Abstract
The genome of the ethanol-producing bacterium Zymomonas mobilis encodes a bd-type terminal oxidase, cytochrome bc1 complex and several c-type cytochromes, yet lacks sequences homologous to any of the known bacterial cytochrome c oxidase genes. Recently, it was suggested that a putative respiratory cytochrome c peroxidase, receiving electrons from the cytochrome bc1 complex via cytochrome c552, might function as a peroxidase and/or an alternative oxidase. The present study was designed to test this hypothesis, by construction of a cytochrome c peroxidase mutant (Zm6-perC), and comparison of its properties with those of a mutant defective in the cytochrome b subunit of the bc1 complex (Zm6-cytB). Disruption of the cytochrome c peroxidase gene (ZZ60192) caused a decrease of the membrane NADH peroxidase activity, impaired the resistance of growing culture to exogenous hydrogen peroxide and hampered aerobic growth. However, this mutation did not affect the activity or oxygen affinity of the respiratory chain, or the kinetics of cytochrome d reduction. Furthermore, the peroxide resistance and membrane NADH peroxidase activity of strain Zm6-cytB had not decreased, but both the oxygen affinity of electron transport and the kinetics of cytochrome d reduction were affected. It is therefore concluded that the cytochrome c peroxidase does not terminate the cytochrome bc1 branch of Z. mobilis, and that it is functioning as a quinol peroxidase.
Introduction
Over the last few decades, the ethanol-producing bacterium Zymomonas mobilis has been an object of ongoing interest in biotechnology (Swings & deLey, 1977; Rogers et al., 1982). Recently, full genome sequences of several Z. mobilis strains have become available (Seo et al., 2005; Kouvelis et al., 2009; Pappas et al., 2011; Desiniotis et al., 2012). The high specific rates of sugar uptake and ethanol fermentation by Z. mobilis, and its relatively small genome size, make it a promising candidate for metabolic engineering of pathways for bioethanol synthesis from agricultural and forestry waste (Dien et al., 2003; Rogers et al., 2007; Lau et al., 2010). However, for wider applications in novel bioprocesses, a more in-depth understanding of its energy metabolism would be needed, in particular concerning its aerobic metabolism.
This bacterium possesses a constitutive electron transport chain with a relatively high rate of oxygen consumption, but a low apparent yield of ATP (Bringer et al., 1984; Kalnenieks et al., 1993). Neither the physiological function of the respiratory chain nor the mechanistic reasons for the low coupling efficiency of oxidative phosphorylation in Z. mobilis have been sufficiently elucidated (Kalnenieks, 2006). In part, this is because the organization of respiratory components and the routes for electron transfer to oxygen remain unresolved. Based on genomic information, there is only one functional respiratory NAD(P)H dehydrogenase in the Z. mobilis electron transport chain, belonging to the type II respiratory dehydrogenase (Ndh) family (Kalnenieks et al., 2008; Yang et al., 2009), and only one terminal cytochrome bd-type quinol oxidase has been identified so far (Kalnenieks et al., 1998; Seo et al., 2005; Sootsuwan et al., 2008). The known Z. mobilis genome sequences also contain genes encoding a cytochrome bc1 complex and several genes for c-type cytochromes, yet lack sequences homologous to any known bacterial cytochrome c oxidase genes.
Recently, mutants of the cytochrome bc1 complex and of the bd terminal oxidase were constructed and studied (Strazdina et al., 2012). Both mutants showed strongly altered respiratory phenotypes. With two functional branches of electron transport chain, the lack of genes for terminal oxidases other than cytochrome bd raises the intriguing problem of what could be the nature of the oxidase terminating the cytochrome bc1 branch. Sootsuwan et al. (2008) and Charoensuk et al. (2011) proposed that the cytochrome bc1 branch is probably terminated by a cytochrome c peroxidase (PerC). The corresponding candidate gene is present in the genome (ZZ60192). We speculated that the cytochrome c peroxidase gene product might indeed substitute for a ‘proper’ alternative oxidase (Strazdina et al., 2012), which in principle could happen in different ways. First, it might have side-activity of an oxidase, although that would be most unusual and, to the best of our knowledge, has never been reported for a cytochrome c peroxidase. Second, it could function in combination with a respiratory peroxide-generating reaction, like the one reported for Escherichia coli fumarate reductase under aerobic conditions (Korshunov & Imlay, 2010). We aimed to test these hypotheses to establish the relevance of PerC to the alternative oxidase activity and the cytochrome bc1 branch in Z. mobilis. For that, construction of a ZZ60192 null mutant was necessary. In the present work, we report construction and study of such a cytochrome c peroxidase mutation in the Z. mobilis centrotype strain ATCC 29191. We set out to: (i) establish the presence of the product of gene ZZ60192 (perC) in membranes, using cytochrome redox differential spectroscopy; (ii) verify the putative role of perC in the electron transport to H2O2 and/or oxygen, and in the protection of cells against exogenous hydrogen peroxide (H2O2); and (iii) determine whether the cytochrome bc1 complex serves as the electron donor for the cytochrome c peroxidase, by comparing the respiratory parameters of the respective mutants.
Methods
Bacterial strains, plasmids, and transformation.
E. coli JM109 and plasmid pGEM-3Zf(+) were purchased from Promega. Strain JM109 was used as the host for cloning of the recombinant plasmids. Z. mobilis ATCC 29191 (Zm6) and its mutant derivative, defective in the cytochrome b subunit of the bc1 complex (strain Zm6-cytB), were maintained and cultivated as described previously (Kalnenieks et al., 1993; Strazdina et al., 2012). The plasmids and Z. mobilis strains constructed and used in the present work are listed in Table 1. E. coli was transformed by the CaCl2 procedure described by Sambrook et al. (1989). Z. mobilis was transformed by electroporation (Liang & Lee, 1998).
Table 1. Plasmids and strains used in the study.
| Plasmid/strain | Characteristics | Source |
| pGEM-3Zf(+) | Ampr | Promega |
| pBT | Cmr | Stratagene |
| pGEMperC | pGEM-3Zf(+) derivative, carrying a 1.34 kb fragment of PCR-amplified genomic DNA with the ORF of the cytochrome c peroxidase gene (perC; ZZ60192) cloned between the BamHI and HindIII sites of the MCS | Present work |
| pGEMperC : : cmr | pGEMperC derivative, carrying in the AgeI site of the perC a 1.3 kb AgeI restriction fragment of pBT, with a 0.7 kb chloramphenicol-resistance ORF | Present work |
| pBBR1MCS-2 | Kanr | NCBI GenBank |
| U23751 | ||
| pBBRperC | pBBR1MCS-2 derivative, carrying a 1.57 kb fragment of PCR-amplified genomic DNA with the cytochrome c peroxidase gene (perC; ZZ60192) and its promoter region, cloned between HindIII and BamHI sites of the MCS | Present work |
| Zm6 | Parent strain | ATCC 29191 |
| Zm6-cytB | Zm6 strain with a Cmr insert in the ORF of the cytochrome b subunit gene (ZMO 0957) of the bc1 complex | Strazdina et al. (2012) |
| Zm6-perC | Zm6 strain with a Cmr insert in the ORF of perC | Present work |
| Zm6-perCpBBRperC | Zm6-perC, carrying plasmid pBBRperC | Present work |
Cloning techniques, PCR and mutant construction.
Genomic and plasmid DNA isolation from Z. mobilis were performed as before (Kalnenieks et al., 2008; Strazdina et al., 2012). The Z. mobilis putative cytochrome c peroxidase gene (perC; Z. mobilis Zm6 genome sequence, locus tag ZZ60192) was amplified by PCR using the primer pair cytperox1 (CTTCTTCTGGGATCCTTGCCAGATTATGGC) and cytperox2 (GCCTATGGGGCAACAAGCTTTTATCTGGTTC). The engineered restriction sites for BamHI and HindIII, respectively, are underlined. To obtain a mutant defective in the perC gene (Zm6-perC), the amplified 1.34 kb region of the chromosomal DNA containing the perC ORF was double-digested with BamHI and HindIII, and directionally cloned between the BamHI and HindIII restriction sites of the multiple cloning site (MCS) of plasmid pGEM-3Zf(+), yielding plasmid pGEMperC (Table 1). The plasmid was used to transform E. coli JM109, and the transformants were plated on Luria–Bertani agar with ampicillin (100 μg ml−1). Plasmid pBT (Table 1) was digested with AgeI, and the 1.27 kb restriction fragment, containing a 659 bp ORF of the chloramphenicol acetyltransferase gene, was inserted into the AgeI site of the cloned perC gene, yielding plasmid pGEMperC : : cmr (Table 1). This plasmid, unable to propagate in Z. mobilis, was used to transform Z. mobilis by electroporation, and homologous recombinants were selected on plates containing chloramphenicol (120 µg ml−1).
Verification and complementation of the mutant strain.
After transformation, several colonies growing on plates with chloramphenicol were screened for the perC : : cmr genotype by PCR on the genomic DNA template with primers cytperox1 and cytperox2, yielding an amplified DNA fragment an extra 1.3 kb in length. Insertion of the chloramphenicol-resistance determinant in the cytochrome c peroxidase gene was further verified by sequencing the PCR product. For complementation of the knockout mutant Zm6-perC, a 1.57 kb chromosomal DNA fragment, containing perC with its promoter region, was amplified, using the primer pair ZZ60192f (TGTTAAGCTTCAATAAATAAAAAGGT) and ZZ60192r (TTAAAGAGGATCCTGATTATTTAGAA). The engineered restriction sites for HindIII and BamHI, respectively, are underlined. The amplified fragment was double-digested with BamHI and HindIII, and directionally cloned between the HindIII and BamHI restriction sites of the MCS of shuttle vector pBBR1MCS-2 yielding plasmid pBBRperC (Table 1). Plasmid pBBRperC was used for transformation of Zm6-perC by electroporation, and transformants were selected on agar plates containing chloramphenicol (120 µg ml−1) and kanamycin (310 µg ml−1). Total DNA of the transformed strains was isolated, and the presence of intact ZZ60192 was verified by PCR with the primer pair cytperox1 and cytperox2.
Primers for PCRs were supplied by Operon and Invitrogen. PCRs were carried out in an Eppendorf Mastercycler, using Fermentas Taq DNA polymerase. Other DNA manipulations were carried out as described previously (Kalnenieks et al., 2008; Strazdina et al., 2012), using Qiagen kits. All DNA constructs were confirmed by DNA sequencing, carried out by Beckman Coulter genomics.
Cultivation, preparation of membranes and cytochrome spectroscopy.
The growth medium contained glucose (20 g l−1), yeast extract (5 g l−1) and mineral salts, as described previously (Kalnenieks et al., 1993). Cultivations were carried out on a shaker at 100 r.p.m. in 200 ml shaken flasks, containing 30 ml of culture. Membranes were prepared by ultrasonic breakage of cells, followed by centrifugation steps, as described previously (Strazdina et al., 2012). Room temperature reduced minus oxidized (‘as prepared’) cytochrome absorption spectra were taken using membrane samples (2.5 ml) at a protein concentration of 5–6 mg ml−1, adding 25 µl of 0.5 M NADH as the reductant. Spectra were recorded with an Olis RSM1000 dual-beam rapid scanning monochromator (Online Instrument Systems), which permits the rapid acquisition of up to 1000 absorbance scans per second over a wavelength range of 300 nm, giving extremely fast time-resolved spectra or it allows the generation of average scans from the many taken over a period of time, greatly increasing the signal-to-noise ratio. The time course of cytochrome d reduction after addition of NADH was recorded by rapid, repetitive scanning in the wavelength range between 400 and 700 nm every 10 s, acquiring 1000 scans s−1 (averaged to give one scan per time point). The degree of cytochrome d reduction was calculated as the mean value of the absorbance differences at wavelength pairs 630/614 and 630/646 nm, while wavelength pairs 550/545 and 560/575 nm were used to calculate the overall degree of c- and b-type cytochrome reduction.
Determination of oxygen affinities.
Oxygen affinity of the Z. mobilis respiratory chain was determined by monitoring the deoxygenation kinetics of sperm-whale oxymyoglobin, essentially following the routine described by D’Mello et al. (1994). A custom-made, sealed optical cuvette (1.3 ml total volume, 1 cm light pathlength) was filled with 100 mM potassium phosphate buffer, previously deoxygenated by gassing with nitrogen. A few microlitres of NADH and oxymyoglobin stock solutions were added to the cuvette via a small hole in the lid using a Hamilton syringe, to yield final concentrations of 2 mM and 10 µM, respectively, and the cuvette was placed on a magnetic stirrer in the SDB-4 dual-wavelength spectrophotometer. Stability of the oxygenated form of myoglobin was checked by recording the absorbance difference between 575 and 560 nm for several minutes (Bergersen & Turner, 1979; Wood, 1984). After addition of membranes (10–100 µl), the 575/560 nm absorbance difference was further monitored, to follow deoxygenation kinetics of the oxygenated myoglobin (Fig. 1a). At least five separate determinations were carried out on each membrane preparation. The rate of oxygen consumption and the concentration of free dissolved oxygen at each time point were calculated according to Bergersen & Turner (1979) using Microsoft Excel software. In brief, the fractional oxygenation of myoglobin at any moment of time, Yt = [MbO2]/([MbO2]+[Mb]), can be estimated from the measured absorbance ratio (At−Ared)/(Aoxy−Ared), where At is the 575/560 nm absorbance difference at the respective time point, Aoxy is the absorbance difference with all myoglobin being in the oxygenated state, and Ared is the same with all myoglobin being deoxygenated (Fig. 1a). Taking 0.786 µM for the oxymyoglobin dissociation constant, K = ([Mb][O2])/[MbO2] (Bergersen & Turner 1979), the explicit relationship between the concentration of free oxygen and the fractional oxygenation of myoglobin would be: [O2] = 0.786Yt/(1−Yt). The total amount of oxygen can be calculated from the equation: O2 total = V(YtC+[O2]), where V is the volume of the cuvette (1.3 ml in our case) and C is the total concentration of myoglobin, estimated from the 435/420 nm absorbance difference in the CO difference spectrum of the dithionite-reduced myoglobin sample (Wood, 1984).
Fig. 1.
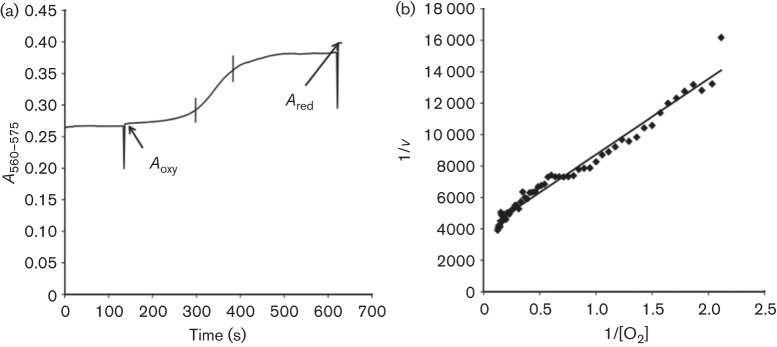
Kinetics of myoglobin deoxygenation resulting from oxygen consumption by cytoplasmic membrane preparation. (a) A typical time course of the absorbance difference at 575/560 nm with Zm6 membranes; Aoxy is the absorbance difference recorded immediately after addition of membranes into the assay with oxygenated myoglobin; Ared is the absorbance difference reached after deoxygenation of myoglobin and addition of a small amount of reductant (dithionite). (b) The absorbance differences [between the two vertical bars in (a)] were taken for calculations and for building of Lineweaver–Burk plots of oxygen consumption rate versus the free oxygen concentration, used to find the Km values (here the calculated Km value is 1.24 µM).
To find the apparent Km for oxygen, the calculated values of free dissolved oxygen concentration were plotted in Lineweaver–Burk coordinates versus the corresponding rates of decrease of the total oxygen amount in the cuvette (Fig. 1b). The values of At for the calculations were taken from the time interval in which the most rapid change of this parameter occurred (between the two vertical bars in Fig. 1a). Such Km values represent average estimates for the entire respiratory chain. The exact number and the nature of terminal oxidases in Z. mobilis remain uncertain, and therefore we did not attempt to identify the contributions of putative individual terminal oxidases by using Eadie–Hofstee plots.
Analytical methods.
H2O2 production by cells was determined fluorimetrically by monitoring Amplex UltraRed fluorescence during its reaction with H2O2, catalysed by horseradish peroxidase (Korshunov & Imlay, 2010). Fluorescence was measured with a FluoroMax-3 spectrofluorimeter (Jobin–Yvon), using 520 nm wavelength for excitation and 620 nm wavelength for emission. To quantify the generated H2O2, fluorescence increase was calibrated by addition of 1 mM H2O2 in 5 µl increments. The NADH oxidase assay for membranes was carried out by monitoring NADH oxidation spectrophotometrically at 340 nm, as previously described (Kalnenieks et al., 2008). Measurement of the NADH peroxidase activity was done generally following the assay described by Yamada et al. (2007). After deoxygenation by gassing the cuvette with oxygen-free nitrogen, the peroxidase reaction was started by addition of 10 mM H2O2 (to a final concentration of 0.01 mM) to the assay mixture containing the membranes, 1 mM glucose and 50 units of glucose oxidase, and NADH oxidation was monitored at 340 nm. For whole-cell oxygen consumption measurements, the concentration of dissolved oxygen was monitored using a Radiometer Clark-type oxygen electrode. Protein concentration in cell-free extracts and membrane samples was determined according to Markwell et al. (1978). Cell concentration was determined as OD550, and dry cell mass of the suspensions was calculated by reference to a calibration curve. Results were means of at least three replicates. Oxygen affinity measurements were done in five to eight replicates. Error bars in the figures represent sem.
Results
H2O2 sensitivity
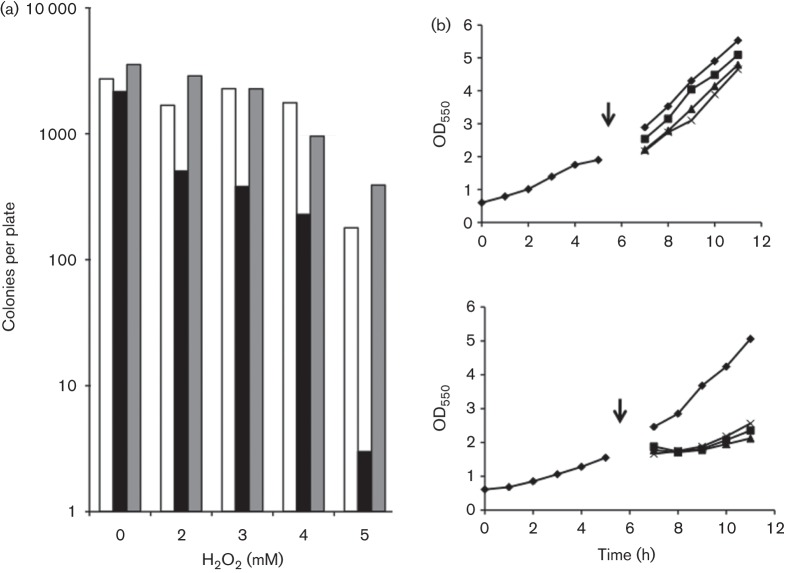
An H2O2 killing assay was carried out with cultures grown in shaken flasks at 200 r.p.m. to an OD550 of 2, corresponding to late exponential phase. Cultures were split into aliquots and transferred to Eppendorf tubes, and H2O2 was added to final concentrations as indicated in Fig. 2(a). After incubation for 30 min at 30 °C, suspensions were diluted 400 times, 6 µl aliquots were spread on agar plates with appropriate antibiotics and colonies were counted after incubation overnight. As seen from Fig. 2(a), the sensitivity of Zm6-perC to H2O2 had significantly increased. At 5 mM H2O2 in the medium the survival rate of the mutant was below 2 % of that of Zm6. However, complementation of the mutation fully restored the initial resistance to H2O2 killing.
Fig. 2.
Effect of various concentrations of H2O2 on aerobic growth and survival. (a) Colony counts in H2O2 killing assay: open bars, Zm6; black bars, Zm6-perC; grey bars, Zm6-perCpBBRperC. (b) Aerobic growth of batch cultures of Zm6 (top) and Zm6-perC (bottom) after transfer into fresh growth media following H2O2 addition (denoted by arrows) at (⧫) 0 mM, (□) 0.5 mM, (▴) 1.0 mM and (×) 1.5 mM final concentration.
In the peroxide sensitivity assay, shown in Fig. 2(b), growing cells were harvested by centrifugation and resuspended in fresh growth medium supplemented with various concentrations of H2O2 in the millimolar range. Also in this assay, sensitivity of growing Zm6-perC to externally added H2O2 appeared to be higher than that seen with Zm6 and surpassed even that of the previously constructed catalase-deficient strain (Strazdina et al., 2012). Its growth was stopped even at 0.5 mM H2O2, although catalase activity in Zm6-perC was the same as in Zm6 (data not shown). Note that, in contrast to Zm6-perC, the H2O2 sensitivity of growing Zm6-cytB culture, using the same sensitivity assay, was previously found to be similar to that of the parent strain (Strazdina et al., 2012).
Function of cytochrome c peroxidase in the respiratory chain
As the ZZ60192 product showed a distinct physiological effect on H2O2 sensitivity, our next step was to relate its function to the respiratory chain. Previously it was found that in Z. mobilis cytochrome c peroxidase could not be detected by the standard assay based on H2O2-dependent oxidation of externally added cytochrome c in cell-free extracts (Charoensuk et al., 2011; Strazdina et al., 2012). We therefore tried to establish whether the null mutation had any effect on the cytochrome content and function of the respiratory chain.
The mutation did not alter the kinetic parameters of oxygen consumption by cytoplasmic membrane preparations. Both Zm6 and Zm6-perC showed NADH oxidase activity very close to 0.5 U per milligram of membrane protein (Table 2). Fig. 1(a) shows a typical time course of 575/560 nm absorbance difference during deoxygenation of myoglobin in the presence of Zm6 membrane preparation, and Fig. 1(b) shows the corresponding Lineweaver–Burk graph used for calculation of the apparent Km for oxygen. The apparent Km values for oxygen in membrane preparations of strains Zm6 and Zm6-perC did not differ significantly, being around 1.2 µM (Table 2). Notably, in Zm6-cytB, the strain with the disrupted gene for the cytochrome b subunit of the bc1 complex, the NADH oxidase activity was in the same range, while the Km for oxygen was substantially lower, close to 0.4 µM. The difference between the mean Km values of Zm6 and Zm6-cytB, as well as between those of Zm6-perC and Zm6-cytB, was statistically significant (P<0.01 for both cases).
Table 2. Kinetics of NADH oxidation in Z. mobilis membrane preparations with H2O2 and O2 as electron acceptors.
Values are shown as mean (±sem).
| Strain | NADH oxidation [U (mg protein)−1] | Km (µM O2) | |
| NADH peroxidase | NADH oxidase | ||
| Zm6 | 0.114 (±0.008) | 0.491 (±0.092) | 1.28 (±0.242) |
| Zm6-perC | 0.072 (±0.010) | 0.499 (±0.023) | 1.18 (±0.365) |
| Zm6-cytB | 0.128 (±0.007) | 0.543 (±0.007) | 0.38 (±0.079) |
‘As prepared’ visible-light absorption spectra of membrane preparations of the parent strain and the mutant were recorded, using NADH as the reductant (Fig. 3). The cytochrome c peroxidase deficiency clearly manifested itself in the difference spectra of the mutant membranes: in the alpha region between 545 and 560 nm, corresponding largely to c-type cytochromes, absorbance was decreased. For Zm6, absorbance in this spectral region 1 min after NADH addition reached a higher level (Fig. 3a) than for Zm6-perC (Fig. 3b), and accordingly the stationary cytochrome reduction levels attained after 3 min markedly differed between the two strains (Fig. 3c). At the same time, perC disruption had much less effect on cytochrome d reduction. The cytochrome d spectral signal around 630 nm 1 min after NADH addition, as well as the final absorbance values reached after 3 min, was similar in both strains.
Fig. 3.
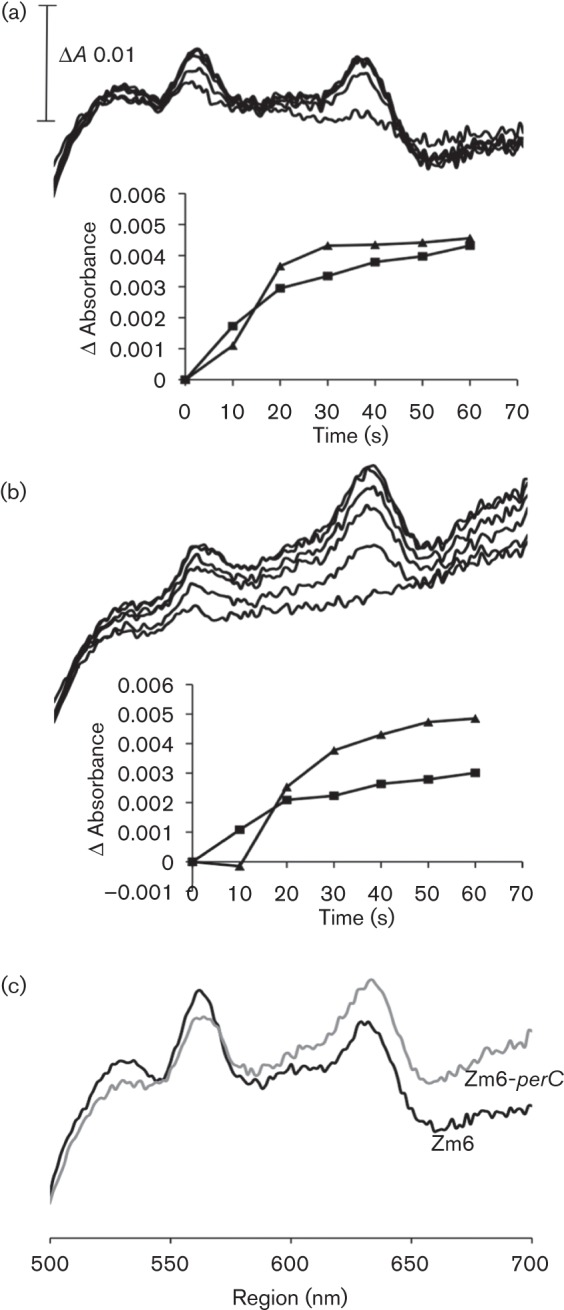
Cytochrome reduction with NADH in membrane preparations. (a) Time course of cytochrome reduction in membranes of Zm6, and (b) the same in membranes of Zm6-perC; spectra were recorded at 10 s intervals during the first minute after NADH addition. Insets: the time course of absorbance of cytochrome d (mean absorbance differences at wavelength pairs 630/614 and 630/646 nm) (▴), and of c- and b-type cytochromes (the wavelength pairs 560/545 and 560/575 nm) (□). (c) The cytochrome spectra 3 min after NADH addition.
NADH-dependent membrane peroxidase activity
Membrane preparations of Zm6 in a cuvette, flushed with nitrogen gas and supplemented with excessive amounts of glucose and glucose oxidase for oxygen removal, were able to oxidize NADH in response to H2O2 addition (Table 2). Similar observations were reported by Charoensuk et al. (2011) for a thermotolerant Z. mobilis strain. Zm6-perC membranes in such an assay showed a significantly lower (P<0.05) rate of NADH oxidation than that of the parent strain. Nevertheless, the perC disruption did not eliminate all of the apparent H2O2-dependent NADH-oxidizing activity. We speculate that at least part of the remaining activity in the mutant membranes could be due to the NADH oxidase activity of the electron transport chain, which might be supplied with trace amounts of oxygen coming from slow decomposition of H2O2. The presence of oxygen in the assay at very low concentration seems plausible also because of low oxygen affinity of the oxygen-removing enzyme: the Km of glucose oxidase for oxygen has been reported to be in the several hundred micromolar range (Nakamura et al., 1976), while for the electron transport chain, according to our data, Km is around 1 µM. In comparison to strain Zm6, however, the NADH peroxidase assay in strain Zm6-cytB indicated no decrease, but rather an increase of activity (Table 2). Accordingly, the difference between strains Zm6-perC and Zm6-cytB was even more marked (P<0.01).
Aerobic growth and respiration
Aerobic growth and respiratory capacity of whole cells was monitored during batch cultivations (Fig. 4). For the respiration assay, 1.5 ml samples were taken after 5, 7, 9 and 11 h of cultivation. Cells were sedimented, washed and resuspended in 100 mM phosphate buffer, pH 7, and oxygen consumption was measured with ethanol (10 g l−1) as electron donor. Mutating perC did not cause any loss of respiratory activity. Instead, a slight increase of respiration rate was noted in Zm6-perC. Likewise, mutation had no effect on H2O2 evolution from cells. Cells of both strains, when suspended in phosphate buffer with 20 g glucose l−1, excreted H2O2 at a low rate, close to 0.02 nmol min−1 (mg dry wt)−1 (data not shown). The mutant strain, nevertheless, grew more slowly and reached a lower biomass concentration at the end of batch growth (Fig. 4).
Fig. 4.
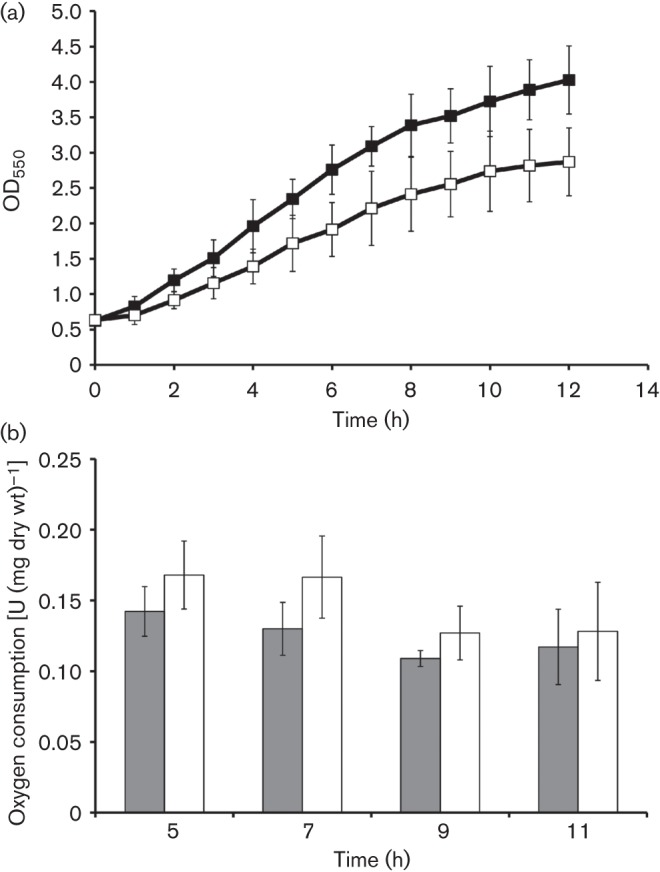
Aerobic growth and oxygen consumption. (a) Aerobic batch cultivation of Zm6 (▪) and Zm6-perC (□). (b) Oxygen consumption in washed cell suspension of Zm6 (filled bars) and Zm6-perC (empty bars) with ethanol (10 g l−1).
Discussion
Our results support the presence of a functional cytochrome c peroxidase, the product of perC (ZZ60192), in the membranes of Z. mobilis Zm6 (ATTC 29191). Cytochrome c peroxidase deficiency in the mutant strain Zm6-perC was detectable in the difference spectra of membranes, and manifested itself via a partial loss of the membrane NADH peroxidase activity, a decrease of aerobic growth rate and an increased sensitivity of cells to externally added H2O2. Elevated H2O2 sensitivity, severe disturbance of aerobic growth at high temperature and loss of respiratory peroxidase activity were reported by Charoensuk et al. (2011) for a thermotolerant Z. mobilis perC mutant strain. Cytochrome c peroxidase is thus physiologically important for Z. mobilis oxidative stress tolerance, and its deficiency is poorly compensated for by alternative stress-protection systems. However, the present work was intended primarily to check Z. mobilis for a novel, non-standard role of a bacterial cytochrome c peroxidase. We were interested in whether PerC could serve as a substitute for cytochrome c oxidase function in the respiratory chain of this bacterium.
Here we demonstrated that the perC mutation had no significant effect upon: (i) the kinetics of cytochrome d reduction, (ii) the apparent Km for oxygen in membrane preparations, (iii) the respiratory capacity of growing cells and (iv) the excretion of H2O2 by the cells. Together these findings indicate that the product of the perC (ZZ60192) gene is not participating in electron transfer to oxygen – neither directly, nor in combination with a putative respiratory H2O2-generating reaction. Hence, perC seems not to be the solution for the puzzle of the ‘hidden’ alternative oxidase of Z. mobilis. At the same time, the cytB mutation did affect the apparent Km value for oxygen and, as reported by Strazdina et al. (2012), also the redox state of cytochrome d. In contrast to perC, the cytB mutation did not cause any loss of membrane NADH peroxidase activity and also, as previously shown (Strazdina et al., 2012), had no effect on the H2O2 sensitivity of cells. Therefore, it seems apparent that: (i) an unidentified oxidase, but not cytochrome c peroxidase, is terminating the cytochrome bc1 branch, and (ii) the cytochrome bc1 complex is not the major supplier of electrons for the Z. mobilis cytochrome c peroxidase.
The cytochrome c peroxidase of Z. mobilis belongs to the family of bacterial peroxidases with three haem-binding motifs. These cytochrome c peroxidases carry an N-terminal extension with a third haem c-binding motif (CXXCH) and a methionine ligand (Atack & Kelly, 2006). The tri-haem cytochrome c peroxidase in Aggregatibacter actinomycetemcomitans has been shown to be a quinol peroxidase (Yamada et al., 2007; Takashima & Konishi, 2008), and we assume the same also for Z. mobilis. Yet, based on inhibitor analysis with antimycin, Charoensuk et al. (2011) concluded that for Z. mobilis enzyme the cytochrome bc1 complex is the major source of electrons, with NADH as the reductant. They found that the H2O2-dependent oxidation of NADH in the membranes was highly sensitive to 50 µM antimycin, while the more rapid H2O2-dependent oxidation of externally added quinol was less sensitive to this inhibitor. It is known, however, that antimycin acts as a competitive inhibitor also for quinone-binding sites of several other bacterial electron transport components apart from the cytochrome bc1 complex. For example, 50 µM antimycin inhibits E. coli cytochrome bd terminal oxidase by 80 % and cytochrome bo terminal oxidase by 18 % (Meunier et al., 1995), even though this bacterium lacks a cytochrome bc1 complex. Accordingly, binding of antimycin directly to the putative quinol-binding site of the cytochrome c peroxidase would be a reasonable alternative explanation for the data of Charoensuk et al. (2011). In that case, at high externally added quinol concentrations competitive inhibition should be less pronounced, while with NADH, presumably generating lower quinol concentration, the inhibitory effect of antimycin should be more marked, as was indeed observed. Hence, quinol is a more likely supplier of electrons to PerC than the bc1 complex, and so the pathway of electrons from the cytochrome bc1 complex to oxygen in this bacterium remains to be elucidated.
Acknowledgements
This work was funded by the Latvian Council of Science (grants 09.1306 and 536/2012), by a Royal Society R3 Grant for Travel for Collaboration (TG102318) and by the Biotechnology and Biological Sciences Research Council (UK).
Abbreviations:
- MCS
multiple cloning site
References
- Atack J. M., Kelly D. J. (2006). Structure, mechanism and physiological roles of bacterial cytochrome c peroxidases. Adv Microb Physiol 52, 73–106. 10.1016/S0065-2911(06)52002-8 [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. (1979). Systems utilizing oxygenated leghemoglobin and myoglobin as sources of free dissolved O2 at low concentrations for experiments with bacteria. Anal Biochem 96, 165–174. 10.1016/0003-2697(79)90569-4 [DOI] [PubMed] [Google Scholar]
- Bringer S., Finn R. K., Sahm H. (1984). Effect of oxygen on the metabolism of Zymomonas mobilis. Arch Microbiol 139, 376–381. 10.1007/BF00408383 [DOI] [Google Scholar]
- Charoensuk K., Irie A., Lertwattanasakul N., Sootsuwan K., Thanonkeo P., Yamada M. (2011). Physiological importance of cytochrome c peroxidase in ethanologenic thermotolerant Zymomonas mobilis. J Mol Microbiol Biotechnol 20, 70–82. 10.1159/000324675 [DOI] [PubMed] [Google Scholar]
- D’Mello R., Hill S., Poole R. K. (1994). Determination of the oxygen affinities of terminal oxidases in Azotobacter vinelandii using the deoxygenation of oxyleghaemoglobin and oxymyoglobin: cytochrome bd is a low-affinity oxidase. Microbiology 140, 1395–1402. 10.1099/00221287-140-6-1395 [DOI] [Google Scholar]
- Desiniotis A., Kouvelis V. N., Davenport K., Bruce D., Detter C., Tapia R., Han C., Goodwin L. A., Woyke T. & other authors (2012). Complete genome sequence of the ethanol-producing Zymomonas mobilis subsp. mobilis centrotype ATCC 29191. J Bacteriol 194, 5966–5967. 10.1128/JB.01398-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien B. S., Cotta M. A., Jeffries T. W. (2003). Bacteria engineered for fuel ethanol production: current status. Appl Microbiol Biotechnol 63, 258–266. 10.1007/s00253-003-1444-y [DOI] [PubMed] [Google Scholar]
- Kalnenieks U. (2006). Physiology of Zymomonas mobilis: some unanswered questions. Adv Microb Physiol 51, 73–117. 10.1016/S0065-2911(06)51002-1 [DOI] [PubMed] [Google Scholar]
- Kalnenieks U., De Graaf A. A., Bringer-Meyer S., Sahm H. (1993). Oxidative phosphorylation in Zymomonas mobilis. Arch Microbiol 160, 74–79. [Google Scholar]
- Kalnenieks U., Galinina N., Bringer-Meyer S., Poole R. K. (1998). Membrane d-lactate oxidase in Zymomonas mobilis: evidence for a branched respiratory chain. FEMS Microbiol Lett 168, 91–97. [DOI] [PubMed] [Google Scholar]
- Kalnenieks U., Galinina N., Strazdina I., Kravale Z., Pickford J. L., Rutkis R., Poole R. K. (2008). NADH dehydrogenase deficiency results in low respiration rate and improved aerobic growth of Zymomonas mobilis. Microbiology 154, 989–994. 10.1099/mic.0.2007/012682-0 [DOI] [PubMed] [Google Scholar]
- Korshunov S., Imlay J. A. (2010). Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol 75, 1389–1401. 10.1111/j.1365-2958.2010.07059.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvelis V. N., Saunders E., Brettin T. S., Bruce D., Detter C., Han C., Typas M. A., Pappas K. M. (2009). Complete genome sequence of the ethanol producer Zymomonas mobilis NCIMB 11163. J Bacteriol 191, 7140–7141. 10.1128/JB.01084-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M. W., Gunawan C., Balan V., Dale B. E. (2010). Comparing the fermentation performance of Escherichia coli KO11, Saccharomyces cerevisiae 424A(LNH-ST) and Zymomonas mobilis AX101 for cellulosic ethanol production. Biotechnol Biofuels 3, 11. 10.1186/1754-6834-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C. C., Lee W. C. (1998). Characteristics and transformation of Zymomonas mobilis with plasmid pKT230 by electroporation. Bioprocess Eng 19, 81–85. [Google Scholar]
- Markwell M. A. K., Haas S. M., Bieber L. L., Tolbert N. E. (1978). A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87, 206–210. 10.1016/0003-2697(78)90586-9 [DOI] [PubMed] [Google Scholar]
- Meunier B., Madgwick S. A., Reil E., Oettmeier W., Rich P. R. (1995). New inhibitors of the quinol oxidation sites of bacterial cytochromes bo and bd. Biochemistry 34, 1076–1083. 10.1021/bi00003a044 [DOI] [PubMed] [Google Scholar]
- Nakamura S., Hayashi S., Koga K. (1976). Effect of periodate oxidation on the structure and properties of glucose oxidase. Biochim Biophys Acta 445, 294–308. 10.1016/0005-2744(76)90084-X [DOI] [PubMed] [Google Scholar]
- Pappas K. M., Kouvelis V. N., Saunders E., Brettin T. S., Bruce D., Detter C., Balakireva M., Han C. S., Savvakis G. & other authors (2011). Genome sequence of the ethanol-producing Zymomonas mobilis subsp. mobilis lectotype strain ATCC 10988. J Bacteriol 193, 5051–5052. 10.1128/JB.05395-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P. L., Lee K. J., Skotnicki M. L., Tribe D. E. (1982). Ethanol production by Zymomonas mobilis. Adv Biochem Eng 23, 37–84. [DOI] [PubMed] [Google Scholar]
- Rogers P. L., Jeon Y. J., Lee K. J., Lawford H. G. (2007). Zymomonas mobilis for fuel ethanol and higher value products. Adv Biochem Eng Biotechnol 108, 263–288. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. (1989). Molecular Cloning: a Laboratory Manual, 2nd edn Cold Spring Harbor: Cold Spring Harbor Laboratory. [Google Scholar]
- Seo J.-S., Chong H., Park H. S., Yoon K.-O., Jung C., Kim J. J., Hong J. H., Kim H., Kim J.-H. & other authors (2005). The genome sequence of the ethanologenic bacterium Zymomonas mobilis ZM4. Nat Biotechnol 23, 63–68. 10.1038/nbt1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sootsuwan K., Lertwattanasakul N., Thanonkeo P., Matsushita K., Yamada M. (2008). Analysis of the respiratory chain in ethanologenic Zymomonas mobilis with a cyanide-resistant bd-type ubiquinol oxidase as the only terminal oxidase and its possible physiological roles. J Mol Microbiol Biotechnol 14, 163–175. 10.1159/000112598 [DOI] [PubMed] [Google Scholar]
- Strazdina I., Kravale Z., Galinina N., Rutkis R., Poole R. K., Kalnenieks U. (2012). Electron transport and oxidative stress in Zymomonas mobilis respiratory mutants. Arch Microbiol 194, 461–471. 10.1007/s00203-011-0785-7 [DOI] [PubMed] [Google Scholar]
- Swings J., De Ley J. (1977). The biology of Zymomonas. Bacteriol Rev 41, 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima E., Konishi K. (2008). Characterization of a quinol peroxidase mutant in Aggregatibacter actinomycetemcomitans. FEMS Microbiol Lett 286, 66–70. 10.1111/j.1574-6968.2008.01253.x [DOI] [PubMed] [Google Scholar]
- Wood P. M. (1984). Bacterial proteins with CO-binding b- or c-type haem. Functions and absorption spectroscopy. Biochim Biophys Acta 768, 293–317. 10.1016/0304-4173(84)90020-X [DOI] [PubMed] [Google Scholar]
- Yamada H., Takashima E., Konishi K. (2007). Molecular characterization of the membrane-bound quinol peroxidase functionally connected to the respiratory chain. FEBS J 274, 853–866. 10.1111/j.1742-4658.2006.05637.x [DOI] [PubMed] [Google Scholar]
- Yang S., Tschaplinski T. J., Engle N. L., Carroll S. L., Martin S. L., Davison B. H., Palumbo A. V., Rodriguez M., Jr, Brown S. D. (2009). Transcriptomic and metabolomic profiling of Zymomonas mobilis during aerobic and anaerobic fermentations. BMC Genomics 10, 34. 10.1186/1471-2164-10-34 [DOI] [PMC free article] [PubMed] [Google Scholar]