Abstract
Some argue that hippocampus supports declarative memory, our capacity to recall facts and events, whereas others view the hippocampus as part of a system dedicated to calculating routes through space, and these two contrasting views are pursued largely independently in current research. Here we offer a perspective on where these views can and cannot be reconciled, and update a bridging framework that will improve our understanding of hippocampal function.
Two experimental and theoretical literatures currently dominate in ideas on what the hippocampus does. One literature began with the characterization of amnesia following hippocampal damage in humans, and has shown that the hippocampus plays a selective and critical role in declarative memory, our ability to remember everyday facts and events (Cohen & Squire, 1980). The other literature began with the discovery of “place cells”, hippocampal principal neurons that fire when a rat is in a particular location in its environment, as well as findings that hippocampal damage causes profound deficits in some types of spatial memory in rodents, leading to the view that the hippocampus is specialized for mapping space (O’Keefe & Nadel, 1978). Studies on declarative memory in humans and on spatial mapping in rodents have been pursued largely separately, but recently interest has grown in merging these literatures.
Declarative memory view
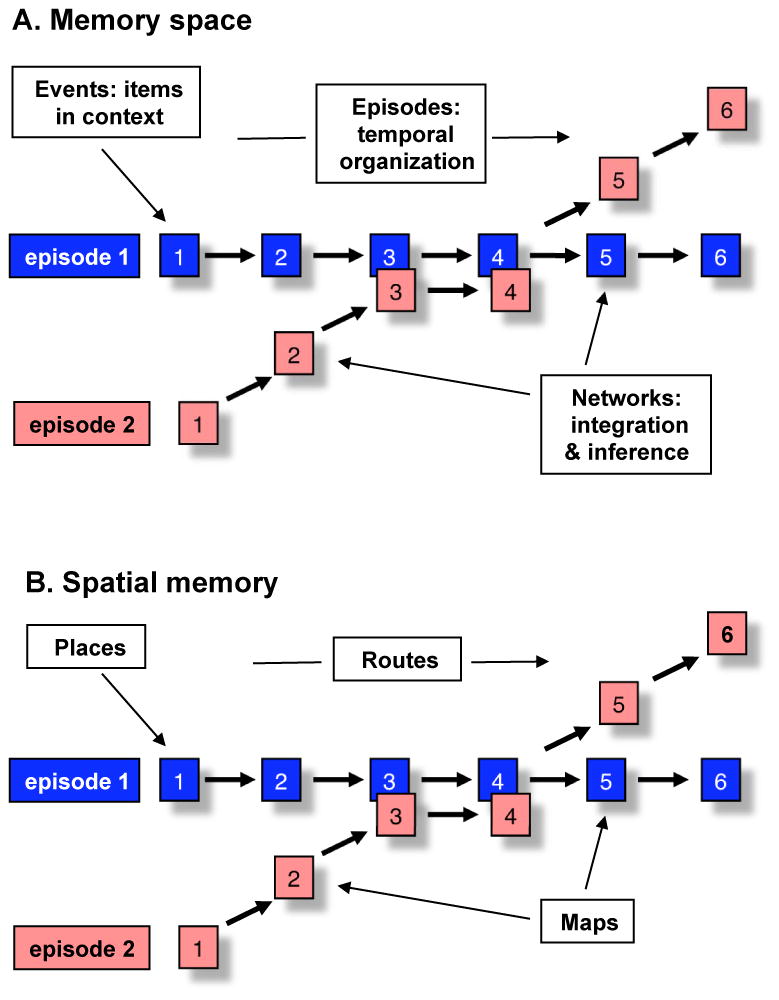
In our extension of the initial description of a selective role of the hippocampal system in declarative memory (Cohen & Squire, 1980), we advanced the idea that the hippocampus supports declarative memory by providing a general relational processing mechanism, across species and across the different tasks, modalities, and domains tested (Cohen & Eichenbaum, 1993). On our view, the hippocampus enables relational representations that bind in memory the elements of experiences and links memories via their common elements, composing a “memory space” (Eichenbaum et al., 1999; Konkel & Cohen, 2009). By rapidly forming associations among any subset of its inputs, and between its inputs and reactivated relational memories, the hippocampus plays a critical role in the generation, recombination, and flexible use of information of all kinds. The representational schemes that underlie relational processing of ongoing experiences are: (1) the representation of events as the relations among objects within the context in which they occur, (2) the representation of episodes as the flow of events across time, and (3) the interleaving of events and episodes into relational networks, supporting the ability to draw novel inferences from memory (Figure 1A; Eichenbaum, 2004).
Figure 1.
A. A conceptual illustration of a memory space, designating the three key types of relational processing. Events are composed as items (object, behaviors, and events) are positioned within the context in which they occurred. Episodes are composed as a temporal organization of events. Relational networks are composed as links between events and episodes, supporting the capacity for inferences between indirectly related events. B. Illustration of our conception of a spatial memory embodied as the same types of relational processing. Adapted from Eichenbaum (2004).
Spatial navigation view
The original account of O’Keefe & Nadel (1978) proposed that the hippocampus supports a cognitive map composed as a Euclidian coordinate space dedicated to allocentric (external world referenced) physical space where place cells identify one’s location of occupancy within the map. Considerable data following this proposal showed that place cells are not well described as signaling coordinates within a unified spatial map - most place cells are independently controlled by subsets of cues (O’Keefe & Burgess, 1996; Gothard et al., 1996; Shapiro et al., 1997) - and place cells encode much more than spatial coordinates, including a broad range of stimulus events and behavioral actions (e.g., Wood et al., 1999; reviewed in Eichenbaum et al., 1999). In the ensuing years, few studies have pursued the original cognitive mapping hypothesis per se. However, following discoveries of additional spatial coding in the neighboring entorhinal cortex and elsewhere, there has been a resurgence of a narrower version of this view that focuses on navigation through physical space (Hartley et al., 2014). For many investigators, path integration by self-motion cues is the fundamental mechanism by which maps are created and position is updated (McNaughton et al., 2006; Burgess et al., 2007). The new view incorporates the larger set of spatial correlates of neural activity found in the hippocampal region (in addition to place cells, there are now grid cells, head direction cells, boundary cells) to support the claim of a spatial representation system (Moser et al., 2008). Like the original view, this view maintains that the system is dedicated to allocentric space and does not support body centered spatial reference, local spatial arrangements on a screen, or non-spatial organizations such as in temporal or semantic organization of memories (Hartley et al., 2014).
What is wrong with the spatial navigation view?
A full reconciliation of the declarative memory and spatial navigation views is not possible if hippocampal function is dedicated solely to spatial processing, as argued by any narrowly framed spatial navigation view. So, it is useful here to evaluate the key tenets of such a view. We believe this examination strongly suggests that hippocampus contributes by supporting memory necessary for successful navigation rather than by performing navigational computations per se.
First, students of real-world navigation recognize that the ability to find our way normally takes advantage of a rich combination of information and includes a critical role for memory, including memories for the spatial layout of the environment, routes taken, and the origin and destination of a journey (Dudchenko, 2010; Huth, 2013; Wolbers & Hegarty, 2010). Accordingly, it is difficult to empirically associate the hippocampus with spatial computations per se, as opposed to memory for the spatial parameters and events relevant to ongoing behavior in space.
Second, outside the hippocampus literature, path integration by self-motion cues is widely held to be the least useful method of navigation because of its inherent rapid accumulation of spatial error (Dudchenko, 2010; Huth, 2013). Considerable evidence indicates that humans and animals have very limited capacity for path integration, barely supporting accurate movement over a few body lengths (Huth, 2013; Kim et al., 2013). Notably, rats cannot employ path integration with sufficient accuracy to solve the Morris water maze problem, the paradigm commonly held as the benchmark of hippocampal spatial navigation function (Benhamou, 1997). These observations call into question the extent to which path integration is relevant to how the hippocampus contributes to spatial navigation.
Third, the evidence that the hippocampus is essential for path integration is mixed, including studies showing intact spatial cognition in humans with hippocampal damage (Teng & Squire, 1999), successful path integration following hippocampal damage in animals (Alyan & McNaughton 1999) and humans (Kim et al., 2013), and failure on the same task both when path integration is required and when it is not (Whishaw & Maaswinkel, 1998). Consistent with descriptions of human navigation, in a review of the animal literature, Etienne & Jeffery (2004, p. 180) concluded that, “path integration exerts a functionally important control over navigation only as long as the animal can combine it with learned strategies and/or spatial cues from a familiar environment”, and many studies have confirmed that environmental cues readily overrule self-motion information (reviewed in Navratilova & McNaughton, 2014). Furthermore, evidence is building that the creation and updating of spatial representations in the hippocampus do not require path integration inputs from the medial entorhinal cortex (whose “grid cells” figure prominently in many theories), calling into question current navigation models that depend on this functional circuitry (Brandon et al., 2014).
Fourth, the empirical evidence for neuronal calculations within the hippocampal region supporting path integration is based largely on observations that place fields are dependent on self motion (Foster et al., 1989) and are briefly stable when dominant environmental cues are removed (O’Keefe & Speakman, 1987; Muller & Kubie, 1987; also observed in adjacent entorhinal cortex; Hafting et al., 2005). These observations are interpreted as reflecting on-line processing of internally generated movement information used to update the place representations (McNaughton et al., 1996, 2006). However, while these observations show that the hippocampus represents a variety of spatial signals when relevant, they do not imply either that the hippocampus is dedicated to representing movement generated spatial representations or that on-line spatial calculations are necessarily performed within the hippocampus. Thus, when animals are not moving, the engagement of the hippocampus in processing both non-spatial and spatial information is readily observed. When animals are immobilized, hippocampal neurons prominently encode non-spatial events (Berger et al., 1983; MacDonald et al., 2013; Naya & Suzuki, 2011) and when animals are still following movement to locations where salient events occur, hippocampal neurons are driven by specific events in particular places (e.g., Moita et al., 2003; Komorowski et al., 2009; Itskov et al., 2011). Furthermore, the relevant spatial calculations that occur on-line during movement may well be performed at earlier stages of cortical processing that provide inputs to the hippocampal system, e.g., parietal cortex (Harvey et al., 2012; Nitz, 2012). Indeed, a recent review acknowledges, “…there is in fact no evidence that the actual computation of trajectories or routes takes place [in the hippocampal system]” (Navratilova & McNaughton, 2014, p. 195). Confirming that the hippocampal contribution to navigation does not occur during movement, Spiers & Maguire (2006) reported that the hippocampus is activated only during the initial planning of a route (which, of course, includes a strong demand for memory) and not during its execution.
Fifth, prominent models of navigation do not actually rely on calculations of vector summation that define path integration. Instead, they are based on neural coding mechanisms and associative networks that just as easily support a general role in memory (Hasselmo, 2012; Buzsaki & Moser, 2013). The combination of these issues cast doubt that the hippocampus contributes in a special way to spatial navigation beyond its role in memory.
So, how can memory and spatial views be reconciled?
Since the outset of our work on declarative memory, we proposed that it is fundamentally a relational processing system, which applies well to the domain of spatial memory, and we have emphasized the role of the hippocampal system in encoding events as relational mapping of objects and actions within spatial contexts, representing routes as episodes defined by sequences of places traversed, and relating spatial episodes to existing semantic maps of space that can support navigation via novel route construction (Cohen & Eichenbaum, 1993; Eichenbaum, 2004; Figure 1B). Recently, other efforts that consider how the same hippocampal circuitry can be applied to support both memory and navigation echo this perspective and extend the evidence supporting the characteristics of relational memory.
Following on the idea that events are represented as items in spatial context, Maguire & Mullaly (2013) argued that the hippocampus constructs spatially coherent scenes (which, notably, are egocentric, rather than the allocentric representations envisioned by O’Keefe and Nadel). Following on the idea that episodes are encoded as sequences of events, Maguire & Mullaly (2013) added that scene representations can be elaborated with temporal or other information to support the full experience of episodic memory. From the perspective of hippocampal circuitry, Hasselmo (2012) argued that the behavioral dynamics of episodic memory can be characterized as spatio-temporal trajectories “not limited to the dimensions of physical time and space” (p. 15). Similarly, Buzsaki & Moser (2013) likened hippocampal coding of routes to episodic memories that also flow in time.
Expanding on the idea that memory networks are composed from the integration of episodes, Buzsaki & Moser (2013) also likened the integration of multiple routes into cognitive maps to the integration of episodic memories into semantic networks. Similar to the idea that these relational networks form a “memory space” (Eichenbaum et al., 1999), Milivojevic & Doeller (2013) proposed that the memory and spatial functions of the hippocampus have in common are the creation of mental maps that can be organized in space, time, and conceptual dimensions. Each of these recent considerations is a welcome extension of our relational memory account, providing useful clarifications about how to reconcile a declarative memory view with cognitive mapping views that do not focus exclusively on spatial navigation.
New experimental evidence supporting our theoretical ideas about hippocampal processing of events, episodes, and relational networks
The memory and spatial functions of the hippocampus can be reconciled by extending hippocampal function beyond navigation in allocentric space to the organization of events in spatial contexts, to non-spatial organizations including time and more, and to the larger sense of “navigation” through a memory space. Here we offer a selection of prominent recent examples that support this view.
Events are represented as objects organized in context
In animals as well as humans, the hippocampus is essential to recalling objects in the context in which they were studied. For example, rats with hippocampal damage can recognize familiar objects in the spatial context in which they were experienced, but fail to recognize familiar objects misplaced in context or in a new context (Eacott & Norman, 2004; Langston & Wood, 2010). Similarly, in the non-spatial domain, rats with hippocampal damage are impaired in remembering objects in the context of a current list, even as they retain a sense of familiarity with the objects (Fortin et al., 2004). Correspondingly, considerable recent evidence indicates that place cells reflect the substrates of the spatial organization of memories, consistent with the original cognitive mapping view, albeit applying to both allocentric space (in rats) and spatial arrangements on a computer screen (in humans). Several studies have shown that, as rats learn about specific objects, the representations of those objects are overlaid onto spatial firing patterns such that place cells become specialized to activate while objects are experienced at specific locations (e.g., Moita et al., 2003; Komorowski et al., 2009; Itskov et al., 2011, 2012).
In parallel, recent studies on human memory implicate a key role for the hippocampus in remembering the locations of objects in spatial scenes. Thus, normal individuals disproportionately scan locations where objects were moved from their originally seen locations in a scene, whereas individuals with hippocampal damage fail to show the normal object-location memory by preferential viewing (Ryan et al., 2000). Correspondingly, neurologically intact participants showed activation of the hippocampus that predicted subsequent successful object-location memory (Hannula & Ranganath, 2008). In another study, Watson et al (2013) found that hippocampal damage elicited disproportionate impairment in swapping the locations of objects while remembering their previously studied positions in a scene. Notably, hippocampal damage did not produce disproportionate error rates on geometric measures involving coordinates, distances and angles among the objects, inconsistent with the spatial navigation view. In none of these behavioral paradigms is there navigation by body movement through allocentric space; rather, performance requires simply shifting gaze to easily visualized targets arranged on a screen.
Episodes are represented as sequences of events organized in temporal context
Beyond spatial context, there is now considerable evidence that memories are represented within the broad temporal context of an entire experience. For example, when rats traverse overlapping routes through a maze, hippocampal place cells fire very differently depending upon the origin and destination of the overall route as much as, or more so than by the current specific location of the rat within the maze (e.g., Wood et al., 2000; Frank et al., 2000; reviewed in Shapiro et al., 2006). Particularly impressive is that the context of a route can be defined not only by spatial information (origin and destination) but also by motivational cues, such as hunger or thirst (Kennedy & Shapiro, 2009). A large variety of studies on humans have shown that memory for temporal context is dependent on the hippocampus (e.g., Konkel et al., 2008) and that the hippocampus is activated when events must be recalled in order or within temporal context (reviewed in Eichenbaum, 2013).
Furthermore, recent evidence indicates that representations of temporally extended episodes are mediated directly by hippocampal representations of time and order independent of place in humans and animals (Eichenbaum, 2013). An early study showed that activity patterns of hippocampal neural ensembles gradually change while rats sampled sequences of odors, and this continuous context signal predicted success in remembering the odor sequence (Manns et al., 2007). Confirming these findings in humans, it was recently reported that pattern similarity in hippocampal activation signaled temporal proximity of successively viewed objects and this signal was correlated with order memory (Ezzyat & Davachi, 2014).
In addition, several other studies using a range of behavioral tasks have now identified hippocampal principal neurons that fire at a particular moments in time of a temporally structured event, composing temporal maps of specific experiences (Eichenbaum, 2013). Because the properties of these neurons parallel those of place cells, we called these neurons “time cells”. Time cells are under the control of temporal cues, such as the beginning and end of episodes, and the temporal structure (duration) of the period, much as place cells are under the control of spatial landmarks and the shape of an environment (MacDonald et al., 2011). Furthermore, time cell patterns are specific to distinct memories and predict memory performance (MacDonald et al., 2013). Confirming the specificity of temporal organization of event memories find in humans, it was recently reported that pattern similarity in hippocampal activation signaled the combination of object and temporal context information in sequence learning (Hsieh et al., 2014). The existence of time cells offers a parallel temporal organizing mechanism to the spatial organizing mechanism offered by place cells, supporting our notion that the hippocampus represents episodes by mapping events within a framework of time as well as space.
Relational networks link related events and episodes and support flexible, inferential memory across spatial and non-spatial dimensions
Tse et al. (2008) showed that when rats learn to find specific food flavors in particular places in an open field, they develop an organized representation of the spatial relations among the objects in a particular environment and rely on the hippocampus for rapid assimilation of new flavor-place associations within the relational representation. Relating these findings to place cells, Mckenzie et al. (2013) reported that hippocampal neurons encode multiple reward locations and rapidly assimilate and reorganize the overall network representation to incorporate new reward locations. In a more ambitious study where rats learned multiple context-dependent object-reward associations, Mckenzie et al. (2014) characterized the neural ensemble representations as a hierarchy of relations among event dimensions, including the identity of the objects, their reward assignments, the positions within a context in which they were experienced, and the context in which they occurred. Furthermore, after initial learning of one set of object associations, new object associations were rapidly assimilated into the relational structure that was established by initial learning. In addition, within the overall representation, items that had in common their reward associations in particular positions had strongly similar representations, even when they were never experienced together. This aspect of relational representations likely underlies the essential role of the hippocampus in the capacity for transitive inferences between indirectly related items in hierarchical (Bunsey et al. 1996) and associative (Dusek et al., 1997) memory networks in animals.
In studies on relational networks in humans (similar to the transitive inference studies in animals cited just above), Zeithamova et al. (2012) trained subjects on overlapping pairwise object associations (e.g., AB and BC) from which they can make inferences between indirectly related elements (AC). They found that the learning of the second, overlapping pair (BC) can reinstate the hippocampal representation of the earlier learned pair (AB), a content-specific hippocampal activation that predicted subsequent success on the inferential judgment. It is notable that the relational network in this paradigm (as used in rats as well) is organized neither by space nor time, but simply by associative links among the elements. More generally, other recent studies have shown that the hippocampus plays a critical role in the generation, recombination, and flexible use of information of all kinds, as revealed by deficits in patients with damage to the hippocampus in tasks across areas as diverse as exploration, imagination, creativity, decision-making, character judgments, empathy, social discourse, and language use (e.g., Duff et al., 2009, 2013).
Conclusions
A central feature of the spatial navigation view, as well as the original cognitive mapping hypothesis, is that the hippocampal system is dedicated to spatial representations. It should be clear from the above discussion that hippocampus encodes a broad range of non-spatial information including all manner of specific objects, behaviors, and events. Furthermore, in addition to both allocentric and egocentric spatial organizations, the hippocampus employs non-spatial organizations, including temporal organization and associative networks. The notion of the hippocampal system as dedicated to space and navigation can only be viewed as a metaphor of its role in a “memory space” (Figure 1).
For one potentially useful way to think about the historical disconnect between the studies on humans that emphasize non-spatial relations among elements of memories and studies on animals that emphasize the representation of spatial organization, consider the context-guided object association task mentioned above (Mckenzie et al., 2014). In the hierarchy of representational dimensions encoded by the hippocampus in rats solving this task, spatial dimensions were represented at the top of the hierarchy whereas representations of object features were embedded at the bottom. Note that this particular behavioral paradigm contained both prominent spatial and non-spatial dimensions. However, most studies of human memory focus on object features while holding space constant, whereas most studies on spatial navigation focus on spatial dimensions while eliminating or randomizing specific events that occur. Closing the gap between these differences in the designs of experimental protocols is likely to confirm the commonalities we suggest in memory and cognitive mapping, and in doing so, lead to a more comprehensive understanding of the nature and organization of hippocampal information processing.
Our recent work in humans has helped to bridge the divide between rodent spatial navigation and human memory literatures by including the same requirements for the active use of memory in on-line processing as has been implicit in rodent navigation studies. Although above we have discussed navigation in the usual, purely spatial sense of moving from place to place, we would argue that the hippocampus plays a critical role in the broader challenge of navigating through life. Humans are active agents, engaging with our world and tailoring our behavior to meet current situational demands, by constantly encoding relations (spatial or otherwise), updating mental representations, and using that information in real-time to guide upcoming actions and choices (see Wang, Cohen, & Voss, 2014; Yee et al., 2014). We believe that additional emphasis in on-line (spatial and non-spatial) relational processing by the hippocampus in humans and animals will further facilitate a merging of currently divergent views of hippocampal function.
Acknowledgments
MH094263, MH051570, MH052090, MH095297
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Alyan S, McNaughton BL. Hippocampectomized rats are capable of homing by path integration. Behav Neurosci. 1999;113:19–31. doi: 10.1037//0735-7044.113.1.19. [DOI] [PubMed] [Google Scholar]
- Benhamou S. Path integration by swimming rats. An Behav. 1997;54:321–327. doi: 10.1006/anbe.1996.0464. [DOI] [PubMed] [Google Scholar]
- Berger TW, Rinaldi PC, Weisz DJ, Thompson RF. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. J Neurophsiol. 1983;50: 1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- Brandon MP, Koenig J, Leutgeb JK, Leutgeb S. New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron. 2014;82:789–796. doi: 10.1016/j.neuron.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Burgess N, Barry C, O’Keefe J. An oscillatory interference model of grid cell firing. Hippocampus. 2007;17:801–812. doi: 10.1002/hipo.20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neurosci. 2013;16: 130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of a pattern- analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. Cambridge, MA: M.I.T. Press; 1993. [Google Scholar]
- Dudchenko PA. Why People Get Lost: The Psychology and Neuroscience of Spatial Cognition. New York: Oxford University Press; 2010. [Google Scholar]
- Duff MC, Kurczek J, Rubin R, Cohen NJ, Tranel D. Hippocampal amnesia disrupts creative thinking. Hippocampus. 2013;23:1143–1149. doi: 10.1002/hipo.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Hippocampal amnesia disrupts verbal play and the creative use of language in social interaction. Aphasiology. 2009;23: 926–939. doi: 10.1080/02687030802533748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc Nat Acad Sci USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Norman G. Integrated memory for object, place, and context in rats, a possible model of episodic-like memory? J Neurosci. 2004;24:1948–1953. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchencko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Memory on time. Trends in Cognitive Sciences. 2013;17:81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne AS, Jeffery KJ. Path Integration in Mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Davachi L. Similarity breeds proximity: Pattern similarity within and across contexts is related to later mnemonic judgments of temporal proximity. Neuron. 2014;81:1179–1189. doi: 10.1016/j.neuron.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Castro CA, McNaughton BL. Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science. 1989;244:1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16: 823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of relational memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, O’Keefe J. Space in the brain: how the hippocampal formation supports spatial cognition. Phil Trans R Soc B. 2014;369:20120510. doi: 10.1098/rstb.2012.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–68. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. How We Remember: Brain Mechanisms of Episodic Memory. Cambridge, MA: MIT Press; 2012. [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal activity patterns carry information about temporal context. Neuron. 2014;81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth JE. The Lost Art of Finding Our Way. Cambridge MA: Harvard University Press; 2013. [Google Scholar]
- Itskov PM, Vinnik E, Diamond ME. Hippocampal representation of touch-guided behavior in rats: Persistent and independent traces of stimulus and reward location. PLoS ONE. 2011;6(1):e16462. doi: 10.1371/journal.pone.0016462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov PM, Vinnik E, Honey C, Schnupp J, Diamond ME. Sound sensitivity of neurons in rat hippocampus during performance of a sound-guided task. J Neurophysiol. 2012;107:1822–1834. doi: 10.1152/jn.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Shapiro ML. Contextual memory retrieval: motivational states activate distinct hippocampal representations. Proc Natl Acad Sci USA. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sapiurkac M, Clark RE, Squire LR. Contrasting effects on path integration after hippocampal damage in humans and rats. Proc Natl Acad Sci USA. 2013;110:4732–4737. doi: 10.1073/pnas.1300869110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens. J Neuroscience. 2009;29:9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Warrant DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Human Neurosci. 2008 doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston RF, Wood ER. Associative recognition and the hippocampus: Differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–1153. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71: 737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, Eichenbaum H. Distinct hippocampal time cell sequences represent odor memories in immobilized rats. J Neurosci. 2013;33: 14607–14616. doi: 10.1523/JNEUROSCI.1537-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mullally SL. The hippocampus: A manifesto for change. J Exptl Psych Gen. 2013;142:1180–1189. doi: 10.1037/a0033650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard M, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser M-B. Path-integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7: 663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Robinson NTM, Herrera L, Churchill JC, Eichenbaum H. Learning Causes Reorganization of Neuronal Firing Patterns to Represent Related Experiences within a Hippocampal Schema. J Neurosci. 2013;33: 10243–10256. doi: 10.1523/JNEUROSCI.0879-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Rivière PD, Eichenbaum H. Hippocampal representation of related and opposing memories develop within distinct, hierarchically-organized neural schemas. 2014;83:202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard M, Jung MW, Knierim JJ, et al. Deciphering the hippocampal polyglot: The hippocampus as a path integration system. J Exper Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the “cognitive map.” Nat. Rev Neurosci. 2006;7: 663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- Milivojevic B, Doeller CF. Mnemonic networks in the hippocampal formation: from spatial maps to temporal and conceptual codes. J Exptl Psych Gen. 2013;142:1231–1241. doi: 10.1037/a0033746. [DOI] [PubMed] [Google Scholar]
- Moita MAP, Moisis S, Zhou Y, LeDoux JE, Blair HT. Hippocampal place cells acquire location specific location specific responses to the conditioned stimulus during auditory fear conditioning. Neuron. 2003;37: 485–497. doi: 10.1016/s0896-6273(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff K, Moser M-B. Place cells, grid cells, and the brain’s spatial representation system. Ann Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;77:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova Z, McNaughton BL. Models of path integration in the hippocampal complex. In: Derdikman D, Knierim JJ, editors. Space, Time and Memory in the Hippocampal Formation. Vienna: Springer-Verlag; 2014. pp. 191–224. [Google Scholar]
- Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333:773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- Nitz DA. Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nature Neurosci. 2012;15: 1365–1367. doi: 10.1038/nn.3213. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford University Press; 1978. [Google Scholar]
- O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68: 1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:6454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Kennedy P, Ferbinteanu J. Representing episodes in the mammalian brain. Curr Opinion Neurobio. 2006;16: 701–709. doi: 10.1016/j.conb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Tanila H, Eichenbaum H. Cues that hippocampal place cells encode: Dynamic and hierarchical representation of local and distal stimuli. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Thoughts, behviour, and brain dynamics during navigation in the real world. Neuroimage. 2006;31:1826–1840. doi: 10.1016/j.neuroimage.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Teng E, Squire LR. Memory for places learned long ago is intact after hippocampal damage. Nature. 1999;400:675–677. doi: 10.1038/23276. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RGM. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Wang JX, Cohen NJ, Voss JL. Covert rapid action-memory simulation (CRAMS): A hypothesis of hippocampal-prefrontal interactions for adaptive behavior. Neurobiol Learn Mem. 2014 Apr 19; doi: 10.1016/j.nlm.2014.04.003. pii: S1074-7427(14)00064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PD, Voss JL, Warren DE, Tranel D, Cohen NJ. Spatial reconstruction by patients with hippocampal damage is dominated by relational memory errors. Hippocampus. 2013;23:570–80. doi: 10.1002/hipo.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Maaswinkel H. Rats with fimbria–fornix lesions are impaired in path integration: A role for the hippocampus in “sense of direction”. J Neurosci. 1998;18:3050–3058. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M. What determines our navigational abilities? Trends Cog. Sci. 2010;14:138–146. doi: 10.1016/j.tics.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397: 613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Wood E, Dudchenko P, Robitsek JR, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yee LT, Warren DE, Voss JL, Duff MC, Tranel D, Cohen NJ. The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014;24:154–164. doi: 10.1002/hipo.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]