Abstract
Background. Human immunodeficiency virus (HIV) infection–induced indoleamine 2,3-dioxygenase-1 (IDO) expression in activated monocytes and dendritic cells catabolizes tryptophan to kynurenine and other downstream catabolites that inhibit T-cell proliferation and interleukin 17 (IL-17) production. The prognostic significance of this pathway in treated HIV disease is unknown.
Methods. We measured systemic IDO activity (calculated as the ratio of plasma levels of kynurenine to tryptophan; hereafter, the “KT ratio”) in HIV-infected Ugandans before and during antiretroviral therapy (ART)–mediated viral suppression and its association with the rate of subsequent CD4+ T-cell count recovery and mortality.
Results. Among 435 participants, a higher pre-ART KT ratio was associated with a higher plasma virus load (P < .001) and lipopolysaccharide level (P = .018), a lower CD4+ T-cell count (P < .001), and female sex (P = .047). Through month 12 of ART-mediated viral suppression, the plasma KT ratio decreased by approximately 50% (P < .001). After adjustment for pre-ART CD4+ T-cell count, virus load, age, and sex, a higher month 12 KT ratio predicted a slower rate of subsequent CD4+ T-cell count recovery (P = .001). Thirty-nine participants died. After adjustment for pre-ART CD4+ T-cell count, virus load, body mass index, sex, and age, a higher pre-ART and month 6 KT ratio predicted increased mortality (P ≤ .016).
Conclusions. The kynurenine pathway of tryptophan catabolism independently predicts poor CD4+ T-cell count recovery and increased mortality among HIV-infected Ugandans initiating ART and may be an important target for interventions.
Keywords: Tryptophan; kynurenine; indoleamine 2,3-dioxygenase-1; HIV; mortality; antiretroviral therapy; Uganda
Immune activation has long been recognized as a key feature of human immunodeficiency virus type 1 (HIV-1) pathogenesis, remains abnormally elevated despite antiretroviral therapy (ART)–mediated viral suppression, and predicts morbidity and mortality in this setting [1]. Thus, developing interventions to reverse immune activation during treated HIV disease has emerged as a major priority for the modern HIV treatment era. Nevertheless, the pathways that are optimal targets for interventions have remained unclear.
One potentially attractive therapeutic target is indoleamine 2,3-dioxygenase-1 (IDO) and the kynurenine pathway of tryptophan catabolism. IDO is induced in dendritic cells and monocytes by interferon γ (IFN-γ), IFN-α, lipopolysaccharide (LPS) and other inflammatory mediators in HIV-infected individuals [2–4]. IDO catabolizes tryptophan to kynurenine and a variety of downstream catabolites that are potentially neurotoxic and immunoregulatory. For example, the downstream catabolite quinolinic acid may contribute to several neurodegenerative diseases, including AIDS dementia complex [5–8]. The tryptophan catabolites kynurenine and picolinic acid also inhibit T-cell proliferation [9, 10], which may play an essential role in maternal tolerance of the fetal allograft during pregnancy [11] and in the evasion of host immune responses by several cancers [12]. Last, the tryptophan catabolite 3-hydroxyanthralinic acid (3-HAA) induces loss of T-helper type 17 (Th17) cells in HIV-infected individuals, potentially contributing to microbial translocation and immune activation [13–18]. Since microbial translocation may further induce IDO expression, a vicious cycle may result that perpetuates Th17 depletion, microbial translocation, and immune activation, even after HIV replication is controlled during ART [19]. The activity of this pathway can be quantified in vivo by measuring the ratio of kynurenine to tryptophan (hereafter, the “KT ratio”), in which a high KT ratio reflects heightened activation of the IDO pathway (and IDO-like enzymes) and greater catabolism of tryptophan to its potentially harmful catabolites. While constitutive activity of the hepatic enzyme tryptophan 2,3-dioxygenase and enrichment for gut bacteria with IDO-like enzymes may also contribute to the high plasma KT ratios in HIV disease [13, 20], IDO is thought to be the primary inducible systemic driver of the kynurenine pathway of tryptophan catabolism.
Abnormally high plasma KT ratios have long been observed in untreated HIV infection and cross-sectionally associated with progressive HIV disease and AIDS dementia complex in the pre-ART era [21–25]. A high KT ratio also predicts earlier mortality in elderly HIV-uninfected individuals [26, 27]. The plasma KT ratio declines during ART-mediated viral suppression but fails to normalize [21, 22]. No study, however, has addressed whether the plasma KT ratio predicts subsequent clinical events in treated HIV disease.
To address this issue, we measured the plasma KT ratio through the first year of ART and assessed its relationship to subsequent CD4+ T-cell count recovery and mortality in HIV-infected individuals starting their first ART regimen in the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort. We chose the UARTO cohort for this initial study on the basis of its relatively large number of deaths during early ART, its very low rates of losses to follow-up, and the availability of T-cell activation data (as a comparator immunologic predictor) [28].
METHODS
Participants
HIV-infected participants were sampled from UARTO, a cohort of HIV-infected adults initiating their first ART regimen at the Immune Suppression Clinic in Mbarara, Uganda. All participants commence ART with 2 nucleoside reverse transcriptase inhibitors and a nonnucleoside reverse transcriptase inhibitor. Participants are seen before commencement of ART and then every 3 months. At each visit, detailed interviews are conducted, and a blood specimen is obtained between 8:00 am and 9:30 am (before a meal) for CD4+ T-cell counts, plasma HIV RNA levels, and biologic specimen archiving. The cohort also features an active vital status ascertainment program for tracking participants who are lost to follow-up. For the present analysis, all UARTO participants who initiated ART before 2011 and had pre-ART plasma specimens available were sampled.
Measurements
Plasma HIV RNA levels were assessed using the Amplicor HIV Monitor 1.5 test (Roche, Branchburg, NJ; lowest level of detection, 400 copies/mL) or the Cobas Taqman HIV-1 test v1.0 (Roche; lowest level of detection, 48 copies/mL). CD4+ T-cell counts were assessed in the Makerere University/Johns Hopkins University laboratory in Kampala, Uganda. Self-reported dietary protein intake was assessed in a consecutive subset of 361 participants, using a dietary diversity scale, which assesses consumption of food in 12 different groups in a reference 24-hour period, as previously described [29]. Plasma tryptophan and kynurenine levels were assessed on cryopreserved ACD-anticoagulated plasma samples from pre-ART time points (all participants) and at months 6 and 12 of ART among those with plasma HIV RNA levels of <400 copies/mL. Liquid chromatography–tandem mass spectrometry was used to assess kynurenine and tryptophan levels as previously described [13, 30], and the KT ratio was expressed in nanomoles per micromoles. Lipopolysaccharide (LPS) levels were also assessed on ACD-anticoagulated plasma with the Limulus amebocyte assay by use of the heat and dilution method for extracting LPS from plasma lipoproteins and subtracting the background absorbance as described previously [31]. The frequency of activated (CD38+HLA-DR+) CD4+ and CD8+ T cells was assessed on fresh whole-blood specimens processed the same day as described previously [28].
Statistical Methods
For cross-sectional comparisons, continuous variables were compared between groups with Wilcoxon rank sum tests. Dichotomous variables were compared between groups by use of χ2 and Fisher exact tests. Relationships between continuous variables were assessed with Spearman's rank order correlation coefficients. Adjusted differences between groups were assessed with linear regression, with calculation of standard errors with heteroskedasticity-consistent covariance matrix estimators and log-transformation of outcomes when necessary to satisfy model assumptions.
The rate and predictors of CD4+ T-cell count recovery among UARTO participants achieving a plasma HIV RNA level of <400 copies/mL by month 6 of ART were assessed with linear mixed models (PROC MIXED in SAS). A 3-piece segmented linear model (0–3 months, 3–12 months, and >12 months) allowed for changing slopes of CD4+ T-cell count recovery over time. Observations were censored at the time of a subsequent plasma HIV RNA level of >1000 copies/mL. Baseline predictors of the rate of CD4+ T-cell count recovery in each segment were assessed with time-by-interaction terms. Factors associated with the rate of CD4+ T-cell count recovery in unadjusted analyses (P < .10) were included as potential confounders in multivariable models. Predictors of mortality were assessed with Kaplan-Meier methods and Cox proportional hazards models for adjusted analyses.
RESULTS
Participant Characteristics Before ART Initiation
A total of 435 treatment-naive HIV-infected adults were included in these analyses. Most (70%) were female, 23 of whom reported being pregnant. At the time of ART initiation, the median age was 34 years (interquartile range [IQR], 28–39 years), the median CD4+ T-cell count was 133 cells/mm3 (IQR, 79–201 cells/mm3), and the median plasma HIV RNA level was 5.0 log10 copies/mL (IQR, 4.5–5.3 log10 copies/mL; Table 1). As reported previously, the median pre-ART frequency of activated (CD38+HLA-DR+) CD8+ T cells was 67% (IQR, 57%–76%) [28]. Before ART, the median plasma KT ratio was 130 nM/µM (IQR, 90–200 nM/µM), and the median plasma LPS level was 16 pg/mL (IQR, 12–24 pg/mL).
Table 1.
Characteristics of Human Immunodeficiency Virus (HIV)–infected Ugandans Initiating Antiretroviral Therapy
| Characteristic | Value |
|---|---|
| Age, years | 34 (28–39) |
| Female sex | 305 (70) |
| CD4+ T-cell count, cells/mm3 | 133 (79–201) |
| Plasma HIV RNA level, log10 copies/mL | 5.0 (4.6–5.5) |
| BMIa | 21 (19–24) |
| CD38+HLA-DR+ CD8+ T cells, % | 67 (56–76) |
| Plasma tryptophan level, µM | 18 (11–23) |
| Plasma kynurenine level, nM | 2157 (1734–2709) |
| Plasma KT ratio, nM/µM | 131 (92–198) |
Data are no. (%) of patients or median (interquartile range).
Abbreviation: KT ratio, ratio of kynurenine to tryptophan.
a Body mass index (BMI) is defined as the weight in kilograms divided by the height in meters squared.
Correlates of the Pre-ART KT Ratio
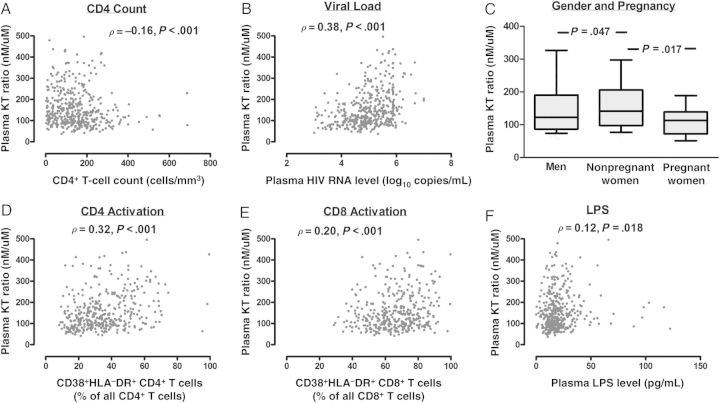
In unadjusted analyses, a higher pre-ART KT ratio was associated with higher plasma HIV RNA levels (ρ = 0.38; P < .001), lower CD4+ T-cell counts (ρ = −0.16; P < .001), higher plasma LPS levels (ρ = 0.13; P = .007), and higher frequencies of activated (CD38+HLA-DR+) CD4+ T cells (ρ = 0.32; P < .001) and CD8+ T cells (ρ = 0.20; P < .001; Figure 1). Nonpregnant women had a higher median pre-ART KT ratio than men (141 vs 122 nM/µM; P = .047) and pregnant women (115 nM/µM; P = .017). In a multivariable model including each of these factors, a higher KT ratio was associated with higher plasma HIV RNA levels (P < .001), lower CD4+ T-cell counts P = .005), higher LPS levels (P = .042), higher frequency of activated CD4+ T cells (P < .001), and female sex (P = .024). There was no evidence for an association between age and pre-ART KT ratio.
Figure 1.
Factors associated with the plasma ratio of kynurenine to tryptophan (hereafter, the “KT ratio”) before antiretroviral therapy (ART) initiation in human immunodeficiency virus (HIV)–infected Ugandans. The relationships between the pre-ART KT ratio and the CD4+ T-cell count (A), the plasma HIV RNA level (B), sex and pregnancy status (C), CD4+ T-cell activation (D), CD8+ T-cell activation (E), and plasma lipopolysaccharide (LPS) level (LPS, F) were assessed in 435 HIV-infected Ugandans. Between-group comparisons were assessed with Wilcoxon rank sum tests, and Spearman correlation coefficients were used for comparing continuous variables.
Impact of Suppressive ART on the Plasma KT Ratio
Among UARTO participants with a plasma HIV RNA level of <400 copies/mL at month 6 of ART, the KT ratio decreased during ART by a mean of 46% (median level at month 6, 72 nM/µM [IQR, 56–94 nM/µM]; Figure 2A). Among those continuing to maintain a plasma HIV RNA level of <400 copies/mL at month 12 of ART, the KT ratio continued to decline—albeit at a slower rate—by a mean of 15% between months 6 and 12 (P < .001) to a median of 62 nM/µM (IQR, 49–78 nM/µM). Despite ART-mediated declines in the KT ratio, the pre-ART KT ratio continued to be a strong predictor of the KT ratio at month 12 of ART-mediated viral suppression (ρ = 0.56; P < .001; Figure 2B).
Figure 2.
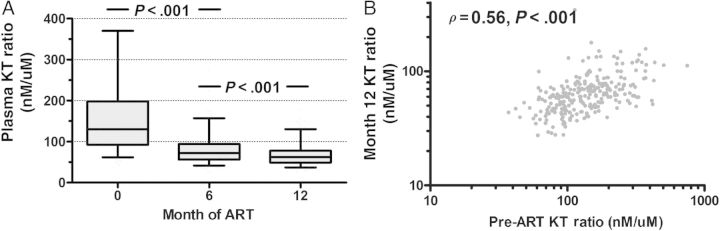
Impact of antiretroviral therapy (ART)–mediated viral suppression on the plasma ratio of kynurenine to tryptophan (hereafter, the “KT ratio”) in human immunodeficiency virus (HIV)–infected Ugandans. Changes in the KT ratio were assessed in HIV-infected Ugandans during the first year of confirmed ART-mediated viral suppression to <400 copies/mL (A). Boxes represent interquartile ranges, and central bars reflect median values. Error bars span the 5th and 95th percentile values. P values reflect changes from baseline levels assessed with linear mixed models. The correlation between the pre-ART KT ratio and the month 12 KT ratio was also assessed (B).
Relationship Between the Plasma KT Ratio and CD4+ T-Cell Count Recovery
We next assessed the relationship between the pre-ART KT ratio and subsequent CD4+ T-cell count recovery among those achieving a plasma HIV RNA level of <400 copies/mL by month 6 of ART (n = 408). Median follow-up during ART-mediated viral suppression was 38 months (IQR, 22–47 months), and participants contributed a median of 12 CD4+ T-cell count observations (IQR, 7–15 observations) before censoring. While there was no evidence for a relationship between the pre-ART KT ratio and the rate of CD4+ T-cell count recovery between months 0–3 and 3–12 of ART, each 2-fold increase in the pre-ART KT ratio was associated with 1 fewer CD4+ T cell gained per month (95% confidence interval [CI], −1.8 to −.01 cells/mm3 per month; P = .046) following month 12 of ART, after adjustment for pre-ART CD4+ T-cell count and plasma HIV RNA level, age, and sex (each with time-interaction terms). Since the pre-ART KT ratio was a strong predictor of month 12 KT ratio, we next assessed the relationship between the month 12 KT ratio and subsequent CD4+ T-cell count recovery among those with plasma HIV RNA levels of <400 copies/mL at month 12 of ART. In unadjusted analyses, a higher month 12 KT ratio during confirmed ART-mediated viral suppression predicted a subsequent mean 1.3 fewer CD4+ T cells/mm3 gained per month (95% CI, −2.2 to −.3 cells/mm3 per month; P = .010; Table 2). Even after adjustment for pre-ART plasma HIV RNA level, age, sex, and both month 12 CD4+ T-cell count and CD8+ T-cell activation, each 2-fold increase in the month 12 KT ratio continued to predict a subsequent mean 2 cells/mm3 fewer CD4+ T cells gained per month (95% CI, −3.1 to −.8 cells/mm3; P = .001). Results were similar when adding the pre-ART CD4+ T-cell count and its time interaction term to the model (mean of 2.2 fewer cells/mm3/month per 2-fold increase in month 12 KT ratio; P < .001). These relationships were essentially unchanged in sensitivity analyses excluding women who were pregnant at month 12 (n = 9). It is also important to note that the pre-ART and month 12 KT ratio are collinear (Figure 2B), such that when both the pre-ART and month 12 KT ratios are included in the same model, the effect of each measurement on the slope of the CD4+ T-cell count recovery was rendered insignificant (data not shown). This may suggest that the impact of a persistently high KT ratio on the CD4+ T-cell count recovery during ART may be largely determined by the extent of immune dysfunction before ART initiation.
Table 2.
Predictors of Late CD4+ T-Cell Count Recovery Among Human Immunodeficiency Virus (HIV)–Infected Ugandans Receiving Suppressive Antiretroviral Therapy
| Characteristic | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|
| Change in CD4+ T-Cell Count | P | Change in CD4+ T-Cell Count | P | |
| Per month of study | +3.0 (2.5–3.4) | <.001 | +2.7 (1.7–3.6) | <.001 |
| Month 12 plasma KT ratio | ||||
| Per 2-fold increase | +47.9 (7.5–88.2) | .02 | +49.5 (11.0–87.8) | .012 |
| Per month of studyb | −1.3 (−2.2 to −.3) | .010 | −2.0 (−3.1 to −0.8) | .001 |
| Month 12 CD4+ T-cell count | ||||
| Per 100 cell/mm3 increase | +72.4 (55.0–89.8) | <.001 | +65.8 (42.1–89.6) | <.001 |
| Per month of studyb | −0.3 (−.7 to .03) | .07 | −0.4 (−.8 to −.01) | .045 |
| Month 12 CD8+ T-cell activation | ||||
| Per 10 percentage-point increase | −34.2 (−51.5 to −16.9) | <.001 | −14.9 (−30.9 to 1.1) | .068 |
| Per month of studyb | +0.1 (−.4 to .5) | .78 | −0.01 (−.5 to .4) | .96 |
| Female sex | ||||
| Overall | +26.3 (−15.7 to 68.2) | .22 | +17.3 (−30.0 to 64.7) | .47 |
| Per month of studyb | +1.1 (.1–2.1) | .028 | +0.9 (−.3 to 2.1) | .15 |
| Age | ||||
| Per 10-year increase | −19.8 (−43.2 to 3.5) | .096 | +3.2 (−2.4 to 3.0) | .82 |
| Per month of studyb | −0.4 (−.9 to .2) | .21 | +0.1 (−.7 to .9) | .90 |
| Pretherapy plasma HIV RNA level | ||||
| Per log10 copies/mL increase | −30.6 (−56.1 to −5.1) | .019 | −23.5 (−46.1 to −.9) | .041 |
| Per month of studyb | +0.1 (−.5 to .7) | .75 | +0.5 (−.2 to 1.1) | .14 |
Data are mean no. of cells/mm3 (95% confidence interval).
a Adjusted models include all listed factors.
b This is a factor-by-time interaction term, which reflects the association between each unit increase in the primary factor on the rate of CD4+ T-cell recovery (in CD4+ T-cells/mm3 per month).
Relationship Between the Plasma KT Ratio and Mortality
Thirty-nine participants died after initiating ART, with nearly half of the deaths occurring within the first 6 months of ART. As most UARTO participants live in a rural resource-limited environment, many of the deaths occurred at home, and the cause of death is unknown for the majority of cases. Nonetheless, a higher pre-ART KT ratio strongly predicted increased mortality during ART (P for trend < .001; Figure 3A). Since a higher pre-ART KT ratio might conceivably be a consequence of a subclinical illness that would subsequently cause death during early ART (eg, via an immune reconstitution inflammatory syndrome [IRIS]), we also assessed the prognostic significance of the month 6 KT ratio among those with plasma HIV RNA levels of <400 copies/mL during ART (n = 348). Each tertile increase in the month 6 KT ratio was also strongly predictive of subsequent mortality (P for trend = .012), with the vast majority of deaths occurring at least 1 year after the measurement (Figure 3B). We next assessed whether these relationships were independent of other known clinical predictors of mortality. Lower pre-ART CD4+ T-cell counts and body mass index, male sex, and higher plasma HIV RNA levels were all associated with increased mortality in unadjusted models (Table 3). Even after adjustment for these factors and age, each 2-fold increase in the pre-ART plasma KT ratio was associated with a 1.8-fold increased risk of mortality (P = .016). As we have previously reported [28], in addition to the factors described above, the extent of CD8+ T-cell activation at month 6 of suppressive ART was also predictive of subsequent mortality in unadjusted models. Even after adjustment for age, sex, pre-ART CD4+ T-cell count, pre-ART plasma HIV RNA level, and extent of CD8+ T-cell activation at month 6 of suppressive ART, each 2-fold increase in the month 6 KT ratio was associated with a 3.7-fold increased risk of subsequent mortality (P = .008). These relationships were essentially unchanged in sensitivity analyses excluding women who were pregnant at baseline (n = 24) or month 6 (n = 6). While a minority of deaths (16 of 39) had available dietary diversity data, we found no evidence that the number of self-reported dietary protein sources modified the association between the KT ratio and mortality (P for interaction = .66).
Figure 3.
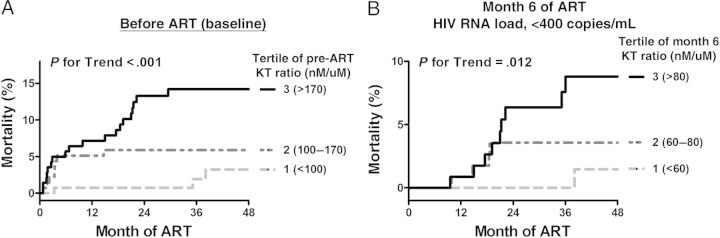
Relationship between the plasma ratio of kynurenine to tryptophan (hereafter, the “KT ratio”) and mortality among human immunodeficiency virus (HIV)–infected Ugandans initiating antiretroviral therapy (ART). The relationship between the KT ratio measured before ART initiation (A) and at month 6 of ART-mediated viral suppression of <400 copies/mL (B) and subsequent mortality was assessed with Kaplan-Meier methods. The KT ratio at each time point was assessed in tertiles, and P values reflect findings from tests for trend.
Table 3.
Predictors of Mortality Among Human Immunodeficiency Virus (HIV)–Infected Ugandans Initiating Antiretroviral Therapy (ART)
| Characteristic | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Pre-ART Factors? | ||||
| Pre-ART KT ratio, per 2-fold increase | 2.0 (1.4–3.0) | <.001 | 1.80 (1.1–2.9) | .016 |
| Pre-ART CD4+ T-cell count, per 100 cells/mm3 increase | 0.58 (.38–.87) | .008 | 0.63 (.38–1.1) | .08 |
| BMI, per kg/m2 increase | 0.78 (.70–.88) | <.001 | 0.79 (.68–.91) | .001 |
| Female sex | 0.53 (.28–.99) | .049 | 1.1 (.48–2.5) | .83 |
| Pre-ART plasma HIV RNA level, per log10 copies/mL increase | 1.75 (1.1–2.9) | .026 | 1.1 (.57–2.1) | .79 |
| Age, per 10-year increase | 1.3 (.92–1.8) | .13 | 1.3 (.82–2.0) | .28 |
| After month 6 of ART for those with an HIV RNA load <400 copies/mL | ||||
| Month 6 KT ratio, per 2-fold increase | 2.8 (1.3–5.8) | .006 | 3.7 (1.4–9.7) | .008 |
| Pre-ART CD4+ T-cell count, per 100 cells/mm3 increase | 0.71 (.38–1.3) | .29 | 0.52 (.22–1.2) | .14 |
| BMI, per kg/m2 increase | 0.84 (.70–1.0) | .053 | 0.83 (.65–1.1) | .14 |
| Female sex | 0.38 (.13–1.08) | .069 | 0.49 (.11–2.1) | .34 |
| Pre-ART plasma HIV RNA level, per log10 copies/mL increase | 1.7 (.75–4.1) | .20 | 1.0 (.24–4.1) | .99 |
| Age, per 10-year increase | 1.8 (.92–3.4) | .091 | 1.5 (.70–3.2) | .30 |
| Month 6 percentage of activated CD8+ T cells, per 2-fold increase | 4.3 (1.01–18.7) | .049 | 4.1 (.68–25) | .12 |
Abbreviations: BMI, body mass index; CI, confidence interval; KT ratio, ratio of kynurenine to tryptophan; OR, odds ratio.
a Adjusted models include all listed factors.
DISCUSSION
While the kynurenine pathway of tryptophan catabolism—induced either by host IDO activity or possibly upon enrichment of gut-resident bacteria with IDO-like enzymes—has been associated with microbial translocation, systemic immune activation, and proliferative T-cell defects during HIV infection [10, 13, 20], no prior study had assessed the relationship between this pathway and clinical outcomes during treated HIV disease. In our current study, we evaluated the prognostic significance of the plasma KT ratio—a marker of systemic induction of IDO and IDO-like enzymes—in a cohort of 435 HIV-infected Ugandans starting their first ART regimen. We found that a higher pre-ART KT ratio (which reflects greater metabolism of tryptophan to its metabolites) was strongly associated with higher plasma HIV RNA levels, lower CD4+ T-cell counts, and female sex and was modestly associated with higher plasma LPS levels. Furthermore, a higher plasma KT ratio—both before ART and during ART-mediated viral suppression—strongly and independently predicted lower late CD4+ T-cell count recovery and increased mortality. While it remains unclear whether the kynurenine pathway is causally associated with CD4+ T-cell count recovery and mortality, these data provide the first evidence supporting the potential clinical relevance of IDO activity and the kynurenine pathway in treated HIV disease.
Consistent with earlier studies in resource-rich settings [13, 21, 22], we observed strong relationships between the extent of HIV replication and CD4+ T-cell depletion in plasma KT ratio and the untreated HIV-infected Ugandans with advanced AIDS in our study. Like our group's recent study in San Francisco, we also observed a modest relationship between the plasma KT ratio and LPS levels, confirming a link between the kynurenine pathway and microbial translocation in an African cohort [13]. Interestingly, we also observed higher plasma KT ratio levels in women than men after adjustment for differences in plasma HIV RNA levels. This is consistent with prior studies suggesting that women have more-robust innate immune responses to Toll-like receptor 7 stimulation by HIV than men [32]. Future studies evaluating the influence of sex hormones on IDO induction, as well as the longitudinal impact of pregnancy on the KT ratio, would also be of significant interest. Like earlier studies from resource-rich settings [22], we also confirmed in a rural African setting that ART-mediated viral suppression results in a robust decline in the plasma KT ratio. Nevertheless, in our Ugandan study, the KT ratio appeared to plateau by month 12 of suppressive ART at levels that are still well above those observed in our cohort of patients in San Francisco with ART-suppressed HIV levels (median, 62 nM/µM vs 37 nM/µM) and in a cohort of North American patients with ART-suppressed HIV levels who had a history of advanced AIDS in the Study of the Ocular Complications of AIDS (median, 50 nM/µM), all assessed in the same laboratory [13, 33]. These levels are also >2-fold higher than reported in other studies of European healthy blood donors or HIV-uninfected control populations (median, 25–28 nM/uM) [22, 34]. While we did not have a matched HIV-uninfected Ugandan control group to determine whether these levels were abnormally high for this setting, and while there are clearly many potential differences between cohorts that could explain differences, these levels did appear to persist at high levels in treated HIV-infected Ugandans.
We also demonstrated that both the pretherapy and month 12 KT ratio strongly and independently predicted late (ie, after 12 months) CD4+ T-cell count recovery. While the impact of the month 12 KT ratio on the adjusted rate of CD4+ T-cell count recovery might seem of modest clinical significance (−2 cells/mm3/month per 2-fold increase), this translates into an absolute difference of −240 cells/mm3 over 10 years and −480 cells/mm3 over 20 years of suppressive ART, which could potentially be clinically significant for individuals starting ART at low CD4+ T-cell counts. Importantly this effect was stronger than and independent of the relationship we previously reported between pretherapy CD8+ T-cell activation and CD4+ T-cell count recovery from this same cohort [28], potentially suggesting that the kynurenine pathway is more closely linked to the biologic pathways mediating CD4+ T-cell count recovery than the extent of T-cell activation. The fact that kynurenine and other downstream catabolites suppress T-cell proliferation in vitro may be one mechanism to explain these findings [9, 10]. Alternatively, reduction in Th17 cell levels in mucosal tissues by the catabolite 3-HAA may lead to decreased tight junctions between gut epithelial cells and decreased bacterial clearance, leading to microbial translocation, which could suppress T-cell proliferation through alternative mechanisms [35]. It is also notable that the pre-ART KT ratio did not predict early (ie, during the first 3 months) ART-mediated CD4+ T-cell count recovery. This may be because those with higher pre-ART KT ratios experience greater declines (on an absolute scale) in the KT ratio (and potentially a greater reversal of proliferative defects) and/or greater declines in plasma HIV RNA levels, resulting in greater redistribution of CD4+ T cells from lymphoid tissues [36].
Even more importantly, we established that plasma KT ratio measured both before ART and at month 6 of suppressive ART strongly and independently predict increased mortality. While many of the deaths occurred in the first few months of ART and may have represented IRIS events against subclinical infections that were contributing to higher pre-ART KT ratio levels, KT ratio levels measured after 6 months of confirmed suppressive ART continued to predict subsequent mortality, with the majority of deaths occurring over a year after the measurement, making it less likely that the higher KT ratio was simply a consequence of a prefatal illness. This relationship was also notably stronger than and independent of CD8+ T-cell activation, which we had previously reported to predict mortality in this same cohort [28], again suggesting that the kynurenine pathway is more closely linked to the mechanistic pathways mediating the risk of mortality than to T-cell activation.
Since this was an observational study, it remains unclear whether the kynurenine pathway is causally associated with clinical outcomes or simply a marker of some other related immunologic process (ie, innate immune activation) that causes poor CD4+ T-cell count recovery and mortality through independent mechanisms. Given the many potentially bidirectional relationships between the kynurenine pathway, microbial translocation, and innate immune activation, future mediation and/or pathway studies with additional immunologic markers will be necessary to further address this question, as will clinical trials of targeted interventions. Indeed, several IDO inhibitors are in clinical development for treatment of cancer [37, 38]. Last, it should be noted that all the participants in our study came from southwestern Uganda and that all had advanced HIV disease at the time of ART initiation. Future studies in other settings and with less advanced populations will be necessary before these observations can be generalized to other populations of HIV-infected individuals.
In summary, our study is the first to demonstrate that kynurenine pathway activity predicts poor CD4+ T-cell count recovery and increased mortality during treated HIV disease. These data provide the first evidence that the kynurenine pathway may be clinically relevant in treated HIV disease. Whether this pathway is causally associated with disease will need to be assessed in future mechanistic studies, but these data highlight IDO and the kynurenine pathway more generally as a potential target for future interventional studies.
Notes
Acknowledgments. We thank the study participants, who made this work possible; the capable UARTO study administrators, research assistants, data managers, laboratory staff, participant trackers, drivers, and volunteers, who were critical to conducting this multidisciplinary research; the Mbarara University of Science and Technology (MUST) administration and the MUST Immune Suppression Syndrome Clinic medical staff, nurses, pharmacists, and counselors, for their helpful assistance; and the members of the Cleveland Immunopathogenesis Consortium, for helpful scientific discussions.
Financial support. This work was supported by the National Institutes of Health (R56AI100765, R21AI078774, K24MH087227, K23MH087228, T32AA007240, R01MH054907, P30AI27763, U19 AI96109, D43 CA153717, and P01 AI076174), the Doris Duke Charitable Foundation (Clinical Scientist Development Award 2008047), and the Sullivan Family Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–47. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–22. [PubMed] [Google Scholar]
- 4.Grant RS, Naif H, Thuruthyil SJ, et al. Induction of indoleamine 2,3-dioxygenase in primary human macrophages by HIV-1. Redox Rep. 2000;5:105–7. doi: 10.1179/135100000101535366. [DOI] [PubMed] [Google Scholar]
- 5.Sardar AM, Reynolds GP. Frontal cortex indoleamine-2,3-dioxygenase activity is increased in HIV-1-associated dementia. Neurosci Lett. 1995;187:9–12. doi: 10.1016/0304-3940(95)11324-p. [DOI] [PubMed] [Google Scholar]
- 6.Gold AB, Herrmann N, Swardfager W, et al. The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J Neuroinflammation. 2011;8:17. doi: 10.1186/1742-2094-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widner B, Leblhuber F, Walli J, Tilz GP, Demel U, Fuchs D. Degradation of tryptophan in neurodegenerative disorders. Adv Exp Med Biol. 1999;467:133–8. doi: 10.1007/978-1-4615-4709-9_19. [DOI] [PubMed] [Google Scholar]
- 8.Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7:103–23. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- 9.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 12.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 13.Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klatt NR, Estes JD, Sun X, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–33. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–85. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–8. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 18.Jenabian MA, Patel M, Kema I, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One. 2013;8:e78146. doi: 10.1371/journal.pone.0078146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt PW. Th17, gut, and HIV: therapeutic implications. Curr Opin HIV AIDS. 2010;5:189–93. doi: 10.1097/COH.0b013e32833647d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem. 1998;44:858–62. [PubMed] [Google Scholar]
- 22.Zangerle R, Widner B, Quirchmair G, Neurauter G, Sarcletti M, Fuchs D. Effective antiretroviral therapy reduces degradation of tryptophan in patients with HIV-1 infection. Clin Immunol. 2002;104:242–7. doi: 10.1006/clim.2002.5231. [DOI] [PubMed] [Google Scholar]
- 23.Heyes MP, Brew BJ, Saito K, et al. Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol. 1992;40:71–80. doi: 10.1016/0165-5728(92)90214-6. [DOI] [PubMed] [Google Scholar]
- 24.Sardar AM, Bell JE, Reynolds GP. Increased concentrations of the neurotoxin 3-hydroxykynurenine in the frontal cortex of HIV-1-positive patients. J Neurochem. 1995;64:932–5. doi: 10.1046/j.1471-4159.1995.64020932.x. [DOI] [PubMed] [Google Scholar]
- 25.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37:684–7. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Pertovaara M, Raitala A, Lehtimaki T, et al. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–9. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25:2123–31. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2013;65:456–62. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Y, Louie A, Yang Q, et al. A simple LC-MS/MS method for determination of kynurenine and tryptophan concentrations in human plasma from HIV-infected patients. Bioanalysis. 2013;5:1397–407. doi: 10.4155/bio.13.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 32.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt P, Rodriguez B, Shive C, et al. Gut epithelial barrier dysfunction, inflammation, and coagulation predict higher mortality during treated HIV/AIDS [abstract 278]. Program and abstracts from the 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. [Google Scholar]
- 34.Raitala A, Pertovaara M, Karjalainen J, Oja SS, Hurme M. Association of interferon-gamma +874(T/A) single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand J Immunol. 2005;61:387–90. doi: 10.1111/j.1365-3083.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- 35.Said EA, Dupuy FP, Trautmann L, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. 2010;16:452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 37.Volgraf M, Lumb JP, Brastianos HC, et al. Biomimetic synthesis of the IDO inhibitors exiguamine A and B. Nat Chem Biol. 2008;4:535–7. doi: 10.1038/nchembio.107. [DOI] [PubMed] [Google Scholar]
- 38.Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–98. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]