Abstract
Objectives This systematic review and meta-analysis examined the effects of psychological therapies for management of chronic pain in children. Methods Randomized controlled trials of psychological interventions treating children (<18 years) with chronic pain conditions including headache, abdominal, musculoskeletal, or neuropathic pain were searched for. Pain symptoms, disability, depression, anxiety, and sleep outcomes were extracted. Risk of bias was assessed and quality of the evidence was rated using GRADE. Results 35 included studies revealed that across all chronic pain conditions, psychological interventions reduced pain symptoms and disability posttreatment. Individual pain conditions were analyzed separately. Sleep outcomes were not reported in any trials. Optimal dose of treatment was explored. For headache pain, higher treatment dose led to greater reductions in pain. No effect of dosage was found for other chronic pain conditions. Conclusions Evidence for psychological therapies treating chronic pain is promising. Recommendations for clinical practice and research are presented.
Keywords: adolescents, children, chronic pain, psychological interventions
Introduction
Chronic or recurrent pain (lasting longer than 3 months) is a common complaint of childhood (Perquin et al., 2000), although in most cases, it is self-limiting and clinically uncomplicated. However, a minority of children and adolescents report pain that interferes with their daily lives (Huguet & Miro, 2008), is associated with functional disability and distress, and has wider impact on social and physical functioning, family life, and parenting (Hunfeld et al., 2002; Jordan, Eccleston, & Osborn, 2007; Logan, Simons, Stein, & Chastain, 2008; Walker, Guite, Duke, Barnard, & Greene, 1998). Many of these children present health care facilities seeking both pain relief and help with disability, depression, anxiety, and social functioning.
Chronic pain problems can arise from many physical health conditions or emerge idiopathically. The most common pain conditions in children are headache, abdominal pain, back pain, complex regional pain syndrome Type 1, pain from disease such as rheumatoid arthritis, and nonspecific complaints such as “growing pain.” Also common are widespread musculoskeletal pain complaints, including fibromyalgia syndrome (FMS) (King et al., 2011). Although pain is commonly defined as chronic after 3 months, patients presenting to specialized pain centers typically have had pain for much longer (Eccleston, Malleson, Clinch, Connell, & Sourbut, 2003; Logan et al., 2012).
Psychological treatments, principally but not exclusively cognitive and behavioral treatments, have been developed with a focus on the self-management of pain and disability (Palermo, 2012). Psychological treatments are well established in the treatment of adult chronic pain (Williams, Eccleston, & Morley, 2012). Typically, cognitive-behavioral treatments focus on the client/patient being actively involved in treatment, which often consists of behavioral strategies for engagement with normal daily activities, an increased awareness and challenge of the role of cognition in exacerbating suffering, a focus on the self-regulation of emotion, and the use of techniques for reducing aversive arousal (e.g., relaxation). This is all delivered within a psychoeducational frame. Treatments that focus specifically on pediatric chronic pain may provide skills training both to children and their parents (Eccleston, Palermo, Fisher, & Law, 2012b). Typically, when parents are included in treatment, the focus of treatment strategies is on operant skills training.
There have been periodic summaries of the evidence base for psychological therapies in children with chronic pain, and this review follows in that tradition. In the previous 1999 Journal of Pediatric Psychology special issue, three reviews focused explicitly on headache (Holden, Deichmann, & Levy, 1999), recurrent abdominal pain (RAP) (Janicke & Finney, 1999), and on disease related pain (including studies with FMS) (Walco, Sterling, Conte, & Engel, 1999). Interestingly, systematic reviews have also been reported outside the psychological press, often with psychological treatments being included in a compound review of treatments for a single condition such as abdominal pain (Weydert, Ball, & Davis, 2003). All of these reviews return either positive or promising conclusions for the effectiveness of psychological treatments, even when data are sparse. However, these earlier reviews were undertaken without any methodological concern for bias and included trials conducted before consensus statements on methodological quality were reported (McGrath et al., 2008). Modern systematic review practices are incorporated in Cochrane Systematic Reviews, which have focused on RAP within the context of irritable bowel syndrome (Huertas-Ceballos, Logan, Bennett, & Macarthur, 2014), and chronic pain in children (Eccleston et al., 2012c). The latter review reports data from a broad range of trials that investigated the effectiveness of psychological therapies in both headache and nonheadache populations. This Cochrane Review was first published in 2003, substantially updated in 2009, and most recently updated in 2012 (Eccleston et al., 2012c). Palermo, Eccleston, Lewandowski, Williams, and Morley (2010) extended the 2009 version of this review to include studies on Internet-delivered treatments. The analyses of 37 included studies showed that psychological therapies were effective in improving pain symptoms for both headache and nonheadache pain conditions posttreatment compared with control conditions, and at follow-up for the headache group. Disability also significantly improved in the nonheadache group posttreatment. Finally, mood (including depression and anxiety) significantly improved in the headache group at follow-up.
In this systematic review, we take the Cochrane Review last updated in 2012 as our starting point. We enhance and extend former reviews in three specific ways:
First, we separate trials into discrete clinical conditions offering evidence summaries of the quantitative effects of psychological treatment in the areas of headache (including migraine), abdominal pain, neuropathic pain (including complex regional pain syndrome Type 1), and musculoskeletal pain (including FMS).
Second, we expand the outcome assessment in several ways. We separate the compound category of “mood” into two separate outcomes: depression and anxiety (including catastrophizing), as they are distinct concepts that may have differential effects from psychological treatments for chronic pain. Furthermore, rumination about possible extreme negative outcomes due to pain, known variously as catastrophizing, awfulizing, or worry, has been identified as a key anxious cognitive construct in adapting to pain (Eccleston, Fisher, Vervoort, & Crombez, 2012a) and has been identified as a possible therapeutic mechanism that is influential to the effectiveness of cognitive-behavioral therapy (CBT) (Burns, Day, & Thorn, 2012). This addition of an anxiety outcome matches that undertaken in the recent Cochrane Review for psychological interventions with adults suffering chronic pain (Williams et al., 2012). We also include sleep as an outcome, for the first time. There is evidence that sleep disturbances are related to mood disturbances and increased functional disability outcomes of chronic pain in children (Palermo & Kiska, 2004), and that insufficient sleep can negatively impact pain management (Lewin & Dahl, 1999).
Third, we examine the relationship of treatment dose (hours of delivered treatment) on effect sizes related to treatment outcomes to understand the optimal dose of therapy for children with chronic pain.
The aims of the review were as follows:
To quantify the effects of psychological interventions and any adverse outcomes from psychological interventions for the management of chronic pain (including all conditions) in children and adolescents.
To summarize the evidence for the efficacy of psychological interventions for four chronic pain conditions: headache, abdominal pain, neuropathic pain, and musculoskeletal pain. Evidence is reported according to each condition, in five outcome domains (pain, functional disability, depression, anxiety, and sleep). Where possible, outcomes are explored both immediately following treatment and at longer-term follow-up (3–12 months).
To examine the relationship of treatment dose (i.e., total minutes of exposure to treatment) on effect sizes related to treatment outcomes to explore the optimal treatment dose for the reduction of pain symptoms for headache and chronic pain (excluding headache) conditions.
Methods
Study Design
Randomized controlled trials (RCTs) of studies comparing the experimental treatment with treatment as usual, or an active, a placebo, or a waiting-list control, were included. Each study needed a minimum of 10 participants in each arm of the trial immediately posttreatment to meet the inclusion criteria, which follows criteria suggested in other pain intervention reviews (Eccleston et al., 2012c; Williams et al., 2012).
Participants
Participants were children and adolescents of ≤18 years of age who report chronic or recurrent pain (>3 months) associated with idiopathic pain conditions, including headache, abdominal pain, neuropathic pain, and musculoskeletal pain.
Interventions
All credible psychological interventions were included. Credible interventions were judged as having recognizable components of psychological therapy (e.g., coping skills, recognizing stress and negative emotions), with content related to theory of behavior change. Typically, interventions are likely to be behavioral (including relaxation training, behavioral scheduling, biofeedback), cognitive-behavioral (including problem-solving, cognitive training, attentional retraining), or from any other tradition of psychotherapy, including psychodynamic psychotherapy, psychoanalysis, or behavioral analysis. Interventions could be individual, group, or class, and could be delivered through a variety of media, including computer-based and Internet delivery. Family therapy or interventions including parents were eligible for inclusion provided the aim of the treatment was to improve child outcomes. Multimodal or complex treatments were included if the psychological component was both primary and >50% of treatment.
Outcomes
Five domains of outcome were extracted: pain, disability, depression, anxiety (including catastrophic thinking about pain), and sleep. All instruments deployed in individual studies were assessed. Where duplicate, overlapping, or multiple tools were used within any one domain, the most appropriate was selected on the basis of its content validity (to reduce heterogeneity of content across trials and within a domain) and the frequency of its use in the field (to improve comparability across trials). Adverse events (e.g., worsening of mental health of clinical concern, hospitalizations, pain flares) were also extracted.
Study Identification and Search
A three-stage search strategy was used. First, all studies from the review of Eccleston et al. (2012c) were identified. Second, a search strategy optimized for each particular online abstracting service (Medline, Embase, and PsycINFO; see Supplementary Appendix A) was created and run. Third, citation searches and reference lists of recovered trials and reviews were searched.
Data Extraction
Two forms of data were extracted. First, information regarding the design of the study, including the child pain condition, sample demographics, setting, outcomes, and characteristics of the treatment and comparison intervention, including its label, content, and dose (total exposure to treatment), was extracted. Second, raw outcome data [e.g., N, means, standard deviations (SD)] from each study were extracted for meta-analysis at each relevant time point (immediately posttreatment and follow-up) for each planned comparison. Follow-up periods were defined as 3–12 months posttreatment. If two or more follow-up periods were included in this period, the longer of the two was extracted and analyzed. Study authors were contacted when data were incomplete. Two authors extracted data and any discrepancies were arbitrated by a third author.
Assessment of Risk of Bias in Included Studies
The Cochrane Collaboration Risk of Bias Tool was used to assess the risk of bias in included studies (Higgins & Green, 2011). There are four categories of bias included in this tool: selection bias, detection bias, attrition bias, and reporting bias. For selection bias, which refers to the introduction of differences at baseline between groups, random sequence generation and allocation concealment were judged. Detection bias refers to differences in how outcomes are measured between groups. Blinding of outcome assessors was judged for detection bias. Attrition bias refers to different rates of withdrawals between groups. This was judged according to incomplete outcome data. Reporting bias describes the systematic differences between reported and unreported outcomes (Higgins & Green, 2011). Reporting bias was judged according to selective reporting. Blinding of participants and personnel was excluded from the risk of bias assessment for this review. It is rarely possible, sensible, or ethical to blind participants and personnel from receiving or delivering a psychological intervention and is therefore redundant. Two review authors reviewed all papers for risk of bias; disagreements were arbitrated by a third author.
Quality of Evidence
Quality of evidence was assessed using the GRADE criteria (Guyatt et al., 2011a). Only the seven most important outcomes are presented in each summary of findings table (Guyatt et al., 2013). The seven most important outcomes were judged to be the outcomes that included the highest number of participants. Pain outcomes posttreatment and at follow-up were included in all GRADE tables. This decision was made post hoc to provide the outcomes that provided the highest possible level of certainty. For each outcome (e.g., pain posttreatment, pain at follow-up, disability posttreatment, etc.), studies included in the analysis are assessed on five categories: limitations in the design and implementation, indirectness, inconsistency, imprecision, and publication bias. Limitations in the design and implementation were assessed by the risk of bias for studies included in each outcome (Guyatt et al., 2011f; Higgins & Green, 2011). Outcomes are downgraded if they include studies that have a high number of unclear or a high risk of bias ratings. Indirectness describes the population, intervention, comparator, or outcomes that are not of interest (Guyatt et al., 2011d). For example, outcomes are downgraded in quality when most included studies use a wait-list control rather than an active control. Inconsistency assesses heterogeneity of the studies included in a given outcome (Guyatt et al., 2011c). Studies that report heterogeneity >45% are downgraded. Imprecision refers to small sample sizes and wide confidence intervals (CI; Guyatt et al., 2011b). Outcomes are downgraded when there is a small number of participants producing an outcome, lowering the confidence of the estimate of effect and contributing to high CIs. Finally, publication bias assesses failure to report studies or outcomes (Guyatt et al., 2011e). Outcomes are downgraded if studies fail to publish null findings or outcomes. This provides an overall rating for each outcome, which ranges from “high” to “very low.” High ratings are given when “further research is very unlikely to change the confidence in the estimate of effect.” Moderate quality is given when “further research is likely to have an important impact on the confidence in the estimate of effect and may change the estimate.” Low ratings are given when “further research is very likely to change our estimate of effect.” Very low quality is given when “we are very uncertain about the estimate” (Balshem et al., 2011).
Results
Results of Search
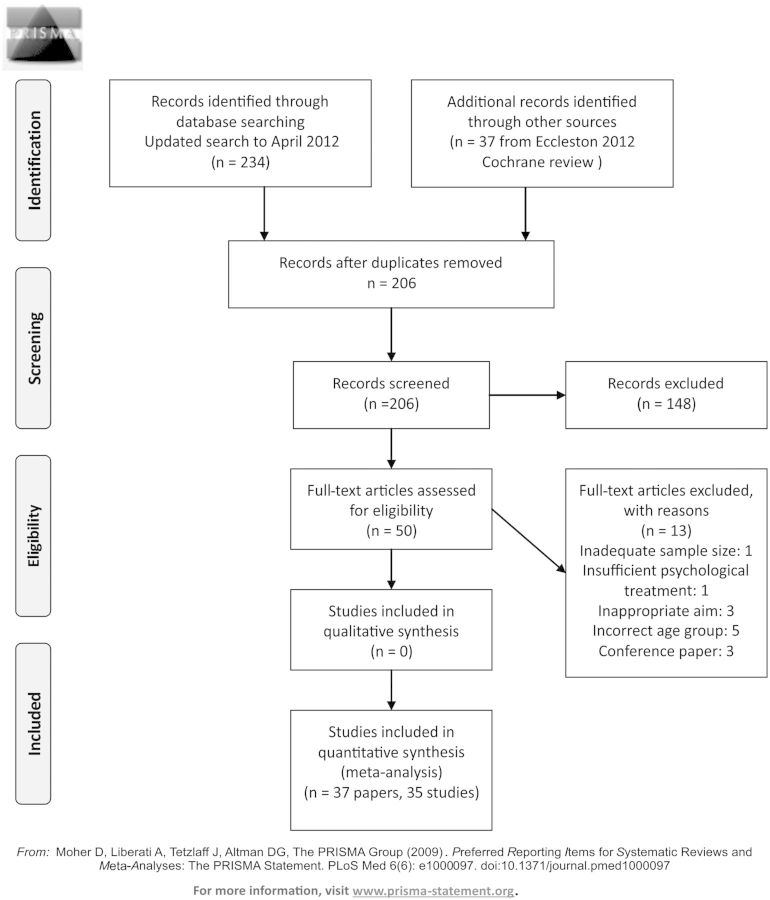
The search of Medline, Embase, and PsycINFO was twofold. First, databases were searched from inception to March 2012 (for a more detailed search report, see Eccleston et al., 2012c). Second, databases were searched from March 2012 to April 2013 for the purposes of this systematic review. Thirty-seven studies (39 papers) were identified in the first search, and outcomes of pain, disability, and mood (anxiety and depression combined) are reported in Eccleston et al. (2012c). For the purposes of this review, 50 papers were read in full, 13 were excluded, and 37 included (35 studies; Figure 1). There were no additional RCTs identified in the updated search. However, two papers identified in the second search (Kashikar-Zuck et al., 2013; Levy et al., 2013) containing additional information from previously published trials (Kashikar-Zuck et al., 2012; Levy et al., 2010) were included in this review. Two studies of sickle cell pain that were included in the Eccleston et al., (2012c) review were excluded for the purposes of this review because they did not meet the inclusion criteria.
Figure 1.
PRISMA 2009 flow diagram.
Included Study Characteristics
Study characteristics have previously been reported in Eccleston et al. (2012c). Thirty-five studies (37 papers) were identified in the search (Table I). The total number of participants completing trials was 1,005 (M = 29/trial). More girls (66%) entered trials than boys. The average age of participants entering trials was 9.40 years (SD = 1.14 years). The mean duration of pain reported in studies (n = 17) was 3.39 years. Of the 35 included studies, 21 treated children and adolescents diagnosed with headache, eight treated abdominal pain, and three treated musculoskeletal pain. There were three studies that included children with multiple pain conditions: Hicks, von Baeyer, and McGrath (2006) treated children and adolescents with RAP and headache; Palermo, Wilson, Peters, Lewandowski, and Somhegyi (2009) treated children and adolescents with headache, abdominal pain, and musculoskeletal pain; and Wicksell, Melin, Lekander, and Olsson (2009) treated children and adolescents with headache, musculoskeletal pain, visceral pain, and complex regional pain syndrome. Each study was included in analyses of individual pain conditions where children with that condition were included in the sample. Characteristics of included studies such as design of the study, demographics of children, characteristics of treatment and control groups, and outcomes measured are reported in Supplementary Appendix B.
Table I.
Characteristics of Included Studies
| Study identifier, year | Pretreatment N | Treatment arm(s) | Control arm(s) | Treatment dose (hours, minutes) |
|---|---|---|---|---|
| Headache | ||||
| Abram et al., 2007 | 50 | Headache Clinical Model: behavioural intervention | Headache Traditional Model: consultation with neurologist | 1 hr 30 mins |
| Barry and von Baeyer, 1997 | 29 | CBT | Waiting list control | 3 hrs |
| Bussone et al., 1998 | 35 | Biofeedback (assisted relaxation) | Relaxation | 7 hrs |
| Connelly et al., 2006 | 36 | CBT CD-ROM | Waiting list control | 4 hrs |
| Fichtel and Larsson, 2001 | 36 | Relaxation | Waiting list control | 7 hrs 30 mins |
| Griffiths and Martin, 1996 | 42 | CBT (clinic based) + (home based)* | Self-monitoring | 12 hrs |
| Hicks et al., 2006 | 42 | Internet CBT (with internet and phone) | Standard medical care | Unknown |
| Kroener-Herwig and Denecke, 2002 | 75 | CBT group/Self-help* | Waiting list control | 12 hrs |
| Labbe and Williamson, 1984 | 28 | Autogenic feedback training | Waiting list control | 6 hrs 40 mins |
| Labbe, 1995 | 46 | Skin temperature biofeedback + autogenic relaxation/Autogenic relaxation* | Waiting list control | 7 hrs 30 mins |
| Larsson et al., 1987a | 46 | Therapist assisted relaxation/Self-help relaxation* | Self-monitoring | 6 hours 45 mins |
| Larsson et al., 1987b | 36 | Self-help relaxation/Problem discussion group* | Self-monitoring | 5hrs |
| Larsson et al., 1990 | 43 | Self-help relaxation | Waiting list control | Unknown |
| Larsson et al., 1996 | 26 | Relaxation treatment | Waiting list control | 2 hrs 20 mins |
| McGrath et al., 1988 | 99 | Relaxation training | Attention control/Own best efforts* | 6 hrs |
| McGrath et al., 1992 | 73 | Therapist administered cognitive behavioural + stress coping + relaxation training/Self-administered cognitive behavioural + stress coping + relaxation training* | Information and support | 8 hrs |
| Osterhaus et al., 1997 | 39 | Behavioural treatment package | Waiting list control | 9 hrs 2 mins |
| Palermo et al., 2009 | 44 | Internet-delivered family cognitive-behavioural therapy | Waiting list control | 4 hrs |
| Passchier et al., 1990 | 119 | Progressive relaxation training | Placebo training | 2 hrs 10 mins |
| Richter, 1986 | 43 | Relaxation training/Cognitive coping* | Attention control | 9 hrs |
| Sartory et al., 1998 | 43 | Cephalic vasomotor training + stress management/Relaxation training + stress management* | Beta-blocker (metoprolol) | Unknown |
| Scharff et al., 2002 | 34 | Hand-warming biofeedback and stress management | Hand-cooling attention control/ Waiting list control* | 4 hrs |
| Trautmann and Kroner-Herwig, 2010 | 55 | CBT, self-help and management/Applied relaxation group* | Education | Unknown |
| Wicksell et al., 2009 | 24 | Exposure and acceptance | Multidisciplinary treatment with amitriptyline | 10 hrs |
| Recurrent Abdominal Pain | ||||
| Alfven and Lindstrom, 2007 | 48 | Psychological treatment and physiotherapy | Physiotherapy alone | Unknown |
| Duarte et al., 2006 | 32 | CBT | Standard medical care | 3 hrs, 20 mins |
| Hicks et al., 2006 | 42 | Internet CBT (with internet and phone) | Standard medical care | Unknown |
| Humphreys et al., 2000 | 61 | CBT + biofeedback + parental support + fibre/CBT + biofeedback + fibre/Biofeedback + fibre** | Fibre | Unknown |
| Levy et al., 2010 | 200 | CBT | Education | 4 hrs |
| Palermo et al., 2009 | 44 | Internet-delivered family cognitive-behavioural therapy | Waiting list control | 4 hrs |
| Robins et al., 2005 | 69 | Short term family CBT plus standard medical care | Standard medical care | 3 hrs 30 mins |
| Sanders et al., 1994 | 44 | CBT | Standard medical care | 6 hrs |
| Wicksell et al., 2009 | 24 | Exposure and acceptance | Multidisciplinary treatment with amitriptyline | 10 hrs |
| Neuropathic Pain | ||||
| Wicksell et al., 2009 | 24 | Exposure and acceptance | Multidisciplinary treatment with amitriptyline | 10 hrs |
| Musculoskeletal Pain | ||||
| Kashikar-Zuck et al., 2005 | 27 | Coping skills training | Self-monitoring | 6 hrs |
| Kashikar-Zuck et al., 2012 | 114 | CBT | Fibromyalgia education | 7 hrs 30 mins |
| Palermo et al., 2009 | 44 | Internet-delivered family CBT | Waiting list control | 4 hrs |
| Stinson et al., 2010 | 39 | Internet treatment | Attention control group | 5 hrs |
| Van Tilburg et al., 2009 | 24 | Guided imagery treatment | Standard medical care | 12 hrs 5 mins |
| Vlieger et al., 2007 | 51 | Gut-directed hypnotherapy | Standard medical care plus supportive therapy | 5 hrs |
| Wicksell et al., 2009 | 24 | Exposure and acceptance | Multidisciplinary treatment with amitriptyline | 10 hrs |
Note. *Three-arm trial; **Four-arm trial. CBT = Cognitive behavioral therapy; TAU = Treatment as usual.
Of the 35 included studies, data could be extracted from 30 posttreatment. Of those 30 studies, 10 provided follow-up data (3–12 months posttreatment). Sleep outcomes were not measured or reported in any of the 35 studies, and therefore could not be included in the analyses. Treatment dose was reported in 28 studies (M = 5 hrs, 42 min, range = 45 min to 12 hrs, 5 min; Table I).
Risk of Bias
Risk of bias results have previously been published in Eccleston et al. (2012c). Risk of bias was assessed using four categories (selection bias, detection bias, attrition bias, and reporting bias), reporting on five judgments (random sequence generation, allocation concealment, detection bias, incomplete outcome data, and selective reporting).
Thirteen studies reported a thorough randomization procedure and were marked as having low risk of bias. In comparison, 22 studies were marked unclear for their randomization procedure, as they did not give an adequate method of randomization. For allocation concealment, 11 studies had a low risk of bias, 20 studies were unclear, and four studies were deemed to have a high risk of allocation concealment bias. Risk of bias for outcome assessment was marked low for only six studies, which explicitly stated a blind outcome assessor. The remaining studies were marked unclear. Study authors had to report attrition completely (no statistical differences between treatment completers and noncompleters) for the study to be judged as low risk of bias. From the 35 studies, 15 reported no statistical differences between completers and noncompleters and had low risk of bias, 15 only partially reported attrition and were judged to be unclear, and five studies did not report attrition and were judged to have high risk of bias. Finally, 20 studies were judged to have low risk of bias, as all data could be extracted from studies, whereas 15 studies were judged to have high risk of bias, as data could not be fully extracted. Only one study (Kashikar-Zuck et al., 2012) scored low risk of bias on all judgments. See Figure 2 and risk of bias tables (Supplementary Appendix B) for individual study summaries.
Figure 2.
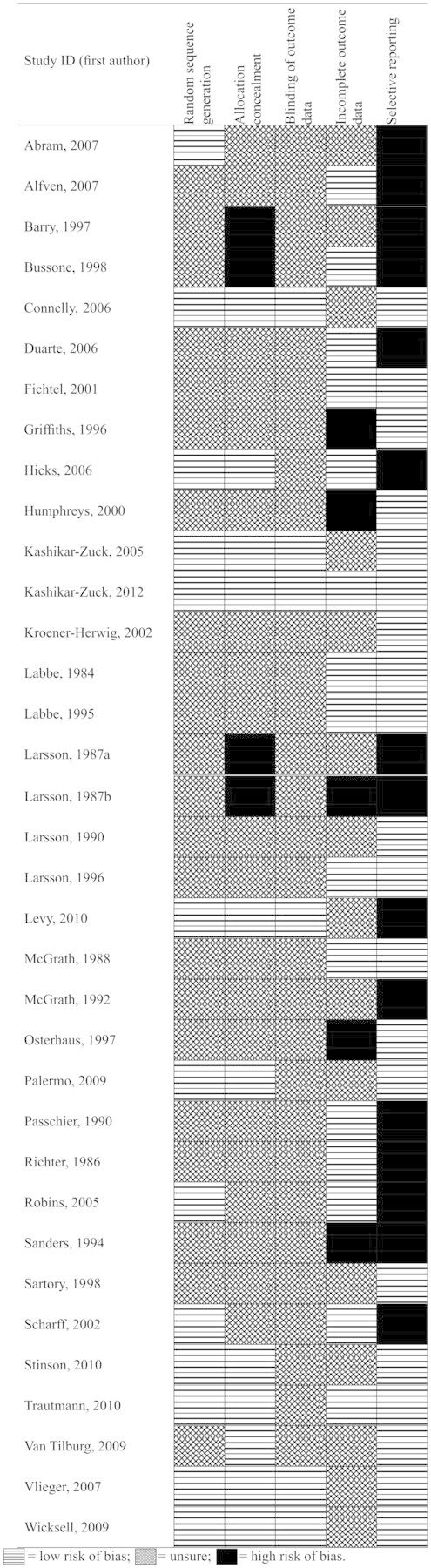
Risk of bias.
Data Analysis
Outcome data were entered and analyzed using RevMan (version 5.2). Dichotomous and continuous data were used to analyze the effect of psychological treatment. Dichotomous data were analyzed using Mantel–Haenszel methods. These methods are commonly used when data are sparse and sample sizes are small. Risk ratios (RR; risk of an event occurring) and number needed to treat for effect (NNT) are reported (Moore, Edwards, Barden, & McQuay, 2003). NNT statistics can only be calculated for dichotomous outcomes. Continuous data were analyzed using an inverse variance method, meaning larger studies are given more weight in comparison with smaller studies. Random-effects analysis was used due to the heterogeneity of measures included in the analyses. Standardized mean differences (SMD) and CIs are reported. Studies investigating children with headache conditions most typically measure pain outcome dichotomously, whereas in studies on chronic pain (excluding headache) conditions, pain is typically measured continuously. Therefore, these conditions are split for the outcome pain when determining the effect of psychological interventions.
A curve fitting estimation was also carried out to investigate the relationship between the treatment dose (exposure to treatment) and pain scores to determine the most effective treatment dose for children with headache and chronic pain (excluding headache) conditions, if one exists. Other outcomes were not tested due to lack of data. We examined the fit of linear and quadratic curves to the relationship between treatment dose and pain score. If there was a relationship between the two, then we would expect a significant fit to a linear model. Furthermore, if there was an optimal treatment dose, after which further treatment was less effective, then we would expect a quadratic curve (allowing for a maximum point in an inverted U-shaped curve) to provide a significantly better fit to the data. If both models fit the data, but there is no significant difference between the two models, the simpler (linear model) was assumed (Motulsky & Christopoulos, 2003).
Results of Analyses
First, the quantitative effects of psychological interventions for children and adolescents with chronic pain (including all conditions) were analyzed on four outcomes, namely, pain, disability, depression, and anxiety, posttreatment and at follow-up. No data could be extracted for sleep outcomes and are therefore not included. Due to the different types of data extracted for pain outcomes, chronic pain (excluding headache) and headache conditions were analyzed separately. Second, pain conditions were split into headache, abdominal pain, neuropathic pain, and musculoskeletal pain (see Supplementary Appendix C, for forest plots of all analyses and Supplementary Appendix D, for the summary of findings). Third, the quality of evidence was assessed using GRADE and is presented for each outcome. Fourth, we examined the relationship between treatment dose and reduction in pain symptoms.
Chronic Pain, Treatment Versus Control
Pain Symptoms
Six hundred seventy-two children with chronic pain (excluding headache; for analysis of headache pain symptoms, please see “Headache” section) from 11 studies were included in the analysis to investigate whether psychological interventions improved pain across therapies posttreatment. Psychological therapies had a moderate beneficial effect on reducing pain intensity in children with chronic pain (excluding headache) compared with control conditions posttreatment (SMD = −0.60, 95% CI −0.91 to −0.29) and this was significant (z = 3.77, p < .001, Figure 3). Using the GRADE criteria, the quality of evidence was judged to be moderate, meaning further research is likely to have an important impact on our confidence in the estimate of effect. At follow-up, 324 children from four studies were included in the analysis. There was no clear evidence of benefit for psychological therapies on pain reduction at follow-up and the analysis was not significant (SMD = −0.30, 95% CI −0.77 to 0.16, z = 1.27, p > .05). The quality of evidence was low, meaning further research is very likely to have an important impact on our confidence in the estimate of effect.
Figure 3.
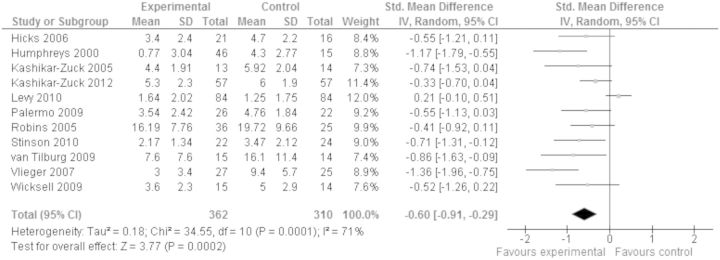
Treatment versus control, chronic pain (excluding headache) conditions, pain posttreatment.
Disability
Six hundred nineteen children reporting chronic pain (all conditions) from 10 studies were included in the analysis to investigate whether psychological interventions improved disability posttreatment. Psychological therapies had a small beneficial effect on reducing disability in children with chronic pain compared with control conditions posttreatment (SMD = −0.27, 95% CI −0.46 to −0.08) and this was significant (z = 2.81, p < .01). The quality of evidence was scored high, meaning further research is very unlikely to change our confidence in the estimate of effect. At follow-up, 292 children from three studies were included in the analysis. There was no clear evidence of benefit for psychological therapies on disability at follow-up, (SMD = −0.19, 95% CI −0.51 to 0.13) and the analysis was not significant (z = 1.14, p > .05). We are moderately confident in the estimate of effect.
Depression
Five hundred thirty-two children reporting chronic pain (all conditions) from eight studies were included in an analysis to investigate whether the psychological interventions improved depression posttreatment. There was no clear evidence of benefit for psychological therapies on depressive symptoms in children compared with control conditions posttreatment (SMD = −0.09, 95% CI −0.32 to 0.14) and the analysis showed the difference to be nonsignificant (z = 0.78, p > .05). The quality of evidence was high, meaning further research is very unlikely to change our confidence in the estimate of effect. At follow-up, 292 children from three studies were included in the analysis, and similarly, the analysis was not significant and there was no clear evidence of benefit for psychological therapies on depressive symptoms (SMD = −0.09, 95% CI −0.32 to 0.14, z = 0.74, p > .05). We are moderately confident in the estimate of effect.
Anxiety
Four hundred three children reporting chronic pain (all conditions) from six studies were included in an analysis to investigate whether psychological interventions improved anxiety posttreatment. There was no clear evidence of benefit for psychological therapies on anxiety compared with control conditions posttreatment (SMD = −0.18, 95% CI −0.44 to 0.07) and the analysis was not significant (z = 1.42, p > .05). We are moderately confident in the estimate of effect. At follow-up, 322 children from five studies were included in the analysis, and similarly, there was no clear evidence of benefit for psychological interventions on anxiety (SMD = −0.22, 95% CI −0.57 to 0.14) and the analysis was not significant (z = 1.20, p > .05). The quality of evidence was low, meaning we have low confidence in the estimate of effect.
Adverse Events
Only one study (Kashikar-Zuck et al., 2012) reported adverse events that were unrelated to the intervention delivered in the trial. No other studies reported either the presence or absence of adverse events.
Headache, Treatment Versus Control
Pain Symptoms
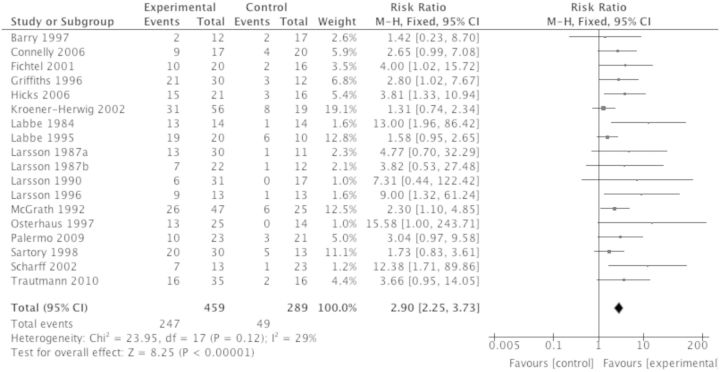
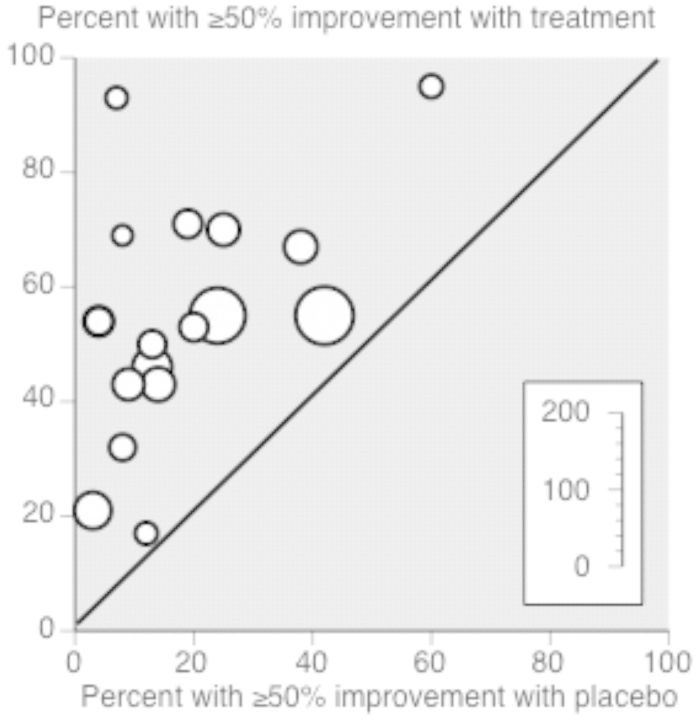
Seven hundred forty-eight participants from 18 studies were included in the analysis to investigate whether psychological interventions improved pain symptoms posttreatment in children with headache. Psychological interventions were beneficial and reduced headache by at least 50% in the experiment condition compared with the control condition posttreatment (RR = 2.90, 95% CI 2.25 to 3.73, z = 8.25, p < .001; Figure 4). The NNT for benefit based on these results is 2.72 (95% CI 2.32 to 3.29; Figure 5). The quality rating based on GRADE guidelines was low for this outcome, meaning further research is very likely to have an important impact on our confidence in the estimate of effect. At follow-up, 196 participants from six studies were included in the analysis, and similar beneficial effects on pain reduction were found (RR = 3.34, 95% CI 2.01 to 5.53, z = 4.68, p < .001). This produced an NNT for benefit at follow-up of 2.01 (95% CI 1.62 to 2.64). However, the quality rating was very low, meaning we are very uncertain of the estimate of effects.
Figure 4.
Treatment versus control, headache pain posttreatment.
Figure 5.
Labbe plot of treatment versus control, headache pain posttreatment.
Disability
One hundred eight participants from three studies were included in the analysis to investigate whether psychological interventions improved disability posttreatment among children with headache. There was no clear evidence of benefit for psychological therapies on disability compared with control conditions posttreatment and the analysis was not significant (SMD = −0.30, 95% CI −0.85 to 0.24, z = 1.09, p > .05). We have low confidence in this estimate of effect. At follow-up, only one study measured disability at follow-up, and therefore, no conclusions can be drawn and we are very uncertain of the estimate of effects.
Depression
One hundred seventy-four participants reporting headache pain from four studies were included in the analysis to investigate whether psychological interventions improved depression posttreatment. There was no clear evidence of benefit for psychological interventions on depression compared with control conditions posttreatment and the analysis was not significant (SMD = −0.14, 95% CI −0.46 to 0.18, z = 0.87, p > .05). Quality was judged to be moderate for this outcome, meaning our confidence in the estimate of effect is moderate. However, at follow-up, only one study could be included in the analysis, and therefore, no conclusions can be drawn and we are very uncertain of the estimate of effects.
Anxiety
One hundred forty participants reporting headache pain from four studies were included in the analysis to investigate whether psychological interventions improved anxiety posttreatment. There was no clear evidence of benefit for psychological interventions on anxiety compared with control conditions posttreatment and the analysis was not significant (SMD = −0.32, 95% CI −0.67 to 0.03, z = 1.77, p > .05). We are very uncertain of the estimate of effects. Three studies with 84 participants were entered into the follow-up analysis, with similar findings (SMD = −0.37, 95% CI −0.93 to 0.19, z = 1.30, p > .05). Similarly, the quality was judged to be very low for this outcome, meaning we are very uncertain of the estimate of effects.
Abdominal Pain, Treatment Versus Control
Pain Symptoms
Four hundred eighty-five participants reporting abdominal pain from eight studies were included in the analysis to investigate whether psychological interventions improved pain symptoms posttreatment. Psychological therapies were beneficial and significantly reduced pain in children compared with control conditions posttreatment with a moderate effect size (SMD = −0.62, 95% CI −1.05 to −0.19, z = 2.83, p < .01). The quality rating based on GRADE guidelines was moderate for this outcome, meaning further research is likely to have an important impact on our confidence in the estimate of effect. At follow-up, 210 participants from three studies were included in the analysis of pain reduction, but there was no clear evidence of the benefit for psychological interventions and the analysis was not significant, (SMD = −0.43, 95% CI −1.23 to 0.38, z = 1.03, p > .05). The quality of evidence was judged as very low, meaning we are very uncertain of the estimate of effects.
Disability
Four hundred one participants reporting abdominal pain from six studies were included in the analysis to investigate whether psychological interventions improved disability posttreatment. Psychological therapies had a small beneficial effect and significantly reduced disability in children compared with control conditions posttreatment (SMD = −0.35, 95% CI −0.66 to −0.05, z = 2.25, p < .05). Our confidence in the estimate of effect was low. At follow-up, 178 participants from two studies were included in the analysis, but there was no evidence of benefit for psychological interventions on disability compared with control conditions and the analysis was not significant (SMD = −0.06, 95% CI −0.45 to 0.33, z = 0.29, p > .05). Furthermore, the quality was very low, meaning we are very uncertain of the estimate of effects.
Depression
Two hundred forty-five participants reporting abdominal pain from three studies were included in the analysis to investigate whether psychological interventions improved depression posttreatment. Psychological interventions did not show any evidence of benefit for depression in children compared with the control conditions of no treatment or placebo posttreatment, (SMD = −0.09, 95% CI −0.59 to 0.42) and the analysis was not significant (z = 0.33, p > .05). The quality of evidence for this outcome was low, meaning our confidence of the estimate of effect was low. At follow-up, 178 participants from two studies were included in the analysis, with similar findings (SMD = −0.13, 95% CI −0.55 to 0.30, z = 0.59, p > .05). We are very uncertain of the estimate of effects for this outcome.
Anxiety
One hundred ninety-five participants reporting abdominal pain from two studies were included in the analysis to investigate whether psychological interventions improved anxiety posttreatment. There was no clear evidence of benefit for psychological therapies on anxiety in children compared with control conditions posttreatment (SMD = 0.11, 95% CI −0.18 to 0.39, z = 0.74, p > .05). Our confidence of the estimate of effect is very low. At follow-up, 170 participants from two studies were included in the analysis, and similarly, there was no clear evidence for the benefit of psychological interventions (SMD = 0.14, 95% CI −0.16 to 0.44, z = 0.91, p > .05). Similar to anxiety posttreatment, our confidence in the estimate of effect was very low.
Neuropathic Pain, Treatment Versus Control
Only one study (Wicksell et al., 2009) treated children with neuropathic pain, and therefore, data could not be entered into a meta-analysis. No conclusions can be drawn.
Musculoskeletal Pain, Treatment Versus Control
Pain Symptoms
Two hundred sixty-four participants from five studies were included in the analysis to investigate whether psychological interventions improved musculoskeletal pain symptoms posttreatment. Psychological therapies had a significant moderate effect on improving pain in children with musculoskeletal pain compared with control conditions, and these effects were beneficial (SMD = −0.50, 95% CI −0.74 to −0.25, z = 3.96, p < .001). The quality rating based on GRADE guidelines was moderate for this outcome, meaning further research is likely to have an important impact on our confidence in the estimate of effect. At follow-up, 138 participants from two studies were included in the analysis. There was no clear evidence of the benefit of psychological interventions on pain reduction (SMD = −0.24, 95% CI −0.58 to 0.09, z = 1.41, p > .05). Furthermore, the quality was low, meaning our confidence of the estimate of effect was low.
Disability
Two hundred sixty-four participants reporting musculoskeletal pain from five studies were included in the analysis to investigate whether psychological interventions improved disability posttreatment. Psychological therapies produced a small beneficial effect on reducing disability in children compared with control conditions posttreatment (SMD = −0.36, 95% CI −0.61 to −0.12) and this was significant (z = 2.90, p < .01). Our confidence in the estimate of the effect is moderate. At follow-up, 138 participants from two studies were included in the analysis. Psychological therapies had a large beneficial effect on reducing disability in children compared with control conditions at follow-up (SMD = −3.86, 95% CI −7.23 to −0.49), which was significant (z = 2.25; p < .05). However, our confidence in the estimate of effect was low.
Depression
Two hundred sixty-seven participants reporting musculoskeletal pain from five studies were included in the analysis to investigate whether psychological interventions improved depression posttreatment. Psychological therapies had a small beneficial effect on improving depression in children compared with control conditions posttreatment (SMD = −0.28, 95% CI −0.52 to −0.04), which was significant (z = 2.28, p < .05). Our confidence in the estimate of effect was moderate. At follow-up, 138 participants from two studies were included in the analysis, and there was no clear evidence of the benefit of psychological interventions on depression (SMD = −0.85, 95% CI −3.01 to 1.31, z = 0.77, p > .05). The quality of evidence was low, meaning our confidence in the estimate of effect was low.
Anxiety
One hundred thirty-two participants reporting musculoskeletal pain from two studies were included in the analysis to investigate whether psychological interventions improved anxiety posttreatment. There was no clear evidence of benefit for psychological interventions on anxiety compared with control conditions posttreatment (SMD = −0.20, 95% CI −0.60 to 0.21, z = 0.96, p > .05). Our confidence in the estimate of effect was low. At follow-up, 132 participants from two studies were included in the analysis, with similar findings (SMD = −0.23, 95% CI −0.66 to 0.20, z = 1.06, p > .05). We are very uncertain of the estimate of effects for anxiety at follow-up.
Quality of Evidence Summary
GRADE was used to assess the quality of evidence. Only two outcomes scored high quality on the assessment, disability and depression posttreatment when combining all conditions (Table II), meaning further research is unlikely to change our confidence in the estimate of effect for these outcomes. Of the remaining ratings, seven scored moderate quality, nine were low quality, and eight were very low quality. No studies reported sleep outcomes, and therefore, they are not included in the quality of evidence tables. Quality of evidence for individual conditions is shown in Tables III, IV, and V (see Supplementary Appendix E). A quality of evidence table could not be created for neuropathic pain due to lack of evidence.
Table II.
Summary of Findings for Chronic Pain in Children and Adolescents (All Conditions)
| Psychological therapies for the management of chronic and recurrent pain in children and adolescents | ||||||
|---|---|---|---|---|---|---|
| Patient or population: Children and adolescents with chronic and recurrent pain | ||||||
| Settings: Community and secondary care | ||||||
| Intervention: Psychological therapies | ||||||
| Outcomes |
Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Psychological therapies | |||||
|
|
|
|
SMD −0.60 (−0.91 to −0.29) | ||
|
|
|
SMD −0.30 (−0.77 to 0.16) | |||
| Pain (headache, posttreatment) | 170 per 1000 |
|
|
|
||
| Pain (headache, follow-up) | 167 per 1000 |
|
|
|
||
|
|
|
|
SMD −0.27 (−0.46 to −0.08) | ||
|
|
|
|
SMD −0.09 (−0.32 to 0.14) | ||
|
|
|
|
SMD −0.18 (−0.44 to 0.07) | ||
Note. *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI = confidence interval; RR = risk ratio; SMD = standardized mean difference.
GRADE Working Group grades of evidence.
High quality ⊕⊕⊕⊕: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality ⊕⊕⊕⊖: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality ⊕⊕⊖⊖: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality ⊕⊖⊖⊖: We are very uncertain about the estimate.
aA number of studies had high risk of bias.
bMost studies use a wait-list control.
cSmall number of participants.
dI squared is >45% (high), heterogeneity Tau = p > 0.05, variation can be explained.
ePublication bias, incomplete reporting of outcomes.
Effectiveness of Treatment Dose
The quadratic and linear curves both fit the data significantly for headache conditions posttreatment [R2 = .50, F(2, 12) = 5.003, MSE = 19.95, p < .05; R2 = .42, F(1,12) = 7.82, MSE = 21.2, p < .05, respectively]. However, the difference between the two models was not significant [F(1,2) = 1.69, p > .05]. At follow-up, neither the quadratic nor linear curve fit the data significantly [R2 = .710, F(2,4) = 2.44, MSE = 15.12, p = .29; R2 = .70, F(1,4) = 7.02, MSE = 10.40, p = .08, respectively]. For chronic pain (excluding headache) conditions, neither the quadratic nor linear curve fit the data significantly posttreatment [R2 = .053, F(2,8) = .168, MSE = .207, p = .85; R2 = .044, F(1,8) = .321, MSE = .179, p = .59, respectively]. Similarly, at follow-up, neither the nor linear curve fit the data significantly [R2 = .060, F(2,8) = .190, MSE = .227, p = .83; R2 = .049, F(1,8) = .357, MSE = .197, p = .57, respectively].
Therefore, we conclude that the higher the treatment dose given to children with headache conditions, the better the pain score posttreatment. The dose of treatment ranged from 3 to 12 hrs. However, we found no evidence for an optimal treatment dose. No pattern was found for follow-up pain scores, or for chronic pain (excluding headache) conditions posttreatment or at follow-up, meaning treatment intensity had no effect on pain score.
Discussion
Summary of Findings
This meta-analysis aimed to determine the effects of psychological therapies and adverse events for children and adolescents with chronic pain. Individual conditions were analyzed separately. Furthermore, we aimed to investigate the relationship between treatment dose of psychological therapy and effect sizes to determine an optimal dose for improving pain symptoms. Psychological therapies can significantly reduce pain and disability in children and adolescents with chronic pain, although there is currently a lack of evidence for some clinical pain conditions and outcomes. However, where there is sufficient evidence, we can reliably conclude the following: headache pain was reduced significantly posttreatment and at follow-up; only two children would need to receive treatment to receive benefit (NNT was 2.72 posttreatment and 2.01 at follow-up). For abdominal pain, significant improvements for pain and disability were found posttreatment. Among children with musculoskeletal pain, psychological interventions produced significant reductions in pain, disability, and depression posttreatment, and a significant effect was found at follow-up for disability, although only two studies were included. No other significant effects were found posttreatment or at follow-up.
The quality of evidence was assessed using the GRADE criteria. When assessing the quality of outcomes for combined pain conditions, the evidence was moderate (Table II). However, quality of evidence was lower when assessing each pain condition individually (see Supplementary Appendix D). This field is still relatively small and with the continuing addition of high-quality trials, new evidence is likely to change our confidence of the estimate of effect for each condition. This is reflected by the large number of outcomes that scored “low” or “very low” ratings. It was not possible to produce quality of evidence tables for neuropathic pain or for sleep outcomes due to the lack of studies providing outcome data.
Another contribution of the meta-analysis is that we were able to explore the relationship between dose and effect of treatment on pain scores. For headache pain, results revealed that higher treatment dose was associated with more pain reduction. For chronic pain (excluding headache) conditions, no identifiable pattern between treatment dose and pain outcome was found.
Completeness of Data
The evidence base for the efficacy of psychological interventions for reducing pain among children with headache is promising, especially for headache pain reduction. Studies primarily used brief behavioral interventions to treat children with headache. Further studies are unlikely to change such findings, although different components could be added and trialed to reduce disability, depression, and anxiety. The strength of the evidence base for psychological treatments for pediatric chronic pain is building, and at present, our findings are inconsistent with UK NICE clinical guidelines (2012) that state, erroneously, there is little evidence to support the use of psychological interventions in managing chronic headache pain. However, this review demonstrates that CBT and behavioral therapies are effective at reducing pain in children and adolescents with chronic headache, and evidence is mounting for efficacy in other pediatric pain conditions as well.
Despite these findings, there are still considerable gaps in our knowledge regarding the effectiveness of psychological interventions for particular outcomes and conditions. No evidence of effect was found for psychological treatments in reducing anxiety levels in children and adolescents with painful conditions. However, only six studies provided extractable data, so findings should be treated with caution, especially when interpreting anxiety outcomes for individual clinical pain conditions. There is substantial evidence from the anxiety literature that shows psychological interventions can improve anxiety outcomes. For example, a review of 41 RCTs (James, James, Cowdrey, Soler, & Choke, 2013) found positive effects of CBT in reducing anxiety symptoms in children and adolescents with an anxiety disorder. Anxiety and chronic pain have been found to be highly comorbid in children and adolescents (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Kashikar-Zuck et al., 2008). The fear-avoidance model supports the role of anxiety and catastrophizing in maintaining chronic pain through avoidance leading to disuse and depression (Simons & Kaczynski, 2012; Vlaeyen & Linton, 2000). It is possible that although anxiety is a measured outcome, treatment content in most psychological treatment protocols is suboptimal for specifically addressing pain anxiety. Further not all measures of anxiety specifically measured pain-related anxiety, meaning specific changes in anxiety toward pain could not be measured. Future therapies should target specific pain-related anxieties elicited from children and adolescents in treatment.
Similarly, we are unable to comment on the effectiveness of psychological interventions for improving sleep. Sleep was not reported as an outcome for any of the 35 included studies, despite growing evidence that it is an important factor contributing to the reduction in functional ability and low mood in children with chronic pain (Palermo & Kiska, 2004). The underreporting of sleep outcomes in trials is more surprising following the recommendation by the Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials, which published guidelines of core outcomes that should be included in trials with children with chronic pain (McGrath et al., 2008). This paper included sleep as a core outcome in pediatric chronic pain trials, however, in the seven included studies that were published post 2009; none reported sleep as an outcome. Recent reviews of evidence-based sleep assessments have been published; for example, Lewandowski, Toliver-Sokol, and Palermo (2011) review self-report assessments of sleep quality and sleep behavior in children and adolescents that may guide future selection of outcome instruments in this domain.
In the present review, we found insufficient studies of psychological treatments for children with neuropathic pain, meaning we cannot present any findings. Only one study investigated children with neuropathic pain (Wicksell et al., 2009) and therefore could not be analyzed.
Finally, this review did not segregate studies by type of control group, active or inactive, so the apparent effectiveness of psychological interventions may also be attributable to nonspecific benefits: doing something, perhaps anything, with a patient, rather than doing nothing. The use of attention control conditions might help to determine whether it is the specific aspects of the psychological therapy that are improving outcomes, rather than nonspecific effects such as interaction with a health professional, or time in reflection.
Trial Design, Methodological Improvements
RCTs are recommended as the gold standard when evaluating therapies in psychology (Boutron, Moher, Altman, Schulz, & Ravaud, 2008). Recent debate on whether the RCT is the best method to trial psychological therapies for patients with chronic pain has emerged with a call to consider new designs of trials to measure clinical efficacy and effectiveness through large-scale observational and translational studies (Morley, Eccleston, & Williams, 2013; Rowbotham et al., 2013). Currently, the population, measures, and outcomes are heterogeneous in pediatric pain trials, which hinders the pooling of data for meta-analysis and reduces confidence in the evidence base due to the low effect sizes and small population.
A clear recommendation stemming from our findings is that trial reporting needs to improve. From the 175 possible risk of bias responses, 65 were low risk, 86 were unclear, and 24 were high risk. The most prevalent threats to bias in these studies are inadequate reporting of outcomes (N, mean, SD) and attrition. Required standards of reporting have increased in recent years, with the introduction of PRISMA, CONSORT, and other guidelines to assist the transparency of research, but only one study (Kashikar-Zuck et al., 2012) scored low risk of bias in all areas. In addition, only one study (Kashikar-Zuck et al., 2012) reported adverse events. No other study reported on adverse events beyond attrition.
Trialists should set hypotheses a priori and should report all outcomes, ensuring that these address all targets of intervention. Findings concerning the efficacy of psychological interventions on improving sleep, anxiety, and depression are limited in this review because authors do not consistently measure or report these outcomes. For example, only eight studies presented extractable data for depression, six studies presented extractable data for anxiety, and none on sleep. Reporting bias has previously been noted in this field (Chan & Altman, 2005), and we suspect that additional outcomes are measured in studies but are not reported at publication for various reasons. Furthermore, the power of many analyses is low because of the small number of participants entered into the trial.
Future Studies and Innovation
The field is in need of more innovative trials to help reduce pain symptoms, anxiety and depression, and improve disability and sleep. Future psychological therapies should be theoretically coherent and use components and strategies that are clearly linked to both theory and the intended outcome or target of the intervention. Therapies should be trialed with different pain conditions to determine whether they are more efficacious than the current CBT and behavioral treatments. CBT was the only therapy to treat abdominal, neuropathic, and musculoskeletal conditions. High-quality trials are needed to determine whether there are other psychological therapies that achieve significant improvements with children and adolescents with other diagnoses that could be applied to children and adolescents with a painful condition (e.g., multisystemic therapy for children with diabetes, see Eccleston et al., 2012b). Recent CBT trials have attempted to innovate in delivery by using technology instead of traditional face-to-face formats (Palermo et al., 2009; Stinson et al., 2010). Such treatments could be compared with treatments delivered face-to-face for comparative effectiveness, or investigated for the unique relationships between model of delivery and treatment response.
To date, the evidence base does not differentiate between generalized anxiety and pain-related anxiety measures. Future trials should aim to investigate whether reductions in general anxiety or pain-related anxiety (or both) show greatest improvements from treatment.
We are currently unable to identify whether there is any “key” component of psychological therapies, as the multiple components are intended to be synergistic and impact several of the target variables, so disaggregating effects is not possible. Studies and protocols of psychological therapies do not contain enough detail to analyze systematically any intervention components. Protocols are becoming more readily available, through the registration of trials, yet they too are lacking in detail to understand what components of therapy are delivered, and how much time is spent in developing each skill. Furthermore, the content of treatment interacts with the skills of therapists in producing effects, and while the protocol can be standardized, and skills and adherence to a manual assessed, patients remain heterogeneous in their levels of pain, disability, and distress at entry to treatment. Similar issues arise concerning maintenance of gains over follow-up.
Morley et al. (2013) suggest that the psychological profile of children and adolescents entering treatment may be more important for determining treatment response than their differing pain conditions. None of the included studies in this review categorized patients based on psychological profile, and it could potentially be a powerful treatment predictor.
Clinical Implications
Psychological interventions, predominantly CBT and behavioral therapies, are effective for reducing pain in children and adolescents with chronic pain. No effect was found at follow-up (3–12 months), suggesting that the maintenance of therapies is limited, with the exception of headache pain. When investigating individual conditions, psychological interventions were effective for reducing pain in headache, abdominal, and musculoskeletal conditions and disability in abdominal and musculoskeletal pain. However, more focus should be placed on reducing distress including depression and anxiety in these populations. There was no evidence to determine the effect of psychological interventions on neuropathic pain and for the outcome sleep.
There is currently little evidence for other psychological treatments (e.g., problem-solving therapy) for children with chronic pain. Clinicians should not be restricted to CBT or behavioral therapy if it does not suit the child or does not result in positive outcomes. Furthermore, clinicians treating pediatric pain should target therapy based on risks identified at baseline assessment. For example, if a child reported high anxiety, delivering coping skills or relaxation techniques targeting anxiety may be more effective and further reduce disability.
Conclusion
Psychological therapies are effective at reducing pain symptoms for children and adolescents with chronic pain immediately after treatment. Psychological therapies are also effective at improving disability and depressive symptoms for some pain conditions. However, insufficient evidence means that limited conclusions can be drawn regarding anxiety and sleep outcomes. The evidence for the maintenance of treatment effects with psychological interventions is weak due to limited follow-up assessments in included RCTs. Further trials are needed in additional pain conditions (e.g., neuropathic pain) to broaden our understanding of whether psychological therapies can improve outcomes for these conditions. Moreover, further investigation is needed to understand whether psychological therapies can produce long-term changes in pain for children.
Supplementary Data
Supplementary data can be found at: http://www.jpepsy.oxfordjournals.org/.
Acknowledgments
The authors thank authors of the Cochrane Review (Emily Law, Amy Lewandowski, and Stephen Morley) for their work. The authors also thank Jane Hayes and Joanne Abbott for running the searches for this review and previous reviews.
Funding
Emma Fisher is a PhD student funded by the University Research Studentship Graduate School Award, University of Bath. Lauren Heathcote is a DPhil student at the University of Oxford, and her research is funded by a Research Training Fellowship with Action Medical Research.
References
- *Note: Studies marked with an asterisk (*) met the inclusion criteria for this review.
- *Abram H S, Buckloh L M, Schilling L S, Armatti Wiltrout S, Ramirez-Garnica G, Turk W R. A randomized, controlled trail of a neurological and psychoeducational group appointment model for pediatric headaches. Children’s Healthcare. 2007;36:249–265. [Google Scholar]
- *Alfven G, Lindstrom A. A new method for the of recurrent abdominal pain of prolonged negative stress origin. Acta Pediatrica. 2007;96:76–81. doi: 10.1111/j.1651-2227.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann H J, Oxman A D, Kunz R, Brozek J, Vist G E, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt G H. GRADE guidelines 3: Rating the quality of evidence – Introduction. Journal of Clinical Epidemiology. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- *Barry J, von Baeyer C L. Brief cognitive-behavioral group treatment for children’s headache. Clinical Journal of Pain. 1997;13:215–220. doi: 10.1097/00002508-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Boutron I, Moher D, Altman D G, Schulz K F, Ravaud P. Methods and processes of the CONSORT Group: Example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008-w1. [DOI] [PubMed] [Google Scholar]
- Burns J W, Day M A, Thorn B E. Is reduction in pain catastrophising a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Translational Behavioral Medicine. 2012;2:22–29. doi: 10.1007/s13142-011-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bussone G, Grazzi L, D’Amico D, Leone M, Andrasik F. Biofeedback-assisted relaxation training for young adolescents with tension-type headache: A controlled study. Cephalalgia. 1988;1:463–467. doi: 10.1111/j.1468-2982.1998.1807463.x. [DOI] [PubMed] [Google Scholar]
- Chan A W, Altman D G. Identifying outcome reporting bias in randomised trials on PubMed: Review of publications and survey of authors. British Medical Journal. 2005;330:753. doi: 10.1136/bmj.38356.424606.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Connelly M, Rapoff M A, Thompson N, Connelly W. Headstrong: A pilot study of a CD-ROM intervention for recurrent pediatric headache. Journal of Pediatric Psychology. 2006;31:737–747. doi: 10.1093/jpepsy/jsj003. [DOI] [PubMed] [Google Scholar]
- Costello E J, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- *Duarte M A, Penna F J, Andrade E M, Cancela C S P, Neto J C A, Barbosa T F. Treatment of nonorganic recurrent abdominal pain: Cognitive-behavioral family intervention. Journal of Pediatric Gastroenterology and Nutrition. 2006;43:59–64. doi: 10.1097/01.mpg.0000226373.10871.76. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Fisher E A, Vervoort T, Crombez G. Worry and catastrophizing about pain in youth: A reappraisal. Pain. 2012a;153:1560–1562. doi: 10.1016/j.pain.2012.02.039. [DOI] [PubMed] [Google Scholar]
- Eccleston C, Malleson P N, Clinch J, Connell H, Sourbut C. Chronic pain in adolescents: Evaluation of a programme of interdisciplinary cognitive behavior therapy. Archives of Disease in Childhood. 2003;88:881–885. doi: 10.1136/adc.88.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Palermo T M, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database of Systematic Reviews. 2012b;8:CD009660. doi: 10.1002/14651858.CD009660.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Palermo T M, Williams A C D C, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews. 2012c;11:CD003968. doi: 10.1002/14651858.CD003968.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fitchel A, Larsson B. Does relaxation treatment have differential effects on migraine and tension-type headache in adolescents. Headache. 2001;41:290–296. doi: 10.1046/j.1526-4610.2001.111006290.x. [DOI] [PubMed] [Google Scholar]
- *Griffiths J D, Martin P R. Clinical versus home-based treatment formats for children with chronic headache. The British Journal of Health Psychology. 1996;1:151–166. [Google Scholar]
- Guyatt G, Oxman A D, Akl E A, Kunz R, Vist G, Brozek J, Norris S, Falck-Yttr Y, Glasziou P, deBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schunemann H J. GRADE guidelines: 1. Introduction-GRADE evidence profile and summary of findings tables. Journal for Clinical Epidemiology. 2011a;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux P, Montori V M, Freyschuss B, Vist G, Jaeschke R, Williams J W, Jr., Murad M H, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann H J. GRADE guidelines: 6. Rating the quality of evidence – imprecision. Journal for Clinical Epidemiology. 2011b;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl E A, Norris S, Vist G, Dahm P, Shukla V K, Higgins J, Falck-Ytter Y, Schunemann H J. GRADE guidelines: 7. Rating the quality of evidence – Inconsistency. Journal for Clinical Epidemiology. 2011c;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl E A, Post P N, Norris S, Meerpohl J, Shukla V K, Nasser M, Schunemann H J. GRADE guidelines: 8. Rating the quality of evidence – Indirectness. Journal for Clinical Epidemiology. 2011d;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, Williams J W, Jr., Meerpohl J, Norris S L, Akl E A, Schunemann H J. GRADE guidelines: 5. Rating the quality of evidence – Publication bias. Journal for Clinical Epidemiology. 2011e;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl E A, Djulbegovic B, Falck-Ytter Y, Norris S L, Williams J W, Jr., Atkins D, Meerpohl J, Schunemann H J. GRADE guidelines: 4. Rating the quality of evidence -- Risk of bias. Journal for Clinical Epidemiology. 2011f;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Guyatt G, Oxman A D, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbecovic B, Alonso-Coello P, Post P N, Busse J W, Glasziou P, Christensen R, Schunemann H J. GRADE guidelines: 12. Preparing summary of findings tables – Binary outcomes. Journal for Clinical Epidemiology. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- *Hicks C L, von Baeyer C L, McGrath P J. Online psychological treatment for pediatric recurrent pain: A randomized evaluation. Journal of Pediatric Psychology. 2006;31:724–736. doi: 10.1093/jpepsy/jsj065. [DOI] [PubMed] [Google Scholar]
- Higgins J P T, Green S, editors. Cochrane handbook for systematic reviews of interventions (Version 5.1.0) 2011 The Cochrane Collaboration. Retrieved from http://www.cochrane-handbook.org. [Google Scholar]
- Holden E W, Deichmann M M, Levy J D. Empirically supported treatments in pediatric psychology: Recurrent pediatric headache. Journal of Pediatric Psychology. 1999;24:91–109. doi: 10.1093/jpepsy/24.2.91. [DOI] [PubMed] [Google Scholar]
- Huertas-Ceballos A A, Logan S, Bennett C, Macarthur C. Psychosocial interventions for recurrent abdominal pain (RAP) and irritable bowel syndrome (IBS) in childhood. Cochrane Database of Systematic Reviews. 2014;2:CD003014. doi: 10.1002/14651858.CD003014.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet A, Miro J. The severity of chronic pediatric pain: An epidemiological study. Journal of Pain. 2008;9:226–236. doi: 10.1016/j.jpain.2007.10.015. [DOI] [PubMed] [Google Scholar]
- *Humphreys P A, Gevirtz R N. Treatment of recurrent abdominal pain: Components analysis of four treatment protocols. Journal of Pediatric Gastroenterology and Nutrition. 2000;31:47–51. doi: 10.1097/00005176-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Hunfeld J A M, Perquin C W, Hazebroek-Kampshreur A A J M, Passchier J, van Suijlekom-Smit L W A, van der Wouden J C. Physically unexplained chronic pain and its impact on children and their families: The mother’s perception. Psychological Psychotherapy. 2002;75:251–260. doi: 10.1348/147608302320365172. [DOI] [PubMed] [Google Scholar]
- James A C, James G, Cowdrey F A, Soler A, Choke A. Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database of Systematic Reviews. 2013;6:CD004690. doi: 10.1002/14651858.CD004690.pub3. [DOI] [PubMed] [Google Scholar]
- Janicke D M, Finney J W. Empirically supported treatments in pediatric psychology: Recurrent abdominal pain. Journal of Pediatric Psychology. 1999;24:115–127. doi: 10.1093/jpepsy/24.2.115. [DOI] [PubMed] [Google Scholar]
- Jordan A L, Eccleston C, Osborn M. Being a parent of the adolescent with complex chronic pain: An interpretative phenomenological analysis. European Journal of Pain. 2007;11:49–56. doi: 10.1016/j.ejpain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kashikar-Zuck S, Parkins I S, Brent Graham T, Lynch A M, Passo M, Johnston M, Johnston M, Schikler K N, Haskes P J, Banez G, Richards M M. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. The Clinical Journal of Pain. 2008;24:620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kashikar-Zuck S, Sil S, Lynch-Jordan A M, Ting T V, Peugh J, Schikler K N, Hashkes P J, Arnold L M, Passo M, Richards-Mauze M M, Powers S W, Lovell D J. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. The Journal of Pain. 2013;14:492–501. doi: 10.1016/j.jpain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kashikar-Zuck S, Swain N F, Jones B A, Graham T B. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. The Journal of Rheumatology. 2005;32:1594–1602. [PubMed] [Google Scholar]
- *Kashikar-Zuck S, Ting T V, Arnold L M, Bean J, Powers S W, Graham B, Passo M H, Schikler K N, Hashkes P J, Spalding S, Lynch-Jordan A M, Banez G, Richards M M, Lovell D J. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia. Arthritis & Rheumatism. 2012;64:297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S, Chambers C T, Huguet A, MacNevin R C, McGrath P J, Parker L, MacDonald A J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- *Kroener-Herwig B, Denecke H. Cognitive-behavioral therapy of pediatric headache: Are there differences inefficacy between a therapist-administered group training and a self-help format? Journal of Psychosomatic Research. 2002;53:1107–1114. doi: 10.1016/s0022-3999(02)00345-8. [DOI] [PubMed] [Google Scholar]
- *Labbe E E. Treatment of childhood migraine with autogenic training and skin temperature biofeedback: A component analysis. Headache. 1995;35:10–13. doi: 10.1111/j.1526-4610.1995.hed3501010.x. [DOI] [PubMed] [Google Scholar]
- *Labbe E E, Williamson D A. Treatment of childhood migraine using autogenic feedback training. Journal of Consulting and Clinical Psychology. 1984;52:968–976. doi: 10.1037//0022-006x.52.6.968. [DOI] [PubMed] [Google Scholar]
- *Larsson B, Carlsson J. A school-based, nurse-administered relaxation training for children with chronic tension-type headache. Journal of Pediatric Psychology. 1996;21:603–614. doi: 10.1093/jpepsy/21.5.603. [DOI] [PubMed] [Google Scholar]
- *Larsson B, Daleflod B, Hakansson L, Melin L. Therapist assisted versus self-help relaxation treatment of chronic headaches in adolescents: A school-based intervention. Journal of Child Psychology. 1987a;28:127–136. doi: 10.1111/j.1469-7610.1987.tb00657.x. [DOI] [PubMed] [Google Scholar]
- *Larsson B, Melin L, Doberl A. Recurrent tension headache in adolescents treated with self-help relaxation training and a muscle relaxant drug. Headache. 1990;30:665–671. doi: 10.1111/j.1526-4610.1990.hed3010665.x. [DOI] [PubMed] [Google Scholar]
- *Larsson B, Melin L, Lamminen M, Ullstedt F. A school-based treatment of chronic headaches in adolescents. Journal of Pediatric Psychology. 1987b;12:553–566. doi: 10.1093/jpepsy/12.4.553. [DOI] [PubMed] [Google Scholar]
- *Levy R L, Langer S L, Walker L S, Romano J M, Christie D L, Youssef N, DuPen M M, Feld A D, Ballard S A, Welsh E M, Jeffery R W, Young M, Coffey M J, Whitehead W E. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. American Journal of Gastroenterology. 2010;105:946–956. doi: 10.1038/ajg.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Levy R L, Langer S L, Walker L S, Romano J M, Christie D L, Youssef N, DuPen M M, Ballard S A, Labus J, Welsh E, Feld L D, Whitehead W E. Twelve-month follow-up cognitive behavioral therapy for children with functional abdominal pain. JAMA Pediatrics. 2013;167:178–184. doi: 10.1001/2013.jamapediatrics.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski A S, Toliver-Sokol M, Palermo T M. Evidence-based review of subjective pediatric sleep measures. Journal of Pediatric Psychology. 2011;36:780–793. doi: 10.1093/jpepsy/jsq119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin D S, Dahl R E. Importance of sleep in the management of pediatric pain. Journal of Developmental and Behavioural Pediatrics. 1999;20:244–252. doi: 10.1097/00004703-199908000-00007. [DOI] [PubMed] [Google Scholar]
- Logan D E, Carpino E A, Chiang G, Condon M, Firn E, Gaughan V J, Hogan M, Leslie D S, Olson K, Sager S, Sethna N, Simons L E, Zurakowski D, Berde C B. A day-hospital approach to treatment of pediatric complex regional pain syndrome: Initial functional outcomes. Clinical Journal of Pain. 2012;28:766–774. doi: 10.1097/AJP.0b013e3182457619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan D E, Simons L E, Stein M J, Chastain L. School impairment in adolescents with chronic pain. The Journal of Pain. 2008;9:407–416. doi: 10.1016/j.jpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- *McGrath P J, Humphreys P, Goodman J T, Keene D, Firestone P, Jacob P, Cunningham S J. Relaxation prophylaxis for childhood migraine: A randomized placebo-controlled trial. Developmental Medicine and Child Neurology. 1988;30:626–631. doi: 10.1111/j.1469-8749.1988.tb04800.x. [DOI] [PubMed] [Google Scholar]
- *McGrath P J, Humphreys P, Keene D, Goodman J T, Lascelles M A, Cunningham S J, Firestone P. The efficacy and efficiency of a self-administered treatment for adolescent migraine. Pain. 1992;49:321–324. doi: 10.1016/0304-3959(92)90238-7. [DOI] [PubMed] [Google Scholar]
- McGrath P J, Walco G, Turk D C, Dworkin R H, Brown M T, Davidson K, Eccleston C, Finley G A, Goldschneider K, Haverkos L, Hertz S H, Ljungman G, Palermo T, Rappaport B A, Rhodes T, Schechter N, Scott J, Sethna N, Svensson O K, Stinson J, von Baeyer C L, Walker L, Weisman S, White R E, Zajicek A, Zeltzer L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9:771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman D G, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6(6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A, Edwards J, Barden J, McQuay H. Bandolier’s little book of pain. New York, NY: Oxford University Press; 2003. [Google Scholar]
- Morley S, Eccleston C, Williams A C D C. Examining the evidence of psychological treatments for chronic pain: Time for a paradigm shift? Pain. 2013;154:1929–1931. doi: 10.1016/j.pain.2013.05.049. [DOI] [PubMed] [Google Scholar]
- Motulsky H J, Christopoulos A. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting. San Diego, CA: GraphPad Software Inc; 2003. [Google Scholar]
- NICE clinical guideline. 2012. Headaches: Diagnosis and management of headaches in young people and adults. Retrieved from http://guidance.nice.org.uk/cg150 (Accessed August 2013) [Google Scholar]
- *Osterhaus S O L, Lange A, Linssen W H J P, Passchier J. A behavioral treatment of young migrainous and non-migrainous headache patients: Prediction of treatment success. International Journal of Behavioral Medicine. 1997;4:378–396. doi: 10.1207/s15327558ijbm0404_8. [DOI] [PubMed] [Google Scholar]
- Palermo T M. Cognitive-behavioural therapy for chronic pain in children and adolescents. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Palermo T M, Eccleston C, Lewandowski A, Williams A C D C, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: An updated meta-analytic review. Pain. 2010;148:387–397. doi: 10.1016/j.pain.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo T M, Kiska R. Subjective sleep disturbances in adolescents with chronic pain: Relationship to daily functioning and quality of life. Journal of Pain. 2004;6:201–207. doi: 10.1016/j.jpain.2004.12.005. [DOI] [PubMed] [Google Scholar]
- *Palermo T M, Wilson A C, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an internet delivered family cognitive behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146(1–2):205–213. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Passchier J, van den Bree M B M, Emmen H H, Osterhaus S O L, Orlebeke J F, Verhage F. Relaxation training in school classes does not reduce headache complaints. Headache. 1990;30:660–664. doi: 10.1111/j.1526-4610.1990.hed3010660.x. [DOI] [PubMed] [Google Scholar]
- Perquin C W, Hazebroek-Kampschreur A A J M, Hunfeld J A M, Bohnen A M, van Suijlekom-Smit L W A, Passchier J, van der Wouden J C. Pain in children and adolescents: A common experience. Pain. 2000;87:51–58. doi: 10.1016/S0304-3959(00)00269-4. [DOI] [PubMed] [Google Scholar]
- *Richter I L, McGrath P J, Humphreys P J, Goodman J T, Firestone P, Keene D. Cognitive and relaxation treatment of paediatric migraine. Pain. 1986;25:195–203. doi: 10.1016/0304-3959(86)90093-X. [DOI] [PubMed] [Google Scholar]
- *Robins P M, Smith S M, Glutting J J, Bishop C T. A randomized controlled trial of a cognitive-behavioral family intervention for pediatric recurrent abdominal pain. Journal of Pediatric Psychology. 2005;30:397–408. doi: 10.1093/jpepsy/jsi063. [DOI] [PubMed] [Google Scholar]
- Rowbotham M C, Gilron I, Glazer C, Rice A S C, Smith B H, Stewart W F, Wasan A D. Can pragmatic trials help us better understand chronic pain and improve treatment? Pain. 2013;154:643–646. doi: 10.1016/j.pain.2013.02.034. [DOI] [PubMed] [Google Scholar]
- *Sanders M R, Shepherd R W, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: A controlled comparison of cognitive-behavioral family intervention and standard pediatric care. Journal of Consulting and Clinical Psychology. 1994;62:306–314. doi: 10.1037//0022-006x.62.2.306. [DOI] [PubMed] [Google Scholar]
- *Sartory G, Muller B, Metsch J, Pothmann R. A comparison of psychological and pharmacological treatment of pediatric migraine. Behaviour Research and Therapy. 1998;36:1155–1170. doi: 10.1016/s0005-7967(98)00081-3. [DOI] [PubMed] [Google Scholar]
- *Scharff L, Marcus D A, Masek B J. A controlled study of minimal-contact thermal biofeedback treatment in children with migraine. Journal of Pediatric Psychology. 2002;27:109–119. doi: 10.1093/jpepsy/27.2.109. [DOI] [PubMed] [Google Scholar]
- Simons L E, Kaczynski K J. The fear avoidance model of chronic pain: Examination for pediatric application. Journal of Pain. 2012;13:827–835. doi: 10.1016/j.jpain.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Stinson J N, McGrath P J, Hodnett E D, Feldman B M, Duffy C M, Huber A M, Tucker L B, Hetherington C R, Tse S M, Spiegel L R, Campillo S, Gill N K, White M E. An internet-based self-management program with telephone support for adolescents with arthritis: A pilot randomized controlled trial. Journal of Rheumatology. 2010;3:1944–1952. doi: 10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- *Trautmann E, Kroner-Herwig B. A randomized controlled trial of internet-based self-help training for recurrent headache in childhood and adolescence. Behaviour Research and Therapy. 2010;48:28–37. doi: 10.1016/j.brat.2009.09.004. [DOI] [PubMed] [Google Scholar]
- *van Tilburg M A L, Chitkara D K, Palsson O S, Turner M, Blois-Martin N. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: A pilot study. Pediatrics. 2009;124:e890–e897. doi: 10.1542/peds.2009-0028. [DOI] [PubMed] [Google Scholar]
- Vlaeyen J, Linton S J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- *Vlieger A M, Menko-Frankenhuis C, Wolfkamp S C, Tromp E, Benninga M A. Hypnotherapy for children with functional abdominal pain or irritable bowel syndrome: A randomized controlled trial. Gastroenterology. 2007;133:1430–1436. doi: 10.1053/j.gastro.2007.08.072. [DOI] [PubMed] [Google Scholar]
- Walco G A, Sterling C M, Conte P M, Engel R G. Empirically supported treatments in pediatric psychology: Disease-related pain. Journal of Pediatric Psychology. 1999;24:155–167. doi: 10.1093/jpepsy/24.2.155. [DOI] [PubMed] [Google Scholar]
- Walker L S, Guite J W, Duke M, Barnard J A, Greene J W. Recurrent abdominal pain: A potential precursor of irritable bowel syndrome in adolescents and young adults. Journal of Pediatrics. 1998;132:1010–1015. doi: 10.1016/s0022-3476(98)70400-7. [DOI] [PubMed] [Google Scholar]
- Weydert J A, Ball T M, Davis M F. Systematic review of treatments for recurrent abdominal pain. Pediatrics. 2003;111:e1–e11. doi: 10.1542/peds.111.1.e1. [DOI] [PubMed] [Google Scholar]
- *Wicksell R K, Melin L, Lekander M, Olsson G L. Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain -- A randomized controlled trial. Pain. 2009;141:248–257. doi: 10.1016/j.pain.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Williams A C D C, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews. 2012;11:CD007407. doi: 10.1002/14651858.CD007407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.