Abstract
Chromium pollution has been historically widespread throughout the world. Most available remediation technologies often require energy consumption. This study is aimed to develop electrochemical remediation for Cr(VI) in chromium-slag leakage with self-generated electricity. Dynamic leaching experiments of chromium-slag samples were conducted to survey the release and leaching behavior of Cr(VI). Based on previous work, a unique urea-Cr(VI) was designed, in which urea was employed as the fuel and Cr(VI) from the leakage of the dichromate slag served as the oxidant. Furthermore, the electrochemical results showed that the removal percent of Cr(VI) was more than 96% after 18 h with the leakage Cr(VI) concentration of 2.69 mM. The open circuit potential (OCP) varied in the range of 1.56 ~ 1.59 V under different initial Cr(VI) leakage concentrations. The approach explores the feasibility of the promising technique without the need of energy input for simultaneous chromium-slag remediation and generation of electricity.
Chromium was widely used in industry for the production of steel and alloys, ore refining, wood preservation, and electroplating. Due to improper treatment of chromium-containing waste and waste-water, chromium pollution was historically widespread across the world in locations where chromium was released to the environment, such as the Death Village in China1. Regardless, it is difficult to remediate polluted sites caused by chromium-containing waste, and thus, most chromium-slag (67%) was simply deposited over a span of decades2,3,4. UK investigators estimated that approximately 2.5 million tons of chromite ore processing residue were found on several sites in the vicinity of Glasgow5,6. Improper dispersal of chromium pollution has now become a serious contamination problem for soil and water around the world.
To avoid further contamination by chromium-slag, the proper technologies should be applied to treat such chromium wastes before their simple deposit. Currently, most available technologies, such as detoxification treatment2,7,8,9,10,11 and utilization12,13,14, are often energy consuming. Calcinations of chromium-slag with cinder had been used for the reduction of Cr(VI), but this technique requires special equipment and produces secondary pollution, such as waste gas9. Bio-consolidation and bio-remediation were regarded as new and promising detoxification methods7,8,10. However, the current bio-remediation techniques could hardly be widely used due to limitations such as the microbial species, microbial sensitivity to environment, and long remediation time7. Moreover, chromium-slag could be used for the preparation of cement, glass, brick, and concrete aggregate material12,13,14. However, current technologies are required to use the existing equipment and nearby resources to reduce cost. Furthermore, given the complicated variety and uncertainty for utilization and detoxification treatment, it is difficult to make sufficient use of chromium-slag without also causing secondary pollution.
Cr(III) and Cr(VI) are both present in chromium-slag, but the latter ionic form is much more toxic and carcinogenic to humans due to its faster mobility, high solubility, and strong oxidation15,16,17. It is known that dichromate possesses the ability to accept electrons and contains considerable chemical energy that could be retrieved from this substance. Dichromate accepts six electrons, and its oxidation potential is 1.33 V under acidic conditions, which is shown in Equation 1.
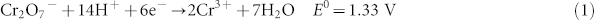 |
Recently, great attention has been paid to energy extraction, instead of energy input, during wastewater treatment by the conversion of chemical energy to electricity. Based on our previous research18, electricity can be harvested directly from an alkaline ethanol fuel cell reactor in which K2Cr2O7 functions as the oxidant. This work aimed to develop electrochemical remediation of Cr(VI) in chromium-slag with the electricity generation to self-accumulate energy. To retrieve the energy, a special urea-Cr(VI) fuel cell device was designed and assembled, in which the reduction of chromium(VI) from the leakage of the dichromate slag was examined. The objectives of this work were (1) to understand the leaching performance of Cr(VI) via a dynamic leaching experiment of chromium-slag and (2) to establish a unique fuel cell to reduce Cr(VI) to Cr(III), which is considered less toxic.
Results
Dynamic leaching test for chromium-slag
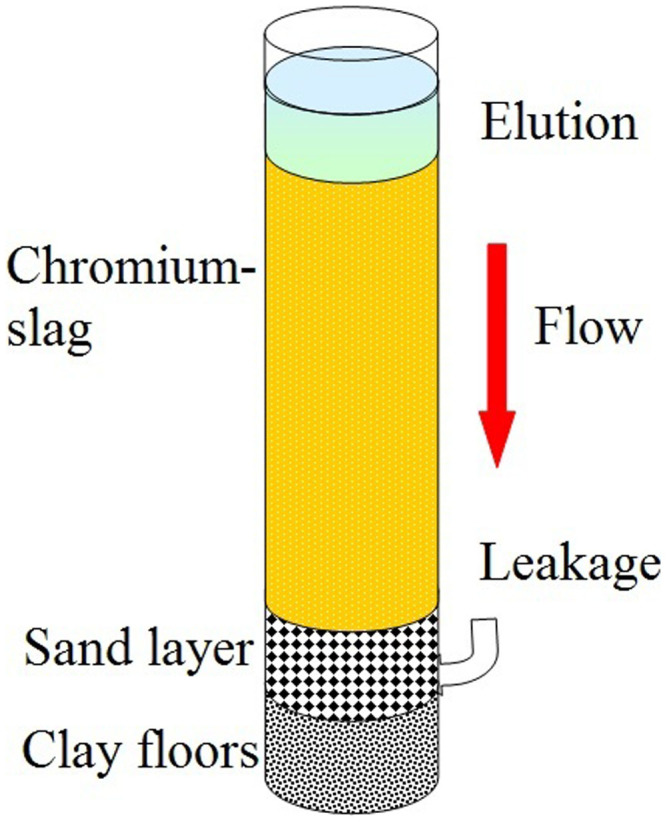
To investigate the leaching procedure and influencing factors for chromium-slag, three samples of chromium-slag, each with a sample size of less than 1000 μm, were collected from the Yunnan province in China. Detailed information on the samples is provided in Table 1. All samples were randomly collected from chemical manufacturers and were typical ore processing residues of chromium-slags that are often largely deposited and waiting for passive treatment. The mass ratio of Cr(VI) in these samples varied from 0.71% to 0.85%. Dynamic leaching tests of chromium-slag were conducted to characterize the Cr(VI) leaching when the samples were seized before the dynamic experiments could be performed uniformly. A setup (Figure 1) was conducted to measure the total Cr(VI) released from the slag by using different eluting solutions.
Table 1. Cr(VI) and total Cr content for the different samples.
| Sample | Color | Source | Particle size μm | The amount of Cr(VI) (mass ratio m/m) | The amount of total Cr (mass ratio m/m) |
|---|---|---|---|---|---|
| 1 | Yellow | Kunming | <150 | 0.71% | 2.2% |
| 2 | Green | Qujing | <150 | 0.72% | 2.0% |
| 3 | Green | Qujing | 250–830 | 0.85% | 1.9% |
Figure 1. Setup of the leaching experiment.
As the chromium-slag was exposed to the ambient environment, it would be rinsed by natural rain with low acidity. Therefore, it is necessary and meaningful to test the effect of stimulated acid rain on the dissolution-release of Cr(VI)19. Several different solutions, including HNO320, HCl21, water22,23, H2SO424, NaCl solution5 and distilled water with CO2 were used as eluting solutions for chromium-slag. Two types of artificial acid rain, including saturated CO2 distilled water (pH = 5.6) and a H2SO4 solution with a fixed pH value, were simulated as the eluting solution in this study and employed to leach the slag. Distilled water was used as the control solution. The main parameters of stimulated rain intensity for the different levels of rainfall are provided in Table 2, according to the weather parameters3.
Table 2. Main parameters of stimulated rain intensity.
| The level of rainfall | Intensity a mm/h | Flow rate of eluting solution b (mL/h) | |
|---|---|---|---|
| 1 | light rain | 1 | 0.31 |
| 2 | moderate rain | 5 | 1.57 |
| 3 | heavy rain | 15 | 4.71 |
| 4 | rainstorm | 30 | 9.42 |
aIntensity is collected based on weather parameters;
bFlow rate = intensity of rainfall × area of column.
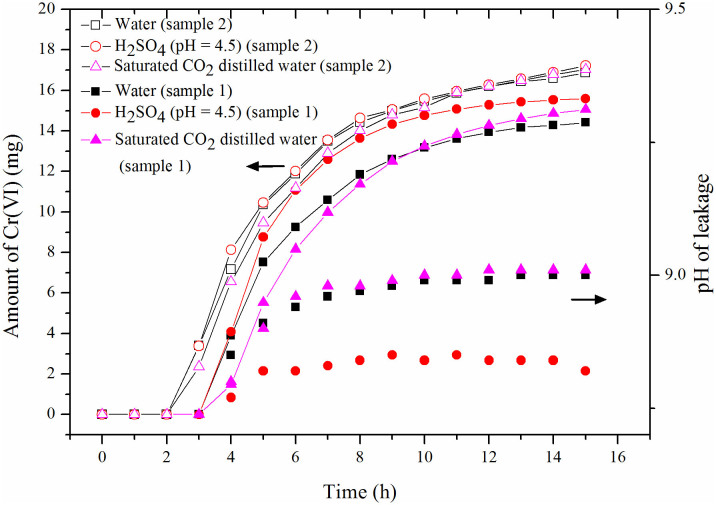
The effect of the eluting solution on Cr(VI) release and pH values in the leachate for the different eluting solutions in the dynamic leaching procedure with a flow rate of 1.5 mL/h (equal to the level of moderate rain) is displayed in Figure 2. The elution time and volume of eluting solution exhibited significant effects on the cumulative amount of Cr(VI) released from the chromium-slag. No leakage was collected within the first 2 h and 3 h for sample 2 and sample 1, respectively, as there were insufficient eluting solutions. During the leaching process, a faster leaching velocity of Cr(VI) within 8 h was obtained, and then the leaching rate gradually slowed. After 15 h of continuous moderate rain, the accumulative amounts of Cr(VI) released from 5 g of sample 1 were 14.4 mg, 15.1 mg and 15.6 mg by the elution of water, saturated CO2 solution, and the H2SO4 solution, respectively, while those of sample 2 were 16.8 mg, 17.0 mg, and 17.2 mg. Therefore, the percent of released Cr(VI) varied from 42% to 45% for sample 1 and changed from 47% to 49% for sample 2. These results indicated that the eluting abilities for the release of Cr(VI) were in the following order: H2SO4 solution with a pH value of 4.5 > saturated CO2 solution > distilled water. In addition, the leakage pH values, shown in Figure 2, indicate that the pH values generally remained constant over the prolonged elution time. The final leakage pH values from sample 1 were 8.82, 9.01, and 9.00 for the H2SO4 solution with a pH value of 4.5, saturated CO2 solution, and distilled water, respectively. Due to the alkaline property of chromium-slag, the elution with low acidity could not significantly change the pH values. Of the three eluting solutions, the H2SO4 solution with a pH value of 4.5 exhibited good capability for accelerating leakage. Hence, the H2SO4 solution was chosen as the elution in the following experiment.
Figure 2. Effect of eluting solution on Cr(VI) release and pH values in the leachate at different eluting solutions in the dynamic leaching procedure.
(5 g sample 1 and 5 g sample 2, flow rate of 1.5 mL/h, equal to the level of moderate rain.).
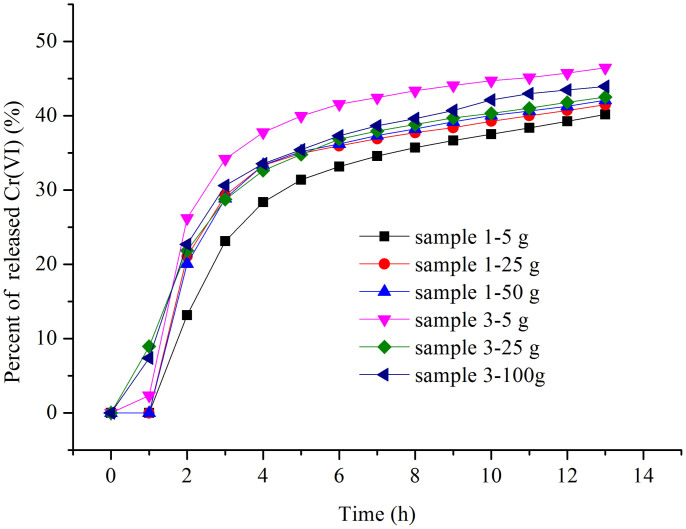
The influence of different amounts of chromium-slags was examined using the H2SO4 solution with a pH value of 4.5 under heavy rain level (Figure 3). The results indicated that the percentage of released Cr(VI) from the same sample was not significantly affected by the amount of sample under the fixed eluting solution. Cr(VI) percentages varying from 40% to 42% were obtained by increasing the amount of sample 1 from 5 g to 50 g after 14 h of continuous heavy rain. Hence, the sample amount of 5 g can be a proper amount in the following experiments.
Figure 3. Percent of released Cr(VI) for different amounts of slag samples in the leaching procedure.
(Flow rate of 4.5 mL/h, equal to the level of moderate rain, H2SO4 with a pH value of 4.5).
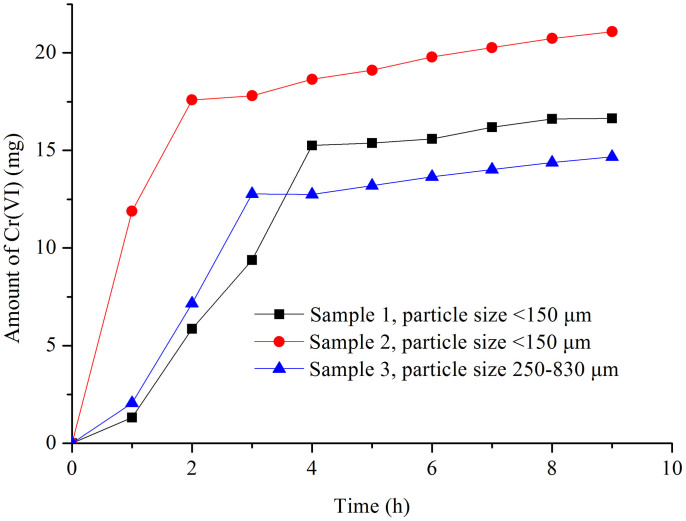
Different chromium-slags were compared with a flow rate of 9.5 mL/h using the H2SO4 solution with a pH value of 4.5 (Figure 4). The dissolution equilibrium was reached after 2–4 h for all slag samples under the level of storm rain. Nevertheless, dissolution from the slag tended to slow with extended time. Cr(VI) percentages of 47%, 65%, and 35% could be dissolved after 9 h of rinsing for sample 1, sample 2, and sample 3, respectively. Obviously, the smaller the particle size, the faster the Cr(VI) dissolution-release velocity was obtained.
Figure 4. Amount of Cr(VI) in the leakage for different slag samples in the leaching procedure.
(5 g samples, flow rate of 9.5 mL/h, equal to the level of storm rain, H2SO4 with a pH value of 4.5.).
Because the pH values of natural rainfall varied (3.5–6.5), the impact of pH values for the H2SO4 solution should be considered. As shown in Figure 5, decreasing pH accelerated the accumulated amount of Cr(VI) in the leachate. However, the final released amounts of Cr(VI) were similar after 11 h under the various pH values, with the content ranging from 15.32 mg to 15.69 mg. With increasing H+ concentration, the oxidative property of Cr(VI) in the chromium-slag increased, which could lead to more redox reactions between Cr(VI) and the reducing substances in the slag and result in less Cr(VI) released in the end. However, the absolute amount of H+ was low in the H2SO4 leaching solution with pH value ranged from 3.5 to 6.5, and the eluting solution was alkaline, and therefore, the amounts of Cr(VI) released were similar regardless of pH. Furthermore, according to the F test of statistics, the differences in the accumulative amounts of Cr(VI) were not statistically significant.
Figure 5. Amount of Cr(VI) in the leakage at different pH values in the dynamic leaching procedure.
(5 g samples, flow rate of 4.5 mL/h, equal to the level of heavy rain.).
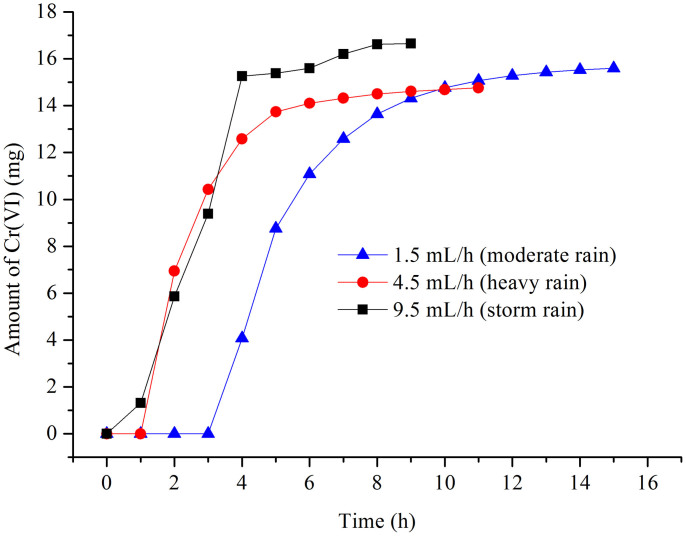
To compare the eluting results under the different levels of rainfall, the effect of different flow rates on the Cr(VI) release was investigated over a range of 1.5 mL/h to 9.5 mL/h (Figure 6). A higher flow rate was a benefit to the rapid dissolution of Cr(VI) and the total amount of released Cr(VI), which could reach equilibrium within 3 h using storm rain. Thus, the experiments took 5 h and 8 h to reach equilibrium for accumulative amounts of Cr(VI) under moderate rain and heavy rain, respectively.
Figure 6. Amount of Cr(VI) concentration in the leachate at different flow rates in the leaching procedure.
(5 g samples, H2SO4 with a pH value of 4.5.).
Cogeneration of electricity and Cr(VI) removal
A special urea-Cr(VI) fuel cell device was designed and assembled to simultaneously generate electricity and reduce Cr(VI) released from chromium-slag. To study the stability of Cr(VI) leakage and examine if reducing substances were obviously existing in the leakage, the concentration of Cr(VI) in the leakage with 0.5 M H2SO4 was determined with prolonged storage time. Table 3 shows that the leakage Cr(VI) concentration decreased slightly during the 112 h, which revealed that few reduction compounds presented in the leakage and the urea-Cr(VI) fuel cell device could theoretically perform well.
Table 3. Concentration of Cr(VI) in the leakage under different storage times with the H2SO4 of 0.5 M (mM).
| Initial concentration of Cr(VI) in leakage | Concentration of Cr(VI) after 40 h | Concentration of Cr(VI) after 64 h | Concentration of Cr(VI) after 112 h |
|---|---|---|---|
| 1.44 | 1.42 | 1.42 | 1.26 |
| 2.88 | 2.60 | 2.57 | 2.34 |
Urea was employed as the anode fuel due to its convenience and low-cost advantages25. Under alkaline conditions, urea can release six electrons and the products of CO2, H2O, and N2 without forming secondary pollution (Equation 2).
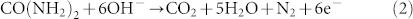 |
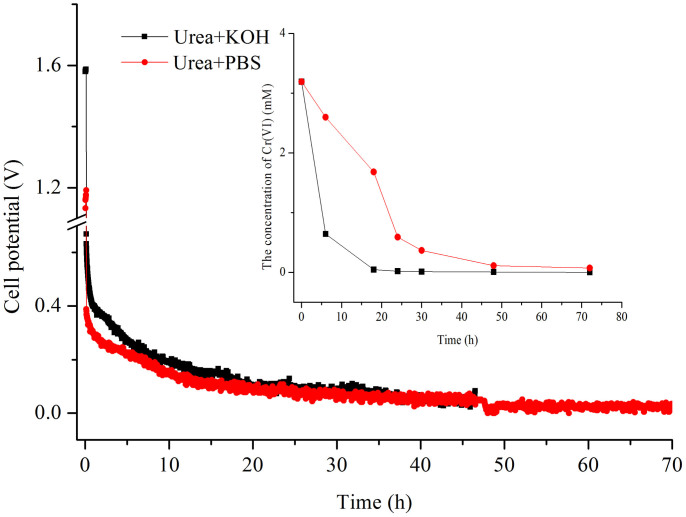
Two types of medium, including PBS (phosphate buffer solution, pH = 7.4) and KOH solution (1 M), were applied to compare the variation of cell voltage with time (Figure 7). Alkaline urea showed higher cell voltage than that of urea in the PBS solution; the open circuit potential (OCP) was 1.58 and 1.17 V. In addition, Cr(VI) exhibited a greater removal efficiency in urea with the KOH solution. After 24 h of operation, the removal efficiency was 98% and 47% in the KOH and PBS systems, respectively.
Figure 7. Variation of cell voltage and Cr(VI) concentration in the leakage with time under different systems.
(1.0 M PBS (red), 1.0 M KOH (black)), containing 1.0 M urea and fixing catholyte Cr(VI) at 3.0 mM in 0.5 M H2SO4, with a constant load of 985 Ω.).
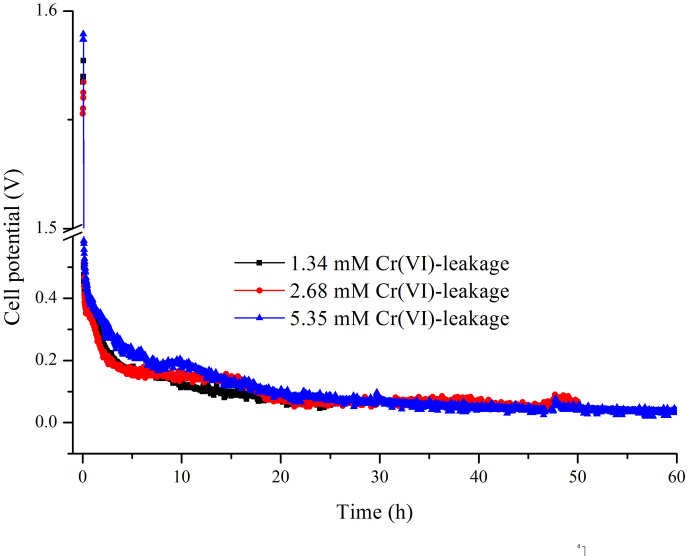
From Figure 8, it can be seen that the OCP varied from 1.55 V to 1.58 V when the initial concentration of Cr(VI) changed from 1.34 mM to 5.35 mM. The preliminary cell potential was laid between 0.476 V and 0.586 V. Then, the value decreased to 0.063 V and 0.083 V after the 24 h process. Moreover, the cell potential could maintain above 0.2 V for the initial 10 h when using Cr(VI) with a concentration of 5.35 mM. Overall, when a leakage with a higher concentration of Cr(VI) was used, higher cell potential was observed. However, the curves of cell potential tended to be the same using different concentrations of Cr(VI) in the leakage after 20 h, which was different from that using pure K2Cr2O7 for oxidation18. The fact might be explained by the presence of some interfering substances, including other metals, and reducing substances that could accelerate the reaction rate.
Figure 8. Variation of cell voltage with time under different initial Cr(VI) concentrations in 0.5 M H2SO4 and fixing the anolyte urea at 1.0 M in 1.0 M KOH, with a constant load of 1 kΩ.
Table 4 presents the calculated electrical parameters. The cathodic efficiency increased from 46.1% to 74.6% when the initial concentration of leakage Cr(VI) was increased from 1.34 to 5.35 mM. Furthermore, the Cr(VI) removal efficiencies were further evaluated using the different systems, which is presented in Table 4. The removal efficiency of Cr(VI) was 96.7% for 1.34 mM Cr(VI) after 12 h, 98.6% for 2.68 mM Cr(VI) after 18 h, and 98.6% for 5.35 mM after 48 h. Remarkably, the efficiencies were above 98% for all systems after 48 h when the initial Cr(VI) was less than 5.35 mM.
Table 4. Cr(VI) removal efficiency and electrical parameters under different electrolytes.
| Systema | C0-Cr(VI) mM | OCP(V) | Cr(VI) removal efficiency | Total generation electricity (C) | Theoretical generation electricity (C) | Cathodic efficiency (%) |
|---|---|---|---|---|---|---|
| Urea + PBS-Cr(VI) t3 | 2.68 | 1.19 | 96.5% | - | - | - |
| Urea + KOH-Cr(VI) t1 | 1.34 | 1.56 | 96.7% | 8.42 | 3.88 | 46.1 |
| Urea + KOH-Cr(VI) t2 | 2.68 | 1.59 | 98.6% | 11.3 | 7.76 | 68.6 |
| Urea + KOH-Cr(VI) t3 | 5.35 | 1.59 | 98.6% | 20.5 | 15.3 | 74.6 |
at1 = 12 h, t2 = 18 h, t3 = 48 h.
Discussion
This dynamic leaching study indicated that the main factors influencing the release of Cr(VI) are flow rate, eluting solution, and properties of the sample. Acidity should be controlled because too much H+ could lead to a vigorous reaction in the chromium-slag and a decreased amount of Cr(VI) released from the slag. The smaller particle size and the larger flow were beneficial for the leaching rate and release of Cr(VI). Based on the parameters gained from the leaching experiments, the total amount of Cr(VI) released from the slag was approximately 15–17 mg within 2–4 hours at a flow rate of 9.5 mL/h, which represented 42–48% of the total amount in the chromium-slag. It could be predicted that most Cr(VI) could be released from chromium-slags by the elution of natural rainwater when the annual rainfall is adequate. This study suggested that Cr(VI) contamination in the surrounding environment cannot be neglected if the chromium-slag was simply deposited. Considering the huge amount of chromium-slag produced by related industries, the device could be easily enlarged and would be an alternative and effective technique for simple and convenient treatment.
The reduction of Cr(VI) released from the contaminated slag via a unique urea-Cr(VI) fuel cell device demonstrates that cogeneration of electricity and chromium-slag remediation could be achieved efficiently at the same time. Nevertheless, this study displayed that most of the Cr(VI) reduction was due to the reaction that mainly produces electricity, while the remaining amount was used for the self oxidation-reduction reaction in the cathode cell due to the complicated interface in the leakage. However, the latter was regarded as an invalid reaction for the fuel cell, as it decreased the cell performance. Compared to the pure K2Cr2O7 solution with the varied OCP in the range of 1.56–1.59 V, the OCP in the leakage solution demonstrated the same range under the different amounts of initial leakage Cr(VI) concentrations. Satisfactory results for Cr(VI) reduction in the leakage were obtained. The removal percent of Cr(VI) was more than 96% after 18 h with the concentration of leakage Cr(VI) at 2.69 mM.
Methods
Leaching experiment of chromium-slag
Dried chromium-slag was put into a glass column (4 glass columns were used with diameters of 2 cm, 4 cm, 6 cm or 8 cm) and eluted by simulated acid rain at a leaching rate (Figure 1). Then, the leakage was collected hourly from the column bottom. The Cr(VI) concentrations in the leakage were determined by using a colorimetric 1,5-diphenylcarbazide method for absorption at 540 nm, and the pH value of the leakage was measured simultaneously. The release percentage of Cr(VI) was calculated according to the following formula (Equation 3):
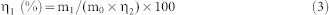 |
where η1 is the release percentage of Cr(VI) from the chromium-slag, m1 is the accumulative content of Cr(VI) in the leakage, m0 is the amount of chromium-slag, and η2 is the mass ratio of Cr(VI) in the chromium-slag.
Preparation of the electrode
For the preparation of the Ni powder, NiSO4 (5.0 mL, 0.4 M) and sodium citrate (10.0 mL, 0.1 M) were added to 250 mL deionized water in a three-neck flask. The solution was treated by aeration of nitrogen gas for 15 min. Then, fresh NaBH4 solution (4 wt%) was added dropwise into the mixed aqueous solution while under violent stirring for 3 h. The final products were collected by filtration and washed several times with deionized water and alcohol. The powder was dried at 45°C for 12 h in a vacuum oven.
Ni powder (100 mg), carbon powder (100 mg, Vulcan XC-72R), deionized water (600 μL), 5% Nafion dispersion (1.2 mL), and isopropyl alcohol (600 μL) were added into the bottle sequentially. Then, the mixture was in ultrasonic dispersion for 30 min until at last a homogeneous solution was obtained, which would be layered on a carbon cloth (Hesen, Shanghai, China) with an effective geometric area of 10 cm2. The special carbon cloth was used as the anode in the urea-KOH solution after it was dried at room temperature for 24 h. The same carbon cloth with the same area was treated as the cathode.
Reduction of Cr(VI) in the leakage via the fuel cell device
A Ni/C catalyst-coated carbon cloth with a loading of 10 mg/cm-2 was used as the anode, while a preprocessed carbon fiber cloth was employed as the cathode. The saturated KNO3 solution blocked by the ceramic core served as the separator. The dichromate leakage with the 0.5 M sulfuric acid was fed as the oxidant into the cathode chamber. A 1.0 M urea in 1.0 M KOH solution was used as the fuel in the anode chamber. All experiments were carried out in a water bath at 25°C. A data acquisition system (PISO-813, ICP DAS) was employed to record cell potentials (U) every minute. The electrical parameters, including the cathodic efficiency, theoretical electricity generation by Cr(VI) reduction, and total generated electricity were calculated on the basis of the recorded cell potential (U) and resistance via the formula in the literature18. The electric quantity (Q) generated during the experiment was calculated by integration of the discharging curve. The theoretical electric quantity at the cathode was calculated according to Equation 1. At the cathode, 50 μL of the chromium solution was removed to determine the remaining Cr(VI) concentration at a predetermined time interval.
Author Contributions
Z.W. directed the research. B.Y., H.Z., W.X. and Z.W. designed the experiments. B.Y., W.X. and G.L. carried out the experiments. B.Y. wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Acknowledgments
The authors are grateful for the financial support of NSFC (Grant No. 21073161, 21173188).
References
- Gao Y. & Xia J. Chromium contamination accident in China: viewing environment policy of China. Environ. Sci. Techno. 45, 8605–8606 (2011). [DOI] [PubMed] [Google Scholar]
- Huang S. H. et al. Spatial distribution of chromium in soils contaminated by chromium-containing slag. Trans. Nonferrous Met. Soc. China 19, 756–764 (2009). [Google Scholar]
- Li H. B., Wang Z. X., Yang Z. H., Chai L. Y. & Liao Y. P. Static and Dynamic Leaching of Chromium(VI) from Chromium-Containing Slag. Environ. Eng. Sci. 29, 426–431 (2012). [Google Scholar]
- Wang Y. Y., Yang Z. H., Chai L. Y. & Zhao K. Diffusion of Hexavalent Chromium in Chromium-Containing Slag as Affected by Microbial Detoxification. J. Hazard. Mater. 169, 1173–1178 (2009). [DOI] [PubMed] [Google Scholar]
- Geelhoed J. S. Modeling of chromium behavior and transport at sites contamination with chromite ore processing residue: implications for remediation methods. Environ. Geochem. Health 23, 261–265 (2001). [Google Scholar]
- Farmer J. G. Assessment and modeling of the environmental chemistry and potential for remediative treatment of chromium-contaminated land. Environ. Geochem. Health 21, 331–337 (1999). [Google Scholar]
- Chai L. Y. et al. Hexavalent chromium reduction by Pannonibacter phragmitetus BB isolated from soil under chromium-containing slag heap. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 44, 615–622 (2009). [DOI] [PubMed] [Google Scholar]
- Chai L. Y. et al. Cr(VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag. J. Hazard. Mater. 167, 516–522 (2009). [DOI] [PubMed] [Google Scholar]
- Beukes J. P.,Van Zyl P. G. & Ras M. Treatment of Cr(VI)-containing wastes in the South African ferrochrome industry-a review of currently applied methods. J. South Afr. Inst. Min. 112, 347–352 (2012). [Google Scholar]
- Achal X., Pan L., Lee D. J., Kumari D. & Zhang D. Y. Remediation of Cr(VI) from Chromium Slag by Biocementation. Chemosphere 93, 1352–1358 (2013). [DOI] [PubMed] [Google Scholar]
- Dhal B., Thatoi H. N., Das N. N. & Pandey B. D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: a review. J. Hazard. Mater. 250, 272–291 (2013). [DOI] [PubMed] [Google Scholar]
- Panda C. R., Mishra K. K., Panda K. C., Nayak B. D. & Nayak B. B. Environmental and technical assessment of ferrochrome slag as concrete aggregate material. Constr. Build. Mater. 49, 262–271 (2013). [Google Scholar]
- Wang T., He M. & Pan Q. A new method for the treatment of chromite ore processing residues. J. Hazard. Mater. 149, 440–444 (2007). [DOI] [PubMed] [Google Scholar]
- Yang Y. G., Xu J. H., Cai B., Wang Q. C. & Xiu D. P. Synthesis and applications of black ceramic from recycled industrial wastes. Adv. Appl. Ceram. 112, 146–148 (2013). [Google Scholar]
- Kamaludeen S., Megharaj M., Juhasz A. L., Sethunathan N. & Naidu R. Chromium-microorganism interactions in soils: Remediation implications. Rev. Environ. Contam. T. 178, 93–164 (2003). [DOI] [PubMed] [Google Scholar]
- Adam V., Quaranta G. & Loyaux-Lawniczak S. Terrestrial and aquatic ecotoxicity assessment of Cr(VI) by the recipe method calculation (Lcia): Application on an old industrial contaminated site. Environ. Sci. Pollut. R. 20, 3312–3321 (2013). [DOI] [PubMed] [Google Scholar]
- Choppala G., Bolan N., Lamb D. & Kunhikrishnan A. Comparative Sorption and Mobility of Cr(III) and Cr(VI) Species in a Range of Soils: Implications to Bioavailability. Water, Air, Soil Pollut. 224, 1699–1711 (2013). [Google Scholar]
- Zhang H. M., Xu W., Wu Z. C., Zhou M. H. & Jin T. Removal of Cr(VI) with cogeneration of electricity by an alkaline fuel cell reactor. J. Phys. Chem. C 117, 14479–14484 (2013). [Google Scholar]
- Wazne M., Jagupilla S. C., Moon D. H., Christodoulatos C. & Koutsospyros A. Leaching mechanisms of Cr(VI) from chromite ore processing residue. J. Environ. Qual. 37, 2125–2134 (2008). [DOI] [PubMed] [Google Scholar]
- Kanchinadham S. B. K., Loganathan V. D. & Kalyanaraman C. A preliminary study on leachability of chromium from a contaminated Site. Environ. Prog. Sustain. Energy 32, 1096–1100 (2013). [Google Scholar]
- Yang Z. H., Chai L. Y., Wang Y. Y., Zhao K. & Shu Y. D. Selective Leaching of Chromium-Containing Slag by HCl. J. Cent. South Univ. Technol. 15, 824–829 (2008). [Google Scholar]
- Unlu K. & Haskok S. Treatability of chromite ore processing waste by leaching. Waste Manag. Res. 19, 217–228 (2001). [DOI] [PubMed] [Google Scholar]
- Yalcin S. & Unlu K. Modeling chromium dissolution and leaching from chromite ore-processing residue. Environ. Engin. Sci. 23, 187–201 (2006). [Google Scholar]
- Geveci A., Topkaya Y. &Ayhan E. Sulfuric acid leaching of Turkish chromite concentrate. Miner. Eng. 15, 885–889 (2002). [Google Scholar]
- Meessen J. H. Ullmann's Encyclopedia of Industrial Chemistry. [Urea] [657–695] (Wiley-VCH, Germany, 2010).