Summary
The role of eosinophils in the progression and resolution of allergic respiratory inflammation is poorly defined despite the commonality of their presence and in some cases their use as a biomarker for disease severity and/or symptom control. However, this ambiguity belies the wealth of insights that have recently been gained through the use of eosinophil-deficient/attenuated strains of mice that have demonstrated novel immunoregulatory and remodeling/repair functions for these cells in the lung following allergen provocation. Specifically, studies of eosinophil-deficient mice suggest that eosinophils contribute to events occurring in the lungs following allergen provocation at several key moments: (i) The initiating phase of events leading to Th2-polarized pulmonary inflammation, (ii) The suppression Th1/Th17 pathways in lung draining lymph nodes, (iii) The recruitment of effector Th2 T cells to the lung, and finally (iv) Mechanisms of inflammatory resolution that re-establish pulmonary homeostasis. These suggested functions have recently been confirmed and expanded upon using allergen provocation of an inducible eosinophil-deficient strain of mice (iPHIL) that demonstrated an eosinophil-dependent mechanism(s) leading to Th2 dominated immune responses in the presence of eosinophils in contrast to neutrophilic as well as mixed Th1/Th17/Th2 variant phenotypes in the absence of eosinophils. These findings highlighted that eosinophils are not exclusively downstream mediators controlled by T cells, dendritic cells (DC), and/or innate lymphocytic cells (ILC2). Instead, eosinophils appear to be more aptly described as significant contributors in complex interrelated pathways that lead to pulmonary inflammation and subsequently promote resolution and the re-establishment of homeostatic baseline. In this review we summarize and put into the context the evolving hypotheses that are now expanding our understanding of the roles eosinophils likely have in the lung following allergen provocation.
Introduction
Eosinophils have been the primary target of asthma therapeutics for decades, in part due to their specific and significant infiltration into the lungs and sputum of greater than 50% of asthmatic patients. A tremendous effort was undertaken by many laboratories to develop model systems (e.g., antigen-induced eosinophilic pulmonary inflammation (OVA/Alum [1] and house dust mite (HDM)[2]) as well as physiological methodologies to measure clinically relevant experimental endpoints (e.g., bronchoconstriction [3]). The majority of this effort was aimed at identifying molecules that targeted eosinophil survival or functions and, in turn, presumably asthma pathologies. Interleukin-5 (IL-5) was identified as critically necessary for eosinophil hematopoiesis, survival, and recruitment in mouse models of asthma and in humans (reviewed in [4]). Moreover, monoclonal antibodies targeting IL-5 were initially shown to deplete eosinophils in peripheral blood and the airways of mouse respiratory models [5] and subsequently in the blood and sputum samples derived from asthma patients [6]. To date various monoclonal antibodies to IL-5 and IL-5 receptors (e.g., Mepolizumab [7, 8], Reslizumab [9], and Benralizumab [10]) are still in clinical trials as a therapy for asthma and asthma exacerbations. Nonetheless, despite both the presence of eosinophils and their suspected deleterious actions, the success of these targeted therapies is controversial (e.g., [11, 12]) and a causative link between eosinophils and asthma symptoms remains elusive (reviewed in [13]).
The origins of the controversies surrounding the use of IL-5 targeted therapies are instructive as they provide the foundation for recent studies exploring the mechanisms of action and the potential role(s) eosinophils in health and disease. The discovery of IL-5 as a mediator of eosinophil hematopoiesis, entry into circulation, survival and activation in allergic respiratory inflammation was first made in mice [14–16]. Subsequent studies in mouse models of allergic respiratory inflammation also demonstrated monoclonal antibodies to IL-5 failed to completely ablate eosinophils in the lung tissue or airways [5, 17, 18] similar to that found in IL-5-deficient mice [19, 20]. This observation was also noted in IL-5 antibody treated asthma patients that retained eosinophils in the lung tissue [8, 10, 21], despite reduced blood and sputum levels. These findings left open the possibility that eosinophil pulmonary activities remained functional in treated asthma patients despite a reduction in the blood and sputum eosinophils in clinical settings. This is underscored in more recent studies where IL-5 therapies led to reduced exacerbations and quality of life scores but not necessarily improvement in lung function or other clinical scores (reviewed in [22, 23]). What are the consequences or potential activities of these remaining pulmonary eosinophils? This has led to the clinical conundrum - How can one deduce the role of eosinophils in asthma patients if they cannot be effectively targeted in all lung compartments and what threshold level of eosinophil depletion in lung tissue, lymphatic, blood, or the airways (sputum) is necessary for significant clinical outcomes? These questions are particularly poignant as asthma is continuously stratified into new phenotypes (e.g., eosinophilic versus neutrophilic [24, 25]) with complex contributing intrinsic and extrinsic agents (e.g., genetic and microbiota, respectively [26–28]) of disease pathology.
Techniques to study eosinophil pathways and functions in mouse models of allergic respiratory inflammation have expanded tremendously over the last decade. We suggest that careful use of these models provides the necessary reductionist approach to answer the clinically relevant questions noted above. Most significant to this enterprise has been the development of various eosinophil-deficient strains of mice. Although no model or mouse is infallible, these strains have provided new foundation from which to discover underappreciated role(s) of eosinophils in both health and diseases. This review summarizes the remarkable number of mouse models targeting eosinophils and the many studies that now indicate that eosinophils are important contributors to the immune/remodeling responses that occur in the lung following allergen provocation.
Eosinophil assessments in mice
Are mouse eosinophils sufficiently “human” in character?
Several review articles have detailed the biology and activities of both mouse and human eosinophils (see for example [29–33]). In particular, our laboratory recently published an extensive review detailing the remarkable similarities between mouse and human eosinophils [34]. These similarities include nearly identical homeostatic baseline levels of eosinophils in tissues as well as at similar concentrations in blood (1–5%) and half-life in blood (~1.2 days). Identification of eosinophils is comparable between mice and humans through the use of cell surface markers (e.g., IL-5Rα, CCR3, Siglec-F (Siglec-8 (human)), F4/80 (EMR1 (human)) yielding a cell surface phenotype whereby human eosinophils are identified as SSChiIL-5Ra+CD16−CD14−Siglec-8+ and mouse eosinophils are identified as SSChiIL-5Ra+CCR3+Gr1medSiglec-F+F4/80medCd11bhi. Unlike humans, CCR3 is uniquely expressed on mouse eosinophils and thus allows for more direct identification/targeting. Recruitment to tissues may rely on species-specific mechanisms as well. For example mice have a pseudogene for eotaxin −3 (CCL26), although both express eotaxins-1 (CCL11) and −2 (CCL24). Conservation of eosinophils secondary granule protein expression is high as both humans and mice highly express and store major basic protein (MBP)-1 and −2, eosinophil peroxidase (EPX), and the eosinophil associated granule ribonucleases (ECP and EDN (human) vs. Ear-1,–2, 1, −2, −6/7, −5/11 (mouse)). Stimulants to induce degranulation in vivo are not entirely defined in either mice or humans, yet human eosinophils degranulate in response to a wider array of molecules in vitro [35]. Finally, both mouse and human eosinophils are characterized by their robust abilities to express inflammation modulating lipid mediators (products of the 5-lipoxygenase and 12/15-lipoxygenase pathways), proteases (e.g., metalloproteases and cathepsins), cytokines (e.g., IL-4 and IL-13) and growth factors (e.g, TGF-β). Thus, while differences between mouse and human eosinophils exist, these appear to be species-specific adaptations with the conservation of fundamental roles of these granulocytes in mice and humans.
Eosinophil-deficient and other eosinophil-specific mouse models
After demonstrating anti-IL-5 administration reduced eosinophil numbers in mice [5, 17], strains of mice were generated that targeted gene knockouts of IL-5 and/or eotaxin1/2 (or its receptor CCR3) [16, 20, 36–39]. Although these initial strains provided an ability to ablate eosinophils in models of allergic respiratory inflammation, these mice varied in reduction of eosinophil levels and activities. However, a significant advancement was made with the generation of mice (i.e., PHIL [40] and ΔdlbGATA-1 [41]) that targeted eosinophils at a level that led to complete ablation in the bone marrow, periphery, and pulmonary compartments (Table 1). These strains of mice have quickly become the hallmark models for assessment of eosinophil functions in allergic respiratory inflammation (Table 2). Despite the value of these models, subsequent studies have demonstrated limitations. For example, ΔdlbGATA-1 mice have been shown to have deficiencies in basophil survival [42] and possibly other cells [43, 44]. Moreover, both PHIL and ΔdlbGATA-1 mice are congenitally eosinophil-deficient models (i.e., the eosinophil deficiency occurs throughout the lifetime of the animal), limiting the ability for assessing eosinophil-specific kinetic and gene-specific roles in allergic respiratory inflammation.
Table 1.
Eosinophil-Attenuated Mouse Strains
| Eosinophil-Deficient Strains |
Genetic Modification |
Congenital or Inducible |
Mechanism of Ablation | Ablation of eosinophils (% relative to wild type) |
Eosinophil Specificity |
Considerations | REF | |
|---|---|---|---|---|---|---|---|---|
| Blood | BAL* | |||||||
| PHIL | Transgenic (plasmid) | Congenital | EPX-promoter driven expression of Diphtheria Toxin A (cell autonomous ablation) | >99% | >99% | YES | Transgenic insertion site is unknown | [40] |
| iPHIL | Knock-In | Inducible | EPX-promoter driven expression of human Diphtheria Toxin Receptor (injection of Diphtheria Toxin targets eosinophils) | >99% | >99% | YES | Can develop humoral resistance to DT with repeated injections over 3 weeks** Endogenous EPX expression is reduced Will not kill differentiated eosinophils, only progenitors. |
[45] |
| eoCRE (X) ROSA26flox-STOP-lox-DTA | Knock-In | Congenital | EPX-promoter driven expression of Cre recombinase. Cre cleaves out a floxed-stop codon to allow translation of Diphtheria Toxin A (cell autonomous ablation) | >99% | >99% | YES | Endogenous EPX expression is reduced | [46] |
| MBP-1−/−/EPX−/− | Knockout | Congenital | Deficiency in granule proteins MBP-1 and EPX leads to apoptosis of eosinophil progenitors (mechanism(s) unknown) | >90% | >80% | YES | A small percent of granule-free eosinophils differentiate in vivo, although the role of these eosinophils is unknown. | [138] |
| ΔdblGATA-1 | Knockout Functional Mutation | Congenital | Knockout mutation of a double GATA DNA binding site with the promoter of the GATA-1 transcription factor gene (GATA-1 is necessary for eosinophil hematopoiesis) | >99% | >99% | Mostly | Mice have anemia. Reduced basophil survival and IL-4 production. Eosinophils can be generated ex vivo Other roles for GATA-1 unknown. |
[42–44, 139, 140] |
| Eosinophil-Attenuated Strains | ||||||||
| epx DTR | Transgenic (BAC)*** | Inducible | EPX-promoter driven expression of human Diphtheria Toxin Receptor (injection of Diphtheria Toxin targets eosinophils) | <75% | ND | YES | Failure to reduce eosinophils by more than 25% in blood and spleen and no reduction in bone marrow. | [141] |
| IL-5−/− | Knockout | Congenital | Targeting of a cytokine significant to eosinophil hematopoiesis, survival and priming. | <20% | >90% | Mostly | Incomplete ablation and other cells may be affected by IL-5 deficiency. LDLN eosinophils remain functional. | [16, 78, 142, 143] |
| IL-5 Rα−/− | Knockout | Congenital | Targeting of a cytokine receptor significant to eosinophil hematopoiesis, survival and priming. | <50% | <10% | Mostly | Incomplete ablation and other cells may be affected by IL-5Rα deficiency | [144] |
| Eotaxin-1−/− | Knockout | Congenital | Targeting of a chemokine (CCL11) that binds CCR3 to induce recruitment into tissues. | 0% | <30% | Mostly | Incomplete ablation. Inhibits recruitment by eotaxin-CCR3 | [36] |
| Eotaxin-2−/− | Knockout | Congenital | Targeting of a chemokine (CCL24) that binds CCR3 to induce recruitment into tissues. | 0% | >70% | Mostly | Incomplete ablation. Inhibits recruitment by eotaxin-CCR3 interactions. | [39] |
| CCR3−/− | Knockout (Neo insert)£ | Congenital | Targeting of the chemokine receptor that binds eotaxins-1,-2, −3. | 0% | >50% | Mostly | Incomplete ablation. Inhibits recruitment by eotaxin-CCR3 interactions. CCR3 may be needed by other cells (e..g, mast cells). Elevated peripheral eosinophilia. | [38] |
| CCR3−/− | Knockout (LacZ insert) £ | Congenital | Targeting of the chemokine receptor that binds eotaxins-1,-2, −3. | 0% | >90% | Mostly | Incomplete ablation. Inhibits recruitment by eotaxin-CCR3 interactions. CCR3 may be needed by other cells (e.g., mast cells). Elevated peripheral eosinophilia. | [37] |
| IL-5−/−/Eotaxin-1 | Knockout | Congenital | Double knockout mice deficient in the cytokine IL-5 and the chemokine eotaxin-2 or eotaxin-1 | <10% | >90% | Mostly | Incomplete ablation. Inhibits recruitment by eotaxin-CCR3 interactions. Elevated peripheral eosinophilia. | [145] |
| Eotaxin-1/-2−/− | Knockout | Congenital | Double knockout mice deficient in the chemokines (CCL11 and CCL24) that bind CCR3 to induce recruitment into tissues. | 0% | >80% | Mostly | Incomplete ablation. Inhibits recruitment by eotaxin-CCR3 interactions. | [37, 52] |
| PIR-B−/− | Knockout | Congenital | Targeting of a paired immunoglobulin-like receptor necessary for survival in response to IL-5 | ~30% | >80% | No | PRI-B is also on myeloid cells. Role in eosinophils is novel. | [146] |
BAL eosinophils in an acute OVA allergy model of asthma;
iPHIL mice are on also available on Ig heavy chain deficient mice to avoid antibody responses to DT;
BAC construct is different EPX sequence than used in PHIL, iPHIL, and eoCRE;
Independently created models; ND; not determined
Table 2.
Eosinophil-deficient Mice Phenotype Responses in Models of Allergic Asthma
| Model | EOS BAL/Lung |
Strain | Allergen | AHR | Mucus | Th2 Cytokines |
Total BAL counts (specific changes) |
Lung T cells |
LDLN T cells |
REF |
|---|---|---|---|---|---|---|---|---|---|---|
| Th2 Phenotype£ | ||||||||||
| PHIL | <0.1%/<0.1% | C57BL/6 | OVA | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | [45] |
| BALB/c | OVA | NA | ↓ | ↓ | ↓ | ↓ | ↓ | [45] | ||
| C57BL/6 | OT-II (i.v.) + OVA | NA | ↓ | ↓ | ↓ | ↓ | ↑ (i.v. OT-II) | [50] | ||
| C57BL/6 | OVA +Eos (i.p.) # | NA | ↓ | ↓ | ↓ | ↓ | ↑ | [78]§ | ||
| C57BL/6 | OVA +Eos (i.t.) # | NA | ↓ | ↓ | ↓ | ↓ | ↓ | § | ||
| C57BL/6 | Ragweed | NA | NA | NA | ↓ | NA | ↓ | [78] | ||
| C57BL/6 | IL-5/hE2 Tg | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | [115] § | ||
| C57BL/6 | OT-II (i.v.)+ OVA +Eos (i.t.)# | NA | ↑ | ↑ | ↑ | ↑ | ↑ (i.v. OT-II) | [50] | ||
| C57BL/6 | OVA +Activated Eos (i.t.) | NA | ↑ | ↑ | ↑ | ↑ | ↑ | § | ||
| iPHIL | <0.1%/<0.1% | C57BL/6 | OVA | ↓ | ↓ | ↓ | ↓ | NA | NA | [45] |
| C57BL/6 | HDM | NA | ↓ | ↓ | ↓ | NA | NA | [45] | ||
| ΔdblGATA-1 | <0.1%/<0.1% | C57BL/6 | OVA | NA | ↓ | ↓ | ↓ | NA | NA | [45] |
| C57BL/6 | OVA | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | [51] | ||
| C57BL/6 | OT-II (i.v.) + OVA | ↓ | ↓ | ↓ | ↓ | ↓ | NA | [51, 94] | ||
| BALB/c | OVA | ↑ | ↑ | ↑ | NA | NA | NA | [94] | ||
| BALB/c | OVA Chronic | ↑ | ↑↓ * | ↑ | ↑ | NA | NA | [41] | ||
| BABL/c | IL-13 Tg | NA | ↓↓ * | ↓ TGF-β1≠ | ↓ (↓ Mac and lymph) | NA | NA | [98] | ||
| C57BL/6 | OVA+ Eos (i.v.) ¥ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [51, 94] | ||
| C57BL/6 | OT-II (i.v.)+ OVA +Eos (i.v.) ¥ | ↑ | ↑ | ↑ | ↑ | ↑ | NA | [94] | ||
| C57BL/6 | OV IL-13−/− Eos (i.v.) ¥ | ↓ | ↓ | ↓ | ↓ | ↓ | NA | [94] | ||
| C57BL/6 | OT-II (i.v.)+ OVA +IL-13-/-+Eos (i.v.) ¥ | ↓ | ↓ | ↓ | ↓ | ↓ | NA | [94] | ||
| MBP-1−/−/EPX−/− | <10%/<10% | C57BL/6 | OVA | ↓ | ↓ | ↓ | ↓ (Φ) | NA | NA | § |
| Neutrophilic Phenotype and Mixed Phenotype£ | ||||||||||
| PHIL | <0.1%/<0.1% | C57BL/6 | OVA | ↑ | ↑ | ↑ | ↓ (Neuts >% ) | NA | ↑ | [45] € |
| BALB/c | OVA | NA | ↑ | ↑ | ↓ (Neuts >30% ) | NA | ↑ | [45]€ | ||
| C57BL/6 | OVA +DC (it.) | NA | ↑ | ↑ | ↓ (Neuts >40% ) | ↑ | ↑ Th1/Th17/Th2 | [50] | ||
| C57BL/6 | OVA +DC(i.t.) +EOS (i.p) # | NA | ↑ | ↑ | ↓ (Neuts >20%) | ↑ | ↑ Th1; ↓Th11/Th17 | [50] | ||
| BALB/c | HDM Chronic | ↑ | ↑*↓* | ↑ | ↑ (↓ Mac ↑ (Neuts 15%) | ↑ | NA | [128] | ||
| iPHIL | <0.1%/<0.1% | C57BL/6 | OVA | ↑ | ↑ | ↑ | ↓ (Neuts >40% )‡ | NA | NA | [45] § |
| C57BL/6 | HDM | NA | ↑ | ↑ | ↓ ( Neuts >30% ) | NA | NA | [45] € | ||
| ΔdblGATA-1 | <0.1%/<0.1% | C57BL/6 | OVA | NA | ↑ | ↑ | ↓ (Neuts >60% ) | NA | NA | [45] € |
| BALB/c | OVA | ↓ | ↓ | TIL-17,IL-5,IL-4≠ | ↓ (Neut >30%; ↓ Mac.) | ↑ Th17 | NA | [82] | ||
| BALB/c | A. Fum. Chronic | NA | ↓ | ↓ | ↓ (Neuts >3-fold, ↓ Mac) | NA | NA | [52] | ||
| BALB/c | HDM Chronic | ↑ | ↑*↑* | ↑ | ↑ (↓ Mac; Neuts NA.) | ↓ | NA | [128] | ||
| MBP-1−/−/EPX−/− | <10%/<10% | C57BL/6 | OVA | NA | ↑ | NA | ↓ (Neuts >15% ) | NA | NA | € |
Th2 Phenotype (Neutrophils <10% of BAL population); Neutrophilic and Mixed Phenotype (Neutrophils calculated to be >15% of total BAL population);
Eosinophils isolated from blood of IL-5 transgenic mice;
Eosinophils isolated from peritoneal cavity IL-5 transgenic intraperitoneally injected with OVA to recruit eosinophils;
IL-13 not measured, other cytokines reported;
Remodeling (collagen deposition);
In press;
Unpublished observation;
Resistant to corticosteroid administration on final day of challenge; NA not available;
↑ up arrow is increased pathologies;
↓ down arrow is decreased pathologies.
The solutions to the needs of next-generation eosinophil-targeting models came with the development of gene knock-in strains of mice exploiting the eosinophil specificity of the endogenous EPX promoter. Specifically, separate knock-in strategies have led to the creation of two strains of mice (iPHIL [45] and eoCRE [46]), expressing the human diphtheria toxin receptor (hDTR) and Cre-recombinase, respectively. In iPHIL mice, only hDTR expressing cells (i.e., eosinophils) are targeted for cell death by DT, lending to its inducible nature. The eoCre strain offers the unique ability of targeting eosinophil-specific gene expression when used in conjunction with strains of animals carrying targeted genes flanked by Cre-recombinase recognition sequences (i.e., loxP repetitive elements). This strategy allows investigators to create strains of mice that modulate gene expression exclusively in the eosinophil lineage. Thus, together these new models are now enabling researchers to refine eosinophil-specific activities in vivo and assess specific roles of eosinophils in both health and disease.
Eosinophil functions in allergen sensitization (primary immune response) of allergen provocation models
The role of eosinophils following allergen sensitization/challenge has been tested in various mouse models of allergic respiratory inflammation using ovalbumin (OVA), house dust mite (HDM), ragweed (RGW), and Aspergillus fumigatus as the targeting allergens. Sensitization is necessary to generate memory T cells that will recognize antigen upon secondary exposure in the airways and mount an adaptive Th2 immune response. The sensitization phases of these protocols are allergen/model specific with intraperitoneal injection of antigen/adjuvant (e.g, OVA/Alum [1]) or through intranasal exposure of antigen alone (e.g., HDM [2]). Several earlier studies suggested eosinophils have the potential to participate in sensitization to allergen to generate the primary immune response (i.e, antigen-specific memory T cells). Eosinophils have the capacity to act as antigen presenting cells [47], they are found at sites of immunization [48, 49], and eosinophil-deficient (PHIL[50] and ΔdlbGATA-1[51]) or eosinophil-attenuated (e.g., IL-5−/−eotaxin-1−/− [20] and CCR3−/− [52]) mice have reduced Th2 T cell responses following allergen provocation. Yet due to the congenital nature of these eosinophil-deficient/attenuated mice, the role of eosinophils during sensitization (i.e., primary immune response) as compared to challenge (i.e, secondary immune responses) could not be determined. However, the development of iPHIL mice has allowed investigators to conditionally depleted eosinophils during the sensitization step and return them to homeostatic levels by the time of allergen challenge [45]. These studies with iPHIL mice showed that the presence of eosinophils was not required during sensitization (primary immune response) to induce Th2 allergic inflammation in both OVA/Alum (intraperitoneal) and HDM (intranasal) models of acute allergen provocation. Interestingly this was confirmed in infection models of Nippostrongylus brasiliensis where primary infection is a lung injury event requiring activation of Th2 responses for worm expulsion. Eosinophils were not required for expulsion of worms upon first infection, yet a secondary infection four weeks after the first exposure in ΔdlbGATA-1 mice demonstrated that both eosinophils and T cells cooperated in expulsions of worms [53]. Collectively, these studies highlight that eosinophils do not participate in generation of the antigen-specific memory T cell pool but rather in secondary immune responses upon allergen provocation in allergic respiratory inflammation.
Eosinophil-deficient mice and adoptive transfer techniques highlight underappreciated roles for eosinophils in the events following allergen challenge (secondary immune responses)
Pulmonary initiating events following allergen provocation
Airway allergen exposure leads to complex downstream pathways that rely on activities of the local stromal and resident cells of the lung to take up antigen and/or produce early innate mediators of inflammation. Allergens are often a mix of innocuous protein, endotoxins, proteases, and/or even pathogens that activate the epithelium, in part, through binding to pattern recognition receptors (PRRs) that recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (reviewed in [54–56]). Activation of PRRs on epithelial cells leads to expression of IL-1α that acts in an autocrine manner to induce expression of cytokines (pro-IL-33, IL-25, and GM-CSF) and chemokines (KC and CCL20) triggering an inflammatory cascade [57]. These early signaling events also lead to the elaboration of other cytokines such as IL-1β and TNF-α that contribute to the early inflammatory phase and activation/recruitment of pro-inflammatory cells. The innate character of these responses was demonstrated in studies where administering recombinant IL-33 to the airways led to recruitment and activation of eosinophils independently of T cells and promoted Th2 cytokine production (IL-4, −5, −13, −9) [58]. IL-33, alone, is sufficient to activate eosinophils, increasing expression of IL-13, TGF-β, and IL-6 as well as adhesion molecules by eosinophils [59, 60] and inducing superoxide production in human eosinophils [61].
These innate interactions at the airway epithelial surface also trigger a complex series of pathways in which eosinophils appear to modulate the accumulating pro-inflammatory cell infiltrate Specifically, epithelial cells release defensins and other antimicrobial factors in response to bacteria, virus, and fungus, which activate macrophages and signal for neutrophils to leave circulation and enter the lung parenchyma [62]. Indeed, kinetic studies in both mouse models of allergic respiratory inflammation [63, 64] and asthma patients [65] have shown that the earliest infiltrating cell to the airways is neutrophils, being recruited within 3–6 hours post allergen challenge. Interestingly, eosinophils are found to recruit in a kinetically similar pattern within 3–6 hours, but unlike neutrophils, maximally peak at 3–4 days after the initial allergen challenge in both mouse models and human subjects. Increasingly, studies have also linked the accumulation of these two granulocytic cell populations along with alternatively activated macrophages as a potentially important regulatory feedback loop to initiate Th2 polarization and ultimately promote resolution of inflammation [66, 67]. For example, the early neutrophil recruitment appears to be an important contributor to the initiation of inflammatory events in the lung as the inhibition of neutrophil recruitment in both mouse models [68] and asthma patients [69] inhibits the evolving Th2 inflammation. Neutrophils likely enhance this cascade by releasing proteases to cleave the pro-form of IL-33 into the active form [70]. For eosinophils in particular, this IL-33 cascade contributes to the production of eotaxins-1 and −2 by endothelial, epithelial, and macrophage cells that are the predominant chemokines for eosinophil recruitment into the lung post allergen challenge [39, 71]. As such mice deficient in IL-33 have attenuated eosinophil recruitment upon allergen challenge [72]. Once eosinophils are localized into the lung they are exposed to survival cytokines IL-5 and GM-CSF, as well as activating cytokines, PRR binding molecules, reactive oxygen species, lipid mediators, growth factors, and proteases. This process does not occur in isolation, eosinophils themselves contribute factors promoting their own recruitment/accumulation. Thus, the number and/or extent of eosinophils accumulation become an important metric that may be a regulatory node influencing the character of the inflammatory response occurring in response to individual provocation events in animal models and in the clinical setting.
Proliferation and polarization of T cells in the lung-draining lymph nodes
Effector T cell production in the lung draining lymph nodes (LDLN) is necessary to induce the pathologies associated with allergen challenge [73]. Adoptive transfer studies utilizing fluorescently tagged antigens have demonstrated that both dendritic cells (DCs) [74, 75] and eosinophils [47, 76] migrate to the LDLN with similar kinetics, are capable of processing antigen, and are capable of inducing Th2 T cell activation in mouse models of respiratory inflammation. DCs have long been presumed to be the central mediators of the secondary T cell activation following allergen challenge. For example, studies showed that DC-deficient mice (e.g., CD11C–DTR [77]) were not able to activate T cells in the LDLN and adoptive transfer of eosinophils did not rescue T cell activation. Yet, recently it was demonstrated that PHIL mice also fail to induce T cell proliferation in LDLN after allergen challenge and have reduced allergen-mediated Th2 pathologies [78]. This suggested a dichotomy of function with both cell types providing significant contributions that lead to allergen-mediated T cell activation following airway provocation.
In vitro studies highlighted that eosinophils were capable of enhancing dendritic cell maturation [79], viral antigen presentation [80], and chemoattraction [81]. Jacobsen, Lee and colleagues showed that eosinophils adoptively transferred into the peritoneal cavity of PHIL mice in an acute OVA provocation model migrated to the LDLN and induced myeloid DC (CD11c+MHCIIhi) accumulation and T cell proliferation in the LDLN after allergen challenge [78]. In these studies, MHC II-deficient eosinophils were equally sufficient as wild type eosinophils in inducing both DC accumulation and T cell proliferation. Similar DC recruitment and MHC II-independent functions of eosinophils have been identified in a model system of parasite infection (J. Appleton, personal communication). The role of eosinophils in these models (parasite and airway allergen challenge) was to not only to recruit DCs and induce proliferation of T cells but also to polarize the immune responses in the LDLN. Significantly, the transfer of bone marrow-derived myeloid DCs into PHIL mice (to bypass DC-deficiency in these animals) led to a mixed production of effector cells Th1 (INF-γ), Th17 (IL-17) and Th2 (IL-13) in the LDLN in response to allergen challenge. This observation was all the more significant as adoptive transfer of eosinophils (intraperitoneal) into these mice led to a suppression of Th1/Th17 responses in the LDLN. Interestingly, increased levels of Th17 cells have also been described in other eosinophil-deficient mice (ΔdblGATA-1/BALB/c) in acute models of allergic respiratory inflammation [82]. These data suggest that eosinophils mediate polarization of effector T cells and in their absence there is an increased Th1/Th17 phenotype. The mechanisms of eosinophil-dependent Th1/Th17 suppression or Th2 amplification is unknown, however eosinophils have been shown to express many molecules capable of polarizing or altering plasticity of effector T cells, which may contribute to asthma phenotype [83]. For example, cell-cell interactions with T cells may occur through MHC II as a third party member [84] or through co-stimulatory molecules (CD28, CD80/86, CD40) to modulate the activation potential and polarization of T cells [47]. Additionally, eosinophils express cytokines (e.g., IL-2 [85], IL-4[86], IDO [87], IL-25 [88] IL-10 [85], and TGF-β [89] (for review [32, 33]) or toll-like receptor ligands [90] (e.g., mitochondrial DNA [91] or granule proteins [79]) that may have demonstrable effects on both DCs and T cells to modulate the Th1/Th17 vs. Th2 polarization of immune responses following airway allergen provocation. These studies suggest a role for eosinophils in the pulmonary compartment of the lung where polarizing events have been demonstrated to occur, such as the BALT or lung draining lymph nodes of patients. As such, incomplete eosinophil ablation from lymphatics in clinical settings (e.g., anti-IL-5 antibody treatment) may not sufficiently abolish important immune regulating functions of eosinophils.
Recruitment of effector T cells to the lung to propagate Th2 inflammation in response to allergen challenge
The pulmonary pathologies associated with asthma inextricably require the recruitment of effector T cells to the lung [92]. Chemokines and lipid mediators are significant inducers of T cell recruitment for both humans and mice. For example, Th2 cells are recruited via CCL17/TARC and CCL22/MDC that bind CCR8 and CCR4, Th1 respond to CCL5/RANTES and CXCL10/IP-10 that bind CCR5 and CXCR3, respectively, while Th17 cells respond to CCL20/MIP3α and bind CCR6 [93]. Consistently, eosinophil-deficient mice (ΔdblGATA-1[52], PHIL [50], iPHIL [45], MBP-1−/−EPX−/− (unpublished observation), CCR3−/− [52], IL-5−/−eotaxin-1−/− [20]), had lower levels of bronchoalveolar (BAL) Th2 cytokine expression, inflammatory cellular infiltrate, mucus production and AHR regardless of background strain (BALB/c [52] vs C57BL/6 [50]) in various mouse models of respiratory inflammation. In part these reduced pathologies are explained by an inability to recruit effector T cells to the lung in response to allergen provocation. The importance of eosinophils in T cell recruitment were made first in PHIL [50] and ΔdblGATA-1 [51] mice using acute OVA models of allergic respiratory inflammation. Specifically, Th2 polarized OVA-specific effector T cells that were transferred intravenously into PHIL mice did not traffic to the lung following OVA airway challenge. However, adoptive transfer of eosinophils into the lungs of mice in conjunction with OVA airway challenge enabled the recruitment of these effector Th2 T cells into the lung through an eosinophil-dependent elaboration of pulmonary TARC and MDC chemokines [50]. In another model, published by Walsh et.al, adoptive transfer of eosinophils intravenously into OVA sensitized ΔdblGATA-1 mice was sufficient to induce T cell recruitment to the lung [51], again demonstrating an eosinophil-dependence on T cell recruitment. In a recent study, Walsh, et.al, have repeated the transfer of polarized Th2 cells into naïve ΔdblGATA-1 mice during OVA airway challenge and showed a requirement for eosinophils to induce pulmonary T cell recruitment [94]. It is also noteworthy that OVA airway challenge of sensitized IL-5−/− mice, which have reduced pulmonary eosinophilia (<90% decrease), was sufficient to induce T cell activation in the LDLN, yet was insufficient to recruit effector T cells to the lung [78]. These studies demonstrate a necessity for pulmonary eosinophils in the recruitment of effector T cells to the lung in mouse models of respiratory inflammation.
One potentially important mechanism of eosinophil-induced effector T cell recruitment is the production of IL-13 and its effects on other resident pulmonary cells [95]. Specifically, IL-13 induces the expression of Th2 chemokines TARC and MDC by several cells (e.g., epithelial cells and monocytes) [96, 97] as well as enhances amplification of eosinophil accumulation through upregulation of eotaxin [39]. In the absence of eosinophils, IL-13 expression is reduced in the BAL or lungs of allergen challenged mice (ΔdblGATA-1[52], PHIL [50], iPHIL [45], MBP-1−/−EPX−/− (unpublished observation), CCR3−/− [52], and IL-5−/−eotaxin-1−/−[20]). Moreover, over-expression of IL-13 was insufficient to bypass eosinophil-dependent Th2 inflammation in eosinophil-deficient mice [98], suggesting IL-13 and eosinophils act in a coordinated manner to induce pulmonary Th2 responses. The direct role of eosinophil-derived IL-13 was highlighted in studies by Walsh, August, and colleagues that demonstrated adoptive transfer of IL-13−/−eosinophils into ΔdblGATA-1 mice did not restore T cell recruitment or Th2 inflammation to the same degree as transfer of wild type eosinophils in a model of allergic respiratory inflammation [94]. Our ongoing studies have shown that the differential activation of eosinophils (e.g., culture with IL-33 or GM-CSF [60]) prior to transfer into the lungs of PHIL mice during allergen challenge led to altered abilities to activate and recruit effector T cells to the pulmonary compartment (unpublished observations). Moreover, in agreement with Walsh and Avery and colleagues [94], our preliminary data also demonstrated, that activated IL-13−/− eosinophils transferred into the lungs of allergen sensitized/challenged PHIL mice failed to induce T cell recruitment to the lung. These data suggest that eosinophil-mediated T cell recruitment to the lung is, in part, occurring via eosinophil-derived IL-13 mediating events. One could hypothesize that targeting both IL-13 and eosinophils may lead to a better response in patient outcome than targeting either alone.
The suggested importance of eosinophil-derived IL-13 must be put in context with recent studies demonstrating the purported importance of IL-13 from innate lymphoid helper cells (ILC2s) following allergen provocation (reviewed in [99, 100]). In T cell-dependent models of allergic respiratory inflammation (i.e., OVA/Alum and HDM models), ILC2s (Lin−, CD90+CD25+IL-7Rα+ ICOS+ST2var [101]) contributed to only 30% of the total BAL and lung-derived IL-13 when compared to other Th2 cells in the airways [102]. In an OVA/Alum model, IL-13 producing ILC2 cells were reduced in numbers as the length between sensitization and challenged increased (12-days post-sensitization (73%) vs 25-days post-sensitization (45%)) [103]. Additionally, the number of eosinophils in the airways and lung is markedly larger than the contribution of ILC2s, suggesting that eosinophils may contribute significantly to the overall level of IL-13 in the airways and lungs upon allergen provocation. The implication of these observations is that ILC2s are not necessarily the primary and/or exclusive source of this important Th2 cytokine in T cell-dependent allergen provocation. This observation must be contrasted, and not confused, with the more abundant studies demonstrating the importance of ILC2-derived IL-13 in innate/primary immune responses (reviewed in [104]). Specifically, these studies rely on IL-13 fluorescent reporter mice, “deleter mice” that kill the cytokine expressing cell, or on transfers of ILC2 into lymphocyte deficient mice. In these models, ILC2s were the majority (upwards to 70%) of non-T cells expressing IL-13 following either airway delivery of recombinant IL-33 and IL-25 or primary N. brasiliensis infection [105, 106]).
Although ILC2s are likely to play a role in innate Th2 events, eosinophils may potentiate ILC2 activities as well as act independently to innate signals. For example, eosinophils cultured with IL-33 prior to transfer into lungs of sensitized PHIL mice were sufficient to induce IL-13 production in the airways after allergen challenge (unpublished observations). It is unlikely that the IL-13 production was dependent solely on ILC2s in this model as both PHIL (unpublished data) and ΔdblGATA-1 [107] mice have similar levels of ILC2s as wild type mice. Additionally, ILC2 activities should remain functional in these mice as both eosinophil-deficient strains have normal pulmonary ST2 receptor (IL-33 receptor) and IL-33 expression in allergen models [82]. This suggests that the IL-33 activation of eosinophils was essential to the production of IL-13 in these mice upon allergen provocation. Additionally, eosinophils may potentiate the activities of ILC2 to induce Th2 events. For example, eosinophils may modulate ILC2-mediated IL-13 production through the release of eosinophil-derived IL-2 [85] and IL-25 [88]. To date the literature suggests differential IL-13 production by eosinophils in T-cell dependent models and by ILC2 in innate T-cell independent models of pulmonary inflammation. Yet these roles do not necessarily need to be exclusionary, but may be intertwined depending on the immune microenvironment in the lung. Moreover, the relative importance of ILC2s and eosinophils may depend on the asthma phenotype in patients (e.g., intrinsic, environmental exposure, or allergen provocation) as well as the respiratory inflammation model used in mice.
Th2 inflammation and lung remodeling events
Eosinophils release of a plethora of mediators (e.g., IL-13, TGF-β, metalloproteases, VEGF, leukotrienes, and granule protein activities [29–33] that not only propagate Th2 inflammation but, also, lead to lung remodeling and dysfunction in chronic settings. Historically, eosinophil granule proteins were considered the primary agents that induced lung remodeling in asthma patients, particularly in the clinical setting where immunohistochemistry of severe asthmatic biopsies demonstrated significant release and deposition of granules and granule constituents around the airways (see for example [108]). Moreover, studies with purified granule proteins administered to cultured human cells [109], non-human primates [110], and animal models [111] showed these that specific proteins (e.g., MBP-1,ECP, EPX) were sufficient to induce cell killing and tissue damage as well as induce AHR (reviewed in [35]). Confirmation for a role in remodeling was suggested in clinical studies where inhibition of eosinophils with antibodies to IL-5 reduced airway remodeling [8] and deposition of extracellular matrix [112]. Yet, as described previously, antibody treatment with IL-5 failed to improve lung function in patients in clinical studies despite reduced exacerbations [7, 8] and remains to be fully characterized in clinical studies. The lack of apparent correlations with lung function may simply reflect an inability to completely deplete eosinophils/eosinophil products in the pulmonary compartment in patients or perhaps the more complex suggestion that the role of eosinophils and the release of their granule proteins have different and previously underappreciated roles that do not have direct/acute links with changes in lung function parameters. Moreover, new insights into the ability of eosinophils to interact through neuroimmune pathways [113] and directly modulate neuronal branching [114], through unknown mechanisms, will provide insight into thier potential to modulate and remodel the peripheral nerve responses in bronchoconstriction, cough, and hyperreactivity.
Although eosinophil degranulation is not evident in acute allergen models, severe asthma models have highlighted a direct role for eosinophils in lung remodeling and dysfunction. In particular, this was highlighted in studies crossing transgenic mouse models of severe asthma with eosinophil-deficient mice. Mice that over-express IL-5 and eotaxin-2 (I5/hE2) develop significant eosinophilia in the lungs and airways that degranulate extensively. The prominent eosinophil airways infiltration and degranulation resulted in significant lung remodeling including smooth muscle thickening, goblet metaplasia/mucus production, basement membrane thickening, and AHR that was all abolished by crossing these mice with PHIL (I5/hE2/PHIL) mice to deplete eosinophils [115]. A similar chronic Th2 inflammation model that crossed IL-13 transgenic mice with ΔdblGATA-1 showed that the eosinophil-deficient mice had reduced mucus, TGF-β, and proteases (cathepsins B, S, and MMP-13) despite the elevated levels of IL-13 [116]. Studies of chronic OVA allergen challenge of ΔdblGATA-1(BALB/c) mice also demonstrated that eosinophils participated in collagen deposition and smooth muscle proliferation [41]. Similarly chronic allergen challenge with A. fumigatus showed a decrease in mucus production in ΔdblGATA-1(BALB/c) mice [52]. Collectively, these studies provide evidence of a causative link between eosinophils and remodeling events in the lung that is now supported through the use of multiple independent mouse models of respiratory inflammation.
Role of eosinophils in resolution of inflammation
Inflammation is a host response to injury or infection that represents a shift from baseline homeostasis that is generally self-limiting due to host inflammation resolving mechanisms. Failure in resolution of inflammation must be viewed as part of the collective causative mechanisms (i.e., exacerbations) leading to chronic respiratory inflammation in diseases such as asthma [67]. Resolution is an active process initiated by the inflammatory cascade that is not immunosuppressive but rather switches inflammatory pathways toward resolving pathways. These resolving pathways essentially blunt the inflammatory cascade by blocking granulocyte entry and enhancing the uptake of inflammatory cells by macrophages [66]. These activities are primarily carried out by the release of products from the 5-lipoxygenase (lipoxin A4) and 12/15-lipoxygenase (resolvins and protectins) pathways [117]. Their importance is highlighted in studies where deficiencies in the 12/15-pathway [118] and in protectin D1 [119, 120] production have been associated with severe asthma in patients and in mouse allergic respiratory models[121]. Significantly, eosinophils produce these lipid mediators and may have a potential role in directly suppressing inflammation [119, 122].
The role of eosinophils in the resolution of inflammation also likely extends beyond cell autonomous events and includes pathways resulting from interactions with other pro-inflammatory and resident cells in the lung. Macrophages, in particular, are significant potential targets of eosinophils for inducing resolution. Macrophages are necessary to clear dead cells (efferocytosis) as part of the resolution process and produce resolution lipid mediators (reviewed in [123, 124]). In brief, it is suggested that IL-4/13 are essential to induce the polarization of macrophage from an M1 to M2 phenotype to then enable the transitions from M2 to a resolution phenotype macrophage (i.e., increased phagocytic activity and production of resolving lipid mediators). IL-4/IL-13 and 12/15-lipoxygenase products induce M2 macrophage to convert to an early phase resolution macrophage rM (rM; F4/80+CD11bhi, arginaseI+, iNOS−) that have high efferocytosis activity at the site of inflammation and are necessary to clear inflammation. The extensive activities of rM macrophage results in an ‘exhausted’ phenotype (Mres; F4/80+CD11blow, arginaseI−, iNOS−) that is propagated by exposure to TGF-β leading to reduced efferocytosis, migration to secondary lymphatics, and a return to systemic homeostasis.
Eosinophils have the potential to modulate macrophage activities in resolution through various mechanisms. For example, activated eosinophils that express IL-4/13 have been demonstrated in vitro to polarize macrophage to an M2 phenotype [60, 71], the precursor to resolution macrophage rM/Mres. Eosinophils have been shown to contribute to increased numbers of M2 macrophage in other disease models as well [125]. Evidence of this eosinophil-macrophage interactions are supported by in vivo studies demonstrating reduced macrophage recruitment in allergen models of ΔdlbGATA-1 [52, 82, 116] and PHIL mice ([126] and unpublished observations). Eosinophil-derived protectin D1 was shown, in a non-allergen model, to induce activities of macrophage to clear apoptotic neutrophils from the site of inflammation [127]. Thus, through the release of lipid mediators as well as TGF-β, eosinophils potentially contribute to the activities of resolution macrophage and therefore contribute to a return to homeostasis. It is interesting to speculate that as eosinophils and neutrophils are both early recruits to the inflammatory processes, eosinophils may aid in the subsequent direct suppression of neutrophils as well as polarization and activation of resolution macrophages. Furthermore, failure to complete this cascade, such as in the absence of eosinophils, a neutrophilic inflammatory phenotype may predominate.
Eosinophils: Causative players or diagnostic metrics of asthma phenotype
Asthma phenotypes are distinguished by the induced cellular infiltrate, patient medical history, and/or responsiveness to available drug therapies such as corticosteroids [24, 25]. Generally neutrophilic and eosinophilic asthma phenotypes are considered distinct entities with unique cytokine pathways (Th1/Th17 versus Th2, respectively). However, our recent studies in eosinophil-deficient mice may have highlighted a previously underappreciated role for eosinophils in modulating the phenotype of asthma. We propose that the roles of eosinophils are complex and are part of homeostasis-maintaining mechanisms initiated by inflammatory pathways. That is, instead of exclusively pathology causing effector cells, eosinophils elicit immune modulating responses that promote local tissue remodeling/repair as part of the return to homeostasis (as reviewed in [31]). This larger perspective of eosinophils activities is highlighted by observations in allergen provocation models on the development of pulmonary Th2 responses and the modulation of airway neutrophil recruitment/accumulation. Specifically, over the last decade using several independently-derived eosinophil-deficient strains of mice we [40, 45] and others [41, 51, 52, 82, 128] have shown that these animals fail to develop an airway eosinophilia in models of allergic respiratory inflammation. However, we have also shown that individual eosinophil-deficient mice may instead develop an allergen-dependent airway neutrophilia; the frequency of which appears to vary in response to extrinsic cues [45]. These variant airway phenotypes are particularly significant because in addition to the neutrophilic character of the airway infiltrate (>15% neutrophils), these animals display significant allergen-induced histopathological changes and elevated BAL IL-13 cytokine levels that are resistant to corticosteroid treatment [45]. The specific mechanisms responsible for these shifts in phenotypes are unknown but gene/environment interactions are likely to be significant contributors [129, 130]. Particularly in mouse models of allergic respiratory inflammation, the commensal bacterial flora has been demonstrated to play significant roles in systemic immunity [131, 132], asthma susceptibility [133, 134], and is easily transmissible even in specific pathogen free facilities [135, 136]. A recent publication by Chu et.al., demonstrated a direct correlation between the composition of commensal bacteria in the gastrointestinal tract and the presence or absence of eosinophils [137]. These data demonstrated an increased load of Bacteriodetes relative to Firmcutes, concomitant with alterations in IgA production in both PHIL and ΔdblGATA-1 mice at baseline homeostasis. We hypothesize this eosinophil-dependent altered microbiome may represent a significant mechanism of immune regulation that potentially may lead to the increased neutrophilic and/or mixed asthma phenotype upon allergen challenge in eosinophil-deficient mice. Commensal bacterial composition as a predictor for asthma phenotype and susceptibility is not unique to animal models of respiratory inflammation and is proposed to be a contributing mechanism in asthma patients as well [26–28]. The appearance of this induced neutrophilic or mixed Th2/Th17 phenotype in the mouse models of respiratory inflammation occurred regardless of strain (C57BL/6 or BALB/C) or mechanism of depletion of eosinophils (iPHIL, ΔdblGATA-1, PHIL, MBP-1−/−/EPX−/−) ([45] and unpublished observation). It is noteworthy that an eosinophil-dependent neutrophilia has been shown by other investigators as well and is not a model/investigator specific observation. Studies with ΔdblGATA-1 mice in an acute OVA model demonstrated these mice had greater than 30% neutrophilia in the airways (detected by differentials as well as flow cytometry) as well as increased IL-17 expression [82]). In a A. fumigatus provocation model ΔdblGATA-1 mice were shown to have increased neutrophils as compared to wild type mice and allergen-induced AHR than saline treated mice [52].
The ability of eosinophils to directly influence the development of a neutrophilic phenotype has been demonstrated in several studies. For example, adoptively transferred eosinophils reduced airway neutrophilia and Th1/Th17 polarization in OVA allergen sensitized/challenged PHIL mice [78]. The inducible nature of iPHIL mice permitted us to determine the consequence of eosinophil depletion on an otherwise wild type mouse. Depletion of eosinophils by DT administration during allergen challenge resulted in airway neutrophilia. This neutrophilic phenotype was reversed to an eosinophilic and Th2 character by stopping DT administration prior to a second allergen challenge at a time when eosinophil levels had returned to baseline levels [45]. These studies showed that the appearance of neutrophilic respiratory variants were not a developmental consequence of a dysregulated immune responses as a result of a congenital eosinophil deficiency (as in ΔdblGATA-1, PHIL, MBP-1−/−/EPX−/− mice) but instead a direct result of losing one or more eosinophil-mediated activities during the immune responses to allergen. In essence eosinophils act as a “check point” for inflammation phenotype. Although much remains to be defined, eosinophils potentially suppress Th1/Th17 polarization events, block neutrophil recruitment, and promote neutrophil clearance through activation of macrophage. The translation of the insights gained from these reductionist approaches to patient studies holds great promise in our understanding of the role eosinophils in the events leading to different asthma phenotypes in patients.
Conclusion
The studies highlighted in this review underline the wider importance of eosinophils as regulators of the immune responses in allergic inflammation, suggesting that they are not simply downstream mediators of other inflammatory cells but important contributors to the evolving responses that follow allergen provocation of sensitized subjects. Specifically, through the use of allergen provocation models and eosinophil-deficient mice, novel roles for eosinophils have been identified that exemplify an underappreciated scope and complexity of effector functions. Most significantly, eosinophils were recently demonstrated to participate in the modulation of immune responses necessary for the induction, propagation, polarization, and potentially resolution of allergic inflammation. Eosinophils perform many of these functions through both direct effects and interactions with other cells. For example, eosinophil-derived IL-13 is associated with eosinophil-dependent recruitment of effector Th2 cells to the lung after allergen provocation. Potentially, eosinophils and ILC2s act in concert to amplify this response as eosinophils also produce ILC2 modulating cytokines (e.g., IL-2 and IL-25). Eosinophils also uniquely promote accumulation of DCs and antigen-specific Th1/Th17 suppression, and thus increased Th2 polarization in the lung draining lymph nodes upon allergen challenge. Finally, eosinophils have been shown to modulate the polarization/phenotype in allergen provocation models of eosinophil-deficient mice. The mechanisms/pathways by which eosinophils mediated these effects on aeroallergen-mediated immune responses remain to be defined. However, through the release of IL-4/13, 12/15-lipoxygenase products (resolvins and protectins), and TGF-β eosinophils may regulate the unique balance necessary between Th1/Th17/Th2 immune responses, inhibit/suppress the development of airway neutrophil accumulation, and promote the activation of M2 and resolution macrophages following allergen provocation. Future studies are clearly required to fully understand the eosinophil-dependent and eosinophil-contributory mechanisms linked with the immune regulation and lung remodeling/repair that occur following allergen provocation. Our recent development of novel eosinophil-inducible and eosinophil-specific Cre-expressing mice will enable further clarification of these eosinophil-dependent and eosinophil–specific functions. Moreover, the use of these strains of mice and the ever-increasing availability of other mouse models will provide the necessary resources to re-define the roles of eosinophils in asthma and other diseases as well as the unique roles of eosinophils necessary to maintain homeostasis in otherwise healthy subjects.
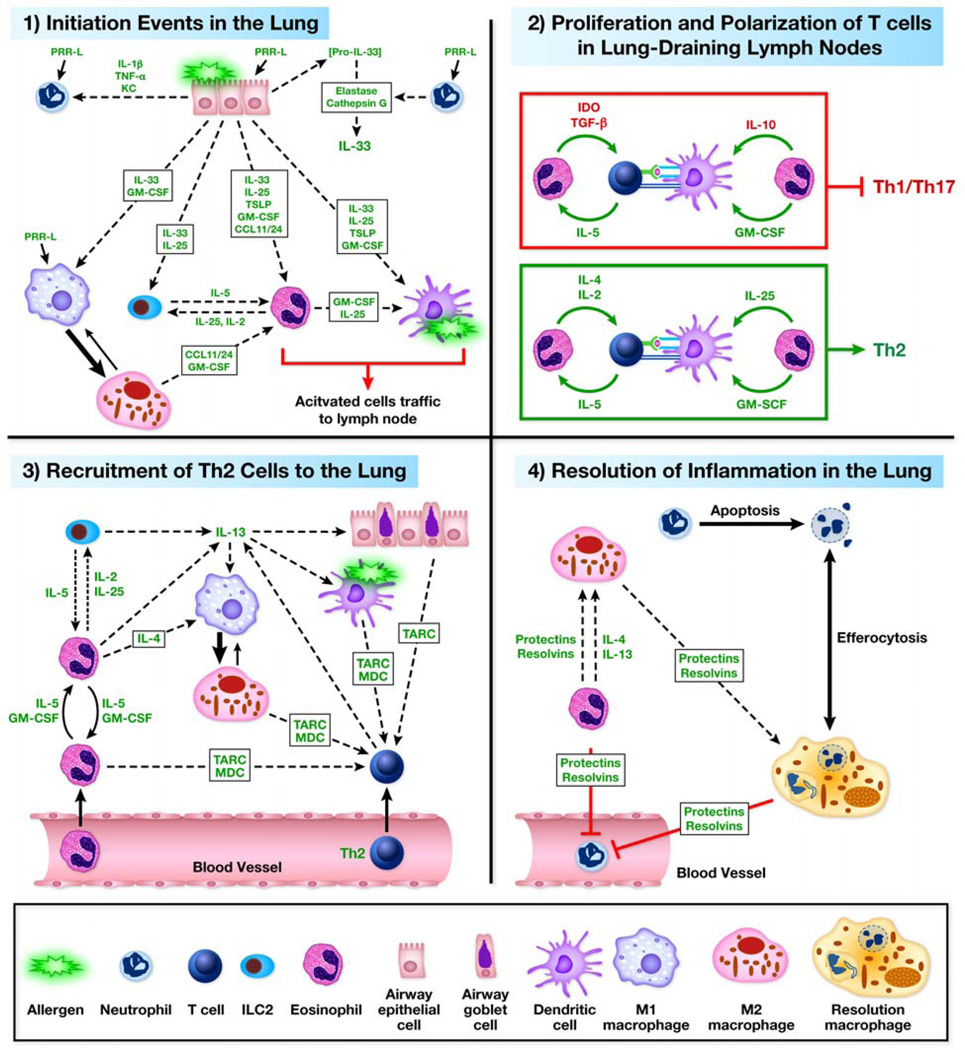
Figure 1. Eosinophil Functions in Allergic Respiratory Inflammation.
Through the use of eosinophil-deficient/attenuated strains of mice and adoptive transfer techniques in models of allergic respiratory inflammation various effector functions of eosinophils have been identified. These effector functions include role(s) in (1) Initiation, (2) Polarization/Proliferation, (3) Recruitment of Th2 cells/Th2 propagation, and (4) Resolution of the inflammatory events. (1) Initiation. Stromal cells and resident leukocytes respond to airway allergens by producing cytokines, chemokines, and other mediators that aid in the recruitment and activation of eosinophils to the lung. Eotaxin-1 (CCL11) and eotaxin-2(CCL24) by epithelial cells, macrophage, and endothelial cells recruit eosinophils to the lung from the periphery. L-33 and GM-CSF aid in activation and survival of eosinophils once in the lung. Eosinophils enhance their survival via autocrine and paracrine release of mediators (e.g., IL-5 and GM-CSF). Eosinophils enhance the activities of other cells through release of mediators (e.g., cytokine, reactive oxygen species, leukotrienes, lipids, and granule proteins). (2) Polarization/Proliferation. Once eosinophils are primed in the lung they migrate to the lung draining lymph node (LDLN) to aid in the secondary immune response. In particular, eosinophils are necessary for antigen-specific T cell proliferation in an MHC II-independent manner in the LDLN through inducing accumulation of DCs. Eosinophils suppress Th1/Th17 polarization and enhance Th2 polarization through release of mediators that are undefined, but may include IDO, TGF-β, and IL-10. (3) Recruitment of Th2 cells/Th2 Propagation. Eosinophils are necessary to induce recruitment of effector Th2 T cells through the production of TARC and MDC. Eosinophils enhance accumulation and activation of DCs. Eosinophils aid in the propagation of inflammatory responses through production of IL-4 and IL-13, as well as other mediators (e.g., cytokine, reactive oxygen species, leukotrienes, lipids, and granule proteins). (4) Resolution. Eosinophils may contribute to the resolution process by polarization of macrophage to M2 (via IL-4/13) and through release of protectins and resolvins (12/15-lipoxygenase products). Failure to polarize toward Th2 and to resolve inflammation may predispose the inflammation toward a neutrophilic/Th1/Th17 phenotype.
Acknowledgements
The authors wish to thank the invaluable contribution of numerous individuals from within Lee Laboratories not listed as authors, including Dr. Sergei Ochkur, Alfred Doyle, Katie Zellner, Dana Colbert, Will LeSuer, Cheryl Protheroe, Huijun Luo, Charlie Kern, and Linda Mardel as well as the tireless efforts of the Mayo Clinic Arizona medical graphic artist Marv Ruona.
Funding
This work was supported by grants from National Institutes of Health (JJL) and (NAL), American Heart Association (EAJ), and Mayo Foundation for Medical Education and Research.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Kumar RK, Herbert C, Foster PS. The "classical" ovalbumin challenge model of asthma in mice. Curr Drug Targets. 2008;9:485–494. doi: 10.2174/138945008784533561. [DOI] [PubMed] [Google Scholar]
- 2.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends In Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 4.Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42:712–737. doi: 10.1111/j.1365-2222.2011.03854.x. [DOI] [PubMed] [Google Scholar]
- 5.Kung TT, Stelts DM, Zurcher JA, Adams GK, Egan RW, Kreutner W, et al. Involvement of IL-5 in a murine model of allergic pulmonary inflammation - prophylactic and therapeutic effect of an anti-IL-5 antibody. Am J Respir Cell Mol Biol. 1995;13:360–365. doi: 10.1165/ajrcmb.13.3.7654390. [DOI] [PubMed] [Google Scholar]
- 6.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 7.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 8.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 10.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–1096. e5. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bochner BS. Verdict in the case of therapies versus eosinophils: The jury is still out. J Allergy Clin Immunol. 2004;113:3–9. doi: 10.1016/j.jaci.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel SE. Eosinophils in asthma--closing the loop or opening the door? N Engl J Med. 2009;360:1026–1028. doi: 10.1056/NEJMe0900334. [DOI] [PubMed] [Google Scholar]
- 13.Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013 doi: 10.1111/all.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model [see comments] J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur M, Herrmann K, Li X, Qin Y, Weinstock J, Elliott D, et al. TRFK-5 reverses established airway eosinophilia but not established hyperresponsiveness in a murine model of chronic asthma. Am J Respir Crit Care Med. 1999;159:580–587. doi: 10.1164/ajrccm.159.2.9712018. [DOI] [PubMed] [Google Scholar]
- 18.Hogan SP, Koskinen A, Foster PS. Interleukin-5 and eosinophils induce airway damage and bronchial hyperreactivity during allergic airway inflammation in BALB/c mice. Immunol Cell Biol. 1997;75:284–288. doi: 10.1038/icb.1997.43. [DOI] [PubMed] [Google Scholar]
- 19.Shen HH, Ochkur SI, McGarry MP, Crosby JR, Hines EM, Borchers MT, et al. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J Immunol. 2003;170:3296–3305. doi: 10.4049/jimmunol.170.6.3296. [DOI] [PubMed] [Google Scholar]
- 20.Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil's role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167:199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 22.Hambly N, Nair P. Monoclonal antibodies for the treatment of refractory asthma. Curr Opin Pulm Med. 2014;20:87–94. doi: 10.1097/MCP.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 23.Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Disc. 2012;11:958–972. doi: 10.1038/nrd3792. [DOI] [PubMed] [Google Scholar]
- 24.Pavord ID. Eosinophilic phenotypes of airway disease. Ann Am Thorac Soc. 2013;10(Suppl):S143–S149. doi: 10.1513/AnnalsATS.201306-168AW. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal MN. Genetic, epigenetic, and environmental factors in asthma and allergy. Ann Allergy Asthma Immunol. 2012;108:69–73. doi: 10.1016/j.anai.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Dulek DE, Peebles RS. Bacteria and asthma: more there than we thought. Exp Rev Resp Med. 2011;5:329–332. doi: 10.1586/ers.11.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell SL, Finlay BB. The impact of gut microbes in allergic diseases. Curr Opin Gastroenterol. 2012;28:563–569. doi: 10.1097/MOG.0b013e3283573017. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2012 doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120:3882–3890. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in Health and Disease: The LIAR Hypothesis. Clin Exp Allergy. 2010;40:563–575. doi: 10.1111/j.1365-2222.2010.03484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- 33.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011;343:57–83. doi: 10.1007/s00441-010-1049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, et al. Human versus mouse eosinophils: “That which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130:572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 36.Rothenberg ME, MacLean JA, Pearlman E, Luster AD, Leder P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J Exp Med. 1997;185:785–790. doi: 10.1084/jem.185.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The Eotaxin Chemokines and CCR3 Are Fundamental Regulators of Allergen-Induced Pulmonary Eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 38.Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, et al. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci U S A. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pope SM, Fulkerson PC, Blanchard C, Saito Akei H, Nikolaidis NM, Zimmermann N, et al. Identification of a cooperative mechanism involving IL-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. Epub 2005 Jan 12. [DOI] [PubMed] [Google Scholar]
- 40.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 41.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 42.Nei Y, Obata-Ninomiya K, Tsutsui H, Ishiwata K, Miyasaka M, Matsumoto K, et al. GATA-1 regulates the generation and function of basophils. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1311668110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majewski IJ, Metcalf D, Mielke LA, Krebs DL, Ellis S, Carpinelli MR, et al. A mutation in the translation initiation codon of Gata-1 disrupts megakaryocyte maturation and causes thrombocytopenia. Proc Natl Acad Sci U S A. 2006;103:14146–14151. doi: 10.1073/pnas.0606439103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiyama D, Tanaka M, Kitajima K, Zheng J, Yen H, Murotani T, et al. Differential context-dependent effects of friend of GATA-1 (FOG-1) on mast-cell development and differentiation. Blood. 2008;111:1924–1932. doi: 10.1182/blood-2007-08-104489. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen EA, Lesuer WE, Willetts L, Zellner KR, Mazzolini K, Antonios N, et al. Eosinophil activities modulate the immune/inflammatory character of allergic respiratory responses in mice. Allergy. 2014;69:315–327. doi: 10.1111/all.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doyle AD, Jacobsen EA, Ochkur SI, Willets L, Shim K, Neely J, et al. Homologous recombination into the eosinophil peroxidase locus generates a strain of mice expressing Cre recombinase exclusively in eosinophils. J Leukoc Biol. 2013;94:17–24. doi: 10.1189/jlb.0213089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akuthota P, Wang H, Weller PF. Eosinophils as antigen-presenting cells in allergic upper airway disease. Curr Opin Allergy Clin Immunol. 2010;10:14–19. doi: 10.1097/ACI.0b013e328334f693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kool M, Soullie T, van Nimwegen M, Willart MA, Muskens F, Jung S, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, et al. Allergic Pulmonary Inflammation in Mice is Dependent on Eosinophil-induced Recruitment of Effector T Cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walsh ER, Sahu N, Kearley J, Benjamin E, Kang BH, Humbles A, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. 2014;151:1–15. doi: 10.1016/j.clim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 55.Minnicozzi M, Sawyer RT, Fenton MJ. Innate immunity in allergic disease. Immunol Rev. 2011;242:106–127. doi: 10.1111/j.1600-065X.2011.01025.x. [DOI] [PubMed] [Google Scholar]
- 56.Lambrecht BN, Hammad H. Asthma: the importance of dysregulated barrier immunity. Eur J Immunol. 2013;43:3125–3137. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 57.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, et al. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J Exp Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 59.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 60.Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 Exacerbates Eosinophil-Mediated Airway Inflammation. J Immunol. 2010;185:3472–3480. doi: 10.4049/jimmunol.1000730. [DOI] [PubMed] [Google Scholar]
- 61.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tecle T, Tripathi S, Hartshorn KL. Review: Defensins and cathelicidins in lung immunity. Innate Immun. 2010;16:151–159. doi: 10.1177/1753425910365734. [DOI] [PubMed] [Google Scholar]
- 63.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal Association between Airway Hyperresponsiveness and Airway Eosinophilia in Ovalbumin-Sensitized Mice. Am J Respir Crit Care Med. 2001;163:721–730. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 64.Ohkawara Y, Lei XF, Stampfli MR, Marshall JS, Xing Z, Jordana M. Cytokine and eosinophil responses In the lung, peripheral blood, and bone marrow compartments In a murine model of allergen-induced airways inflammation. Am J Respir Cell Mol Biol. 1997;16:510–520. doi: 10.1165/ajrcmb.16.5.9160833. [DOI] [PubMed] [Google Scholar]
- 65.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmscher S, et al. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–97. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 66.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levy BD, Vachier I, Serhan CN. Resolution of inflammation in asthma. Clin Chest Med. 2012;33:559–570. doi: 10.1016/j.ccm.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koga H, Miyahara N, Fuchimoto Y, Ikeda G, Waseda K, Ono K, et al. Inhibition of neutrophil elastase attenuates airway hyperresponsiveness and inflammation in a mouse model of secondary allergen challenge: neutrophil elastase inhibition attenuates allergic airway responses. Resp Res. 2013;14:8. doi: 10.1186/1465-9921-14-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gauvreau GM, Boulet LP, Schmid-Wirlitsch C, Cote J, Duong M, Killian KJ, et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Resp Res. 2011;12:140. doi: 10.1186/1465-9921-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, et al. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci U S A. 2012;109:1673–1678. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 72.Oboki K, Ohno T, Kajiwara N, Arae K, Morita H, Ishii A, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci U S A. 2010;107:18581–18586. doi: 10.1073/pnas.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008;26:205–232. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 74.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H-B, Ghiran I, Matthaei K, Weller PF. Airway eosinophils: allergic inflammation recruited professional antigen-presenting cells. J Immunol. 2007;179:7585–7592. doi: 10.4049/jimmunol.179.11.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ. Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation. J Immunol. 2011;187:6059–6068. doi: 10.4049/jimmunol.1102299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davoine F, Cao M, Wu Y, Ajamian F, Ilarraza R, Kokaji AI, et al. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69–77. e1–e2. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 81.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 82.Cho KA, Suh JW, Sohn JH, Park JW, Lee H, Kang JL, et al. IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L429–L440. doi: 10.1152/ajplung.00252.2011. [DOI] [PubMed] [Google Scholar]
- 83.Prussin C, Yin Y, Upadhyaya B. TH2 heterogeneity: Does function follow form? J Allergy Clin Immunol. 2010;126:1094–1098. doi: 10.1016/j.jaci.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niu N, Laufer T, Homer RJ, Cohn L. Cutting edge: Limiting MHC class II expression to dendritic cells alters the ability to develop Th2- dependent allergic airway inflammation. J Immunol. 2009;183:1523–1527. doi: 10.4049/jimmunol.0901349. [DOI] [PubMed] [Google Scholar]
- 85.Woerly G, Roger N, Loiseau S, Capron M. Expression of Th1 and Th2 immunoregulatory cytokines by human eosinophils. Inter Arch Allergy Immunol. 1999;118:95–97. doi: 10.1159/000024038. [DOI] [PubMed] [Google Scholar]
- 86.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 87.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, et al. Cutting Edge: Human Eosinophils Regulate T Cell Subset Selection through Indoleamine 2,3-Dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 88.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wong DT, Elovic A, Matossian K, Nagura N, McBride J, Chou MY, et al. Eosinophils from patients with blood eosinophilia express transforming growth factor beta 1. Blood. 1991;78:2702–2707. [PubMed] [Google Scholar]
- 90.Kvarnhammar AM, Cardell LO. Pattern-recognition receptors in human eosinophils. Immunology. 2012;136:11–20. doi: 10.1111/j.1365-2567.2012.03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 92.Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126:1081–1091. doi: 10.1016/j.jaci.2010.06.025. quiz 92–3. [DOI] [PubMed] [Google Scholar]
- 93.MacLeod MK, Kappler JW, Marrack P. Memory CD4 T cells: generation, reactivation and reassignment. Immunology. 2010;130:10–15. doi: 10.1111/j.1365-2567.2010.03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walsh ER, Thakar J, Stokes K, Huang F, Albert R, August A. Computational and experimental analysis reveals a requirement for eosinophil-derived IL-13 for the development of allergic airway responses in C57BL/6 mice. J Immunol. 2011;186:2936–2949. doi: 10.4049/jimmunol.1001148. [DOI] [PubMed] [Google Scholar]
- 95.Grunig G, Corry DB, Reibman J, Wills-Karp M. Interleukin 13 and the evolution of asthma therapy. Am J Clin Exp Immunol. 2012;1:20–27. [PMC free article] [PubMed] [Google Scholar]
- 96.Crapster-Pregont M, Yeo J, Sanchez RL, Kuperman DA. Dendritic cells and alveolar macrophages mediate IL-13-induced airway inflammation and chemokine production. J Allergy Clin Immunol. 2012;129:1621–1627. e3. doi: 10.1016/j.jaci.2012.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berin MC, Eckmann L, Broide DH, Kagnoff MF. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am J Respir Cell Mol Biol. 2001;24:382–389. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]
- 98.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006;169:2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nature Immunology. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 100.Li BW, Hendriks RW. Group 2 innate lymphoid cells in lung inflammation. Immunology. 2013;140:281–287. doi: 10.1111/imm.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 102.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 103.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, et al. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198. e1–e4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 104.Walker JA, McKenzie AN. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol. 2013;25:148–155. doi: 10.1016/j.coi.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley RM. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–1194. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filley WV, Holley KE, Kephart GM, Gleich GJ. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet. 1982;2:11–16. doi: 10.1016/s0140-6736(82)91152-7. [DOI] [PubMed] [Google Scholar]
- 109.Young JD, Peterson CG, Venge P, Cohn ZA. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986;321:613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- 110.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991;87:1470–1473. doi: 10.1172/JCI115155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coyle AJ, Ackerman SJ, Burch R, Proud D, Irvin CG. Human eosinophil granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J Clin Invest. 1995;95:1735–1740. doi: 10.1172/JCI117850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol. 2001;107:211–218. doi: 10.1067/mai.2001.112940. [DOI] [PubMed] [Google Scholar]
- 114.Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One. 2011;6:e22029. doi: 10.1371/journal.pone.0022029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, et al. Co-Expression of IL-5 and Eotaxin-2 in Mice Creates an Eosinophil-Dependent Model of Respiratory Inflammation with Characteristics of Severe Asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 116.Fulkerson PC, Fischetti CA, Rothenberg ME. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am J Pathol. 2006;169:2117–2126. doi: 10.2353/ajpath.2006.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, et al. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32:1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 119.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miyata J, Fukunaga K, Iwamoto R, Isobe Y, Niimi K, Takamiya R, et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 121.Hajek AR, Lindley AR, Favoreto S, Jr, Carter R, Schleimer RP, Kuperman DA. 12/15-Lipoxygenase deficiency protects mice from allergic airways inflammation and increases secretory IgA levels. J Allergy Clin Immunol. 2008;122:633–639. e3. doi: 10.1016/j.jaci.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Isobe Y, Kato T, Arita M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol. 2012;3:270. doi: 10.3389/fimmu.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ariel A, Serhan CN. New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Fronti Immunol. 2012;3:4. doi: 10.3389/fimmu.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am J Respir Crit Care Med. 2011;183:179–188. doi: 10.1164/rccm.200905-0736OC. [DOI] [PubMed] [Google Scholar]
- 127.Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB Journal. 2011;25:561–568. doi: 10.1096/fj.10-170027. [DOI] [PubMed] [Google Scholar]
- 128.Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, et al. Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am J Respir Crit Care Med. 2011;183:179–188. doi: 10.1164/rccm.200905-0736OC. [DOI] [PubMed] [Google Scholar]
- 129.Lewis CC, Yang JY, Huang X, Banerjee SK, Blackburn MR, Baluk P, et al. Disease-specific gene expression profiling in multiple models of lung disease. Am J Respir Crit Care Med. 2008;177:376–387. doi: 10.1164/rccm.200702-333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Willis-Owen SA, Valdar W. Deciphering gene-environment interactions through mouse models of allergic asthma. J Allergy Clin Immunol. 2009;123:14–23. doi: 10.1016/j.jaci.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 131.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 132.Hansen CH, Nielsen DS, Kverka M, Zakostelska Z, Klimesova K, Hudcovic T, et al. Patterns of early gut colonization shape future immune responses of the host. PLoS ONE. 2012;7:34043. doi: 10.1371/journal.pone.0034043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herbst T, Sichelstiel A, Schar C, Yadava K, Burki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184:198–205. doi: 10.1164/rccm.201010-1574OC. [DOI] [PubMed] [Google Scholar]
- 134.Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun. 2004;72:4996–5003. doi: 10.1128/IAI.72.9.4996-5003.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sczesnak A, Segata N, Qin X, Gevers D, Petrosino JF, Huttenhower C, et al. The genome of th17 cell-inducing segmented filamentous bacteria reveals extensive auxotrophy and adaptations to the intestinal environment. Cell Host Microbe. 2011;10:260–272. doi: 10.1016/j.chom.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma BW, Bokulich NA, Castillo PA, Kananurak A, Underwood MA, Mills DA, et al. Routine habitat change: a source of unrecognized transient alteration of intestinal microbiota in laboratory mice. PLoS ONE. 2012;7:e47416. doi: 10.1371/journal.pone.0047416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, et al. Eosinophils promote generation and maintenance of immunoglobulin-a-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582–593. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 138.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DTC, et al. Expression of the Secondary Granule Proteins Major Basic Protein (MBP)-1 and Eosinophil Peroxidase (EPX) is Required for Eosinophilopoiesis in Mice. Blood. 2013;122:781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsuoka K, Shitara H, Taya C, Kohno K, Kikkawa Y, Yonekawa H. Novel basophil- or eosinophil-depleted mouse models for functional analyses of allergic inflammation. PLoS ONE. 2013;8:e60958. doi: 10.1371/journal.pone.0060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 143.Purkerson JM, Isakson PC. Interleukin 5 (IL-5) provides a signal that is required in addition to IL-4 for isotype switching to immunoglobulin (Ig) G1 and IgE. J Exp Med. 1992;175:973–982. doi: 10.1084/jem.175.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoshida T, Ikuta K, Sugaya H, Maki K, Takagi M, Kanazawa H, et al. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5Ra-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 145.Pope SM, Brandt EB, Mishra A, Hogan SP, Zimmermann N, Matthaei KI, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol. 2001;108:594–601. doi: 10.1067/mai.2001.118600. [DOI] [PubMed] [Google Scholar]
- 146.Baruch-Morgenstern NB, Shik D, Moshkovits I, Itan M, Karo-Atar D, Bouffi C, et al. Paired immunoglobulin-like receptor A is an intrinsic, self-limiting suppressor of IL-5-induced eosinophil development. Nat Immunol. 2014;15:36–44. doi: 10.1038/ni.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]