Abstract
Background. Previous studies have established that antibody titer measured by the hemagglutination-inhibiting (HAI) assay is correlated with protection against influenza virus infection, with an HAI titer of 1:40 generally associated with 50% protection.
Methods. We recruited index cases with confirmed influenza virus infection from outpatient clinics, and followed up their household contacts for 7–10 days to identify secondary infections. Serum samples collected from a subset of household contacts were tested by HAI and microneutralization (MN) assays against prevalent influenza viruses. We analyzed the data using an individual hazard-based transmission model that adjusted for age and vaccination history.
Results. Compared to a reference group with antibody titers <1:10, we found that HAI titers of 1:40 against influenza A(H1N1) and A(H3N2) were associated with 31% (95% confidence interval [CI], 13%–46%) and 31% (CI, 1%–53%) protection against polymerase chain reaction (PCR)–confirmed A(H1N1) and A(H3N2) virus infection, respectively, while an MN titer of 1:40 against A(H3N2) was associated with 49% (95% CI, 7%–81%) protection against PCR-confirmed A(H3N2) virus infection.
Conclusions. An HAI titer of 1:40 was associated with substantially less than 50% protection against PCR-confirmed influenza virus infection within households, perhaps because of exposures of greater duration or intensity in that confined setting.
Keywords: antibody, immunity, influenza, transmission
(See the editorial commentary by Peters and Poehling on pages 671–3.)
The classic study by Hobson et al in 1972 [1] and more recent studies [2–4] have established that antibody titer measured by the hemagglutination-inhibition (HAI) assay is correlated with protection against influenza virus infection, with an HAI titer of 1:40 generally associated with 50% protection against infection compared to HAI titers <1:10. One of the immunogenicity criteria for licensure of new vaccines is that at least 70% of vaccine recipients achieve postvaccination HAI titers ≥1:40 [5, 6]. Moreover, there are few studies exploring the correlation of other measures of humoral immunity, such as from viral-neutralization assays, with protection against influenza virus infection in community settings [7–9].
The objective of our study was to estimate the protection conferred by different antibody titers against influenza virus infection during household outbreaks, when there is defined exposure to influenza virus infection. Based on a prospective study of transmission of influenza A virus in households in Hong Kong conducted in the influenza seasons of 2008 and 2009 [10, 11], we assessed the degree of protection against influenza virus infection associated with specific HAI and microneutralization (MN) assay titers.
METHODS
Study Subjects
Our study was conducted from January through September 2008 and from January through August 2009 in Hong Kong. During these periods of influenza virus circulation, we recruited subjects that presented to outpatient clinics with acute respiratory illness within 48 hours of onset of illness, and lived in a household with at least 2 other persons. Index cases with positive influenza A or B results on a QuickVue Influenza A + B rapid diagnostic test (Quidel, San Diego, CA) were further followed up along with their household contacts. Daily symptoms for index cases and all household contacts were recorded in symptom diaries. Pooled nasal and throat swabs were collected from all household contacts regardless of whether each person was ill via home visits within 48 hours of enrolment, and at 2 additional follow-up home visits approximately 3 and 6 days after the initial visit. Serum samples were collected at the initial home visit from a subgroup of index cases and household contacts that consented to venipuncture. From January 2008 through August 2009, subjects were recruited as part of a trial of face masks and hand washing; cases subsequently recruited in the summer of 2009 were followed up without random allocation to interventions as part of a comparative study of pandemic and seasonal influenza virus transmission in households. Additional details of the study design were previously reported [10, 11]. Only households in which the index case had polymerase chain reaction (PCR)–confirmed influenza virus infection were included in the present analyses.
Laboratory Methods
Nasal and throat swabs were tested by a quantitative reverse-transcriptase PCR assay to detect the presence of the matrix gene of influenza A or B virus. The subtype of the influenza virus in PCR-positive specimens was determined by subtype-specific primers. Serum specimens were tested with a hemagglutination-inhibition assay for antibody responses to 2 seasonal influenza viruses: the A/Brisbane/59/2007 (H1N1) virus and an A/Brisbane/10/2007 (H3N2)–like virus, A/Uruguay/716/2007. A subset of serum specimens from the summer of 2009 were also tested with a viral-neutralization assay for antibody responses to the prevalent A/Perth/16/2009 (H3N2)–like virus, A/HK/1985/2009.
Statistical Analysis
We defined household contacts to have PCR-confirmed influenza virus infection if they had a positive result on testing of 1 or more pooled nasal and throat specimens collected during the follow-up. We defined the illness onset time for PCR-confirmed influenza virus infection as the first day when the subject reported at least 2 of the following 7 signs or symptoms: runny nose, cough, sore throat, headache, phlegm, myalgia, and fever [12].
To characterize influenza transmission dynamics within households and estimate the effects of factors affecting transmission, we used an individual-based hazard model [13, 14]. The model described the risk of PCR-confirmed infection among household contacts as depending on time since illness onset in other infected persons in the household. We extended the model to allow a time-varying risk of influenza virus infection from outside the household, by assuming that the risk from outside was directly proportional to influenza incidence rates in the general community, proxied by local surveillance data [15–17], and estimating the constant of proportionality for each subtype.
We used this model to estimate the serial interval distribution, allowing for infections from outside the household (“community infections”) or infections via other household contacts rather than the index case (“tertiary infections”). We estimated the protection associated with certain antibody titer levels within the transmission model. Age and vaccination status were considered as confounders in the relationship between antibody titer level and risk of PCR-confirmed influenza virus infection, and were adjusted accordingly. We also estimated the age-specific relative susceptibility from this transmission model by comparing the average hazard of household contacts who were 18 years of age or younger (who were above 50 years of age) to that of household contacts who were 19–50 years of age (Supplementary Appendix). The same approach was used to estimate the age-specific vaccine effectiveness.
To account for household contacts with missing data on antibody titers and retain all available data on transmission dynamics in our analysis, we performed our statistical analysis in a Bayesian framework and constructed a Markov Chain Monte Carlo algorithm [18] that allowed us to impute missing antibody titer levels based on the estimated individual posterior distribution of the antibody titer level for every individual. Simulation studies demonstrated that this algorithm could give unbiased parameter estimates (Supplementary Appendix). The reduction in risk of PCR-confirmed influenza virus infection associated with higher antibody titer levels, defined as 1 minus the hazard ratio, was estimated relative to the hazard at an antibody titer level <1:10 [4, 19, 20]. We assumed a log-linear model to estimate the relationship between protection against influenza infection and antibody titer [3, 4]. Other models (including logistic and nonparametric models) were also fitted to determine whether the results were robust with respect to model selection (Supplementary Appendix). The deviance information criterion was used for the comparison of models [21]. The adequacy of model fit was assessed with simulation-based χ2 tests, comparing observed and expected distributions of the number of secondary cases in households of different sizes [13]. All statistical analyses were conducted using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) and MATLAB 7.8.0 (MathWorks Inc, Natick, MA).
RESULTS
A total of 4279 potential index cases consented to participate in the study and met the inclusion criteria. We focused our analysis on the households of index cases with PCR-confirmed seasonal (prepandemic) influenza A(H1N1) or seasonal influenza A(H3N2) virus infections because the numbers of cases with PCR-confirmed seasonal B and pandemic A(H1N1)pdm09 virus infections were insufficient to allow robust parameter estimates. In 139 households, the index case had PCR-confirmed influenza A that was not subtyped; none of the household contacts in those 139 households provided serum specimens, and data from those households were not included here. In total, 182 index cases with PCR-confirmed seasonal A(H1N1) and 115 index cases with PCR-confirmed seasonal A(H3N2) were included in our analyses (Figure 1). These subjects were recruited from January 2008 through August 2009, during 4 local influenza A virus epidemics (Figure 2).
Figure 1.
Flow chart of enrollment of index cases with PCR-confirmed influenza virus infection and their household contacts. Abbreviations: PCR, polymerase chain reaction; RT, reverse-transcriptase.
Figure 2.
Timeline of subject recruitment and local influenza virus activity. A, Number of index cases with seasonal influenza A(H1N1) and A(H3N2) recruited over time. B, Proxy measure of seasonal influenza A(H1N1) and A(H3N2) virus activity in Hong Kong based on local surveillance data on influenza-like illness and laboratory detections of influenza.
In the households of these 297 index cases, 146/558 (26%) household contacts of the index cases with seasonal A(H1N1) and 52/337 (15%) household contacts of the index cases with seasonal A(H3N2) provided serum specimens. We compared the characteristics of seasonal A(H1N1) and seasonal A(H3N2) index cases and their household contacts in 297 households (Table 1). Index cases with seasonal A(H1N1) were on average significantly younger than those with seasonal A(H3N2) (P < .01, χ2 test). The proportion of vaccinated household contacts with seasonal A(H3N2) was slightly higher than that of vaccinated household contacts with seasonal A(H1N1). Other characteristics of household contacts were similar for index cases with seasonal A(H1N1) and those with seasonal A(H3N2) virus infection.
Table 1.
Characteristics of Index Cases With PCR-confirmed Seasonal Influenza A(H1N1) or A(H3N2) Virus Infection, and Their Household Contacts
| Characteristic | Seasonal A(H1N1) | Seasonal A(H3N2) |
|---|---|---|
| Index cases | ||
| No. of cases | 182 | 115 |
| Age | ||
| ≤5 y | 34 (19%) | 21 (18%) |
| 6–15 y | 102 (56%) | 44 (38%) |
| 16–30 y | 25 (14%) | 16 (14%) |
| 31–50 y | 14 (8%) | 21 (18%) |
| >50 y | 7 (4%) | 13 (11%) |
| Male sex | 93 (51%) | 66 (57%) |
| Prior vaccination | 25 (14%) | 24 (21%) |
| Household contacts | ||
| No. of contacts | 558 | 337 |
| Age | ||
| ≤5 y | 37 (7%) | 18 (5%) |
| 6–15 y | 72 (13%) | 60 (18%) |
| 16–30 y | 59 (11%) | 47 (14%) |
| 31–50 y | 304 (54%) | 161 (48%) |
| >50 y | 86 (15%) | 51 (15%) |
| Male sex | 206 (37%) | 136 (40%) |
| Prior vaccination | 61 (11%) | 60 (18%) |
Abbreviation: PCR, polymerase chain reaction.
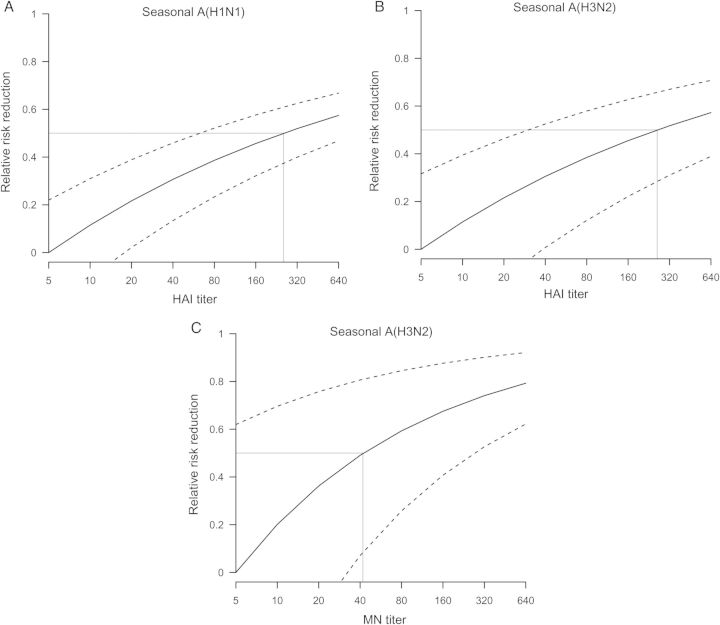
Adjusting for age and prior receipt of vaccination, higher antibody titers against the influenza A subtype identified in the household index case were statistically significantly associated with a reduction in the risk of PCR-confirmed infection in household contacts (Figure 3). HAI titers of 1:40 against seasonal A(H1N1) and A(H3N2) were associated with 31% (95% credible interval, confidence interval [CI], 13%–46%) and 31% (CI, 1%–53%) protection against PCR-confirmed seasonal A(H1N1) and A(H3N2) virus infection, respectively, while a MN titer of 1:40 against A(H3N2) was correlated with 49% (95% CI, 7%–81%) protection against PCR-confirmed A(H3N2) virus infection. We estimated that the level of HAI titers against seasonal A(H1N1) and A(H3N2) that were associated with 50% protection against PCR-confirmed seasonal A(H1N1) and A(H3N2) virus infection were 1:255 (CI, 1:62–1:917) and 1:260 (CI, 1:30–1:2009), respectively, while the level of MN titer against seasonal A(H3N2) that was associated with 50% protection against PCR-confirmed seasonal A(H3N2) virus infection was 1:42 (CI, 1:7–1:266).
Figure 3.
Correlation between reciprocal antibody titers and protection against PCR-confirmed influenza virus infection. A, Protection among household contacts of index cases with seasonal influenza A(H1N1) virus infection associated with reciprocal HAI titers against seasonal A(H1N1) virus. B, Protection among household contacts of index cases with seasonal influenza A(H3N2) virus infection associated with reciprocal HAI titers against seasonal A(H3N2) virus. C, Protection among household contacts of index cases with seasonal influenza A(H3N2) virus infection associated with reciprocal MN titers against seasonal A(H3N2) virus. Reciprocal antibody titers corresponding to 50% protection are indicated with gray lines. Abbreviations: HAI, hemagglutination-inhibiting; MN, microneutralization; PCR, polymerase chain reaction.
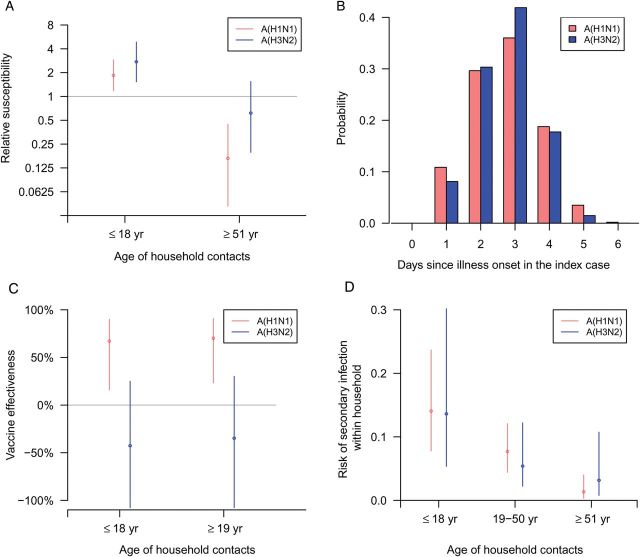
We estimated that household contacts who were 18 years of age or younger were more susceptible to PCR-confirmed influenza virus infection by a factor of 1.85 compared to those who were 19–50 years of age for PCR-confirmed seasonal A(H1N1) infection (relative susceptibility, 1.85; 95% CI, 1.18–2.90; P = .004) and by a factor of 2.75 for PCR-confirmed seasonal A(H3N2) infection (relative susceptibility, 2.75; 95% CI, 1.53–4.87; P < .001). We also estimated that household members who were older than 50 years of age were less susceptible than those who were 19–50 years of age for PCR-confirmed seasonal A(H1N1) infection (relative susceptibility, 0.17; 95% CI, .04–.45; P < .001) but there was no significant difference for PCR-confirmed seasonal A(H3N2) infection (relative susceptibility, 0.62; 95% CI, .20–1.54; P = .134) (Figure 4A). We estimated that the mean serial intervals for seasonal A(H1N1) virus and seasonal A(H3N2) virus were 2.7 days (95% CI, 2.3–3.4) with a standard deviation of 1.0 days (95% CI, .7–1.7), and 2.6 days (95% CI, 2.2–3.5) with a standard deviation of 0.8 days (95% CI, .4–1.9), respectively (Figure 4B).
Figure 4.
Factors affecting household transmission and the household serial interval. A, Estimates of the relative susceptibility of household contacts who were <19 years of age and of those who were >50 years of age (compared to the reference group of contacts who were 19–50 years of age). B, Serial interval distributions estimated under the transmission model, accounting for tertiary and community infections. C, Age-specific estimates of vaccine effectiveness against infection for household contacts. D, Age-specific risk of infection for household contacts, including the risk of secondary and tertiary transmission and infection from outside the household.
We estimated that the vaccine effectiveness for household members who were 18 years of age or younger for seasonal A(H1N1) and A(H3N2) were 67% (95% CI, 16%–90%) and −43% (95% CI, −158%–25%) respectively. The vaccine effectiveness for household members who were 19 years of age or older for seasonal A(H1N1) and A(H3N2) were 70% (95% CI, 23%–91%) and −35% (95% CI, −138%–30%), respectively (Figure 4C). The risk of infection within the household for household contacts for seasonal A(H1N1) virus for children, middle-age adults, and older-age adults was 14.1% (95% CI, 7.8%–23.7%), 7.7% (95% CI, 4.4%–12.1%), and 1.3% (95% CI, .3%–4.0%), respectively. We also estimated that the risk of infection for seasonal A(H3N2) virus for children, middle-age adults, and older-age adults was 13.6% (95% CI, 5.4%–30.2%), 5.4% (95% CI, 2.2%–12.2%), and 3.2% (95% CI, .8%–10.7%), respectively (Figure 4D).
Our statistical model included a time-varying term for the risk of infection of household contacts from outside the household, where the pattern in risk over time was assumed to be proportional to the pattern in influenza activity shown in local surveillance data and we estimated the scaling factor (Supplementary Appendix). The estimates of the cumulative risk of seasonal A(H1N1) and A(H3N2) virus infections across 3 separate periods of increased influenza activity in Hong Kong in 2008–2009 based on the fitted model are shown in Table 2. Our results suggested substantial risk of infection during each of the 4 epidemics, particularly among children.
Table 2.
Age-specific Estimates (With 95% Credibility Intervals) of the Cumulative Community Risk of Infectiona for 4 Influenza Seasons in 2008–2009
| Subtype, Age Group | 11 Feb–30 Mar 2008 | 7 Jul–24 Aug 2008 | 19 Jan–15 Mar 2009 | 6 Jul–6 Sep 2009 |
|---|---|---|---|---|
| Seasonal A(H1N1) | ||||
| ≤18 y | 0.111 (0.065, 0.176) | 0.118 (0.070, 0.188) | 0.239 (0.145, 0.363) | 0.042 (0.024, 0.068) |
| 19–50 y | 0.060 (0.039, 0.088) | 0.064 (0.042, 0.094) | 0.133 (0.089, 0.192) | 0.022 (0.015, 0.033) |
| >50 y | 0.010 (0.003, 0.029) | 0.011 (0.003, 0.031) | 0.024 (0.006, 0.066) | 0.004 (0.001, 0.011) |
| Seasonal A(H3N2) | ||||
| ≤18 y | 0.071 (0.033, 0.136) | 0.160 (0.077, 0.293) | 0.074 (0.035, 0.143) | 0.316 (0.160, 0.531) |
| 19–50 y | 0.028 (0.012, 0.053) | 0.065 (0.029, 0.122) | 0.029 (0.013, 0.056) | 0.137 (0.061, 0.247) |
| >50 y | 0.016 (0.005, 0.045) | 0.038 (0.011, 0.104) | 0.017 (0.005, 0.048) | 0.081 (0.025, 0.213) |
aWe assumed that the risk of infection from outside the household was directly proportional to influenza incidence rates reflected in local surveillance data, and estimated the constant of proportionality for each subtype in our model. The estimates here reflect the cumulative incidence across 3 specific influenza seasons, where influenza seasons were defined using a method previously described [16].
We performed a simulation study to confirm that our estimation procedure was able to estimate the model parameters despite having a substantial amount of missing data on antibody titers (Supplementary Appendix). We also conducted sensitivity analyses to verify that our key findings on the protection against infection associated with certain HAI titers was robust to the modeling assumptions and the relationship assumed between HAI titers and protection, and also the additional adjustment for any interventions that some households were randomly allocated to (face masks or hand washing) in the transmission model (Supplementary Appendix).
DISCUSSION
While data from experimental challenge studies and observational studies in community settings found that 50% protection against influenza infection associated with an HAI titer of 1:40 compared to an HAI titer < 1:10 was approximately 50% [1–3], we found that the degree of protection against infection associated with a titer of 1:40 appeared to be considerably less than 50% in the household setting (Figure 3). One possible explanation for this observation is that influenza virus infections in a household index case result in more intense exposures among susceptible household contacts [22–24]. Recently, 1 study estimated low vaccine effectiveness among household contacts of index cases with influenza and proposed a similar explanation [25]. While there can be some degree of variability between laboratories in HAI titer measurements on the same samples [26], we conducted one of the other studies identifying a correlation between a titer of 1:40 and 50% protection using the same HAI assays in the same laboratory [4]. Another possible explanation for this observation is that attenuated viruses with less pathogenicity, compared to viruses in natural setting, were used in experimental challenge studies [1].
We demonstrated that the MN titer was also correlated with protection against seasonal influenza A(H3N2) virus infection. While antibody titers measured by MN may not be directly compared with those measured by HAI, our results on the protection associated with HAI and MN titers suggest that an MN titer of 1:42 is equivalent to an HAI titer of 1:260 for people with naturally acquired seasonal A(H3N2) influenza virus infection. This is consistent with a previous study that found that HAI titers are significantly higher than corresponding MN titers for people with naturally acquired pandemic A(H1N1) influenza infection [9]. However, 1 previous study found that MN titers were higher on average than the corresponding HAI titers [7] for people with naturally acquired pandemic influenza A(H1N1) virus infection, and this is inconsistent with our observation that HAI titers are generally higher than corresponding MN titers. Possible explanations include that the relationship between HAI titers and corresponding MN titers may be different for different influenza virus strains [27] and that there may be variability in MN assay results conducted in different laboratories [26]. Our study is one of the first studies in a natural setting to demonstrate that the MN titer correlates with protection.
We found that the estimated risks of infection from both community and household for children were higher than for adults (Figure 4). While one of the explanations for higher susceptibility among children was that children had lower antibody titers on average, age remained a significant predictor of risk of infection after accounting for antibody titer. Explanations for this include a greater intensity of exposure for children due to closer contact with the index case [28], and perhaps lower hygiene standards. In addition, antibody titers measured by HAI or MN may not fully capture the strength of immunity against infection. Our estimates of the serial interval distributions (Figure 4) were consistent with results from other studies [29, 30], and slightly shorter than earlier estimates from subsets of these data [10, 31] after accounting for tertiary transmission in this model.
Our estimates of the vaccine effectiveness for seasonal A(H1N1) were consistent with other observational studies [32] and higher than one other recent study in households [25]. However, the estimated vaccine effectiveness for seasonal A(H3N2) was suboptimal (Figure 4). Whereas the index cases with A(H1N1) were predominantly recruited in the months of January and March 2009 shortly after the seasonal influenza vaccination campaigns in October–December each year in Hong Kong, the majority of the index cases with A(H3N2) were recruited in the months of June–August 2009 (Figure 2) and vaccine effectiveness could have waned after this longer delay [33, 34]. In addition, there was an imperfect match between the vaccine strain A/Brisbane/10/2007(H3N2)–like, and the circulating antigenically drifted A/Perth/16/2009-like (H3N2) viruses in our study period [10, 11, 35].
While the design of our study with a relatively short duration of follow-up of households allowed us to focus on the risks of within-household transmission, we were also able to make inference on the risk of infection from the general community in our analysis (Table 2). We do not have any other estimates of the risk of infection in 2008 for comparison, but we did conduct a separate community-based cohort study in 2009, and estimated that the cumulative risk of seasonal influenza A(H1N1) was 21% (95% CI, 9%–32%) in children in the winter of 2009 [35], similar to the estimate of 23.9% (95% CI, 14.5%–36.3%) here.
Our study had a number of limitations. First, index cases were recruited from outpatient clinics and these patients were screened for influenza virus infection by using a rapid test. Index cases therefore had more serious illness that warranted medical attention, and had higher levels of virus shedding required for a positive result on a rapid test [36]. If more severe illness was associated with greater infectiousness, then our estimated risk of infection within households may be overestimated. Second, our analysis controlled for age and vaccination, which were considered as potential confounders, when we estimated the association between antibody titer and risk of influenza virus infection. However, we could not rule out the risk of unidentified confounders. Third, in our study, the duration of follow-up was from 7 to 12 days after illness onset in the index case, hence it was possible that some secondary cases with long serial intervals or some tertiary cases were missed. However, with average serial intervals below 3 days, the effect of such censoring should be minimal. In addition, we collected 3 home visits at 3-day intervals, and nose and throat swabs were collected during the home visits. It was therefore possible that some PCR-positive infections were missed if the peak viral shedding occurred between these visits. Finally, we did not perform antigenic characterization of each virus detected in our study, and we used the same HAI assays in 2 consecutive years during which the vaccine strains remained the same. It is possible, therefore, that there was a mismatch between the antibody titers measured in household contacts and the circulating viruses.
In conclusion, we demonstrated that an HAI titer of 1:40 was not sufficient to provide 50% protection against PCR-confirmed influenza virus infection in households, potentially because of exposures of greater duration or intensity in that confined setting. We also demonstrated that MN titers were statistically significantly correlated with protection against infection. Further work could examine how information on HAI titers and MN titers could be combined to provide a better immune correlate than the HAI or MN titer alone.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all the doctors, nurses, and staff members at the participating centers for facilitating recruitment; the dedicated team of healthcare workers who conducted the home visits; and Chan Kit Man, Calvin Cheng, Rita Fung, Ho Yuk Ling, Lam Yiu Pong, Lincoln Lau, Tom Lui, Tong Hok Leung, Edward Ma, and Teresa So for research support.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases under contract no. HHSN266200700005C; ADB No. N01-AI-70005 (National Institute of Allergy and Infectious Diseases Centers for Excellence in Influenza Research and Surveillance), a commissioned grant from the Health and Medical Research Fund from the Government of the Hong Kong Special Administrative Region, the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (grant no. U54 GM088558), and the Area of Excellence Scheme of the Hong Kong University Grants Committee (grant no. AoE/M-12/06). T. K. T. was supported by a Research Scholarship from L'Oreal Hong Kong.
Potential conflicts of interest. G. M. L. has received speaker honoraria from HSBC and CLSA. J. S. M. P. receives research funding from Crucell NV and serves as an ad hoc consultant for GlaxoSmithKline and Sanofi. B. J. C. has received research funding from MedImmune Inc and Sanofi Pasteur, and consults for Crucell NV. D. K. M. I. has received research funding form Hoffmann-La Roche Inc. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox JP, Cooney MK, Hall CE, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol. 1982;116:228–42. doi: 10.1093/oxfordjournals.aje.a113408. [DOI] [PubMed] [Google Scholar]
- 3.Coudeville L, Bailleux F, Riche B, et al. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a Bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng S, Fang VJ, Ip DK, et al. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J Infect Dis. 2013;208:1320–4. doi: 10.1093/infdis/jit372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox RJ. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother. 2013;9:405–8. doi: 10.4161/hv.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown F, Haaheim LR, Wood JM, Schild GC. Laboratory Correlates of Immunity to Influenza—A Reassessment. New York: S Karger Pub; 2003. [Google Scholar]
- 7.Veguilla V, Hancock K, Schiffer J, et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol. 2011;49:2210–5. doi: 10.1128/JCM.00229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H, Ding X, Chen X, et al. Neutralizing antibody but not hemagglutination antibody provides accurate evaluation for protective immune response to H5N1 avian influenza virus in vaccinated rabbits. Vaccine. 2011;29:5421–3. doi: 10.1016/j.vaccine.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 9.Chan KH, To KK, Hung IF, et al. Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin Vaccine Immunol. 2011;18:867–73. doi: 10.1128/CVI.00555-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151:437–46. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 12.Lau LL, Cowling BJ, Fang VJ, et al. Viral shedding and clinical illness in naturally acquired influenza virus infections. J Infect Dis. 2010;201:1509–16. doi: 10.1086/652241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauchemez S, Donnelly CA, Reed C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361:2619–27. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boelle PY. A Bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat Med. 2004;23:3469–87. doi: 10.1002/sim.1912. [DOI] [PubMed] [Google Scholar]
- 15.Wu P, Cowling BJ, Wu JT, et al. The epidemiological and public health research response to 2009 pandemic influenza A(H1N1): experiences from Hong Kong. Influenza Other Respir Viruses. 2013;7:367–82. doi: 10.1111/j.1750-2659.2012.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu P, Goldstein E, Ho LM, et al. Excess mortality associated with influenza A and B virus in Hong Kong, 1998–2009. J Infect Dis. 2012;206:1862–71. doi: 10.1093/infdis/jis628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu P, Goldstein E, Ho L-M, et al. Excess mortality impact of two epidemics of pandemic influenza A(H1N1pdm09) virus in Hong Kong. Influenza Other Respir Viruses. 2014;8(1):1–7. doi: 10.1111/irv.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilks WR, Richardson S, Spiegelhalter D. Markov chain Monte Carlo in practice. London: Chapman & Hall; 1996. [Google Scholar]
- 19.Dunning AJ. A model for immunological correlates of protection. Stat Med. 2006;25:1485–97. doi: 10.1002/sim.2282. [DOI] [PubMed] [Google Scholar]
- 20.Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081–5. doi: 10.1097/INF.0b013e3182367662. [DOI] [PubMed] [Google Scholar]
- 21.David J, Spiegelhalter NGB, Bradley P. Carlin and Angelika van der Linde Bayesian measures of model complexity and fit. J R Stat Soc. 2002;64:583–639. [Google Scholar]
- 22.Foy HM, Cooney MK, Allan I. Longitudinal studies of types A and B influenza among Seattle schoolchildren and families, 1968–74. J Infect Dis. 1976;134:362–9. doi: 10.1093/infdis/134.4.362. [DOI] [PubMed] [Google Scholar]
- 23.Fox JP, Hall CE, Cooney MK, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol. 1982;116:212–27. doi: 10.1093/oxfordjournals.aje.a113407. [DOI] [PubMed] [Google Scholar]
- 24.Longini IM, Jr., Koopman JS, Monto AS, Fox JP. Estimating household and community transmission parameters for influenza. Am J Epidemiol. 1982;115:736–51. doi: 10.1093/oxfordjournals.aje.a113356. [DOI] [PubMed] [Google Scholar]
- 25.Ohmit SE, Thompson MG, Petrie JG, et al. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis. 2014;58(3):319–27. doi: 10.1093/cid/cit736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephenson I, Heath A, Major D, et al. Reproducibility of serologic assays for influenza virus A (H5N1) Emerg Infect Dis. 2009;15:1252–9. doi: 10.3201/eid1508.081754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okuno Y, Tanaka K, Baba K, et al. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J Clin Microbiol. 1990;28:1308–13. doi: 10.1128/jcm.28.6.1308-1313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLOS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrie JG, Ohmit SE, Cowling BJ, et al. Influenza transmission in a cohort of households with children: 2010–2011. PLOS One. 2013;8:e75339. doi: 10.1371/journal.pone.0075339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy JW, Cowling BJ, Simmerman JM, et al. The serial intervals of seasonal and pandemic influenza viruses in households in Bangkok, Thailand. Am J Epidemiol. 2013;177:1443–51. doi: 10.1093/aje/kws402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cowling BJ, Fang VJ, Riley S, Malik Peiris JS, Leung GM. Estimation of the serial interval of influenza. Epidemiology. 2009;20:344–7. doi: 10.1097/EDE.0b013e31819d1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 33.Pebody R, Andrews N, McMenamin J, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Euro Surveill. 2013;18:25–32. doi: 10.2807/ese.18.05.20389-en. [DOI] [PubMed] [Google Scholar]
- 34.Kissling E, Valenciano M, Larrauri A, et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case-control study. Euro Surveill. 2013;18:2–6. doi: 10.2807/ese.18.05.20390-en. [DOI] [PubMed] [Google Scholar]
- 35.Cowling BJ, Ng S, Ma ES, et al. Protective efficacy of seasonal influenza vaccination against seasonal and pandemic influenza virus infection during 2009 in Hong Kong. Clin Infect Dis. 2010;51:1370–9. doi: 10.1086/657311. [DOI] [PubMed] [Google Scholar]
- 36.Cheng CK, Cowling BJ, Chan KH, et al. Factors affecting QuickVue Influenza A + B rapid test performance in the community setting. Diagn Microbiol Infect Dis. 2009;65:35–41. doi: 10.1016/j.diagmicrobio.2009.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.