Abstract
While Marijuana continues to be the most widely used illicit drug, abuse of synthetic cannabinoid (SCB) compounds in ‘Spice’ or ‘K2’ herbal incense products has emerged as a significant public health concern in many European countries and in the USA. Several of these SCBs have been declared Schedule I controlled substances but detection and quantification in biological samples remain a challenge. Therefore, we present a liquid chromatography–tandem mass spectrometry method after liquid–liquid extraction for the quantitation of CP-47,497, CP-47,497-C8 and JWH-250 in mouse brain. We report data for linearity, limit of quantification, accuracy/bias, precision, recovery, selectivity, carryover, matrix effects and stability experiments which were developed and fully validated based on Scientific Working Group for Forensic Toxicology guidelines for forensic toxicology method validation. Acceptable coefficients of variation for accuracy/bias, within- and between-run precision and selectivity were determined, with all values within ±15% of the target concentration. Validation experiments revealed degradation of CP-47, 497 and CP-47,497-C8 at different temperatures, and significant ion suppression was produced in brain for all compounds tested. The method was successfully applied to detect and quantify CP-47,497 in brains from mice demonstrating significant cannabimimetic behavioral effects as assessed by the classical tetrad paradigm.
Introduction
Abuse of synthetic cannabinoids (SCBs) in herbal incense products (HIPs) has recently emerged as a significant national public health and safety concern. In the USA, emergency bans were swiftly mandated by the Drug Enforcement Administration to regulate the barrage of SCB compounds available to the public (1). To date, dozens of SCBs have been detected in HIPs, as their structures are continually modified to circumvent legislative efforts (2). Anecdotal accounts from user forums (www.bluelight.ru; erowid.org) suggest that SCB effects are reminiscent of the effects of marijuana (3) and while published user reports of SCB effects are limited, studies indicate SCBs produce alterations in perception, conjunctival injection, xerostomia and heightened pulse rate similar to that of marijuana (4, 5).
SCB compounds were incipiently developed as tools to gain insight on marijuana's effects on the brain and to investigate potential therapeutic applications of the endogenous cannabinoid receptor system (CB1 and CB2) modulation. The cyclohexylphenol (CP) series were synthesized by Pfizer beginning in the 1970s, with CP-47,497 ((2-[(1R,3S)-3-hydroxycyclohexyl]-5-(2-methyloctan-2-yl)phenol)) serving as a prototypical bicyclic analgesic compound (6). Research from scientists at the Winthrop-Sterling company over two decades ago led to the development of the novel aminoalkylindole SCB, WIN55,212-2 ((+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1napthalenylmethanone), which behaves as a full agonist at both CB1 and CB2 receptors (7, 8). Stemming from this research, John W. Huffman continued development of an aminoalkylindole (or JWH) class of SCBs, which include compounds such as JWH-250 (2-(2-methoxyphenyl)-1-(1-pentylindol-3-yl)ethanone) (9). Over the last several years, these SCB compounds, intended solely for research purposes, have been sold as a ‘legal high’ alternative to marijuana, with the first identification of CP 47,497 in 2008 from the Wisconsin State Crime Laboratory (10).
Marijuana remains the most widely used illicit substance worldwide (11), but the fact that standard drug screens do not typically include SCBs, may contribute to the recent popularity of these substances. The sudden spike in SCB abuse will likely continue to threaten public health and safety. Repeated abuse of SCBs has led to tolerance and dependence symptoms after attempted drug cessation (12, 13). Thus, there is a need for validated methods to detect these SCB substances from biological material. In the present study, we demonstrate a high-performance liquid chromatography–tandem mass spectrometry (HPLC/MS/MS) method for the detection and quantification of JWH-250, CP-47,497 and CP-47,497-C8 (2-[(1R,3S)-3-hydroxycyclohexyl]-5-(2-methylnonan-2-yl)phenol), also referred to as cannabicyclohexanol, in brain. We chose to include CP-47,497, CP-47,497-C8 and JWH-250 in this method as they are among the first SCB compounds to be positively detected in herbal products (3, 4, 14–17). Of relevance to their abuse, these three SCBs have also been identified in SCB exposure case reports (18, 19).
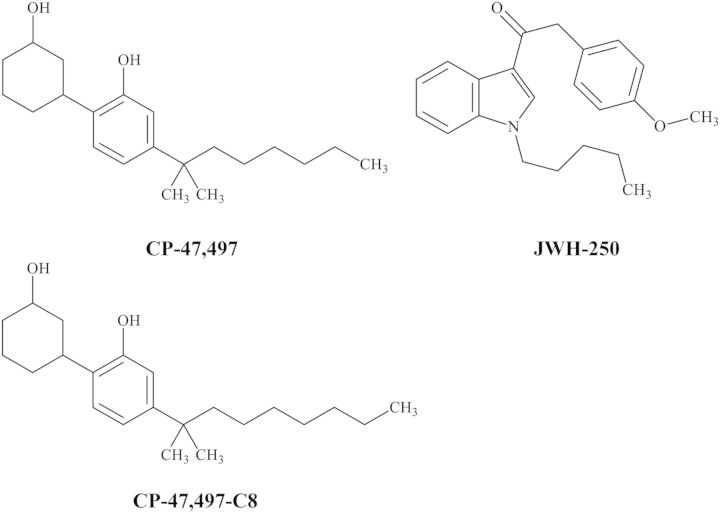
It is hypothesized that the desired subjective effects of SCBs are mediated through activation of CB1 receptors in the central nervous system (CNS) similar to those of Δ-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of marijuana (19–21). In contrast to the partial-agonist activity of THC, many SCBs including CP-47,497, CP-47,497-C8 and JWH-250 (Figure 1) possess enhanced binding potency at CB1 receptors (22). Despite this proposed mechanism of action, few published studies have identified or quantified the presence of SCBs in brain after drug exposure (23–25). Of clinical significance, it has been previously demonstrated that THC can be detected in brain after the window of detection in blood/plasma has lapsed (26). The method presented here demonstrates that SCBs from distinct CP and aminoalkylindole structural classes can be simultaneously detected in brain.
Figure 1.
Structures of CP-47,497, CP-47,497-C8 and JWH-250 analytes.
Materials and methods
Drugs and reagents
CP-47,497, CP-47,497-C8 and JWH-250 drugs and CP-47,497-d11, CP-47,497-C8-d7 and JWH-250-d5 deuterated internal standards (ISTDs) were purchased from Cayman Chemical Company (Ann Arbor, MI). HPLC grade or better acetonitrile, methanol and ammonium formate were obtained from Fisher Scientific (Waltham, MA). Deionized (DI) water was obtained from an in-house water system (Millipore, Bedford MA). Nitrogen gas (medical grade) was obtained from National Welders Supply Company (Richmond, VA).
Animals, behavioral measures and tissue collection
Drug naïve, male, ICR (CD-1) mice (Harlan Laboratories, Dublin, VA) weighing 25–35 g were obtained and housed in Virginia Commonwealth University's animal care facilities at 22 ± 2°C on a 12-h light/dark cycle with ad libitum water and standard rodent chow (7012 Teklad Mouse/Rat Diet, Harlan Laboratories). For proof-of-method experiments, mice received intraperitoneal (i.p.) injections of either vehicle (ethanol : emulphor : saline in a ratio of 1:1:18) or 30 mg/kg CP-47,497 administered at a volume of 0.1 mL per 10 g of body mass, as this SCB dose produced measurable cannabimimetic effects in previous dose–response studies in vivo (27).
For tetrad behavioral testing, mice were acclimated to the test environment for at least 1 h prior to testing for spontaneous activity, catalepsy, antinociception and hypothermia (28, 29). Locomotor activity was assessed by placing mice into individual photocell activity boxes (28 × 16.5 cm chambers containing two sets of eight photocells; Omnitech, Columbus, OH) 5 minutes after vehicle or drug treatment. Photocell beam interruptions were recorded for 10 min using Digiscan Animal Activity Monitor software (Med Associates, Inc., St. Albans, VT) with data expressed as the average number of photocell beam breaks. Mice were assessed for catalepsy, antinociception and hypothermia 30 minutes after vehicle or drug treatment. Catalepsy was measured using the bar test in which both forelimbs of the mouse were placed on a horizontal bar, with the duration of a fixed and motionless posture recorded over a 60-s interval. Antinociception was determined using a warm water (52°C) tail immersion test. The distal end (∼1 cm) of the tail was immersed in the water bath and the latency of the mouse to withdrawal its tail was recorded (to the nearest tenth of a second) using a 10-s cut-off to minimize tail damage. Antinociception data were transformed to represent a maximum percent effect (%MPE) by the following formula: %MPE = [(test latency − pretreatment latency)/(10 − pretreatment latency)] × 100. Body temperature measurements were collected by inserting a rectal probe attached to a telethermometer (Yellow Spring Industries, Inc., Yellow Springs, OH) to a 2-cm depth with measurements recorded to the nearest 0.1°C.
One hour post-injection, mice were deeply anesthetized via 3% isoflurane-containing oxygen induction and humanely sacrificed by cervical dislocation. Whole brain tissue was harvested and stored at −80°C until analysis. All experiments were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Brain homogenate preparation
Brain tissue from ICR and C57BL/J6 mice was harvested, pooled and used to obtain drug-free tissue for the method validation. Brain homogenates, created from whole brain tissue, were prepared in a 4 : 1 ratio (w/w) using DI water with a stand homogenizer (Janke and Kunkel Ultra Turrax T25). For selectivity experiments, 10 individual lots of tissue (each lot required two pooled brains) were used to prepare each of 10 separate homogenate tissue lots. For mouse studies, after homogenization of whole brain tissue, 0.5 mL aliquots of homogenate were used from drug-treated and vehicle-treated mice to isolate CP-47,497 using the liquid–liquid extraction (LLE) method described herein.
Internal standard and stock solution preparation
Separate working stock solutions containing each CP-47,497, CP-47,497-C8 and JWH-250 at 250 ng/mL, 2,500 ng/mL and 10,000 ng/mL were prepared by dilution with methanol of commercially purchased drug compounds. The deuterated ISTD mixture containing 2,500 ng/mL of CP-47,497-d11, CP-47,497-C8-d7 and JWH-250-d5 was prepared by dilution of purchased ISTDs with methanol. Working stock and ISTD stock solutions were stored at −20°C.
Calibrator and QC preparation
Calibrators were prepared by fortifying 0.5 mL aliquots of drug-free mouse brain homogenates with appropriate drug and ISTD concentrations for each calibrator. Prior to every analytical run, fresh calibrators containing CP-47,497, CP-47,497-C8 and JWH-250 were prepared in duplicate at 20, 40, 80, 200, 400, 1,000 and 2,000 ng/g concentrations and used to construct a seven-point calibration curve. A single lot of quality control (QC) specimens containing CP-47,497, CP-47,497-C8 and JWH-250 were prepared at the start of the method validation and analyzed at the following levels: limit of quantification (LOQ) QC, target concentration of 20 ng/g; low QC (LQC), target concentration 60 ng/g; medium QC (MQC), target concentration 300 ng/g; and high QC (HQC), target concentration 1,600 ng/g. A dilution QC (DQC), target concentration 400 ng/g upon dilution (1 : 10), was included to ensure accurate quantification of authentic samples in instances where quantified specimen concentrations surpass the established calibration range, or when the sample volume is insufficient for testing. Calibrators and QC specimens each contained all three SCB analytes along with their respective deuterated ISTDs (final concentration 800 ng/g). A negative control (drug-free) containing ISTD and a double negative control (drug-free without ISTD) were also included in each analytical run. All QC lots and standards were stored at 4°C for the duration of the method validation.
SCB extraction procedure
CP-47,497, CP-47,497-C8 and JWH-250 were isolated using a LLE method for isolation of cannabinoids from blood as described by Foltz et al. (30) and previously adapted specifically for cannabinoid isolation from brain tissue (31). To each set of calibrators, QCs or treated mouse tissue, 10 µL of ISTD mix consisting of 2,500 ng/mL of each CP-47,497-d11, CP-47,497-C8-d7 and JWH-250-d5 was added and vortex-mixed thoroughly. Samples were then refrigerated overnight at 4°C to ensure equilibration. The next day, 2 mL of ‘ice-cold’ acetonitrile was added to homogenates in a drop-wise manner while samples were constantly vortex-mixed. Samples were then centrifuged for 10 min (2,500×g) to separate solid and liquid phases. Extracted samples were stored overnight at −20°C to allow for complete phase resolution. The following day, the organic layer was isolated using a glass pipet, transferred to a clean glass tube and evaporated to dryness in a Savant Speed Vac Concentrator (SPD1010, Thermo Scientific). Samples were reconstituted in 100 µL of 20/80 water/acetonitrile and transferred to auto-sampler vials. The vials were stored on the sample tray maintained at 4°C until analyzed.
HPLC/MS/MS conditions
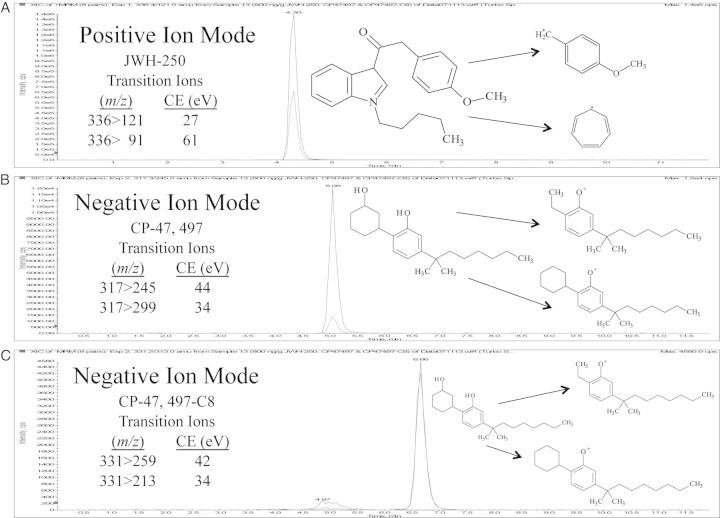
The HPLC/MS/MS system was an Applied Bio Systems 3,200 Q Trap with Turbo V source for TurbolonSpray coupled to a Shimadzu SCL HPLC system with data acquisition controlled by Analyst 1.4.2 software. Chromatographic separation was achieved with a Zorbax Eclipse XBD-C18 (4.6 × 75 mm, 3.5 µm) column (Agilent Technologies). The mobile phase consisted of water/acetonitrile (20:80 v/v) with 0.1 mM ammonium formate and had an isocratic flow rate of 0.5 mL/min. The ionspray voltage was set at 5,000 V with ion source gases 1 and 2 maintained at a flow rate of 60 mL/min. The source temperature was programmed at 650°C, with a curtain flow rate of 30 mL/min. Multiple reaction monitoring (MRM) acquisition mode was used. Transition ions monitored along with the corresponding deprotonation and collision energies (CE) utilized for each analyte are presented in Table I. The injection volume was 10 µL and the method had a total chromatographic run time of 12 min. Figure 2 shows the chromatographic method successfully resolved CP-47,497, CP-47,497-C8 and JWH-250.
Table I.
SCB Retention Time, MRM Transition Ions and Corresponding CE
| Analyte | RT (min) | Quant ion (m/z) | CE (eV) | Qual ion (m/z) | CE (eV) |
|---|---|---|---|---|---|
| Positive mode | |||||
| JWH-250 | 4.3 | 336 > 121 | 27 | 336 > 91 | 61 |
| JWH-250-d5 | 4.2 | 341 > 121 | 27 | 341 > 91 | 61 |
| Negative mode | |||||
| CP-47,497 | 5.1 | 317 > 245 | 44 | 317 > 299 | 34 |
| CP-47,497-d11 | 4.9 | 328 > 245 | 44 | 328 > 299 | 34 |
| CP-47,497-C8 | 6.7 | 331 > 259 | 42 | 331 > 313 | 34 |
| CP-47,497-C8-d7 | 6.3 | 338 > 266 | 42 | 338 > 320 | 34 |
Figure 2.
Chromatographic separation and fragmentation pattern for JWH-250 (A), CP-47,497 (B) and CP-47,497-C8 (C).
Quantitative assay performance
The presented HPLC/MS/MS method validation for the detection and quantification of SCBs in brain was based on recommendations of the Scientific Working Group for Forensic Toxicology (SWGTOX) Standard Practices for Method Validation in Forensic Toxicology (32). The assay was evaluated over 6 days with each run including calibrators prepared in duplicate, a negative control, a double negative control and batch prepared LOQ, LQC, MQC, HQC and DQC samples in replicates of 3 or 6.
Linearity and LOQ
A seven-point calibration curve was prepared in duplicate at 20, 40, 80, 200, 400, 800 and 2,000 ng/g in homogenized brain tissue for determination of SCB drug concentrations. The linear-regression calibration model was verified using the ratio of the peak area of the abundance quantification ion of SCBs to the peak area abundance quantification ion of each SCBs' deuterated ISTD versus the concentration. The lower limit of quantification (LOQ) and lower limit of detection (LOD) were administratively set at 20 ng/g. LOQ QCs were prepared for each run to verify that the LOQ was within ±20% of the target value and had a response at least five times greater than the signal to noise ratio of drug-free brain.
Accuracy/bias and precision
Accuracy/bias and precision (within- and between-run) were determined for the method by analysis of prepared QC samples over six separate analytical runs. The method bias was determined by calculating the ratio of the mean SCB concentration of six aliquots of each QC (LOQ, LQC, MQC, HQC and DQC) to the target concentration of the QC specimen, then multiplying by 100 to obtain a percentage. Data was considered acceptable when the accuracy/bias results were within ±20% of the QC's target value. Precision was assessed at five concentrations (LOQ, LQC, MQC, HQC and DQC) in triplicate over six analytical runs. Within-run and between-run precision were calculated after performing a one-way Analysis of Variance (ANOVA) using GraphPad Prism® statistical software (Version 5.0; LA Jolla, CA) with calculated values from the analysis reported.
Absolute recovery and matrix effects
Absolute recovery was determined at 60, 300 and 1,600 ng/g covering the linear range of the assay by comparing the mean SCB peak area of six aliquots where drug was added to homogenates prior to extraction (i.e., before, B) to six blank homogenate extracts to which drug was added to the extracted matrix (i.e., after, A). To calculate absolute recovery, the following equation was used: (mean area of B/mean area of A) × 100. For determination of matrix effects of LQC, MQC and HQC specimens, the mean SCB peak area of unextracted (i.e., neat, N) samples were compared with the SCB peak area of blank homogenate samples fortified with drug after extraction (A). Percent ion suppression/enhancement was determined by use of the following equation: [(mean area of N/mean area of A) − 1] × 100.
Stability studies
Since little is known regarding the stability of SCB compounds in biological samples, SCB stability experiments were conducted to assess benchtop stability, freeze/thaw stability, storage parameters and post-preparative stability using LQC (60 ng/g), MQC (300 ng/g) and HQC (1,600 ng/g) specimens. LOQ (20 ng/g) and DQC (400 ng/g) samples were also examined for post-preparative stability. For benchtop stability studies, the effects of routine specimen transportation and laboratory handling were investigated. Six aliquots of each LQC, MQC, and HQC specimens were left exposed at room temperature for 72 h, extracted as described herein, then analyzed. Brain tissue is commonly stored frozen prior to analysis so the consequences of freeze/thaw treatment were also evaluated. To account for reanalysis of frozen specimens, three freeze/thaw cycles were performed on six aliquots of each LQC, MQC and HQC. QC samples were frozen at −20°C, allowed to thaw at room temperature unassisted, and refrozen for a minimum of 24 h before the next thaw cycle. Upon the third thaw cycle, the QC specimens were extracted then analyzed using freshly prepared calibrators. Short-term storage was also assessed. Thirty days after QC batches were prepared for the validation study, six aliquots from each LQC, MQC, and HQC lots were extracted and analyzed using freshly prepared calibrators. Post-preparative stability of processed samples was examined after 72 h, to simulate situations where prepared samples cannot be immediately analyzed. LQC, MQC, and HQC samples were extracted normally and quantified using freshly prepared calibrators. These same QC extracts were kept at room temperature, reinjected after 72 h and quantified against the original calibration curve. SCB analytes were considered stable if the concentrations were within ±20% of the target concentrations.
Analyte and ISTD stock stability was assessed at LQC, MQC, and HQC concentrations after room temperature exposure over a 6 h period as previously recommended (33), with stability determined by comparing instrument response of the stocks kept at room temperature to freshly prepared analyte and ISTD stocks. Stock standards were considered stable if analyte or ISTD peak area was within ±20% of freshly prepared stock peak areas.
Results
For each analytical run, the calibrators analyzed in duplicate were back calculated and determined to be within ±15% of their nominal value. Additionally, linear-regression correlation coefficient (r2) means (n = 21) across runs were 0.9964 ± 0.003 (CP-47,497), 0.9963 ± 0.003 (CP-47,497-C8) and 0.9960 ± 0.003 (JWH-250), with ranges of 0.9984–0.9992, 0.9904–0.9992 and 0.9932–0.9996, respectively. It was verified that the LOD of each SCB demonstrated at least five times the signal-to-noise ratio of the response seen for both qualifier and quantifier ions in drug-free brain.
Accuracy/bias shown in Table II of the tested SCBs ranged at the LOQ (20 ng/g) from 90 to 98%, the LQC (60 ng/g) from 87 to 100%, the MQC (300 ng/g) from 93 to 101%, the HQC (1,600 ng/g) from 96 to 100% and the DQC (400 ng/g) from 86 to 97%. Values for the coefficient of variation (CV), expressed as a percentage, for LOQ, LQC, MQC, HQC and DQC were all within ±15% of the target concentration, with a range of 3–12%. Accuracy/bias determined over the linear range varied from a low of 86% exhibited by CP-47,497-C8 at the DQC target value of 400 ng/g to a high of 101% for CP-47,497 at the MQC target value of 300 ng/g.
Table II.
Accuracy/bias (n = 6) for JWH-250, CP-47,497 and CP-47,497-C8 in Mouse Brain; Values represent Mean ± SD (CV)
| LOQ | LQC | MQC | HQC | DQC | |
|---|---|---|---|---|---|
| Murine brain (ng/g) | (20 ng/g) | (60 ng/g) | (300 ng/g) | (1,600 ng/g) | (400 ng/g) |
| JWH-250 | 22 ± 1.2 (6) | 68 ± 2.3 (3) | 319 ± 35 (11) | 1,601 ± 150 (9) | 411 ± 22 (5) |
| CP-47,497 | 20 ± 0.7 (4) | 60 ± 3.6 (6) | 303 ± 26 (9) | 1,598 ± 161 (10) | 369 ± 40 (11) |
| CP-47,497-C8 | 19 ± 1.5 (8) | 56 ± 3.6 (6) | 278 ± 17 (6) | 1,536 ± 182 (12) | 344 ± 33 (10) |
LOQ, limit of quantitation; LQC, low quality control; MQC, medium quality control; HQC, high quality control; DQC, dilution quality control; CV, coefficient of variation.
Within-run and between-run precision is shown in Table III for LOQ, LQC, MQC, HQC and DQC samples. The within-run precision was acceptable with all CV values within ±15% of the expected concentration and a range of 4.8–9.1% CV for JWH-250, 7.7–13.4% CV for CP-47,497 and 6.3–11.2% CV for CP-47,497-C8. The between-day precision across each analyte at five QC levels was within ±15% of the expected CV, with a range of 5.2–15.3% across the SCB analytes tested.
Table III.
Within-run (n = 3) and Between-run (n = 21) Precision for JWH-250, CP-47,497 and CP-47,497-C8 in Mouse Brain
| Analyte | Concentration | Within-run CV (%) | Between-run CV (%) |
|---|---|---|---|
| JWH-250 | LOQ (20 ng/g) | 7.1 | 8.0 |
| LQC (60 ng/g) | 4.8 | 5.2 | |
| MQC (300 ng/g) | 5.2 | 11.9 | |
| HQC (1,600 ng/g) | 6.0 | 11.6 | |
| DQC (400 ng/g) | 9.1 | 10.1 | |
| CP-47,497 | LOQ (20 ng/g) | 11.2 | 9.9 |
| LQC (60 ng/g) | 7.9 | 7.9 | |
| MQC (300 ng/g) | 7.7 | 10.7 | |
| HQC (1,600 ng/g) | 8.4 | 12.2 | |
| DQC (400 ng/g) | 13.4 | 15.3 | |
| CP-47,497-C8 | LOQ (20 ng/g) | 11.2 | 12.5 |
| LQC (60 ng/g) | 7.5 | 8.9 | |
| MQC (300 ng/g) | 9.0 | 9.6 | |
| HQC (1,600 ng/g) | 6.3 | 10.5 | |
| DQC (400 ng/g) | 9.7 | 12.4 |
LOQ, limit of quantitation; LQC, low quality control; MQC, medium quality control; HQC, high quality control; DQC, dilution quality control; CV, coefficient of variation.
Absolute recovery shown in Table IV for the SCBs at the LQC ranged from 94 to 117%, at the MQC from 98 to 116%, and at the HQC from 115 to 118%. Recovery determined over the linear range varied from a low of 94% exhibited by CP-47,497-C8 at a target concentration of 60 ng/g (LQC) to a high of 118% for JWH-250 and CP47-497 both at target concentrations of 1,600 ng/g (HQC). Matrix effects shown in Table IV demonstrated significant ion suppression across the SCBs tested. Matrix effects for JWH-250 ranged from 35 to 45%, CP47,497 from 32 to 69% and CP47,497-C8 from 27 to 69%.
Table IV.
Recovery and Ion Suppression/Enhancement of SCBs (n = 6) and Their Respective Internal Standards (n = 18) in Brain
| Murine brain | Mean ± CV |
||
|---|---|---|---|
| % Recovery | Ion suppression | ||
| SCB | |||
| JWH-250 | LQC (60 ng/g) | 117 ± 20 | 35 ± 10 |
| MQC (300 ng/g) | 116 ± 18 | 43 ± 12 | |
| HQC (1,600 ng/g) | 118 ± 2 | 45 ± 8 | |
| CP-47,497 | LQC (60 ng/g) | 96 ± 16 | 48 ± 9 |
| MQC (300 ng/g) | 105 ± 10 | 32 ± 17 | |
| HQC (1,600 ng/g) | 118 ± 11 | 69 ± 28 | |
| CP-47,497-C8 | LQC (60 ng/g) | 94 ± 11 | 44 ± 20 |
| MQC (300 ng/g) | 98 ± 11 | 27 ± 18 | |
| HQC (1,600 ng/g) | 115 ± 5 | 69 ± 26 | |
| ISTD | |||
| JWH-250-d5 | 800 ng/g | 120 ± 19 | 42 ± 15 |
| CP-47,497-d11 | 800 ng/g | 102 ± 19 | 47 ± 18 |
| CP-47,497-C8-d7 | 800 ng/g | 93 ± 19 | 43 ± 26 |
LQC, low quality control; MQC, medium quality control; HQC, high quality control; ISTD, internal standard.
Selectivity was assessed using 10 separate lots of drug-free brain. Samples from each lot included a drug-free aliquot with and without ISTD, and three aliquots fortified with 60 ng/g of each SCB analyte. Each fortified tissue lot was run in triplicate and calculated CVs were within ± 15% for CP-47,497, CP-47,497-C8 and JWH-250. The selectivity bias range was 100–116% for CP47,497, 80–116% for CP47,497-C8 and 104–119% for JWH-250. Bias for all samples were ±20% of the target value with the exception of one tissue lot which demonstrated 130% bias for CP47,497.
Sample carryover was assessed using two different procedures. First, immediately following the injection of the 2,000 ng/g calibrator, a negative control was injected. No carryover was detected in the negative control. Second, an injection of the HQC (1,600 ng/g) was immediately followed by an injection of the LQC (60 ng/g) sample. This procedure was routinely applied each time HQC and LQC samples were analyzed. Lack of carryover was confirmed, as the SCB LQC concentration bias was <20% of the LQC's target concentration.
Data in Table V demonstrate that CP-47,497 is unstable after 72 h at room temperature, with the MQC (300 ng/g) and LQC (60 ng/g) samples exhibiting only 56 and 74% of their target values, respectively. The stability of CP-47,497, CP-47,497-C8 and JWH-250 was unaffected by freeze/thaw treatment, and was considered acceptable as all QC samples were within ±20% of the target concentration. JWH-250 and CP-47,497 QC samples were stable short-term (4°C), demonstrating values ±20% of the target concentration. However, CP-47,497-C8 fortified QCs exhibited significant analyte loss at all levels tested (Table V). All processed QC samples tested satisfied this criterion of ±20% of the target concentration as shown in Table V. Finally, both ISTD and drug stock solutions were stable at room temperature for up to 6 h, with all CV values within ±15%.
Table V.
Post-Preparative, Benchtop, Freeze/Thaw and Short-Term Stability of CP-47,497, CP-47,497-C8 and JWH-250 in Mouse Brain
| Analyte | Sample | Stability Parameter |
|||
|---|---|---|---|---|---|
| Post-preparative (72 h) | Benchtop (72 h) | Freeze/thaw (3X) | Month (4°C) | ||
| JWH-250 | LQC (60 ng/g) | 98 ± 6.7 (11) | 116 ± 1.9 (3) | 118 ± 1.0 (2) | 116 ± 6.5 (9) |
| MQC (300 ng/g) | 102 ± 41 (13) | 111 ± 25 (7) | 113 ± 18 (5) | 105 ± 16 (5) | |
| HQC (1,600 ng/g) | 80 ± 141 (11) | 107 ± 107 (6) | 113 ± 73 (4) | 91 ± 67 (5) | |
| DQC (400 ng/g) | 87 ± 31 (9) | ||||
| LOQ (20 ng/g) | 95 ± 2.3 (12) | ||||
| CP-47,497 | LQC (60 ng/g) | 111 ± 5 (7) | 85 ± 14 (27) | 107 ± 2.2 (3) | 105 ± 3.5 (6) |
| MQC (300 ng/g) | 117 ± 18 (5) | 56 ± 44 (26) | 103 ± 26 (8) | 92 ± 33 (12) | |
| HQC (1,600 ng/g) | 113 ± 134 (7) | 74 ± 101 (9) | 100 ± 140 (9) | 92 ± 137 (9) | |
| DQC (400 ng/g) | 102 ± 53 (13) | ||||
| LOQ (20 ng/g) | 100 ± 2.7 (13) | ||||
| CP-47,497-C8 | LQC (60 ng/g) | 102 ± 6 (9) | 84 ± 7.3 (15) | 98 ± 8.3 (14) | 50 ± 8.1 (27) |
| MQC (300 ng/g) | 105 ± 33 (11) | 97 ± 43 (12) | 98 ± 25 (8) | 58 ± 18 (10) | |
| HQC (1,600 ng/g) | 98 ± 167 (11) | 91 ± 98 (7) | 102 ± 150 (9) | 68 ± 115 (11) | |
| DQC (400 ng/g) | 95 ± 43 (11) | ||||
| LOQ (20 ng/g) | 97 ± 2.2 (11) | ||||
Results are expressed as the % target value ± SD (%CV); n = 6.
LOQ, limit of quantitation; LQC, low quality control; MQC, medium quality control; HQC, high quality control; DQC, dilution quality control; CV, coefficient of variation.
Application of the method to animal samples
Table VI shows locomotor activity (5–15 min post-injection), catalepsy, antinociception and temperature data (each taken at 30 min post-injection) after a 30 mg/kg challenge of CP-47,497; with corresponding brain concentrations (60 min post-injection) determined. Drug-treated mice exhibited significant (P < 0.05) decreases in locomotor activity, expressed as a number of beam breaks, compared with vehicle-treated mice. Catalepsy and antinociception (%MPE) were not detected after vehicle treatment, whereas CP-47,497-treated mice displayed statistically significant catalepsy and antinociception (P < 0.0001 and P < 0.05, respectively). Pre-treatment (i.e., baseline) means ± SEM for body temperature (°C) were 37.0 ± 0.3 for vehicle-treated and 37.5 ± 0.3 for CP-47,497 treated mice, demonstrating no significant differences in baseline measurements between vehicle- or drug-treated mice. CP-47,497-treated mice showed significant (P < 0.001) hypothermia (i.e., a 6.9 ± 0.6°C drop in temperature) compared with vehicle-treated (i.e., a 1.5 ± 0.4°C increase in temperature) mice. Table VI illustrates the cannabimimetic behavioral effects observed from the same group of mice with corresponding brain concentrations of CP-47,497 (Table VII) ranging from 1,660 ng/g to 1,890 ng/g (mean 1,804 ± 93 ng/g), with no analyte peaks detected in brains from vehicle-treated mice.
Table VI.
Significant Cannabimimetic Effects in Mice After CP-47,497 (30 mg/kg) Treatment in Measures of Spontaneous Activity, Catalepsy, Antinociception and Body Temperature
| Treatment | Spontaneous activity (beam breaks), mean ± SEM | Catalepsy (s), mean ± SEM | Antinociception (MPE), mean ± SEM | Body temperature (°C), mean ± SEM |
|---|---|---|---|---|
| Vehicle (1 : 1 : 18) | 601 ± 78 | ND | ND | 1.3 ± 0.3 |
| CP-47,497 (30 mg/kg) | 194 ± 120 | 50.7 ± 2.2 | 51 ± 14 | −6.9 ± 0.3 |
| P-value | <0.05 | <0.0001 | <0.05 | <0.0001 |
MPE, maximum percentage effect (10 s cut-off); ND, none detected.
Values represent the mean (±SEM); n = 4–5 mice per group.
Pre-treatment (i.e., baseline) means ± SEM for temperature (°C) = 37.4 ± 0.2 for vehicle-treated and 37.9 ± 0.2 for CP-47,497-treated mice.
Baseline tail withdrawal latencies (s) for vehicle-treated mice were 1.5 ± 0.4 and 1.6 ± 0.3 for CP-47,497-treated mice.
Table VII.
Quantification of CP-47,497 (ng/g) in Mouse Brain 1 h After Vehicle or CP-47,497 (30 mg/kg) Injection (i.p.)
| Animal | Treatment |
|
|---|---|---|
| Vehicle | CP-47,497 | |
| 1 | ND | 1,890 |
| 2 | ND | 1,660 |
| 3 | ND | 1,840 |
| 4 | ND | 1,780 |
| 5 | ND | 1,850 |
ND, none detected; i.p., intraperitoneal.
Discussion
We have previously quantified JWH-018 in blood of mice exposed to SCBs in ‘Buzz’ smoke (23, 34). Using the same LLE and HPLC/MS/MS parameters as the presented method, prior experiments demonstrated that this analytical approach is capable of concurrently detecting THC, its carboxylic acid metabolite, JWH-073, JWH-250, JWH-398, CP-47,497 and HU-210 in whole blood (34). Dresen et al. (35) reported an HPLC/MS/MS method for detection of aminoalkylindole compounds in human serum using an LLE, with sensitivity ranging from 0.1 to 2.0 ng/mL. They concluded that cyclohexylphenyl-containing cannabimimetics should be analyzed by gas chromatography–mass spectrometry methods, as both electrospray ionization and chemical ionization showed poor responses during method optimization. While our method used a less sensitive linear range, it was shown to be suitable for the objective of this study. Additionally, Kacinko et al. (36) published an HPLC/MS/MS method validation for quantification of JWH-018, JWH-073, JWH-019, but with only one transition ion found for JWH-250, they reported that this method was only appropriate for qualitative identification of this analyte. An HPLC/MS method was determined for the detection of 25 SCB compounds, including CP-47,497, CP-47,497-C8 and JWH-250 in blood which required only a 100-µL sample volume (37). Similar to results presented in this study, significant matrix effects were seen with both CP-47,497 (82–89%) and CP-47,497-C8 (73–84%) in blood. Matrix-effect experiments, summarized in Table IV, demonstrate that there were significant effects of ion suppression produced in brain specimens tested.
We have previously reported the phenomenon of ion suppression in brain (31) without adverse effects on the method validation, but a literature search provided no other data for ion suppression/enhancement effects of brain in HPLC systems. Data obtained from accuracy/bias studies, shown in Table II, along with assessment of SCB selectivity using 10 separate tissue lots (fortified with 60 ng/g of each analyte), for a total sample size of 30, provide evidence that the observed ion suppression does not affect other validation parameters. No peaks were detected that co-eluted with the analyte or ISTD peaks of interest in any of the 10 lots of brain, which demonstrates a lack of interference from endogenous components and solidifies selectivity of the assay. Importantly, the same pattern and magnitude of ion suppression were observed for each of the deuterated ISTDs (Table IV). Taken together, the ion suppression produced by endogenous brain components does not appear to affect the overall method validation.
Adverse effects of SCBs in humans are communicated primarily as case reports. Thus, there is a gap in knowledge pertaining to SCB disposition and related toxicities. Mice were used for the present validation study in order to procure biological tissue and assess the pharmacological effects due to exposure of the SCB, CP-47,497. Following a 30 mg/kg injection of CP-47,497, mice displayed cannabimimetic behavioral effects, consistent with CB1 receptor activation (38), including a decrease in spontaneous locomotor activity with the presentation of catalepsy, antinociception and hypothermia as previously reported in our laboratory (23) and in other laboratories (39). These cannabimimetic behavioral results are in agreement with previously published reports of quantified SCB concentrations in brain after inhalation exposure (23–25, 34).
Conclusion
The HPLC/MS/MS method validation presented herein is a straightforward, selective and accurate method for the determination of three SCB drugs belonging to the CP and aminoalkylindole structural classes. The assay required a simple LLE procedure before HPLC/MS/MS analysis. Importantly, this is the first full method validation of CP-47,497, CP-47,497-C8 and JWH-250 SCB compounds in tissue. This study has important implications for preclinical research settings where it is pertinent to demonstrate that SCBs are present in the brain, bolstering support that cannabimimetic effects result from a CNS-mediated mechanism of action. Additionally, providing a method to detect and quantify SCBs in brain may assist in further understanding the complex interplay between behavioral responses and SCB drugs, which is of particular interest in post-mortem or impaired driving cases where SCB use is suspected (40). The development and validation of analytical methods for the detection and quantification of SCBs in complex biological matrices are critical for future investigation of the pharmacokinetic, pharmacodynamic and toxicologic profiles of SCBs. Information garnered from animal studies will provide insight and lay the foundation necessary for approval of controlled human SCB studies, both which will provide invaluable knowledge for forensic toxicologists and law enforcement.
Funding
The research included in this publication was supported by the National Institute of Drug Abuse (NIDA) of the National Institutes of Health (NIH) under award number(s): T32DA007027 (WLD), P30DA033934 (WLD), P01DA009789 (AHL) and F31DA033183 (KLS).
References
- 1.Department of justice schedules of controlled substances: temporary placement of three synthetic cannabinoids into Schedule I. Federal Register. 2013;78:17–21. [PubMed] [Google Scholar]
- 2.Choi H., Heo S., Choe S., Yang W., Park Y., Kim E., et al. Simultaneous analysis of synthetic cannabinoids in the materials seized during drug trafficking using GC-MS. Analytical and Bioanalytical Chemistry. 2013;405:3937–3944. doi: 10.1007/s00216-012-6560-z. [DOI] [PubMed] [Google Scholar]
- 3.Vardakou I., Pistos C., Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicology Letters. 2010;197:157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Auwärter V., Dresen S., Weinmann W., Müller M., Pütz M., Ferreirós N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? Journal of Mass Spectrometry. 2009;44:832–837. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 5.Teske J., Weller J.-P., Fieguth A., Rothämel T., Schulz Y., Tröger H.D. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography–tandem mass spectrometry. Journal of Chromatography B. 2010;878:2659–2663. doi: 10.1016/j.jchromb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Weissman A., Milne G.M., Melvin L.S. Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol. The Journal of Pharmacology and Experimental Therapeutics. 1982;223:516–523. [PubMed] [Google Scholar]
- 7.Haubrich D.R., Ward S.J., Baizman E., Bell M.R., Bradford J., Ferrari R., et al. Pharmacology of Pravadoline: a new analgesic agent. The Journal of Pharmacology and Experimental Therapeutics. 1990;255:511–522. [PubMed] [Google Scholar]
- 8.Compton D.R., Gold L.H., Ward S.J., Balster R.L., Martin B.R. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. The Journal of Pharmacology and Experimental Therapeutics. 1992;263:1118–1126. [PubMed] [Google Scholar]
- 9.Huffman J.W., Szklennik P.V., Almond A., Bushell K., Selley D.E., He H., et al. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorganic & Medicinal Chemistry Letters. 2005;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 10.The drug enforcement administration CP-47,497 seized in Wisconsin. Microgram Bulletin. 2009;42:89–102. [Google Scholar]
- 11.UNDOC. World Drug Report. 2013;3:11–14. [Google Scholar]
- 12.Zimmermann U.S., Winkelmann P.R., Pilhatsch M., Nees J.A., Spanagel R., Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of ‘spice gold. Deutsches Arzteblatt International. 2009;106:464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nacca N., Vatti D., Sullivan R., Sud P., Su M., Marraffa J. The synthetic cannabinoid withdrawal syndrome. Journal of Addictive Medicine. 2013;7:296–298. doi: 10.1097/ADM.0b013e31828e1881. [DOI] [PubMed] [Google Scholar]
- 14.Lindigkeit R., Boehme A., Eiserloh I., Luebbecke M., Wiggermann M., Ernst L., et al. Spice: a never ending story? Forensic Science International. 2009;191:58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Hudson S., Ramsey J., King L., Timbers S., Maynard S., Dargan P.I., et al. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in ‘herbal high’ products. Journal of Analytical Toxicology. 2010;34:252–260. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- 16.Uchiyama N., Kikura-Hanajiri R., Kawahara N., Haishima Y., Goda Y. Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chemical and Pharmaceutical Bulletin. 2009;57:439–441. doi: 10.1248/cpb.57.439. [DOI] [PubMed] [Google Scholar]
- 17.Uchiyama N., Kikura-Hanajiri R., Ogata J., Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Science International. 2010;198:31–38. doi: 10.1016/j.forsciint.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Hermanns-Clausen M., Kneisel S., Szabo B., Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 19.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) Thematic paper- Understanding the ‘Spice’ phenomenon. 2009:1–34. http://www.emcdda.europa.eu/publications/thematic-papers/spice . [Google Scholar]
- 20.Huffman J.W., Thompson A.L.S., Wiley J.L., Martin B.R. Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorganic Medicinal Chemistry. 2008;16:322–335. doi: 10.1016/j.bmc.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atwood B.K., Lee D., Straiker A., Widlanski T.S., Mackie K. CP47,497-C8 and JWH073, commonly found in `Spice’ herbal blends, are potent and efficacious CB1 cannabinoid receptor agonists. European Journal of Pharmacology. 2011;659:139–145. doi: 10.1016/j.ejphar.2011.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melvin L.S., Milne G.M., Johnson M.R., Subramaniam B., Wilken G.H., Howlett A.C. Structure–activity relationships for cannabinoid receptor-binding and analgesic activity: studies of bicyclic cannabinoid analogs. Molecular Pharmacology. 1993;44:1006–1015. [PubMed] [Google Scholar]
- 23.Wiebelhaus J.M., Poklis J.L., Poklis A., Vann R.E., Lichtman A.H., Wise L.E. Inhalation exposure to smoke from synthetic ‘marijuana’ produces potent cannabimimetic effects in mice. Drug Alcohol Dependence. 2012;126:316–323. doi: 10.1016/j.drugalcdep.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poklis J.L., Amira D., Wise L.E., Wiebelhaus J.M., Haggerty B.J., Poklis A. Detection and disposition of JWH-018 and JWH-073 in mice after exposure to ‘Magic Gold’ smoke. Forensic Science International. 2012;220:91–96. doi: 10.1016/j.forsciint.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poklis J.L., Amira D., Wise L.E., Wiebelhaus J.M., Haggerty B.J., Lichtlman A.H., et al. Determination of naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in mouse blood and tissue after inhalation exposure to ‘buzz’ smoke by HPLC/MS/MS. Biomedicinal Chromatography: BMC. 2012;26:1393–1398. doi: 10.1002/bmc.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mura P., Kintz P., Dumestre V., Raul S., Hauet T. THC can be detected in brain while absent in blood. Journal of Analytical Toxicology. 2005;29:842–843. doi: 10.1093/jat/29.8.842. [DOI] [PubMed] [Google Scholar]
- 27.Samano K.S., Ignatowska-Jankowska B.M., Lichtman A.H., Poklis A. Preclinical Investigation of CP47,497: a widely abused synthetic cannabinoid. 2013 Abstract #K24, American Academy of Forensic Science 65th Annual Meeting, February 18-23, Washington, D.C. [Google Scholar]
- 28.Wiley J. Cannabinoid pharmacological properties common to other centrally acting drugs. European Journal of Pharmacology. 2003;471:185–193. doi: 10.1016/s0014-2999(03)01856-9. [DOI] [PubMed] [Google Scholar]
- 29.Martin B.R., Compton D.R., Thomas B.F., Prescott W.R., Little P.J., Razdan R.K., et al. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacology, Biochemistry and Behavior. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- 30.Foltz R.L., McGinnis K.M., Chinn D.M. Quantitative measurement of delta 9-tetrahydrocannabinol and two major metabolites in physiological specimens using capillary column gas chromatography negative ion chemical ionization mass spectrometry. Biomedical Mass Spectrometry. 1983;10:316–323. doi: 10.1002/bms.1200100503. [DOI] [PubMed] [Google Scholar]
- 31.Poklis J.L., Thompson C.C., Long K.A., Lichtman A.H., Poklis A. Disposition of cannabichromene, cannabidiol, and Δ9-tetrahydrocannabinol and its metabolites in mouse brain following marijuana inhalation determined by high-performance liquid chromatography–tandem mass spectrometry. Journal of Analytical Toxicology. 2010;34:516–520. doi: 10.1093/jat/34.8.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology report. Journal of Analytical Toxicology. 2013;37:452–474. doi: 10.1093/jat/bkt054. [DOI] [PubMed] [Google Scholar]
- 33.US Department of Health and Human Services. Bioanalytical method validation: a guidance for industry. US Department of Health and Human Services; 2001. pp. 7–8. [Google Scholar]
- 34.Haggerty B.J., Poklis J.L., Wolf C.E., Wise L.E., Wiebelhaus J.M., Lichtman A.H., Poklis A. Detection of cannabimimetic compounds in mice blood after exposure to Buzz smoke. 2010 Abstract. Society of Forensic Toxicologists 40th Annual Meeting, October 19-22, Richmond, VA. [Google Scholar]
- 35.Dresen S., Kneisel S., Weinmann W., Zimmermann R., Auwärter V. Development and validation of a liquid chromatography–tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. Journal of Mass Spectrometry. 2011;46:163–171. doi: 10.1002/jms.1877. [DOI] [PubMed] [Google Scholar]
- 36.Kacinko S.L., Xu A., Homan J.W., McMullin M.M., Warrington D.M., Logan B.K. Development and validation of a liquid chromatography—tandem mass spectrometry method for the Identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. Journal of Analytical Toxicology. 2011;35:386–393. doi: 10.1093/anatox/35.7.386. [DOI] [PubMed] [Google Scholar]
- 37.Ammann J., McLaren J.M., Gerostamoulos D., Beyer J. Detection and quantification of new designer drugs in human blood: part 1—synthetic cannabinoids. Journal of Analytical Toxicology. 2012;36:372–380. doi: 10.1093/jat/bks048. [DOI] [PubMed] [Google Scholar]
- 38.Compton D.R., Rice K.C., De Costa B.R., Razdan R.K., Melvin L.S., Johnson M.R., et al. Cannabinoid structure–activity relationships: correlation of receptor binding and in vivo activities. 1993;265:218–226. [PubMed] [Google Scholar]
- 39.Wiley J.L., Marusich J.a., Martin B.R., Huffman J.W. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Dependence. 2012;123:148–153. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeakel J.K., Logan B.K. Blood synthetic cannabinoid concentrations in cases of suspected impaired driving. Journal of Analytical Toxicology. 2013;37:547–551. doi: 10.1093/jat/bkt065. [DOI] [PubMed] [Google Scholar]