies95,97.
Decreased expression of FcγRIIB has been observed in memory B cells from patients with SLE: this is perhaps due to a failure to upregulate FcγRIIB expression as B cells develop a memory phenotype113. Furthermore, low FcγRIIB expression by memory B cells and plasmablasts from patients with SLE with active, but not quiescent, disease has been described96. Decreased expression of FcγRIIB by DCs has also been observed in patients with SLE, together with an increased expression of activating FcγRs, thus substantially altering the A/I ratio in favour of cell activation114. These differences in expression may, at least in part, occur secondary to inflammation.
Thus, there is a consistent association between decreased FcγRIIB function and SLE, as determined by genetic association or cell surface expression, which may be due to various mechanisms (summarized in FIG. 5).
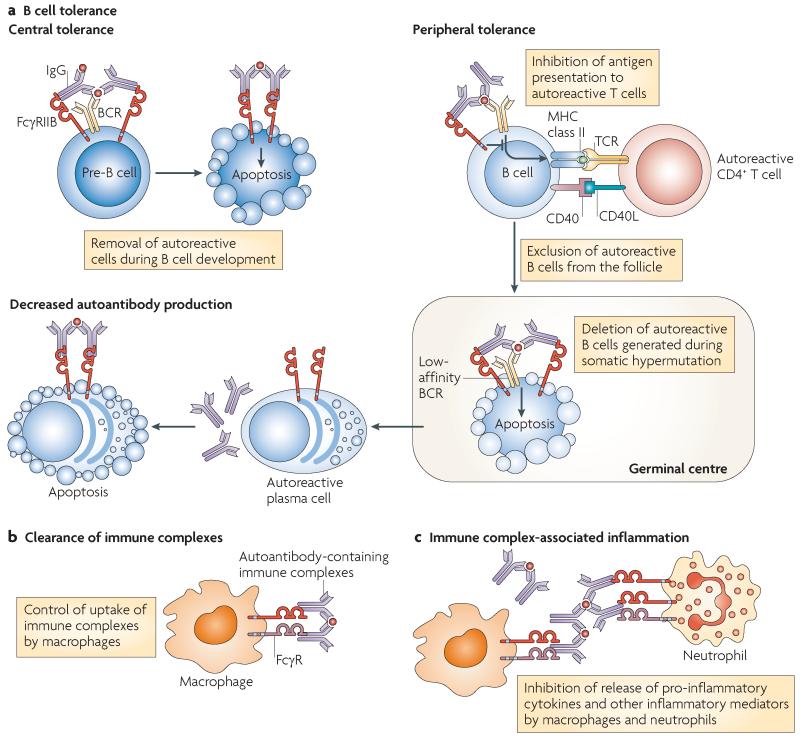
Figure 5. Potential mechanisms by which FcγRIIB might contribute to the pathogenesis of systemic lupus erythematosus.
a | Fc receptor IIB for IgG (FcγRIIB) is important for the maintenance of B cell tolerance through inhibition of B cell activation, promotion of the exclusion of autoreactive cells from follicles and perhaps by mediating apoptosis of autoreactive B cells and plasma cells. In vitro, FcγRIIB cross-linking in pre-B cells can result in apoptosis. This has been proposed as a possible mechanism for the removal of autoreactive B cells that arise during development; however, studies in FcγRIIB-deficient mice do not confirm the importance of this mechanism in central tolerance in vivo. FcγRIIB operates at several stages during later peripheral B cell development, potentially inhibiting B cell receptor (BCR)-mediated activation of autoreactive B cells. In addition, it may be important for the follicular exclusion of low-affinity autoreactive B cells. Autoreactive B cells may arise during somatic hypermutation and affinity maturation. It has been proposed that FcγRIIB cross-linking in the absence of BCR ligation may mediate apoptosis of such autoreactive follicular B cells and may also be important for deleting autoreactive plasma cells. b | Breakdown of B cell tolerance and the emergence of autoantibodies does not necessarily result in autoimmune disease, as there are mechanisms that function to clear circulating immune complexes, preventing them from becoming deposited in tissues. Immune complex clearance is, in part, mediated by binding and internalization by activating FcγRs (particularly on macrophages of the reticuloendothelial system of the liver and spleen). FcγRIIB may enhance or inhibit FcγR-mediated immune complex internalization by phagocytes (depending on the isoform expressed) thus modulating the amount of circulating immune complex present. c | Tissue-deposited immune complexes may initiate inflammation owing to ligation of activating FcγRs on infiltrating macrophages and neutrophils. Pro-inflammatory cytokine release and neutrophil degranulation in response to immune complexes is negatively regulated by FcγRIIB. Thus, FcγRIIB dysfunction might contribute to the pathogenesis of systemic lupus erythematosus at several stages, including the breakdown of self-tolerance, decreased disposal of immune complexes and a failure to modulate the inflammatory response to deposited immune complexes. TCR, T cell receptor.
Rheumatoid arthritis
Collagen-induced arthritis (CIA) is a B cell-dependent mouse model of inflammatory arthritis that shares some features with rheumatoid arthritis. FcγRIIB deficiency increases both collagen-specific IgG titres and disease severity in CIA52. Consistent with this, FcγRIIB B cell transgenic mice show a reduction in disease severity and in collagen-specific IgG titres; however, this effect was not observed in FcγRIIB macrophage transgenic mice42. In addition to influencing CIA through it effects on antibody production, FcγRIIB may also limit cartilage destruction by inhibiting the production of matrix metalloproteinases115.
Rheumatoid arthritis is a chronic inflammatory disease that results in a progressive, deforming polyarthropathy. Many patients have serum autoantibodies, particularly rheumatoid factor (an IgM autoantibody directed against the Fc portion of IgG), as well as antibodies directed against other autoantigens such as cyclic citrullinated peptide (CCP)116; it is thought these antibodies may contribute to disease pathogenesis through interactions with FcγRs. Several investigators have sought to identify an association between rheumatoid arthritis and the nonfunctioning variant receptor FcγRIIBT232; Radstake et al. found no difference in the frequency of the polymorphism encoding FcγRIIBT232 between patients with rheumatoid arthritis and controls, but they did observe an association of the FcγRIIBT232 variant with increased radiological joint damage117. DCs from rheumatoid arthritis patients with the FcγRIIBT232 variant also showed an increase in immune complex-mediated inflammation in vitro. other investigators have observed higher expression of FcγRIIB by DCs from a subset of patients with low rheumatoid arthritis disease activity (in the absence of treatment) than patients with high disease activity118.
Other autoimmune diseases
Anti-glomerular basement membrane (anti-GBM) disease, also known as Goodpasture’s disease, is an antibody-mediated glomerulo nephritis that often leads to irreversible renal failure. one small study has shown an association between the polymorphism encoding FcγRIIBT232 and susceptibility to anti-GBM disease86, which is consistent with findings in mouse models67 but requires independent confirmation.
Multiple sclerosis is an autoimmune disease characterized by demyelination in the central nervous system. In a mouse model of multiple sclerosis, FcγRIIB deficiency was associated with an increase in disease severity and enhanced activation of myelin-specific T cells119. However, there are no studies to date examining the effect of the polymorphism encoding FcγRIIBT232 on susceptibility to multiple sclerosis in humans. Chronic inflammatory demyelinating polyneuropathy is characterized by recurrent episodes of an inflammatory polyneuropathy, and is thought to be mediated by autoantibodies. A recent study found that untreated patients expressed lower levels of FcγRIIB on monocytes and B cells120.
Idiopathic thrombocytopenic purpura (ITP) is characterized by an often chronic antibody-mediated destruction of platelets. In a multivariate logistic regression analysis of data from 60 children with ITP, the presence of the FcγRIIBT232 variant predicted a chronic disease course121. of interest, a subset of patients with ITP was found to be infected with Helicobacter pylori, and this was associated with low surface expression of FcγRIIB on monocytes. H. pylori eradication led to an increase in platelet numbers and in the expression of FcγRIIB by monocytes, suggesting an intriguing link between infection and autoimmunity through the regulation of FcγRIIB expression122.
Thus, to summarize, FcγRIIB affects susceptibility to several autoimmune diseases, particularly SLE, but also rheumatoid arthritis, anti-GBM disease and ITP.
FcγRIIB and infection
Many consider the primary role of the immune system to be defence against infection. Antibodies are a crucial component of this protective system; the interaction between pathogen-bound IgG and FcγR directly mediates pathogen clearance by ADCC, degranulation and phagocytosis and indirectly through the release of cytokines and other inflammatory mediators. FcγRIIB would be expected to control these functions, as well as the production of IgG by the B cell response itself, and it should thus have a notable, and perhaps complex, role in defence against infection.
Most work on FcγRIIB and infection has involved the study of Streptococcus pneumoniae, an encapsulated, gram positive organism, which is a major cause of pneumonia, peritonitis and meningitis in humans. The main defence against S. pneumoniae is through capsular polysaccharide- and cell wall phosphocholine-specific antibodies, which bind pneumococci and mediate FcγR-dependent phagocytosis by neutrophils and macrophages123. In vitro, macrophages from FcγRIIB-deficient mice incubated with opsonized S. pneumoniae show increased phagocytosis and production of cytokines such as TNF, and infected FcγRIIB-deficient mice showed reduced mortality from streptococcal peritonitis53. Mice that overexpress FcγRIIB on macrophages (but not B cells) are more susceptible to pneumococcal peritonitis and pneumonia42,53. However, if FcγRIIB-deficient mice were first immunized with a pneumococcal vaccine and then challenged with high doses of S. pneumoniae, mortality rates were increased and this was associated with an increase in pro-inflammatory cytokine production53. This suggests a role for FcγRIIB in the balance between efficient pathogen clearance and the prevention of the cytokine-mediated effects of sepsis.
FcγRIIB deficiency is also associated with an increased resistance to Staphylococcus aureus in mice66, and following nasal infection with Mycobacterium tuberculosis, FcγRIIB-deficient mice show decreased bacterial burden in lungs and spleen and enhanced protective pulmonary T helper 1 cell responses124. By contrast, FcγRIIB does not seem to be important in modulating phagocytosis of serum-opsonized Salmonella enterica subsp. enterica serovar Typhimurium (an intracellular bacterium) in vitro125. The role of FcγRIIB in defence against viral infections has not been examined in detail. one study using human papilloma virus-like particles (highly immunogenic, nonreplicative structures that mimic their virus counterparts in morphology and immunogenicity) showed decreased uptake of particles by DCs and decreased antiviral antibody and T cell responses in FcγRIIB-deficient mice compared with wild-type mice126.
Therefore, FcγRIIB has a major effect on defence against some, but not all, microorganisms in mice. However, this effect is complex and can lead to different outcomes in different contexts even when the animals are infected by the same organism, as typified by the response to S. pneumoniae; in naive animals with primary infection, FcγRIIB ligation may dampen the immune response sufficiently to impair pneumococcal clearance resulting in a negative effect on survival. By contrast, in immune animals with pre-formed antibody, FcγRIIB ligation may be crucial to limit immune complex-associated inflammation and an excessive, pro-inflammatory cytokine storm, thus enhancing survival. This leads to the conclusion that regulation of FcγRIIB modulates not only pathogen clearance but also the inflammatory response to that pathogen, balancing the risks of infection with those of uncontrolled inflammation and septic shock.
In humans, in vitro studies have shown that expression of the functionally impaired FcγRIIBT232 variant is associated with increased phagocytosis of antibodyopsonized S. pneumoniae by monocyte-derived macrophages100. However, genetic studies have not shown an association between the polymorphism encoding FcγRIIBT232 and septicaemia in a large study of Kenyan children with bacterial sepsis99. This does not exclude the possibility that FCGR2B polymorphisms alter susceptibility to bacterial infection, and indeed genetic variants in FCGR2B have been associated with aggressive periodontitis127. Given the mouse data on FcγRIIB and pneumococcal infection53, future genetic studies should use carefully stratified clinical cohorts, as FcγRIIB may have different effects on the immune and inflammatory response to different organisms in different clinical contexts, which could confound clinically heterogenous cohort studies. Moreover, the effect of the polymorphism encoding FcγRIIBT232 on disease may be influenced by epistatic interactions and linkage disequilibrium with genetic variants in other FCGR genes, particularly the neighbouring FCGR3B gene, which has common and functionally important CNV62.
Ethnic differences in SLE, malaria and evolution
The incidence of SLE is at least two-fourfold higher in populations of African or Asian descent than in Caucasians112 and, consistent with this, the frequency of the homozygous SLE-associated variant FcγRIIBT232 is higher in these populations; for example, in 10% of Kenyans and 6% of Vietnamese but 1% of europeans99. Thus, FcγRIIBT232 is common in areas where malaria is endemic (FIG. 4b), raising the possibility that decreased FcγRIIB function may provide a survival advantage against this disease102.
Consistent with this hypothesis, FcγRIIB-deficient mice are resistant to Plasmodium chabaudi chabaudi, a model of the erythrocytic stage of human malarial infection102. Similarly, in humans, the expression of FcγRIIBT232 increased phagocytosis of antibodyopsonized Plasmodium falciparum-parasitized erythrocytes by monocyte-derived macrophages in vitro102. A large study in two cohorts has shown that homozygous expression of FcγRIIBT232 is associated with substantial protection against severe malaria in Kenyan children99. We propose that several mechanisms may contribute to this protective effect, including increased phagocytosis, malarial-specific antibody titres and TNF production102,128,129 (FIG. 6).
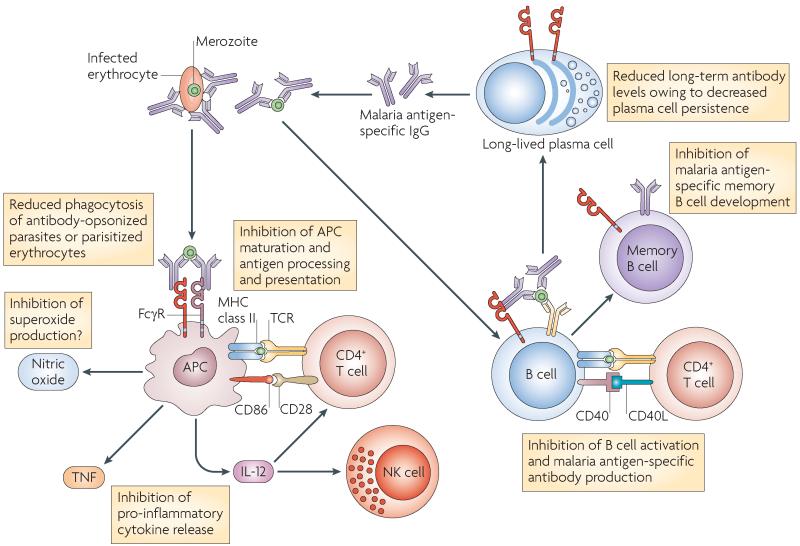
Figure 6. The proven and potential roles of FcγRIIB in defence against malarial infection.
Fc receptor IIB for IgG (FcγRIIB) regulates phagocytosis of parasites and parasitized erythrocytes, antigen presentation to T cells and the production of malarial antibodies by B cells. Macrophages are important in the immune response to malaria, both as antigen-presenting cells (APCs) and phagocytes. In particular, FcγR-mediated phagocytosis is crucial for the elimination of parasitized erythrocytes in mouse malarial models. FcγRIIB inhibits FcγR-mediated phagocytosis of antibody-opsonized parasitized erythrocytes or opsonized merozoites. Subsequent presentation of malarial antigens to T cells may also be potentially modulated by FcγRIIB. Cytokines are important in the immune response to malarial parasites. Tumour necrosis factor (TNF) promotes macrophage phagocytosis and enhances killing of intra-erythrocytic Plasmodium falciparum in humans. FcγRIIB inhibits the production of TNF from macrophages following FcγR-mediated phagocytosis of antibody-opsonized parasitized erythrocytes. IL-12 is crucial for the development of interferon-γ (IFNγ)-mediated protection to mouse malarial infections and for the production of TNF, and its release may also be modulated by FcγRIIB. Antibodies form an important defence to the erythrocytic stages of malaria (as shown by the passive transfer of protective IgG in mice and humans). FcγRIIB-deficient mice generate enhanced levels of malaria-specific IgG following infection with Plasmodium chabaudi chabaudi, suggesting that FcγRIIB may have a role in regulating antibody responses to malarial parasites in humans. IL, interleukin; NK, natural killer; TCR, T cell receptor.
The high mortality rate associated with malarial infection has resulted in its recognition as the strongest known force for evolutionary selection in the recent history of the human genome130. The most well recognized examples of this are the retention of sickle cell anaemia and thalassaemia traits: in Kenyan children from the Coast region of Kilifi, heterozygous and homozygous α-thalassaemia confers a protective effect against malarial infection131. Homozygous FcγRIIBT232 has a protective effect of similar magnitude to heterozygous α-thalassemia99, and it thus could explain the higher frequency of FcγRIIBT232 in Africans and Southeast Asians. This might provide the first example of an immune poly morphism predisposing to polygenic autoimmunity, selected and retained by virtue of its protective effect in malaria infection, and beginning to provide an explanation for the ethnic differences seen in SLE susceptibility (FIG. 4b).
Therapeutic agents
Modification of FcγRIIB expression
Even a modest increase in the expression of FcγRIIB on B cells can ameliorate CIA and spontaneous SLE in mice42. In vitro, IL-4 treatment of monocytes from patients with rheumatoid arthritis altered the FcγR expression balance in favour of FcγRIIB and prevented IgG-induced TNF production by these cells132. These studies raise the possibility that careful cell-specific modulation of FcγRIIB expression may offer a new treatment strategy in autoimmune diseases (TABLE 1). There is also some evidence that two therapeutic agents in widespread use, IVIG and infliximab (Remicade; Centocor/Merck), may owe their efficacy, at least in part, to their effect on FcγRIIB expression.
Table 1. Current and future therapeutic exploitation of FcγRIIB.
| Therapeutic strategy | Clinical effect | Refs |
|---|---|---|
| Modulation of FcγRIIB expression on effector cells | IVIG indirectly increases FcγRIIB expression on ‘effector’ cells | 138,139 |
| Infliximab (Remicade; Centocor/Merck) blocks TNF, leading to increased FcγRIIB expression on monocytes | 142 | |
| Modulation of IgG-binding to FcγRIIB | Decrease in affinity of depleting monoclonal antibodies for FcγRIIB increases efficacy | 45,47 |
| Use of FcγRIIB as a direct therapeutic target | Monoclonal antibody directed against FcγRIIB allowing co-crosslinking of FcγRIIB may induce apoptosis of B cells and plasma cells in lymphoma, myeloma or autoimmunity | 40,150,151 |
| A bispecific antibody that binds antigen-specific BCR and FcγRIIB potentially allows targeted inhibition of autoreactive B cells | - | |
| Therapeutic antibody targeting IgE receptor can inhibit mast cell degranulation and may be useful in the treatment of allergy | 152-155 | |
| Soluble FcγRIIB may inhibit immune complex-mediated immune activation and has shown efficacy in a mouse model of SLE | 156 |
BCR, B cell receptor; FcγR, Fc receptor for IgG; IVIG, intravenous immunoglobulin; SLE, systemic lupus erythematosus; TNF, tumour necrosis factor.
FcγRIIB and IVIG
High dose IVIG is increasingly used to treat autoimmune diseases133. Proposed immunomodulatory mechanisms include the neutralization of pathogenic autoantibodies by anti-idiotypic antibodies, the modulation of cytokine production and the neutralization of the complement components C3a and C5a133. These effects are mainly F(ab′)2 dependent, but studies in patients with ITP and in mouse models of auto immunity suggest that the Fc fragment may be as potent as whole IVIG preparations134. There is increasing evidence that FcγRIIB may have a central role in mediating this Fc-dependent effect. In FcγRIIB-deficient mice, IVIG is not effective in treating autoimmunity81,135-137. Following IVIG treatment of wild-type mice, FcγRIIB expression is upregulated on splenic macrophages135, which, together with a decrease in expression of activating FcγRIV137, might reduce macrophage activation and associated inflammation. More recent data extends this observation to humans, demonstrating an upregulation of FcγRIIB expression by monocytes and B cells following IVIG treatment of patients with chronic inflammatory demyelinating polyneuropathy120.
Studies have suggested that IVIG indirectly increases FcγRIIB expression on effector macrophages through its direct effect on colony-stimulating factor 1 (CSF1)-dependent macrophages138,139. IVIG is ineffective in treating autoimmunity in Csf1-knockout mice, which are deficient in some subpopulations of monocytes and macrophages138. Furthermore, IVIG treatment of Csf1-knockout mice does not upregulate FcγRIIB expression on effector macrophages, suggesting that CSF1-dependent macrophages are required to mediate this effect138. other investigators have suggested that CD11c+ splenocytes (a population that may be separate from, or contained in, the CSF1-dependent population) are required to ‘sense’ IVIG and that FcγRIII is required for ‘sensing’140. However, once ‘sensed’, FcγRIIB expression was required for IVIG-mediated disease inhibition. Alternatively, IVIG may be sensed by DC-SIGN related 1 (SIGNR1), a C-type lectin that preferentially binds sialylated IgG (unlike FcγRs, which have decreased affinity for sialylated IgG). SIGNR1 is expressed by splenic marginal zone macrophages, and is a mouse homologue of human DC-SIGN139. It is estimated that ~5% of IVIG is fully glyco sylated141. De-glycosylation of IVIG abrogates its anti-inflammatory activity, whereas enrichment of IVIG for sialylated forms of IgG increases its anti-inflammatory activity effect 10-fold81. These intriguing results, which await full confirmation in human disease, are potentially of therapeutic importance. In an era where the demand for IVIG significantly outweighs supply, increasing the sialylation levels of IVIG may be a means of substantially improving efficacy.
FcγRIIB and infliximab
TNF upregulates the expression of activating FcγRIIA on neutrophils in vitro, and amplifies immune complex-induced activation of neutrophils in vivo. TNF blockade with infliximab in patients with rheumatoid arthritis led to a decrease in expression of FcγRIIA and an increased expression of FcγRIIB on neutrophils in some patients142. The increased expression of FcγRIIB was associated with a decrease in disease severity, suggesting that the efficacy of TNF blockade may be in part due to modulation of FcγRIIB expression levels.
Modification of antibody binding to FcγRIIB
FcγR interactions predict the efficacy of therapeutic monoclonal antibodies, such as the B cell depleting CD20-specific antibody rituximab (Rituxan/Mabthera; Genentech/Roche/Biogen Idec)143. Studies in Fcγ-chain-deficient mice have shown that the effect of many monoclonal antibodies that deplete immune cells depends on the presence of activating FcγRs, and that inhibitory FcγRIIB can be an important negative regulator of their effect in vivo144-146. The efficacy of cytotoxic antibodies may be improved by decreasing their interaction with FcγRIIB45,47 or by increasing their binding affinity for activating FcγRs147,148. Modulation of the Fc glycosylation levels of monoclonal antibodies may provide a means of manipulating FcγR interaction and efficacy43,80,149.
FcγRIIB as a therapeutic target
A monoclonal antibody against FcγRIIB (hu2B6-3.5; MacroGenics) has been used to direct monocyte- or macrophage-induced cytotoxicity against B cell lymphoma cells150 and plasma cells from patients with systemic lightchain amyloidosis151. FcγRIIB cross-linking on B cells, plasma cells and myeloma cell lines40 can induce apoptosis in vitro, and thus FcγRIIB-specific antibodies might show efficacy in B and plasma cell malignancies. These antibodies might also provide a means of targeting plasma cells in antibody-driven autoimmune diseases.
Cross-linking FcγRIIB activating receptors could therapeutically harness its inhibitory effect. This is being investigated in allergy, where FcγRIIB cross-linking with IgE-antigen complexes or FcεRI has been used to inhibit IgE-mediated histamine release from human mast cells and basophils in vitro and in mouse models152,153. These observations have recently extended to non-human primates154,155. Moreover, the possibility that bispecific antibodies could co-ligate specific antigen and FcγRIIB raises the prospect of autoantigen-specific suppression of humoral immunity.
The soluble FcγRIIB3 isoform has been shown to inhibit the effects of immune complexes in vitro24,26. A more recent study of soluble FcγRIIB in (NZB × NZW) F1 mice showed therapeutic efficacy, slowing the progression of nephritis and prolonging survival156 and prompting its use in human trials in SLE.
Conclusion and future direction
FcγRIIB, identified as the mediator of Fc fragment-induced B cell suppression over 30 years ago, is now known to be widely expressed and to control key aspects of immunity. Variation in its complex and often subtle regulation is crucially involved in determining susceptibility to autoimmunity and defence against infection. Better understanding of this variation is beginning to provide insight into the evolution of disease susceptibility and to open new opportunities for therapy.
Acknowledgements
We thank M. Espeli for review of the manuscript. K.G.C.S is supported by the NIHR Cambridge Biomedical Research Centre and the Wellcome Trust (programme grant number 083650/Z/07/Z) and is a Lister prize fellow. M.R.C. is supported by a Wellcome Trust Intermediate Fellowship (WT081020).
Glossary
- Immune complexes
Complexes of antigen bound to antibody and, sometimes, components of the complement system. The number of circulating immune complexes is increased in some autoimmune disorders (particularly SLE) in which they may be deposited in tissues causing inflammation and tissue damage.
- Antibody-dependent cell-mediated cytotoxicity (ADCC)
A mechanism by which FcR-expressing cells kill other cells, such as virus-infected target cells, that are coated with antibodies. The Fc portions of the coating antibodies interact with the FcγR, thereby initiating a signalling cascade that results in the induction of apoptosis of the antibody-coated cell.
- Acute-phase proteins
A group of proteins, the plasma concentration of which changes in response to trauma, inflammation and infection. C-reative protein is the prototypical acute-phase protein and binds phosphocholine on pathogens and apoptotic cells, opsonizing them for disposal by phagocytes by FcγR binding.
- FcR common γ-chain
A membrane-associated signal adaptor protein that contains an ITAM. It is shared by FcγRI and FcγRIIIA, as well as other receptors, including collagen receptor glycoprotein IV, NKp46, ILT1 (also known as LIR7) and osteoclast-associated immunoglobulin-like receptor (OSCAR).
- Follicular DCs (FDCs)
Cells with a dendritic morphology that are present within B cell follicles in secondary lymphoid tissues such as lymph nodes and spleen. They display intact antigens that are held in immune complexes on their surface, accessible to B cells. FDCs are of non-haematopoietic origin and are not related to ‘conventional’ DCs.
- Germinal centre
A lymphoid structure that arises in B cell follicles in secondary lymphoid organs, such as spleen and lymph node, after immunization with, or exposure to, a T cell-dependent antigen. It is specialized for facilitating the development of high-affinity, long-lived plasma cells and memory B cells.
- Intravenous immunoglobulin (IVIG)
A preparation of human polyclonal IgG obtained from pooled plasma samples taken from thousands of healthy blood donors. It is used as a replacement therapy for individuals with hypogamma-globulinaemia, but is also increasingly used, at much higher doses, to treat autoimmune diseases. IVIG is licensed for the treatment of idiopathic thrombocytopenic purpura, Guillain–Barré syndrome, chronic inflammatory demyelinating polyneuropathy and Kawasaki disease, and is also used in the treatment of other autoimmune diseases, including multiple sclerosis and ANCA-associated vasculitis.
- Central tolerance
Immune tolerance to self antigens that is imposed during lymphocyte development in the thymus (T cells) or the bone marrow (B cells). B cells expressing a BCR that recognizes self antigen must undergo further rearrangement of antigen-receptor genes to become self tolerant or they are deleted or rendered anergic. This process does not eliminate all autoreactive lymphocytes and therefore mechanisms exist in the periphery to maintain tolerance in mature lymphocytes (peripheral tolerance).
- T cell-dependent antigens
Antigens that require T cell–B cell interactions to activate B cells and produce an antibody response.
- Ubiquitin E3 ligase
An enzyme that is required to attach the molecular tag ubiquitin to proteins. Depending on the position and number of ubiquitin molecules that are attached, the ubiquitin tag can target proteins for degradation in the proteasomal complex, sort them to specific subcellular compartments or modify their biological activity.
- Copy number variant (CNV)
A genomic variant characterized by differences in the number of copies of specific repeated DNA fragments that range from 1 kb to several mb long and can contain entire genes.
- Haplotype
A set of single nucleotide polymorphisms (SNPs) or other genetic variants in a gene or locus that are inherited together.
- Systemic lupus erythematosus (SLE)
A multisystem autoimmune disease characterized by hypergamma-globulinaemia and the development of autoantibodies, including those specific for nuclear self antigens such as DNA. These antibodies form immune complexes that become deposited in tissues, including the kidneys and skin. Deposited immune complexes initiate an inflammatory response causing tissue damage. SLE is a polygenic disease, that is multiple, common polymorphisms confer disease susceptibility.
- (NZB × NZW) F1 mice
The F1 generation of the cross between NZB mice and NZW mice. (NZB × NZW) F1 mice spontaneously develop a disease that closely resembles the human disease SLE.
- MRL–lpr mouse
A mouse strain that spontaneously develops glomerulonephritis and other symptoms of SLE. The lpr mutation causes a defect in CD95 (also known as FAS), preventing apoptosis of activated lymphocytes. The MRL background contributes other disease-associated genetic variants.
- Collagen-induced arthritis (CIA)
A model of rheumatoid arthritis. CIA develops in susceptible rodents and primates after immunization with cartilage-derived type II collagen.
- Sickle cell anaemia
Sickle cell anaemia is an autosomal recessive disease in which the gene encoding the β-globin chain has a mutation resulting in an amino acid change (a valine for a glutamic acid at position 6), which leads to erythrocytes that are rigid and sickle shaped. Patients develop anaemia and sickle crises, which significantly shorten life expectancy. Heterozygotes are protected from malaria infection, resulting in the retention of this mutation in populations exposed to malaria.
- Thalassaemia
Thalassaemia is an autosomal recessive genetic disorder, in which there is a mutation in the gene encoding either the α- or β-globin chains that make up haemoglobin. This leads to reduced globin chain (and hence haemoglobin) synthesis, causing anaemia of variable severity. Carriers of the thalassaemia mutations (thalassaemia trait) are protected from malaria infection. This selective advantage is thought to contribute to the persistence of this potentially harmful mutation in the human genetic pool.
- Anti-idiotypic antibody
An antibody that is directed against the antigen-specific binding site of an immunoglobulin or a T cell receptor and therefore may compete with antigen for binding.
Footnotes
Competing interests statement: The authors declare no competing financial interests.
Databases: OMIM: http://www.ncbi.nlm.nih.gov/omim ; uniprotKb: http://www.uniprot.org
Kenneth G. C. Smith’s homepage: http://www.cimr.cam.ac.uk/investigators/smith/index.html
References
- 1.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Perez LG, Costa MR, Todd CA, Haynes BF, Montefiori DC. Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: a specific role for antibodies against the membrane-proximal external region of gp41. J. Virol. 2009;83:7397–7410. doi: 10.1128/JVI.00656-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma A, et al. Analysis of the Fcγ receptor-dependent component of neutralization measured by anthrax toxin neutralization assays. Clin. Vaccine Immunol. 2009;16:1405–1412. doi: 10.1128/CVI.00194-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcγ receptor II. J. Exp. Med. 1999;190:585–590. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J, et al. Structural recognition and functional activation of FcγR by innate pentraxins. Nature. 2008;456:989–992. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinclair NR. Regulation of the immune response. I. Reduction in ability of specific antibody to inhibit long-lasting IgG immunological priming after removal of the Fc fragment. J. Exp. Med. 1969;129:1183–1201. doi: 10.1084/jem.129.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidman CL, Unanue ER. Control of B-lymphocyte function. I. Inactivation of mitogenesis by interactions with surface immunoglobulin and Fc-receptor molecules. J. Exp. Med. 1976;144:882–896. doi: 10.1084/jem.144.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips NE, Parker DC. Cross-linking of B lymphocyte Fcγ receptors and membrane immunoglobulin inhibits anti-immunoglobulin-induced blastogenesis. J. Immunol. 1984;132:627–632. [Papers 6–8 describe some of the early experiments establishing the immunosuppressive effect of the Fc region of IgG and show that this effect is mediated through an FcR.] [PubMed] [Google Scholar]
- 9.Amigorena S, Bonnerot C, Choquet D, Fridman WH, Teillaud JL. Fcγ RII expression in resting and activated B lymphocytes. Eur. J. Immunol. 1989;19:1379–1385. doi: 10.1002/eji.1830190805. [DOI] [PubMed] [Google Scholar]
- 10.Ravetch JV, Kinet JP. Fc receptors. Annu. Rev. Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 11.Daeron M, et al. Distinct intracytoplasmic sequences are required for endocytosis and phagocytosis via murine Fcγ RII in mast cells. Int. Immunol. 1993;5:1393–1401. doi: 10.1093/intimm/5.11.1393. [DOI] [PubMed] [Google Scholar]
- 12.Qin D, et al. Fcγ receptor IIB on follicular dendritic cells regulates the B cell recall response. J. Immunol. 2000;164:6268–6275. doi: 10.4049/jimmunol.164.12.6268. [DOI] [PubMed] [Google Scholar]
- 13.Barrington RA, Pozdnyakova O, Zafari MR, Benjamin CD, Carroll MC. B lymphocyte memory: role of stromal cell complement and FcγRIIB receptors. J. Exp. Med. 2002;196:1189–1199. doi: 10.1084/jem.20021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravetch JV, et al. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986;234:718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- 15.Brooks DG, Qiu WQ, Luster AD, Ravetch JV. Structure and expression of human IgG FcRII (CD32). Functional heterogeneity is encoded by the alternatively spliced products of multiple genes. J. Exp. Med. 1989;170:1369–1385. doi: 10.1084/jem.170.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart SG, et al. Human IgG Fc receptor (hFcRII; CD32) exists as multiple isoforms in macrophages, lymphocytes and IgG-transporting placental epithelium. EMBO J. 1989;8:3657–3666. doi: 10.1002/j.1460-2075.1989.tb08540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogarth PM, et al. Structure of the mouse βFcγ receptor II gene. J. Immunol. 1991;146:369–376. [Together with 14, this is an important early paper describing FcγRs.] [PubMed] [Google Scholar]
- 18.Joshi T, Ganesan LP, Cao X, Tridandapani S. Molecular analysis of expression and function of hFcγRIIbl and b2 isoforms in myeloid cells. Mol. Immunol. 2006;43:839–850. doi: 10.1016/j.molimm.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen HM, Rose JK, Mellman I. Fc receptor isoforms exhibit distinct abilities for coated pit localization as a result of cytoplasmic domain heterogeneity. Cell. 1989;58:317–327. doi: 10.1016/0092-8674(89)90846-5. [DOI] [PubMed] [Google Scholar]
- 20.Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J. Cell Biol. 1994;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J. 1994;13:2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miettinen HM, Matter K, Hunziker W, Rose JK, Mellman I. Fc receptor endocytosis is controlled by a cytoplasmic domain determinant that actively prevents coated pit localization. J. Cell Biol. 1992;116:875–888. doi: 10.1083/jcb.116.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartour E, et al. Identification, in mouse macrophages and in serum, of a soluble receptor for the Fc portion of IgG (Fcγ R.) encoded by an alternatively spliced transcript of the Fcγ RII gene. Int. Immunol. 1993;5:859–868. doi: 10.1093/intimm/5.8.859. [DOI] [PubMed] [Google Scholar]
- 24.Esposito-Farese ME, et al. Membrane and soluble Fcγ RII/III modulate the antigen-presenting capacity of murine dendritic epidermal Langerhans cells for IgG-complexed antigens. J. Immunol. 1995;155:1725–1736. [PubMed] [Google Scholar]
- 25.Sautes C, et al. Soluble Fcγ receptors II (FcγRII) are generated by cleavage of membrane FcγRII. Eur. J. Immunol. 1991;21:231–234. doi: 10.1002/eji.1830210135. [DOI] [PubMed] [Google Scholar]
- 26.Varin N, et al. Recombinant soluble receptors for the Fcγ portion inhibit antibody production in vitro. Eur. J. Immunol. 1989;19:2263–2268. doi: 10.1002/eji.1830191213. [DOI] [PubMed] [Google Scholar]
- 27.Amigorena S, et al. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–1812. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 28.Muta T, et al. A 13-amino-acid motif in the cytoplasmic domain of Fcγ RIIB modulates B-cell receptor signalling. Nature. 1994;369:340. doi: 10.1038/369340a0. [DOI] [PubMed] [Google Scholar]
- 29.Malbec O, et al. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fcγ receptor IIB during negative regulation of mast cell activation. J. Immunol. 1998;160:1647–1658. [PubMed] [Google Scholar]
- 30.Katsumata O, et al. Association of FcγRII with low-density detergent-resistant membranes is important for cross-linking-dependent initiation of the tyrosine phosphorylation pathway and superoxide generation. J. Immunol. 2001;167:5814–5823. doi: 10.4049/jimmunol.167.10.5814. [DOI] [PubMed] [Google Scholar]
- 31.Kwiatkowska K, Sobota A. The clustered Fcγ receptor II is recruited to Lyn-containing membrane domains and undergoes phosphorylation in a cholesterol-dependent manner. Eur. J. Immunol. 2001;31:989–998. doi: 10.1002/1521-4141(200104)31:4<989::aid-immu989>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor FcγRIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [This report shows that the inhibitory effect of FcγRIIB is mediated by SHIP.] [DOI] [PubMed] [Google Scholar]
- 33.Ono M, et al. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 34.Pearse RN, et al. SHIP recruitment attenuates Fcγ RIIB-induced B cell apoptosis. Immunity. 1999;10:753–760. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 35.Tzeng SJ, Bolland S, Inabe K, Kurosaki T, Pierce SK. The B cell inhibitory Fc receptor triggers apoptosis by a novel c-Abl family kinase-dependent pathway. J. Biol. Chem. 2005;280:35247–35254. doi: 10.1074/jbc.M505308200. [DOI] [PubMed] [Google Scholar]
- 36.Carter NA, Harnett MM. Dissection of the signalling mechanisms underlying FcγRIIB-mediated apoptosis of mature B-cells. Biochem. Soc. Trans. 2004;32:973–975. doi: 10.1042/BST0320973. [DOI] [PubMed] [Google Scholar]
- 37.Brauweiler AM, Cambier JC. Autonomous SHIP-dependent FcγR signaling in pre-B cells leads to inhibition of cell migration and induction of cell death. Immunol. Lett. 2004;92:75–81. doi: 10.1016/j.imlet.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 38.Kato I, Takai T, Kudo A. The pre-B cell receptor signaling for apoptosis is negatively regulated by Fcγ RIIB. J. Immunol. 2002;168:629–634. doi: 10.4049/jimmunol.168.2.629. [DOI] [PubMed] [Google Scholar]
- 39.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcγ receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat. Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 40.Xiang Z, et al. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 2007;8:419–429. doi: 10.1038/ni1440. [This, and reference 34, shows that FcγRIIB can mediate B cell apoptosis.] [DOI] [PubMed] [Google Scholar]
- 41.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fcγ RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [This reference describes FcγRIIB-deficient mice, confirming the inhibitory function of this receptor.] [DOI] [PubMed] [Google Scholar]
- 42.Brownlie RJ, et al. Distinct cell-specific control of autoimmunity and infection by FcγRIIb. J. Exp. Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [A paper showing that modest over-expression of FcγRIIB on B cell can suppress autoimmunity (see also reference 107).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 44.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcγ receptors on dendritic cells. J. Exp. Med. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boruchov AM, et al. Activating and inhibitory IgG Fc receptors on human DCs mediate opposing functions. J. Clin. Invest. 2005;115:2914–2923. doi: 10.1172/JCI24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhodapkar KM, et al. Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl Acad. Sci. USA. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhodapkar KM, et al. Selective blockade of the inhibitory Fcγ receptor (FcγRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J. Exp. Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schifferli JA, Taylor RP. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989;35:993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- 50.Bergtold A, Desai DD, Gavhane A, Clynes R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity. 2005;23:503–514. doi: 10.1016/j.immuni.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Clynes R, et al. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J. Exp. Med. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuasa T, et al. Deletion of fcγ receptor IIB renders H-2b mice susceptible to collagen-induced arthritis. J. Exp. Med. 1999;189:187–194. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clatworthy MR, Smith KGC. FcγRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J. Exp. Med. 2004;199:717–723. doi: 10.1084/jem.20032197. [A paper showing that FcγRIIB has a complex effect on responses to infection, balancing the capacity to clear pathogen with the risk of excessive inflammation and septic shock.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, et al. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fcγ RIIb-dependent PGE2 production. J. Immunol. 2009;182:554–562. doi: 10.4049/jimmunol.182.1.554. [DOI] [PubMed] [Google Scholar]
- 55.van Lent P, et al. The inhibitory receptor FcγRII reduces joint inflammation and destruction in experimental immune complex-mediated arthritides not only by inhibition of FcγRI/III but also by efficient clearance and endocytosis of immune complexes. Am. J. Pathol. 2003;163:1839–1848. doi: 10.1016/s0002-9440(10)63543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daeron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J. Clin. Invest. 1995;95:577–585. doi: 10.1172/JCI117701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fong DC, et al. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated FcγRIIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol. Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 58.Kepley CL, et al. Negative regulation of FcεRI signaling by FcγRII costimulation in human blood basophils. J. Allergy Clin. Immunol. 2000;106:337–348. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 59.Malbec O, Fridman WH, Daeron M. Negative regulation of c-kit-mediated cell proliferation by Fcγ RIIB. J. Immunol. 1999;162:4424–4429. [PubMed] [Google Scholar]
- 60.Pricop L, et al. Differential modulation of stimulatory and inhibitory Fcγ receptors on human monocytes by Th1 and Th2 cytokines. J. Immunol. 2001;166:531–537. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 61.van Mirre E, et al. Neutrophil responsiveness to IgG, as determined by fixed ratios of mRNA levels for activating and inhibitory FcγRII (CD32), is stable over time and unaffected by cytokines. Blood. 2006;108:584–590. doi: 10.1182/blood-2005-12-4997. [DOI] [PubMed] [Google Scholar]
- 62.Willcocks LC, et al. Copy number of FCGR3B, which is associated with systemic lupus erythematosus, correlates with protein expression and immune complex uptake. J. Exp. Med. 2008;205:1573–1582. doi: 10.1084/jem.20072413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosales C, Brown EJ. Signal transduction by neutrophil immunoglobulin G Fc receptors. Dissociation of intracytoplasmic calcium concentration rise from inositol 1,4,5-trisphosphate. J. Biol. Chem. 1992;267:5265–5271. [PubMed] [Google Scholar]
- 64.Zhou MJ, Brown EJ. CR3 (Mac-1, αM β2, CD11b/CD18) and FcγRIII cooperate in generation of a neutrophil respiratory burst: requirement for FcγRIII and tyrosine phosphorylation. J. Cell Biol. 1994;125:1407–1416. doi: 10.1083/jcb.125.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coxon A, et al. FcγRIII mediates neutrophil recruitment to immune complexes. A mechanism for neutrophil accumulation in immune-mediated inflammation. Immunity. 2001;14:693–704. doi: 10.1016/s1074-7613(01)00150-9. [DOI] [PubMed] [Google Scholar]
- 66.Gjertsson I, Kleinau S, Tarkowski A. The impact of Fcγ receptors on Staphylococcus aureus infection. Microb. Pathog. 2002;33:145–152. [PubMed] [Google Scholar]
- 67.Nakamura A, et al. Fcγ receptor IIB-deficient mice develop Goodpasture’s syndrome upon immunization with type IV collagen: a novel murine model for autoimmune glomerular basement membrane disease. J. Exp. Med. 2000;191:899–906. doi: 10.1084/jem.191.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snapper CM, Hooley JJ, Atasoy U, Finkelman FD, Paul WE. Differential regulation of murine B cell FcγRII expression by CD4+ T helper subsets. J. Immunol. 1989;143:2133–2141. [PubMed] [Google Scholar]
- 69.Rudge ER, Cutler AJ, Pritchard NR, Smith KGC. Interleukin-4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and FcγRII-mediated B cell suppression. J. Exp. Med. 2002;195:1079–1085. doi: 10.1084/jem.20011435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tridandapani S, et al. Regulated expression and inhibitory function of Fcγ RIIb in human monocytic cells. J. Biol. Chem. 2002;277:5082–5089. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 71.Shushakova N, et al. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcγRs in immune complex-induced lung disease. J. Clin. Invest. 2002;110:1823–1830. doi: 10.1172/JCI200216577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pritchard NR, et al. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcγRII. Curr. Biol. 2000;10:227–230. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 73.Bonnerot C, et al. Methylation in the 5′ region of the murine βFcγ R gene regulates the expression of Fc γ receptor II. J. Immunol. 1988;141:1026–1033. [PubMed] [Google Scholar]
- 74.Bonnerot C, Choukroun V, Marloie MA, Fridman WH. Two distinct regions of the mouse βFcγ R gene control its transcription. Immunobiology. 1992;185:222–234. doi: 10.1016/s0171-2985(11)80643-1. [DOI] [PubMed] [Google Scholar]
- 75.Hor S, Ziv T, Admon A, Lehner PJ. Stable isotope labeling by amino acids in cell culture and differential plasma membrane proteome quantitation identify new substrates for the MARCH9 transmembrane E3 ligase. Mol. Cell Proteomics. 2009;8:1959–1971. doi: 10.1074/mcp.M900174-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-A crystal structure of the human IgG1 Fc fragment-FcγRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 77.Bruhns P, et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 78.Willcocks LC, Smith KG, Clatworthy MR. Low-affinity Fcγ receptors, autoimmunity and infection. Expert Rev. Mol. Med. 2009;11:e24. doi: 10.1017/S1462399409001161. [DOI] [PubMed] [Google Scholar]
- 79.Matsumiya S, et al. Structural comparison of fucosylated and nonfucosylated Fc fragments of human immunoglobulin G1. J. Mol. Biol. 2007;368:767–779. doi: 10.1016/j.jmb.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 80.Boyd PN, Lines AC, Patel AK. The effect of the removal of sialic acid, galactose and total carbohydrate on the functional activity of Campath-1H. Mol. Immunol. 1995;32:1311–1318. doi: 10.1016/0161-5890(95)00118-2. [DOI] [PubMed] [Google Scholar]
- 81.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 82.Warmerdam PA, Nabben NM, van de Graaf SA, van de Winkel JG, Capel PJ. The human low affinity immunoglobulin G. Fc receptor IIC gene is a result of an unequal crossover event. J. Biol. Chem. 1993;268:7346–7349. [PubMed] [Google Scholar]
- 83.Bailey JA, et al. Recent segmental duplications in the human genome. Science. 2002;297:1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- 84.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv. Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 85.Rogers KA, Scinicariello F, Attanasio R. IgG Fc receptor III homologues in nonhuman primate species: genetic characterization and ligand interactions. J. Immunol. 2006;177:3848–3856. doi: 10.4049/jimmunol.177.6.3848. [DOI] [PubMed] [Google Scholar]
- 86.Zhou XJ, et al. Copy number variation of FCGR3A rather than FCGR3B and FCGR2B is associated with susceptibility to anti-GBM disease. Int. Immunol. 2010;22:45–51. doi: 10.1093/intimm/dxp113. [DOI] [PubMed] [Google Scholar]
- 87.Hastings PJ, Lupski JR, Rosenberg SM, Ira G. Mechanisms of change in gene copy number. Nature Rev. Genet. 2009;10:551–564. doi: 10.1038/nrg2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Traherne JA, et al. Mechanisms of copy number variation and hybrid gene formation in the KIR immune gene complex. Hum. Mol. Genet. 2010;19:737–751. doi: 10.1093/hmg/ddp538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luan JJ, et al. Defective FcγRII gene expression in macrophages of NOD mice: genetic linkage with up-regulation of IgG1 and IgG2b in serum. J. Immunol. 1996;157:4707–4716. [PubMed] [Google Scholar]
- 90.Jiang Y, et al. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 2000;51:429–435. doi: 10.1007/s002510050641. [DOI] [PubMed] [Google Scholar]
- 91.Xiu Y, et al. Transcriptional regulation of Fcgr2b gene by polymorphic promoter region and its contribution to humoral immune responses. J. Immunol. 2002;169:4340–4346. doi: 10.4049/jimmunol.169.8.4340. [DOI] [PubMed] [Google Scholar]
- 92.Rahman ZS, et al. Expression of the autoimmune Fcgr2b NZW allele fails to be upregulated in germinal center B cells and is associated with increased IgG production. Genes Immun. 2007;8:604–612. doi: 10.1038/sj.gene.6364423. [DOI] [PubMed] [Google Scholar]
- 93.Hibbs ML, Hogarth PM, McKenzie IF. The mouse Ly-17 locus identifies a polymorphism of the Fc receptor. Immunogenetics. 1985;22:335–348. doi: 10.1007/BF00430917. [DOI] [PubMed] [Google Scholar]
- 94.Slingsby JH, Hogarth MB, Walport MJ, Morley BJ. Polymorphism in the Ly-17 alloantigenic system of the mouse FcgRII gene. Immunogenetics. 1997;46:361–362. doi: 10.1007/s002510050287. [DOI] [PubMed] [Google Scholar]
- 95.Su K, et al. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcγRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J. Immunol. 2004;172:7186–7191. doi: 10.4049/jimmunol.172.11.7186. [DOI] [PubMed] [Google Scholar]
- 96.Su K, et al. Expression profile of FcγRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J. Immunol. 2007;178:3272–3280. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blank MC, et al. Decreased transcription of the human FCGR2B gene mediated by the −343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum. Genet. 2005;117:220–227. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 98.Kyogoku C, et al. Fcγ receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–1254. doi: 10.1002/art.10257. [The paper is the first to describe an association between a naturally occurring polymorphism in FcγRIIB and human disease.] [DOI] [PubMed] [Google Scholar]
- 99.Willcocks L, et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc. Natl Acad. Sci. USA. 2010 Apr 27;107:7881–7885. doi: 10.1073/pnas.0915133107. doi:10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Floto RA, et al. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nature Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 101.Kono H, et al. FcγRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum. Mol. Genet. 2005;14:2881–2892. doi: 10.1093/hmg/ddi320. [Papers 100 and 101 provide a mechanism to explain the association of the FcγRIIBT232 polymorphism with disease.] [DOI] [PubMed] [Google Scholar]
- 102.Clatworthy MR, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcγRIIb reduce susceptibility to malaria. Proc. Natl Acad. Sci. USA. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [This paper, together with reference 99, describes how the SLE-associated FcγRIIB T232 polymorphism protects children from severe malaria and together are beginning to explain the ethnic differences in SLE susceptibility.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Siriboonrit U, et al. Association of Fcγ receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 2003;61:374–383. doi: 10.1034/j.1399-0039.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 104.Goodnow CC, Sprent J, Fazekas de St. Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435:590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki Y, et al. Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int. 1998;54:1166–1174. doi: 10.1046/j.1523-1755.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 106.Bolland S, Ravetch JV. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 107.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [This paper shows that restoring FcγRIIB expression in (NZB × NZW) F1 mice can prevent SLE (see also reference 42).] [DOI] [PubMed] [Google Scholar]
- 108.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 109.Jiang Y, et al. Genetically determined aberrant down-regulation of FcγRIIB1 in germinal center B cells associated with hyper-IgG and IgG autoantibodies in murine systemic lupus erythematosus. Int. Immunol. 1999;11:1685–1691. doi: 10.1093/intimm/11.10.1685. [DOI] [PubMed] [Google Scholar]
- 110.Paul E, Nelde A, Verschoor A, Carroll MC. Follicular exclusion of autoreactive B cells requires FcγRIIb. Int. Immunol. 2007;19:365–373. doi: 10.1093/intimm/dxm002. [DOI] [PubMed] [Google Scholar]
- 111.Li X, et al. A novel polymorphism in the Fcγ receptor IIB (CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. 2003;48:3242–3252. doi: 10.1002/art.11313. [DOI] [PubMed] [Google Scholar]
- 112.Lau CS, Yin G, Mok MY. Ethnic and geographical differences in systemic lupus erythematosus: an overview. Lupus. 2006;15:715–719. doi: 10.1177/0961203306072311. [DOI] [PubMed] [Google Scholar]
- 113.Mackay M, et al. Selective dysregulation of the FcγIIB receptor on memory B cells in SLE. J. Exp. Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carreno LJ, Pacheco R, Gutierrez MA, Jacobelli S, Kalergis AM. Disease activity in systemic lupus erythematosus is associated with an altered expression of low-affinity Fcγ receptors and costimulatory molecules on dendritic cells. Immunology. 2009;128:334–341. doi: 10.1111/j.1365-2567.2009.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blom AB, van Lent PL, Holthuysen AE, Jacobs C, van den Berg WB. Skewed balance in basal expression and regulation of activating v inhibitory Fcγ receptors in macrophages of collagen induced arthritis sensitive mice. Ann. Rheum. Dis. 2003;62:465–471. doi: 10.1136/ard.62.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti-CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2008;1143:268–285. doi: 10.1196/annals.1443.013. [DOI] [PubMed] [Google Scholar]
- 117.Radstake TR, et al. The functional variant of the inhibitory Fcγ receptor IIb (CD32B) is associated with the rate of radiologic joint damage and dendritic cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54:3828–3837. doi: 10.1002/art.22275. [DOI] [PubMed] [Google Scholar]
- 118.Wenink MH, et al. The inhibitory FcγIIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J. Immunol. 2009;183:4509–4520. doi: 10.4049/jimmunol.0900153. [DOI] [PubMed] [Google Scholar]
- 119.Iruretagoyena MI, et al. Activating and inhibitory Fcγ receptors can differentially modulate T cell-mediated autoimmunity. Eur. J. Immunol. 2008;38:2241–2250. doi: 10.1002/eji.200838197. [DOI] [PubMed] [Google Scholar]
- 120.Tackenberg B, et al. Impaired inhibitory Fcγ receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc. Natl Acad. Sci. USA. 2009;106:4788–4792. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bruin M, et al. Platelet count, previous infection and FCGR2B genotype predict development of chronic disease in newly diagnosed idiopathic thrombocytopenia in childhood: results of a prospective study. Br. J. Haematol. 2004;127:561–567. doi: 10.1111/j.1365-2141.2004.05235.x. [DOI] [PubMed] [Google Scholar]
- 122.Asahi A, et al. Helicobacter pylori eradication shifts monocyte Fcγ receptor balance toward inhibitory FcγRIIB in immune thrombocytopenic purpura patients. J. Clin. Invest. 2008;118:2939–2949. doi: 10.1172/JCI34496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Musher DM, et al. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 124.Maglione PJ, Xu J, Casadevall A, Chan J. Fcγ receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J. Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 125.Uppington H, et al. Effect of immune serum and role of individual Fcγ receptors on the intracellular distribution and survival of Salmonella enterica serovar Typhimurium in murine macrophages. Immunology. 2006;119:147–158. doi: 10.1111/j.1365-2567.2006.02416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Da Silva DM, Fausch SC, Verbeek JS, Kast WM. Uptake of human papillomavirus virus-like particles by dendritic cells is mediated by Fcγ receptors and contributes to acquisition of T cell immunity. J. Immunol. 2007;178:7587–7597. doi: 10.4049/jimmunol.178.12.7587. [DOI] [PubMed] [Google Scholar]
- 127.Yasuda K, Sugita N, Kobayashi T, Yamamoto K, Yoshie H. FcγRIIB gene polymorphisms in Japanese periodontitis patients. Genes Immun. 2003;4:541–546. doi: 10.1038/sj.gene.6364021. [DOI] [PubMed] [Google Scholar]
- 128.Kumaratilake LM, et al. A synthetic tumor necrosis factor-α agonist peptide enhances human polymorphonuclear leukocyte-mediated killing of Plasmodium falciparum in vitro and suppresses Plasmodium chabaudi infection in mice. J. Clin. Invest. 1995;95:2315–2323. doi: 10.1172/JCI117923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Muniz-Junqueira MI, dos Santos-Neto LL, Tosta CE. Influence of tumor necrosis factor-α on the ability of monocytes and lymphocytes to destroy intraerythrocytic Plasmodium falciparum in vitro. Cell. Immunol. 2001;208:73–79. doi: 10.1006/cimm.2001.1770. [DOI] [PubMed] [Google Scholar]
- 130.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 2005;77:171–192. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams TN, et al. Both heterozygous and homozygous α+ thalassemias protect against severe and fatal Plasmodium falciparum malaria on the coast of Kenya. Blood. 2005;106:368–371. doi: 10.1182/blood-2005-01-0313. [DOI] [PubMed] [Google Scholar]
- 132.Wijngaarden S, et al. A shift in the balance of inhibitory and activating Fcγ receptors on monocytes toward the inhibitory Fcγ receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum. 2004;50:3878–3887. doi: 10.1002/art.20672. [DOI] [PubMed] [Google Scholar]
- 133.Negi VS, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J. Clin. Immunol. 2007;27:233–245. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- 134.Debre M, et al. Infusion of Fcγ fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342:945–949. doi: 10.1016/0140-6736(93)92000-j. [This paper, together with 81, implicates Fc region sialylation and FcγRIIB in the immunosuppressive action of IVIG.] [DOI] [PubMed] [Google Scholar]
- 135.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 136.Crow AR, et al. IVIg-mediated amelioration of murine ITP via FcγRIIB is independent of SHIP1, SHP-1, and Btk activity. Blood. 2003;102:558–560. doi: 10.1182/blood-2003-01-0023. [DOI] [PubMed] [Google Scholar]
- 137.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J. Exp. Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 139.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl Acad. Sci. USA. 2008;105:19571–19578. doi: 10.1073/pnas.0810163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siragam V, et al. Intravenous immunoglobulin ameliorates ITP via activating Fcγ receptors on dendritic cells. Nature Med. 2006;12:688–692. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]
- 141.Routier FH, et al. Quantitation of the oligosaccharides of human serum IgG from patients with rheumatoid arthritis: a critical evaluation of different methods. J. Immunol. Methods. 1998;213:113–130. doi: 10.1016/s0022-1759(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 142.Belostocki K, et al. Infliximab treatment shifts the balance between stimulatory and inhibitory Fcγ receptor type II isoforms on neutrophils in patients with rheumatoid arthritis. Arthritis Rheum. 2008;58:384–388. doi: 10.1002/art.23200. [DOI] [PubMed] [Google Scholar]
- 143.Cartron G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 144.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 145.Zhang Z, Zhang M, Ravetch JV, Goldman C, Waldmann TA. Effective therapy for a murine model of adult T-cell leukemia with the humanized anti-CD2 monoclonal antibody, MEDI-507. Blood. 2003;102:284–288. doi: 10.1182/blood-2002-11-3601. [DOI] [PubMed] [Google Scholar]
- 146.Uchida J, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J. Exp. Med. 2004;199:1659–1669. doi: 10.1084/jem.20040119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J. Biol. Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 148.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferrara C, Stuart F, Sondermann P, Brunker P, Umana P. The carbohydrate at FcγRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 2006;281:5032–5036. doi: 10.1074/jbc.M510171200. [DOI] [PubMed] [Google Scholar]
- 150.Rankin CT, et al. CD32B, the human inhibitory Fc-γ receptor IIB, as a target for monoclonal antibody therapy of B-cell lymphoma. Blood. 2006;108:2384–2391. doi: 10.1182/blood-2006-05-020602. [DOI] [PubMed] [Google Scholar]
- 151.Zhou P, et al. CD32B is highly expressed on clonal plasma cells from patients with systemic light-chain amyloidosis and provides a target for monoclonal antibody-based therapy. Blood. 2008;111:3403–3406. doi: 10.1182/blood-2007-11-125526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tam SW, Demissie S, Thomas D, Daeron M. A bispecific antibody against human IgE and human FcγRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004;59:772–780. doi: 10.1111/j.1398-9995.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 153.Allen LC, Kepley CL, Saxon A, Zhang K. Modifications to an Fcγ-Fcε fusion protein alter its effectiveness in the inhibition of FcεRI-mediated functions. J. Allergy Clin. Immunol. 2007;120:462–468. doi: 10.1016/j.jaci.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 154.Mertsching E, et al. A mouse Fcγ-Fcepsilon protein that inhibits mast cells through activation of FcγRIIB, SH2 domain-containing inositol phosphatase 1, and SH2 domain-containing protein tyrosine phosphatases. J. Allergy Clin. Immunol. 2008;121:441–447. e445. doi: 10.1016/j.jaci.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 155.Van Scott MR, et al. Systemic administration of an Fcγ-Fcε-fusion protein in house dust mite sensitive nonhuman primates. Clin. Immunol. 2008;128:340–348. doi: 10.1016/j.clim.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 156.Werwitzke S, et al. Treatment of lupus-prone NZB/NZW F1 mice with recombinant soluble Fcγ receptor II (CD32) Ann. Rheum. Dis. 2008;67:154–161. doi: 10.1136/ard.2006.068981. [DOI] [PubMed] [Google Scholar]
- 157.Wandstrat AE, et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 158.Jorgensen TN, et al. Development of murine lupus involves the combined genetic contribution of the SLAM and FcγR intervals within the Nba2 autoimmune susceptibility locus. J. Immunol. 2010;184:775–786. doi: 10.4049/jimmunol.0901322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bygrave AE, et al. Spontaneous autoimmunity in 129 and C57BL/6 mice-implications for autoimmunity described in gene-targeted mice. PLoS Biol. 2004;2:e243. doi: 10.1371/journal.pbio.0020243. [This paper describes how the telomeric region of chromosome 1 from the 129 mouse strain predisposes to autoimmunity, highlighting the complex nature of the interaction between the region as a whole and autoimmunity, which must be taken into account when considering the role of FcγRIIB in mouse models of SLE.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carlucci F, et al. Genetic dissection of spontaneous autoimmunity driven by 129-derived chromosome 1 loci when expressed on C57BL/6 mice. J. Immunol. 2007;178:2352–2360. doi: 10.4049/jimmunol.178.4.2352. [DOI] [PubMed] [Google Scholar]
- 161.Bickerstaff MC, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 162.Wu X, et al. A role for the Cr2 gene in modifying autoantibody production in systemic lupus erythematosus. J. Immunol. 2002;169:1587–1592. doi: 10.4049/jimmunol.169.3.1587. [DOI] [PubMed] [Google Scholar]
- 163.Botto M. C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp. Clin. Immunogenet. 1998;15:231–234. doi: 10.1159/000019076. [DOI] [PubMed] [Google Scholar]
- 164.Sohn HW, Pierce SK, Tzeng SJ. Live cell imaging reveals that the inhibitory FcγRIIB destabilizes B cell receptor membrane-lipid interactions and blocks immune synapse formation. J. Immunol. 2008;180:793–799. doi: 10.4049/jimmunol.180.2.793. [DOI] [PubMed] [Google Scholar]
- 165.Liu W, Sohn HW, Tolar P, Meckel T, Pierce SK. Antigen-induced oligomerization of the B cell receptor is an early target of FcγRIIB inhibition. J. Immunol. 2010;184:1977–1989. doi: 10.4049/jimmunol.0902334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.El Shikh ME, El Sayed R, Szakal AK, Tew JG. Follicular dendritic cell (FDC)-FcγRIIB engagement via immune complexes induces the activated FDC phenotype associated with secondary follicle development. Eur. J. Immunol. 2006;36:2715–2724. doi: 10.1002/eji.200636122. [DOI] [PubMed] [Google Scholar]