Abstract
Background
General anaesthesia facilitates surgical operations and painful interventions in millions of patients every year. Recent observations of anaesthetic-induced neuronal cell death in newborn animals have raised substantial concerns for young children undergoing anaesthesia. However, it remains unclear why some brain regions are more affected than others, why certain neurones are eliminated while neighbouring cells are seemingly unaffected, and what renders the developing brain exquisitely vulnerable, while the adult brain apparently remains resistant to the phenomenon.
Methods
Neonatal (P7), juvenile (P21), and young adult mice (P49) were anaesthetized with 1.5% isoflurane. At the conclusion of anaesthesia, activated cleaved caspase 3 (AC3), a marker of apoptotic cell death, was quantified in the neocortex (RSA), caudoputamen (CPu), hippocampal CA1 and dentate gyrus (DG), cerebellum (Cb), and olfactory bulb (GrO) and compared with that found in unanaesthetized littermates.
Results
After anaesthetic exposure, increased AC3 was detected in neonatal mice in RSA (11-fold, compared with controls), CPu (10-fold), CA1 (three-fold), Cb (four-fold), and GrO (four-fold). Surprisingly, AC3 continued to be elevated in the DG and GrO of juvenile (15- and 12-fold, respectively) and young adult mice (two- and four-fold, respectively).
Conclusions
The present study confirms the findings of previous studies showing peak vulnerability to anaesthesia-induced neuronal cell death in the newborn forebrain. It also shows sustained susceptibility into adulthood in areas of continued neurogenesis, substantially expanding the previously observed age of vulnerability. The differential windows of vulnerability among brain regions, which closely follow regional peaks in neurogenesis, may explain the heightened vulnerability of the developing brain because of its increased number of immature neurones.
Keywords: anaesthesia, paediatric; anaesthetics volatile, isoflurane; brain, injury; safety, drug; toxicity
Editor's key points.
Pre-clinical studies of various species demonstrate anaesthetic-induced neonatal neurotoxicity.
In the current study, mice of different ages were anaesthetized with isoflurane.
Thereafter, neuroapoptosis was quantified in brain regions which have different age-related time courses of neurogenesis.
Susceptibility to apoptosis appeared to parallel peaks of neurogenesis, and continued into adulthood in some areas.
For more than one-and-a-half centuries, general anaesthesia has facilitated surgical operations and painful interventions in millions of patients, from premature infants into old age.1 While hypnosis, analgesia, and immobility are transient effects of anaesthesia, recent findings from newborn and ageing animals have raised concerns that some anaesthetic effects may be undesired and longer lasting. One of the most concerning discoveries has been a widespread neuronal cell death observed in newborn animals after exposure to all common clinically used anaesthetics (reviewed in Istaphanous and colleagues2 and Jevtovic-Todorovic).3
Neurones die via a process known as programmed cell death or apoptosis. Neuroapoptosis is a normal part of brain development in both animals and humans, and is important for eliminating excess and inappropriately integrated neurones. Exposure to anaesthetics, however, dramatically increases the rate of neuroapoptosis in developing animals, and subsequent neurocognitive impairment in these animals has heightened concerns for human patients. Reports of learning abnormalities and language impairments in children after surgery with anaesthesia early in life support these concerns.4–6 However, whether similar neuronal cell death occurs during clinical anaesthesia practice in humans remains uncertain.
Anaesthetic neuroapoptosis has been previously thought to be limited to animals before 14 days of life, best characterized in small rodents for the cerebral cortex and thalamus.7,8 Moreover, studies in adult animals have thus far failed to detect any measurable cell death during anaesthetic exposure,9 which has led to the interpretation that the phenomenon represents an inherent vulnerability of the developing brain.
However, it remains unclear why anaesthesia-induced neuroapoptosis is limited to this very early postnatal age and whether anaesthetic exposure affects all neurones similarly, regardless of their location in the brain. Brain regions develop on distinct trajectories of maturation and neurogenesis peaks at separate time points, relative to gestation, for different regions. Accordingly, we hypothesized that peak vulnerability to anaesthesia-induced neuroapoptosis among brain regions varies by age during exposure. This would predict that neuronal populations developing earlier in life are also more vulnerable to anaesthesia-induced neurodegeneration at an earlier developmental stage, whereas delayed vulnerability would be expected for brain regions with peaks of neurogenesis later in development. Accordingly, the present study quantified the expression of cleaved caspase 3, a marker of apoptotic cell death, in several brain regions experiencing peaks in neurogenesis early in development, such as retrosplenial cortex, caudoputamen, and hippocampal cornu ammonis layer I, and also regions with later peaks, such as cerebellum, dentate gyrus, and olfactory bulb in newborn, juvenile, and young adult mice after a 6 h anaesthetic exposure to isoflurane, comparing them with their respective, unanaesthetized littermates.
Methods
Animals
C57BL6/J mouse breeding pairs were housed on a 14/10 h light/dark cycle at 22°C and allowed free access to food and water. Offspring either remained with the parents until treatment on P7 (n=18) or P21 (n=17), or were weaned from the dam on P28, housed as gender-matched pairs and allowed to spontaneously exercise in running wheels for 3 weeks before treatment on P49 (n=28). P0 was considered as the day of birth. Immediately after anaesthetic exposure or fasting in room air, animals were killed and brains removed for further analysis. All experiments followed the National Institutes of Health guidelines, were approved by the Institutional Animal Care and Use Committee of the Cincinnati Children's Research Foundation, and aimed to minimize the number of animals used. The protocol was in accordance with the criteria of ARRIVE guidelines.
Anaesthesia exposure
On P7, P21, or P49, mice were randomly assigned to either a 6 h exposure to 1.5% isoflurane in 30% oxygen (Anaesthesia) or to fasting in room air (No Anaesthesia) using an online pseudo-random number generator. Both female and male littermates were used, since preliminary experiments did not demonstrate any sex differences in neuroapoptosis (data not shown). Anaesthetic and oxygen concentrations were monitored using a gas analyser (RGM 5250, Datex-Ohmeda, Inc., Louisville, CO, USA). For treatment, animals were placed in padded acrylic containers inside incubators warmed to 35.5°C. Immediately after treatment, mice were killed with an intraperitoneal injection of ketamine (20 mg kg−1), acepromazine (0.5 mg kg−1), and xylazine (1 mg kg−1). Animals were then transcardially perfused by using phosphate-buffered saline with 5% glycerol and 5% sucrose, followed by 4% paraformaldehyde in phosphate-buffered saline (PBS; pH = 7.4), containing 5% glycerol and 5% sucrose (PFA-GS). Brains were removed, fixed in PFA, and cryoprotected in 20% and 30% sucrose solutions in PBS.
Histology
Brains were cryosectioned in the sagittal plane at 40 μm using a Cryotome-SME (Thermo Electronics, Kalamazoo, MI, USA). Sections were mounted to charged slides and stored at −80°C until use. Sections between 0.60 and 0.84 mm lateral to the midline corresponding to figures 106–108 in Paxinos and Franklin's Mouse Brain Atlas10 were then stained for activated, cleaved caspase 3 (AC3), neuronal nuclei (NeuN), and propidium iodide (PI). AC3 is the executioner caspase and a marker of commitment to apoptotic cell death. NeuN represents a neurone-specific protein signifying a post-mitotic stage of development. PI is a red-fluorescent nuclear and chromosomal counterstain, and labels all cells.
All sections were incubated for 5 min at 100°C in a 1:10 dilution of sodium citrate buffer pH 6.0 (CB910 m; Biocare, Concorde, CA, USA) in Coplin jars for antigen retrieval. After this step, slides were incubated in 0.025% Trypsin for 3 min and then in 2 M HCl at room temperature for 30 min. Then, the slides were washed twice in phosphate buffer (pH=8.5), after which sections were blocked in 5% donkey serum, 0.5% glycine, 0.5% non-fat dry milk, and 5% Tween-20 in PBS for 1 h at room temperature before primary antibodies were added.
Slide-mounted sections were incubated overnight at room temperature with two primary antibodies to accommodate combinations of compatible secondary antibodies. Sections were stained with a 1:200 dilution of rabbit cleaved caspase 3 antibody (9661L; Cell Signaling Technology, Danvers, MA, USA) and 1:200 mouse anti-NeuN monoclonal antibodies (MAB377; Millipore, Temecula, CA, USA) in blocker. After primary antibody incubation, slides were rinsed with 5% Tween-20 in PBS followed by a 4 h incubation in 1:250 dilutions of Alexa Fluor 488 donkey anti-rabbit (A21206; Invitrogen, Eugene, OR, USA) and 647 donkey anti-mouse (A31571; Invitrogen), as appropriate for primary antibody species. Slides were then incubated in coplin jars filled with 0.01% propidium iodide (P3566; Invitrogen) in PBS for 5 min. After immunostaining, sections were dehydrated in an ascending ethanol series, washed in PBS, and mounted with Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA, USA).
Quantification of neuroapoptosis
To quantify neuronal apoptosis, three-channel confocal image stacks of AC3, PI, and NeuN immunostaining were collected through the z depth of the tissue from the cerebral cortex, hippocampus, caudoputamen, olfactory bulb, or cerebellar white matter and granular layers in sagittal brain sections using a confocal microscope (Leica TCS SP5 with LAS software, LeicaMicrosystems, Wetzlar, Germany) equipped with a 63× oil-immersion, 1.4 NA objective. Images were collected with a ×1 optical zoom, in 1 µm steps, format 1024×1024, scan speed 200 Hz, and a field size of 246×246 µm. AC3 immunostaining was excited using the 488 nm laser line, and emission wavelengths between 495 and 550 nm were captured. NeuN immunostaining was excited using the 633 nm laser line, and emission wavelengths between 650 and 710 nm were collected. Immunostaining for PI was excited using the 543 nm laser line, and emission wavelengths between 583 and 622 nm were collected. The resulting image stacks were transferred to image analysis software (Neurolucida v10.31, MBF Bioscience, Williston, VT, USA) for assessment by an observer unaware of group assignment. The number of apoptotic, AC3-positive neurones was quantified using the optical dissector method, as previously described.11–13 Densities of apoptotic brain cells were quantified as caspase 3+ cells mm−3 of examined tissue for each animal and then group averaged for the respective brain regions.
Statistical analysis
Data were analysed using Stata v10.1. Normality was tested with the Kolmogorov–Smirnov test and statistical analysis was performed using Student's t-test for parametric data and the Mann–Whitney U-test for non-parametric data. Data are presented as the median and percentiles for all groups if any of the data cells violated normality. Significance was accepted at P<0.05.
Results
Mouse littermates were randomly assigned to receive 6 h of isoflurane anaesthesia or to fast without anaesthesia at one of the three different ages: as newborns (P7), as juveniles (P21), or as young adults (P49). Apoptotic neuronal cell death, as measured by the density of cells expressing AC3, was quantified in anaesthetized and unanaesthetized controls, collected immediately after exposure for each age group. Seven brain regions were examined, as follows: dentate gyrus (DG), the pyramidal layer of the hippocampal cornu ammonis 1 (CA1) region, the superficial layers 2/3 of the dorsal part of retrosplenial agranular cortex (RSA), the caudoputamen (CPu), the granule cell layer of the olfactory bulb (GrO), cerebellar cortex (Cb), and white matter (Cb wm). Caspase 3+ neurones were observed at low levels in several brain regions of unanaesthetized animals at all three ages, indicating the presence of naturally occurring neuronal cell death (Fig. 1).
Fig 1.
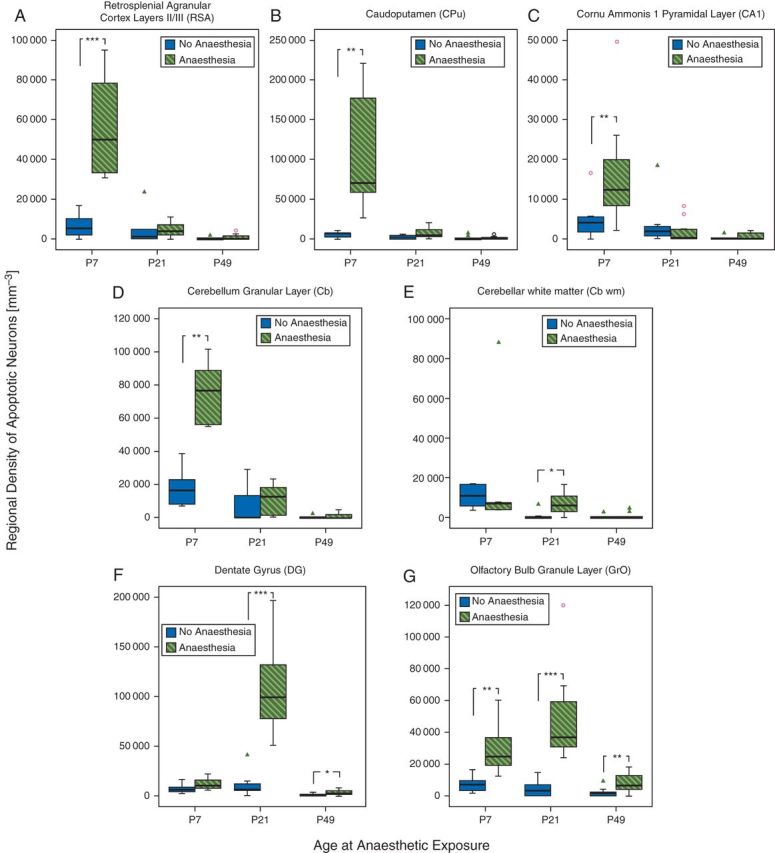
Prolonged exposure to isoflurane triggers neuronal apoptotic cell death in several brain regions in newborn, juvenile, and young adult mice. Boxplots represent density counts of dying cells, as assessed by expression of cleaved caspase 3, a marker of apoptotic cell death. Thick horizontal lines signify respective group medians, boxes are 25th–75th percentiles, whiskers are 10th–90th percentiles, open circles and triangles depict outliers. Littermates were randomly assigned to 6 h of 1.5% isoflurane (Anaesthesia) or 6 h of room air (No Anaesthesia) at three different ages: as newborns [post-natal day (P)7], as juveniles (P21), or as young adults (P49). Density of cleaved caspase 3-positive cells was assessed in superficial layers II/III of the retrosplenial agranular cortex (RSA; a), caudoputamen (CPu; b), the pyramidal layer of cornu ammonis 1 (CA1; c), cerebellar cortex (Cb; d), and white matter (Cb wm; e), the subgranular zone and granule cell layer of dentate gyrus (DG; f), and the granule layer of the olfactory bulb (GrO; g). P7, Anaesthesia (n=10), No Anaesthesia (n=8); P21, Anaesthesia (n=8), No Anaesthesia (n=7); P49, Anaesthesia (n=16), No Anaesthesia (n=13). *P<0.05, **P<0.01, ***P<0.001, compared with the respective No Anaesthesia group.
Newborn mice exhibit significant anaesthesia-induced neuroapoptosis in all brain regions examined except dentate gyrus
In 7-day-old mice, isoflurane exposure dramatically increased the density of apoptotic neurones in RSA, CPu, and CA1 relative to unanaesthetized age-matched littermates, as previously reported. In addition, anaesthesia caused significant neuroapoptosis in Cb and GrO (Figs 1 and 2). However, in these newborn animals, no significant increase in neuroapoptosis was observed in DG.
Fig 2.
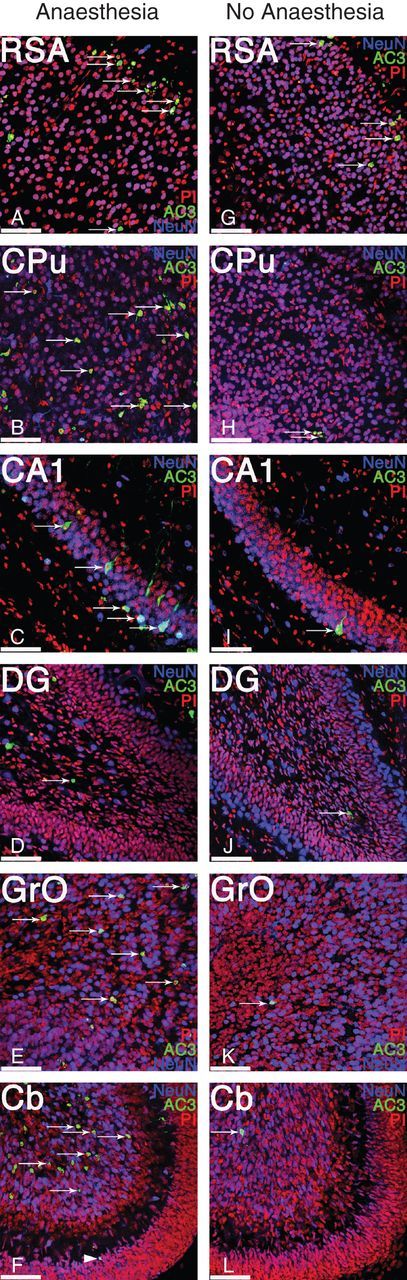
Exposure to prolonged anaesthesia induces significant neuroapoptosis in several brain regions in newborn mice. Representative high-power magnification photomicrographs of layers II/III of the retrosplenial agranular cortex (RSA), caudoputamen (CPu), the pyramidal layer of cornu ammonis region 1 (CA1), subgranular zone and granule cell layer of dentate gyrus (DG), the granule layer of the olfactory bulb (GrO) and cerebellar cortex (Cb), and both internal granule layer (arrows) and external granule layer (arrow head) from a newborn mouse (P7) anaesthetized for 6 h with 1.5% isoflurane (Anaesthesia, a–f) and from an unanaesthetized and fasted littermate (No Anaesthesia, g–l). Sections were stained with propidium iodide (PI; red) to label all cells, for neuronal nuclei (NeuN; blue) to label post-mitotic neurones, and for activated, cleaved caspase 3 (AC3; green) to label apoptotic cells (arrows). All brain regions shown demonstrated statistically significantly increased neuroapoptosis after anaesthetic exposure, compared with unanaesthetized littermates, except for the dentate gyrus, which revealed no significantly increased cell death in newborn animals. For quantification, see Figure 1. Scale bars=50 µm.
Juvenile mice demonstrate significant anaesthesia-induced neuroapoptosis in the olfactory bulb, dentate gyrus, and cerebellum
In juvenile animals (P21), increased anaesthesia-induced cell death was not observed in RSA, CPu, and CA1 when compared with unanaesthetized controls. Surprisingly, however, at this age, dentate granule cells became exquisitely sensitive to isoflurane exposure and neuroapoptosis was substantially increased in this brain region, compared with unanaesthetized littermates (Figs 1 and 3). Moreover, the olfactory bulb neurones continued to demonstrate vulnerability at this age. Increased apoptotic cell death was also still present in the cerebellum; however, the affected region shifted from the internal granular layer to Cb wm, the deeper structures in the white matter tracks (Fig. 3f).
Fig 3.
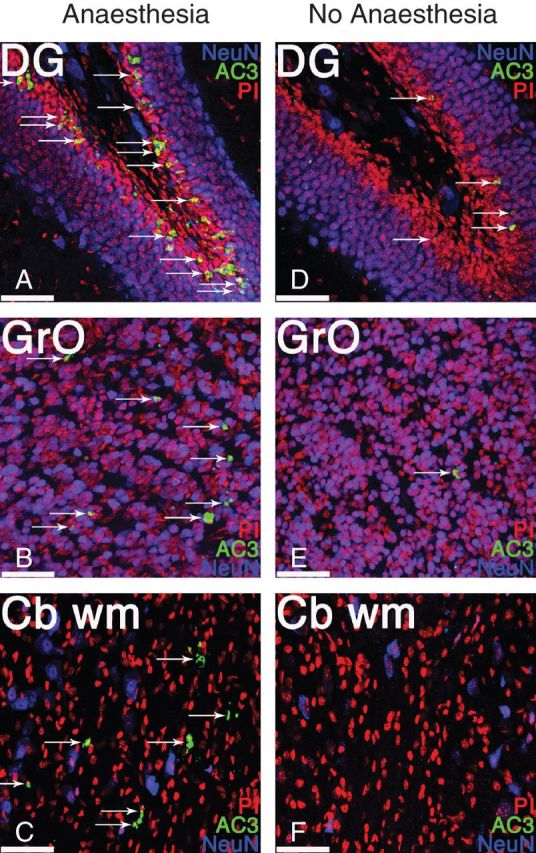
Exposure to prolonged anaesthesia induces significant neuroapoptosis in the dentate gyrus, olfactory bulb, and cerebellum of juvenile mice. Representative high-power magnification photomicrographs of the subgranular zone and granule cell layer of dentate gyrus (DG), the granule layer of the olfactory bulb (GrO) and cerebellar white matter (Cb wm) from a juvenile mouse (P21) anaesthetized for 6 h with 1.5% isoflurane (Anaesthesia; a–c) and from an unanaesthetized, fasted littermate (No Anaesthesia; d–f). Sections were stained with propidium iodide (PI; red) to label all cells, for neuronal nuclei (NeuN; blue) to label post-mitotic neurones, and for activated, cleaved caspase 3 (AC3; green) to label apoptotic cells (arrows). Of all the brain regions examined, the dentate gyrus, olfactory bulb, and cerebellar white matter were the only regions demonstrating significantly increased neuroapoptosis in juvenile animals, compared with unanaesthetized littermates. For quantification, see Figure 1. Scale bars=50 µm.
Young adult mice exhibit persistent vulnerability to anaesthesia-induced neuroapoptosis in the dentate gyrus and olfactory bulb
Similar to juvenile animals, anaesthetic exposure in 49-day-old, young adult animals did not lead to significant increases in anaesthesia-induced neuroapoptosis in RSA, CPu, or CA1. In addition, no increase in neuronal apoptosis was detected in Cb or Cb wm (Fig. 1). Conversely, neurones in DG and GrO remained vulnerable to anaesthesia-induced cell death even at this older age; albeit at decreased levels compared with juvenile animals (Figs 1 and 4).
Fig 4.
Exposure to prolonged anaesthesia induces significant neuroapoptosis in the dentate gyrus and olfactory bulb of young adult mice. Representative high-power magnification photomicrographs of the subgranular zone and granule cell layer of the dentate gyrus (DG) and the granule layer of the olfactory bulb (GrO) from a young adult mouse (P49) anaesthetized for 6 h with 1.5% isoflurane (Anaesthesia; a and b) and from an unanaesthetized and fasted littermate (No Anaesthesia; c and d). Sections were stained with propidium iodide (PI; red) to label all cells, for neuronal nuclei (NeuN; blue) to label post-mitotic neurones, and for activated, cleaved caspase 3 (AC3; green) to label apoptotic cells (arrows). Of all the brain regions examined, the dentate gyrus and olfactory bulb were the only regions in young adult animals demonstrating significantly increased neuroapoptosis, compared with unanaesthetized littermates. For quantification, see Figure 1. Scale bars=50 µm.
Differential age windows of vulnerability to anaesthesia-induced neuroapoptosis dependent on brain region
Combined, our results demonstrate that many, but not all brain regions examined were vulnerable to anaesthetic neuroapoptosis in neonatal animals, that neurotoxicity can extend beyond this period, at least into young adulthood, for some regions, and that, accordingly, differential windows of vulnerability to the phenomenon exist among brain regions (Fig. 5).
Fig 5.
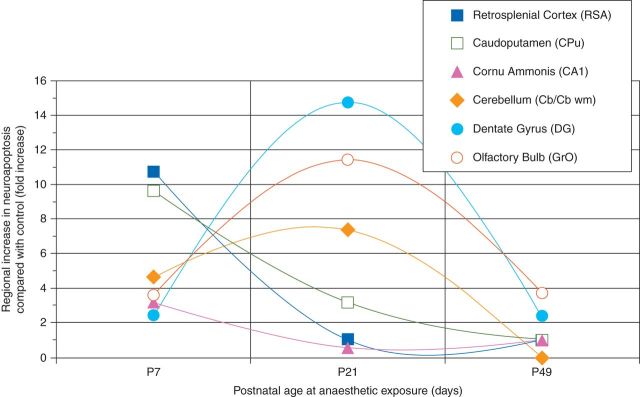
Age windows of vulnerability to anaesthesia-induced neuroapoptosis differ by brain region. Graph indicates the severity of anaesthesia-induced neuroapoptosis depicted as the median fold-increase over physiological apoptotic cell death, as observed in the respective littermate controls. Apoptotic neuronal density was quantified for superficial layers II/III of the retrosplenial agranular cortex (RSA), caudoputamen (CPu), the pyramidal layer of cornu ammonis 1 (CA1), cerebellum (Cb at P7, CB wm at P21), the subgranular zone and granule cell layer of dentate gyrus (DG), and the granule layer of the olfactory bulb (GrO) after a 6 h exposure to 1.5% isoflurane in newborn (P7), juvenile (P21), and adult mice (P49), compared with fasted, unanaesthetized littermates. Maximum vulnerability was observed in the neocortex, caudoputamen, and CA1 at P7, whereas the number of vulnerable neurones peaked at P21 for the cerebellum, dentate gyrus, and olfactory bulb.
Discussion
After the initial discovery more than a decade ago that anaesthetic exposure results in brain cell losses during the early stages of central nervous system development,14 numerous laboratory studies have now confirmed this phenomenon for all routinely utilized anaesthetics in a wide variety of animal species (for reviews, see Istaphanous and colleagues2 and Jevtovic-Todorovic).3 Concerns have therefore been raised that similar effects might also occur in humans, particularly in very young children.15 Several important aspects of anaesthesia-induced neuroapoptosis remain unresolved, however, such as what factors determine which neurones are going to die during exposure and why certain brain regions are more affected than others. The present study attempts to address some of these questions by demonstrating that (i) regional susceptibility to anaesthesia-induced neuroapoptosis varies dependent on the age during exposure, (ii) these windows of vulnerability follow the regional peaks in neurogenesis and natural cell death, and (iii) susceptibility in some areas extends at least into young adulthood.
Anaesthesia-induced neuroapoptosis not limited by age of the animal
Previous studies have established that neuronal survival in the developing animal brain is dramatically affected by anaesthetic exposure. Jevtovic-Todorovic's group has convincingly demonstrated that cortical and subcortical neurones of the forebrain are eliminated in substantial numbers in 7-day-old rats during exposure to an anaesthetic combination of isoflurane, nitrous oxide, and midazolam, but are not affected beyond 10 days of age.7 The present results confirm this window of susceptibility in mice exposed to isoflurane as a single agent by demonstrating that neurones in RSA, CPu, and CA1 are subject to widespread neuroapoptosis after anaesthetic exposure on P7, but not in juvenile (P21) and young adult (P49) animals.
Surprisingly, however, we found that other neuronal populations followed a different trajectory, which extended beyond this early window of vulnerability. Specifically, while dentate granule cells were not significantly affected by anaesthetic exposure at P7, substantial vulnerability was observed at P21. Similarly, granule cells in the olfactory bulb and brain cells in cerebellar white matter were still vulnerable in these juvenile animals. Shifting regional patterns of neuronal injury have also previously been observed after ketamine exposure in fetal compared with newborn rhesus monkeys,16 and in P1–3 vs P4–7 rats.14 Importantly, however, the present study further extends this finding to adult animals by demonstrating that anaesthesia-induced neuroapoptosis is not limited to newborn animals, but can still be observed in dentate granule cells and olfactory bulb neurones, two brain regions with adult neurogenesis, of young adult mice (P49).
Previous studies have not detected anaesthesia-induced neurotoxicity in adult rats after 4 h of isoflurane;9 however, impaired survival has recently been found in adult mice after a 6 h propofol exposure,17 and after 6 h of isoflurane.18 These discrepancies most likely reflect the relatively low rate of granule cell neurogenesis in adult animals, compared with neonates, which renders the detection of increased apoptosis in this population more difficult. To address this problem, adult animals in the present study were allowed to voluntarily exercise in running wheels before the anaesthetic exposure. Physical exercise stimulates granule cell neurogenesis, thereby increasing the number of newly born neurones, functionally and phenotypically indistinguishable from adult-born granule cells generated under normal housing conditions.19,20 Physical activity, however, does not stimulate neurones in the olfactory bulb,20 suggesting that the anaesthesia-induced neuroapoptosis observed in adult animals in the present study was not limited to neurones induced by exercise.
Differential regional windows of vulnerability to anaesthesia-induced neuroapoptosis
Our findings strongly support the existence of multiple windows of susceptibility for anaesthesia-induced neuroapoptosis, with different brain regions being vulnerable at distinct developmental times (Fig. 5). A potential explanation for this novel finding would be the underlying differential timetable of neurogenesis and natural apoptosis in the various brain regions. In small rodents, peak neurogenesis in RSA, CPu, and hippocampal CA1 occurs prenatally.21 Accordingly, we observed peak vulnerability to anaesthesia-induced apoptosis in these regions at P7, 1–2 weeks after these regions' peak in neurogenesis. This early window of vulnerability also applies to neurones in the thalamus and amygdala, which are predominantly formed before birth,21 which were not studied here, but have previously been found to undergo substantial cell death after exposure at P7.7,8,22,23
Conversely, neuronal populations that experience their neurogenic peak post-natally, such as the dentate gyrus and olfactory bulb granule cells around P0–P11,21 were found in the present study to exhibit substantial vulnerability beyond the previously observed window of vulnerability, around P21, again around 2 weeks after their regional neurogenic peak. Importantly, since dentate granule cell and olfactory bulb neurogenesis continues throughout life, albeit at a lower rate,24 vulnerability in these regions would be expected to extend into adulthood, which was indeed observed in our young adult animals, where it was still detected at P49.
The notable exception to this window of vulnerability after 1–2 weeks after the respective brain region's peak in neurogenesis was the cerebellum, where granule cell neurogenesis peaks between P4 and P14,21 but anaesthetic vulnerability was already observed in P7 animals. This seeming discrepancy may be explained by the vastly different rates of neuronal maturation and migration among brain regions, ranging from 2 days for cerebellar granule cells to several weeks for dentate granule cells.25,26
Neuronal cell death is a normal step of brain development, eliminating 50–70% of some neuronal populations during early ontogeny,27,28 peaking at early stages of neuronal development.29 Intriguingly, the present results suggest that the regional progression of isoflurane-induced cell death parallels the progression of naturally occurring cell death. Natural apoptosis peaks in rats between P1 and P7 for neocortex, at P10 in the cerebellar internal granule cell layer, and in the cerebellar white matter and dentate gyrus by P21,29 very similar to the isoflurane-induced neuroapoptosis observed in the present study. These temporal and regional similarities between physiological and anaesthesia-induced apoptosis suggest that anaesthetics may trigger cell death in immature neurones of a specifically vulnerable age, maturational stage, or both throughout the brain, similar to our recent findings in dentate granule cells,18 when their survival relies on trophic factors and network activity, potentially by activating the existing apoptotic machinery.
Limitations
The clinical implications of the present findings are unclear.30 While studies such as ours using small animal models are crucial for examining the effects of anaesthetics in the developing brain, they are also subject to several limitations.31 Because of the limited size of these animals, physiological monitoring could not be performed as stringently as in large animal studies or during the perioperative care in humans. Mortality and abnormalities in blood gas and glucose homeostasis are common during prolonged anaesthetic exposures in small rodents,11,23 yet are not routinely observed in humans, raising the possibility that exposures may not be entirely comparable between species. We have, however, previously found that hypoglycaemia, which has been observed in neonatal mice, did not substantially affect anaesthetic neuroapoptosis. Moreover, the neuronal injury pattern observed in the present study did not appear different from that observed in previous studies of newborn rats, which lacked these abnormalities,22,32 and those in non-human primates with tighter control of physiological variables.33 This suggests that physiological abnormalities did not substantially influence the observed outcome. Another dissimilarity compared with common clinical practice was the lack of painful or inflammatory stimuli in the present study, which are frequently part of operative procedures in humans. Painful stimuli induce a variety of physiological changes, which may or may not affect anaesthesia-induced neuroapoptosis.34,35
Regional and temporal patterns of vulnerability in animals may direct targeted human studies into potential anaesthetic susceptibility
Even though it remains entirely unclear whether this phenomenon also occurs in humans, the present findings of age-dependent regional differences in vulnerability may suggest a framework for future clinical studies. Small rodents are born at a stage of considerable neurodevelopmental prematurity and maturation progresses rapidly during the first 2 weeks of life. Conversely, in humans, many critical steps of brain development occur in utero and post-natal brain maturation progresses much slower than in any other animal species.36 Contemporary studies suggest for brain maturation in 7-day-old rodents to correspond to human fetuses around mid-gestation, while the P21 mouse brain resembles the newborn or infant human brain.21,37 If our present results were transferrable to humans, neocortex, caudoputamen, CA1, thalamus, and amygdala may potentially be most vulnerable during fetal surgery, while the hippocampal dentate gyrus, parts of the cerebellum, and olfactory bulb could potentially be susceptible during anaesthetic exposures in human newborns, children, but also in adults, given the protracted neurogenesis observed in these regions. Since dentate plays an integral role in learning and memory,38,39 while highly speculative, previously observed cognitive abnormalities involving school performance and language function after surgery with anaesthesia may potentially represent this region's vulnerability in young children.4–6 Similarly, memory impairment observed after anaesthetic exposures in adult patients may also be the functional correlate of a lesion in the dentate gyrus.40 Clearly, future studies are warranted to elucidate the mechanisms of this potentially clinically relevant phenomenon and to further investigate the functional correlates of anaesthetic exposure early in life.
Authors’ contributions
M.D.: data collection, data analysis and interpretation, manuscript preparation, and final manuscript approval; R.D.H.: data collection, manuscript preparation, and final manuscript approval; C.J.: data collection, drafting of the article, and final manuscript approval; B.J.: data collection, manuscript preparation, and final manuscript approval; E.A.H.: data collection, manuscript preparation, and final manuscript approval; B.J.: manuscript preparation and final manuscript approval. S.C.D.: data collection, manuscript preparation, and final manuscript approval; A.W.L: study design, data analysis and interpretation, manuscript preparation, and final manuscript approval.
Declaration of interest
None declared.
Funding
This study was supported in part by a Masimo-China Pediatric Anesthesia Research Fellow Program Grant by the Masimo Foundation to M.D. (Mentor A.W.L.), by a Summer Undergraduate Research Fellowship (SURF) by the Center for Clinical & Translational Science & Training (CCTST) at the University of Cincinnati to C.J. (Mentor A.W.L.), and by departmental funds. S.C.D. is supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS).
Acknowledgement
The authors thank Jeffery D. Molkentin, PhD, Division of Molecular Cardiovascular Biology, Cincinnati Children's Hospital Medical Center, for providing the exercise equipment.
References
- 1.Weiser TG, Regenbogen SE, Thompson KD, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet. 2008;372:139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 2.Istaphanous GK, Ward CG, Loepke AW. The impact of the perioperative period on neurocognitive development, with a focus on pharmacological concerns. Best Pract Res Clin Anaesthesiol. 2010;24:433–49. doi: 10.1016/j.bpa.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Jevtovic-Todorovic V. Anesthesia and the developing brain: are we getting closer to understanding the truth? Curr Opin Anaesthesiol. 2011;24:395–9. doi: 10.1097/ACO.0b013e3283487247. [DOI] [PubMed] [Google Scholar]
- 4.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMaggio C, Sun L, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ing C, DiMaggio C, Whitehouse A, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–8. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 7.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–27. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Istaphanous GK, Ward CG, Nan X, et al. Characterization and quantification of isoflurane-induced developmental apoptotic cell death in mouse cerebral cortex. Anesth Analg. 2013;116:845–54. doi: 10.1213/ANE.0b013e318281e988. [DOI] [PubMed] [Google Scholar]
- 9.Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 10.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Edn. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 11.Istaphanous GK, Howard J, Nan X, et al. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–87. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 12.Peterson DA. Quantitative histology using confocal microscopy: implementation of unbiased stereology procedures. Methods. 1999;18:493–507. doi: 10.1006/meth.1999.0818. [DOI] [PubMed] [Google Scholar]
- 13.Howell K, Hopkins N, McLoughlin P. Combined confocal microscopy and stereology: a highly efficient and unbiased approach to quantitative structural measurement in tissues. Exp Physiol. 2002;87:747–56. doi: 10.1113/eph8702477. [DOI] [PubMed] [Google Scholar]
- 14.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 15.Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364:1387–90. doi: 10.1056/NEJMp1102155. [DOI] [PubMed] [Google Scholar]
- 16.Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–84. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krzisch M, Sultan S, Sandell J, Demeter K, Vutskits L, Toni N. Propofol anesthesia impairs the maturation and survival of adult-born hippocampal neurons. Anesthesiology. 2013;118:602–10. doi: 10.1097/ALN.0b013e3182815948. [DOI] [PubMed] [Google Scholar]
- 18.Hofacer RD, Deng M, Ward CG, et al. Cell age-specific vulnerability of neurons to anesthetic toxicity. Ann Neurol. 2013;73:695–704. doi: 10.1002/ana.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–72. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown J, Cooper-Kuhn CM, Kempermann G, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–6. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 21.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 22.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratmann G, May LD, Sall JW, et al. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 24.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 25.Komuro H, Yacubova E, Yacubova E, Rakic P. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–40. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–34. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 28.Blaschke AJ, Weiner JA, Chun J. Programmed cell death is a universal feature of embryonic and postnatal neuroproliferative regions throughout the central nervous system. J Comp Neurol. 1998;396:39–50. doi: 10.1002/(sici)1096-9861(19980622)396:1<39::aid-cne4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.White LD, Barone S., Jr Qualitative and quantitative estimates of apoptosis from birth to senescence in the rat brain. Cell Death Differ. 2001;8:345–56. doi: 10.1038/sj.cdd.4400816. [DOI] [PubMed] [Google Scholar]
- 30.Soriano SG, Loepke AW. Let's not throw the baby out with the bath water: potential neurotoxicity of anesthetic drugs in infants and children. J Neurosurg Anesthesiol. 2005;17:207–9. doi: 10.1097/01.ana.0000178113.72714.4b. [DOI] [PubMed] [Google Scholar]
- 31.Mintz CD, Wagner M, Loepke AW. Preclinical research into the effects of anesthetics on the developing brain: promises and pitfalls. J Neurosurg Anesthesiol. 2012;24:362–7. doi: 10.1097/ANA.0b013e31826a0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson SA, Young C, Olney JW. Isoflurane-induced neuroapoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–8. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 33.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shu Y, Zhou Z, Wan Y, et al. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–50. doi: 10.1016/j.nbd.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Liu JR, Liu Q, Li J, et al. Noxious stimulation attenuates ketamine-induced neuroapoptosis in the developing rat brain. Anesthesiology. 2012;117:64–71. doi: 10.1097/ALN.0b013e31825ae693. [DOI] [PubMed] [Google Scholar]
- 36.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108 ((Suppl. 3)):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- 38.Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci USA. 2009;106:17980–5. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxe MD, Battaglia F, Wang JW, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]



