Abstract
Background
There is an increasing appreciation of the deleterious effects of antibody and B cells on acute and chronic transplant outcomes. Many effector functions of antibody are mediated by a family of receptors (FcγRs) that are expressed on most immune cells, including neutrophils, natural killer cells, and B cells. Most FcγRs are activating and controlled by a single inhibitory receptor, FcγRIIB (CD32B), which also regulates some aspects of B-cell activation and antibody production. FcγRIIB-deficient mice develop severe chronic arteriopathy in a murine cardiac allograft model. A single nucleotide polymorphism in human FcγRIIB (rs1050501) results in profound receptor dysfunction and is associated with systemic lupus erythematosus. The frequency of this FcγRIIB-I/T232 polymorphism also shows significant racial variation.
Methods
In the present study, we sought to determine whether the FcγRIIB-I/T232 single nucleotide polymorphism rs1050501 affected susceptibility to renal allograft rejection or loss and transplant recipient survival. FcγRIIB-I/T232 genotype was determined in 2,851 Caucasian and 570 Afro-Caribbean renal transplant recipients, and in 236 transplant recipients with a primary diagnosis of systemic lupus erythematosus, all of whom were enrolled into the Collaborative Transplant Study.
Results
We found no significant difference in pretransplant panel reactive antibodies, acute rejection at 1-year nor in 10-year transplant or patient survival in individuals with differing FcγRIIB-I/T232 genotype.
Conclusion
This negative result is surprising, given the importance of this receptor in modulating antibody effector function.
Keywords: Antibodies, IgG, Fcγ receptors, FcγRIIB, CD32B, Renal transplantation, Chronic antibody-mediated rejection
Renal transplantation represents the optimal treatment of most patients with end-stage renal failure. In the last three decades, improvements in immunosuppressive regimens have led to an incremental reduction in the severity and frequency of acute T cell–mediated (cellular) rejection such that 1-year kidney allograft survival is currently approximately 95%. However, the treatment of acute antibody-mediated rejection (AMR) remains challenging, and there is an increasing appreciation of the role played by antibody in chronic allograft attrition (1, 2). Chronic AMR is particularly prevalent after human leukocyte antigen (HLA) antibody-incompatible transplantation, despite desensitization protocols centered on removing donor-specific immunoglobulin (Ig) G (3). The occurrence and severity of antibody-mediated graft pathology in both acute and chronic settings is variable, and it is likely that genetic polymorphisms, which affect the magnitude of the B-cell response and of the effector functions of antibody in the recipient, might give rise to such pathologic variation. An additional unresolved challenge to long-term allograft survival is that of recipient death with a functioning graft. This most frequently occurs in the context of infection, malignancy, or cardiovascular disease, all of which are heavily influenced by immunologic factors.
Fc gamma receptors (FcγR) bind the Fc portion of IgG antibodies and are expressed by most immune cells, including natural killer (NK) cells, dendritic cells (DCs), macrophages, and neutrophils (4). Immunoglobulin G binding to activating FcγR receptors (FcγRIIA [CD32A], FcγRIIIA [CD16A], and FcγRIIIB [CD16B]) results in neutrophil and macrophage activation and immunogenic antigen presentation to T cells by DCs. The activating effects of IgG on these myeloid cells are controlled by a single inhibitory receptor, FcγRIIB (CD32B). FcγRIIC and FcγRIIIA expressions by NK cells is required for antibody-dependent cellular cytotoxicity, but these cells do not express the inhibitory FcγRIIB (5, 6). FcγRIIB is expressed by B cells and plasma cells, regulating the B-cell receptor activation threshold on encounter with IgG-opsonized antigen and plasma cell apoptosis (4, 7-9). Thus, the inhibitory IgG receptor FcγRIIB plays a critical role in controlling both antibody generation and its immune activating and inflammatory effects. Manipulation of this receptor in mouse models emphasizes its importance in immune modulation; FcγRIIB-deficient mice are prone to inducible and spontaneous antibody-associated autoimmune disease (10, 11) but have heightened cytotoxic responses to tumor (12) and are protected from some infections (13, 14). In murine cardiac allograft models, FcγRIIB had no effect on acute allograft rejection, but chronic arteriopathy and autoantibody production were increased in FcγRIIB-deficient recipients (15).
In humans, a single nucleotide polymorphism (SNP, rs1050501) has been identified in the FCGR2B gene which encodes an amino acid substitution (a threonine for an isoleucine at position 232) within the transmembrane domain of the receptor. FcγRIIB-T232 is associated with receptor dysfunction (16, 17) and is found at increased frequency in patients with systemic lupus erythematosus (SLE), an autoimmune disease characterized by hypergammaglobulinemia and mediated by IgG immune complexes (18). The prevalence of this polymorphism shows considerable racial variation (7%–13% of Africans are homozygous for FcγRIIB-T232 but only 1%–2% of Caucasians (18)). Such racial variation in SNP frequency may have arisen because of enhanced protective immune responses to some pathogens in FcγRIIB-T232 homozygotes (14, 16, 18). There is currently no information on the effect of the FCGR2B SNP on outcomes in transplantation. Given the importance of FcγRIIB in controlling antibody responses and antibody effector function, we sought to determine whether the defunctioning polymorphism might be associated with altered long-term allograft function or with patient or allograft survival posttransplant. We genotyped the FCGR2B SNP rs1050501 in three cohorts of renal transplant recipients enrolled into the Collaborative Transplant Study (CTS); Cohort A comprised 2851 Caucasian patients, cohort B, 570 Afro-Caribbean patients, and cohort C, 236 patients with a primary diagnosis of SLE. We found no statistically significant difference in long-term transplant or patient survival in patients with differing FcγRIIB-I/T232 genotype.
RESULTS
FcγRIIB-I/T232 Frequency in Transplant Recipients
Baseline characteristics of the three patient cohorts are shown in Table 1. In cohort A, the frequency of FcγRIIB-T232 homozygotes was 2.2% (Table 1), which is broadly similar to previously published data for Caucasian control populations (18). The frequency of FcγRIIB-T232 homozygotes was 6.8% in cohort B (Table 1), in keeping with previous reports of a higher frequency of this genotype in individuals of African ancestry compared with Caucasians (18, 19). In a cohort of predominantly Caucasian patients with a diagnosis of SLE (cohort C), the frequency of FcγRIIB-T/T232 genotype was higher than that observed in cohort A (3.8% vs. 2.2%).
TABLE 1.
Patient demographics and FCGR2B (rs1050501) genotype for patient cohorts investigated
| Cohort A (Caucasian) | Cohort B (Afro-Caribbean) | Cohort C (SLE patients) | |
|---|---|---|---|
| Number of patients | 2851 | 570 | 236 |
| Median follow-up time, yr | 7 | 3.6 | 7 |
| Race | |||
| Caucasian | 2851 (100%) | — | 190 (80.5%) |
| Afro-Caribbean | — | 570 (100%) | 16 ( 6.8%) |
| Other | — | — | 30 (12.7%) |
| Continent | |||
| Europe | 2426 (85.1%) | 51 (8.9%) | 123 (52.1%) |
| North America | 425 (14.9%) | 136 (23.9%) | 84 (35.6%) |
| Africa | — | 355 (62.3%) | 9 (3.8%) |
| Other | — | 28 (4.9%) | 20 (8.5%) |
| Age, yr | |||
| <18 | — | 27 (4.7%) | 7 (3.0%) |
| 18–64 | 2565 (90.0%) | 536 (94.0%) | 225 (95.3%) |
| 65+ | 286 (10.0%) | 7 (1.2%) | 4 (1.7%) |
| Mean (range) | 47.6 (18–76) | 39.4 (4–73) | 37.1 (10–72) |
| Sex | |||
| Male | 1763 (61.8%) | 333 (58.4%) | 41 (17.4%) |
| Female | 1088 (38.2%) | 237 (41.6%) | 195 (82.6%) |
| Transplant year | |||
| 1988–1997 | 1865 (65.4%) | 498 (87.4%) | 155 (65.7%) |
| 1998–2010 | 986 (34.6%) | 72 (12.6%) | 81 (34.3%) |
| Transplant number | |||
| 1 | 2433 (85.3%) | 517 (90.7%) | 214 (90.7%) |
| >1 | 418 (14.7%) | 35 (9.3%) | 22 (9.3%) |
| Donor type | |||
| Deceased | 2851 (100%) | 529 (92.8%) | 180 (76.3%) |
| Living | — | 41 (7.2%) | 56 (23.7%) |
| HLA mismatch | |||
| 0–1 | 419 (14.7%) | 24 (4.2%) | 49 (20.8%) |
| 2–4 | 2031 (71.2%) | 322 (56.5%) | 156 (66.1%) |
| 5–6 | 401 (14.1%) | 224 (39.3%) | 31 (13.1%) |
| FCGR2B genotype | |||
| I/I | 2219 (77.8%) | 319 (56.0%) | 188 (79.7%) |
| I/T | 568 (19.9%) | 212 (37.2%) | 39 (16.5%) |
| T/T | 64 (2.2%) | 39 (6.8%) | 9 (3.8%) |
| Calcineurin inhibitors | |||
| Cyclosporine | 519 (91.1%) | 170 (72.0%) | |
| Tacrolimus | 2415 (84.7%) | 30 (5.3%) | 50 (21.2%) |
| None | 436 (15.3%) | 21 (3.7%) | 16 (6.8%) |
| Antiproliferative | |||
| Azathioprine | 1297 (45.5%) | 492 (86.3%) | 129 (54.7%) |
| Mycophenolate | 903 (31.7%) | 45 (7.9%) | 72 (30.5%) |
| None | 651 (22.8%) | 33 (5.8%) | 35 (14.8%) |
| Steroids | |||
| Yes | 2803 (98.3%) | 565 (99.1%) | 233 (98.7%) |
| No | 48 (1.7%) | 5 (0.9%) | 3 (1.3%) |
HLA, human leukocyte antigen; SLE, systemic lupus erythematosus.
FcγRIIB-I/T232 Polymorphism and Transplant Outcomes in Caucasians
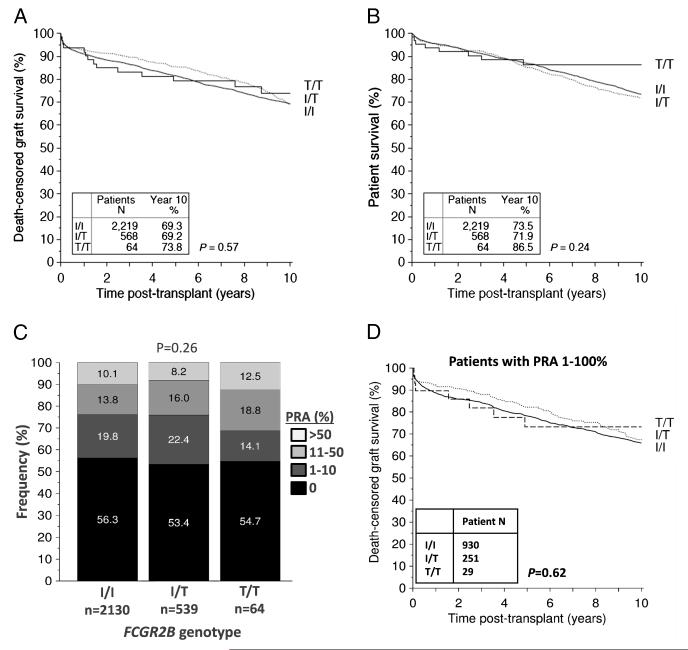
In cohort A (n=2,851 Caucasian transplant recipients), death-censored allograft survival did not significantly differ between FCGR2B genotypes (Fig. 1A and Table 2) at 1 year (93.6%, 92.9%, and 91.1% in those with FcγRIIB-T/T232, FcγRIIB-T/I232, and FcγRIIB-I/I232 genotypes, respectively), 5 years (79.2%, 85.5%, 81.5%, respectively), or 10 years (73.8%, 69.2%, and 69.3%, respectively) after transplantation. All survival results presented were adjusted for potential confounders using multivariable Cox regression. In particular, there were no significant differences in the immunosuppression regimens used in the different genotype groups (see Table 1 and Table S1, SDC, http://links.lww.com/TP/B23). The frequency of treatment of rejection was not statistically significantly different at 1 year in individuals with differing FCGR2B-T232 genotype (Table 3), although the number of patients on whom we had data about rejection episodes was limited, reducing our power to detect a small effect size of genotype on rejection. However, a significantly greater number of patients with the FcγRIIB-T/T232 genotype had a creatinine lower than 130 Kmol/L (1.5 mg/dL) at 1 year compared with patients with the FcγRIIB-T/I232, and FcγRIIB-I/I232 genotypes (Table 3; 73.2%, 54.9%, and 59.1% respectively, P=0.01). Patient survival was similar in all FcγRIIB-I/T232 genotype groups (Fig. 1B). The proportion of patients with a pretransplant panel reactive antibody (PRA) greater than 10% was highest in the FcγRIIB-T/T232 genotype group (31.1%) versus 24.2% and 23.9% in the subjects with the FcγRIIB-T/I232 and FcγRIIB-I/I232 genotypes, respectively, but this did not reach statistical significance (Fig. 1C). We also performed an analysis of graft survival, limited to patients with detectable HLA antibodies pretransplant (PRA, 1%–100%). This showed no significant difference in death-censored graft survival between genotype groups (Fig. 1D).
FIGURE 1.
A, Death-censored allograft survival and (B) patient survival in Caucasian renal transplant recipients. Kaplan-Meier survival curves shown for patients with differing FCGR2B genotype up to 10 years after transplantation. Patients with FcγRIIB-T/T232 (solid black line), with FcγRIIB-I/T232 (dashed line), and FcγRIIB-I/I232 (solid gray line). P values generated using the Mantel-Cox log rank test. C, Pretransplant HLA antibody status in patients with differing FCGR2B genotype, as reflected by the % of patients with each genotype with different levels of PRA. D, Death-censored allograft survival in Caucasian renal transplant recipients with a pretransplant PRA of 1%–100%. Kaplan-Meier survival curves shown for patients with differing FCGR2B genotype up to 10 years after transplantation. PRA, panel-reactive antibodies; HLA, human leukocyte antigen; PRA, panel reactive antibody.
TABLE 2.
Multivariate analysis of allograft and patient survival by FCGR2B genotype in cohorts A–C
| FCGR2B genotype | Cohort A (Caucasian) | Cohort B (Afro-Caribbean) | Cohort C (SLE patients) | |
|---|---|---|---|---|
| Death censored allograft survival | P=0.66 | P=0.22 | P=0.92 | |
| I/I | Reference | Reference | Reference | |
| I/T | 0.92 (P=0.38) | 1.16 (P=0.32) | 1.15 (P=0.69) | |
| T/T | 1.05 (P=0.85) | 0.67 (P=0.22) | 0.98 (P=0.98) | |
| Patient survival | P=0.21 | P=0.25 | P=0.32 | |
| I/I | Reference | Reference | Reference | |
| I/T | 1.09 (P=0.36) | 1.25 (P=0.10) | 0.52 (P=0.21) | |
| T/T | 0.60 (P=0.15) | 0.99 (P=0.98) | 1.70 (P=0.42) |
TABLE 3.
Outcome at 1 year after transplantation
| I/I | I/T | T/T | P a | |
|---|---|---|---|---|
| Cohort A (Caucasian) | ||||
| Serum creatinine at year 1 | ||||
| <130 μmol/L (1.5 mg/dL) | 1135 (59.1%) | 277 (54.9%) | 41 (73.2%) | 0.018 |
| ≥130 μmol/L (1.5 mg/dL) | 786 (40.9%) | 228 (45.1%) | 15 (26.8%) | |
| Rejection treatment during year 1 | ||||
| Yes | 273 (25.1%) | 65 (22.4%) | 9 (29.0%) | 0.51 |
| No | 813 (74.9%) | 225 (77.6%) | 22 (71.0%) | |
| Hospitalization because of infection during year 1 | ||||
| Yes | 73 (17.3%) | 19 (17.1%) | 3 (25.0%) | 0.71 |
| No | 348 (82.7%) | 92 (82.9%) | 9 (75.0%) | |
| Cohort B (Afro-Caribbean) | ||||
| Serum creatinine at year 1 | ||||
| <130 μmol/L (1.5 mg/dL) | 137 (56.8%) | 87 (57.6%) | 16 (53.3%) | 0.90 |
| ≤130 μmol/L (1.5 mg/dL) | 104 (43.2%) | 64 (42.4%) | 14 (46.7%) | |
| Rejection treatment during year 1 | ||||
| Yes | 43 (29.7%) | 33 (29.7%) | 3 (15.8%) | 0.49 |
| No | 102 (70.3%) | 78 (70.3%) | 16 (84.2%) | |
| Hospitalization because of infection during year 1 | ||||
| Yes | 3 (6.7%) | 2 (6.7%) | 1 (12.5%) | 0.68 |
| No | 42 (93.3%) | 28 (93.3%) | 7 (87.5%) | |
| Cohort C (SLE patients) | ||||
| Serum creatinine at year 1 | ||||
| <1.5 mg/dL (130 μmol/L) | 116 (71.2%) | 18 (56.2%) | 6 (100%) | 0.052 |
| ≤1.5 mg/dL (130 μmol/L) | 47 (28.8%) | 14 (43.8%) | 0 (0.0%) | |
| Rejection treatment during year 1 | ||||
| Yes | 19 (25.3%) | 1 (5.9%) | 0 (0.0%) | 0.24 |
| No | 56 (74.7%) | 16 (94.1%) | 3 (100%) | |
| Hospitalization because of infection during year 1 | ||||
| Yes | 4 (12.1%) | 0 (0.0%) | — | 0.56 |
| No | 29 (87.9%) | 11 (100%) |
Fisher test.
Hazard ratios and P values calculated with multivariable Cox regression. SLE, systemic lupus erythematosus.
FcγRIIB-I/T232 Polymorphism and Transplant Outcomes in Afro-Caribbean Transplant Recipients
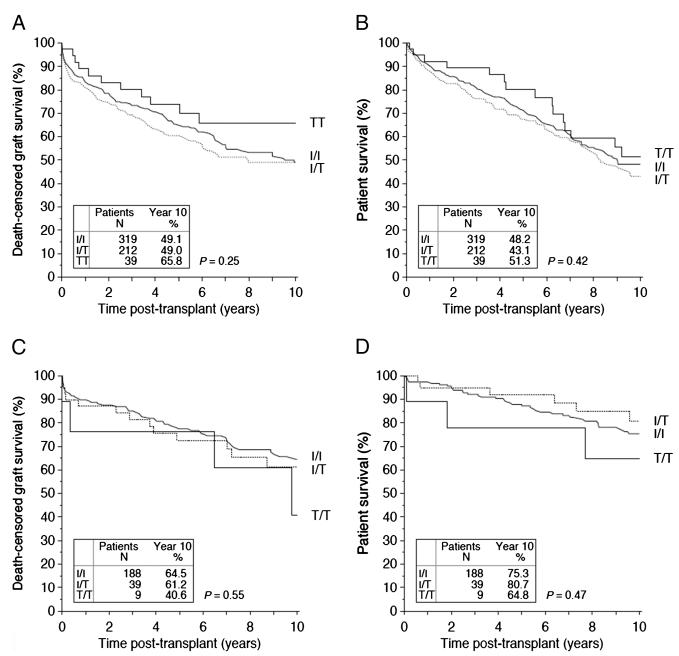
In cohort B (n=570 Afro-Caribbean transplant recipients), there is no significant difference in death-censored allograft survival in FcγRIIB-T232 homozygotes compared with heterozygotes and FcγRIIB-I232 homozygotes, both at 1 year (89.0% vs. 81.4% and 83.4% respectively), 5 years (73.8% vs. 60.5% and 65.3%, respectively), and 10 years after transplantation (65.8% vs. 49.0% and 49.1%, respectively; P=0.25; Fig. 2A and Table 2). There was no statistically significant difference in serum creatinine at 1 year, the frequency of treatment of rejection in the first year after transplantation (Table 3) nor in patient survival (Fig. 2B) between the individuals with different FCGR2B genotype.
FIGURE 2.
A, Death-censored allograft survival and B, Patient survival in renal transplant recipients of Afro-Caribbean origin. C, Death-censored allograft survival and (D) Patient survival in renal transplant recipients with an underlying diagnosis of SLE. Kaplan-Meier survival curves shown for patients with differing FCGR2B genotype up to 10 years after transplantation. Patients with FcγRIIB-T/T232 (solid black line), with FcγRIIB-I/T232 (dashed line), and FcγRIIB-I/I232 (solid gray line). SLE, systemic lupus erythematosus.
FcγRIIB-I/T232 Polymorphism and Transplant Outcomes in Patients With SLE
In cohort C (n=236 patients with a diagnosis of SLE), death-censored allograft survival was lower in FcγRIIB-T232 homozygotes compared with heterozygotes and FcγRIIB-I232 homozygotes at 1 year (76.2% vs. 87.0% and 89.8%, respectively) and at 10 years (40.6%, 61.2%, and 64.5% respectively), but this did not reach statistical significance given the small numbers available for analysis in this cohort (P=0.55; Fig. 2C and Table 2). Allograft function (as measured by serum creatinine) and frequency of treatment of rejection was not statistically significantly different at 1 year in individuals with differing FCGR2B genotype (Table 3). Posttransplant patient survival was similar in individuals of differing FCGR2B genotype (Fig. 2D).
DISCUSSION
We hypothesized that a defunctioning, autoimmune-associated SNP in the IgG inhibitory receptor FcγRIIB might significantly impact allograft survival by altering susceptibility to rejection, particularly chronic AMR (as has been demonstrated in a murine cardiac allograft model (15)). There is an increasing appreciation that the deleterious effects of donor-specific antibodies (DSA) on renal allografts may occur by means of complement-independent pathways, as evidenced by the existence of C4d-negative AMR (20, 21). Such complement-independent effects would likely be mediated by means of FcγRs expressed on effector cells, such as neutrophils and NK cells. Of note, FcγRIIB regulates IgG-mediated activation of neutrophils, a cell type observed within the capillaries of biopsies with AMR (22). There is also increasing evidence that DSA activate NK cells (presumably by means of activating FcγRs), causing chronic allograft pathology (21, 23, 24). We did not detect any statistically significant difference in long-term allograft loss (in which chronic AMR is believed to play a role (1, 2)), which is in contrast to the murine data available (15) and emphasizes the importance of human studies to confirm the relevance of such experimental observations. It may also have implications for our understanding of the pathogenesis of chronic antibody-mediated graft damage; NK cells express only activating FcγR and do not normally express FcγRIIB. Therefore, although the defunctioning FCGR2B SNP would affect neutrophil, macrophage, DC and B-cell activation, it would not normally influence NK cell–mediated allograft damage. Thus, our results support a role for NK cells in mediating the noncomplement-dependent effects of antibody on the allograft, although we have not examined any aspects of NK cell function in this study. Alternatively, it may be that the deleterious effects of the FCGR2B SNP on alloantibodyassociated pathology on long-term graft survival are offset by other beneficial effects, for example, reduced susceptibility to polyoma virus, because antibodies are important in defense against viral infection, and BK neutralizing antibodies have been identified in human sera (25).
Our second hypothesis was that FCGR2B genotype might influence transplant patient survival by virtue of its importance in controlling the immune response to infections and tumors (7, 12). In particular, FcγRIIB has been shown to control defense against bacterial (13, 26), mycobacterial (27), viral (28), and parasitic (14) infections, and previous studies suggest that the FcγRIIB-T/T232 genotype may confer increased resistance to some infections (16, 18). In the present study, patient survival at 10 years after transplantation was higher in Caucasian patients with the FcγRIIB-T/T232 genotype (87%, compared with 72% and 74% in patients with the FcγRIIB-T/I232, and FcγRIIB-I/I232 genotypes, respectively; Fig. 1), but this did not reach statistical significance.
There are a number of caveats worth noting when interpreting our data; failure to detect an association between FCGR2B genotype and allograft or patient survival may be because of the fact that the effect size of this SNP is smaller than estimated by our power calculations, and therefore we were underpowered to detect any differences. Larger studies with an increased number of patients may reveal such associations. In addition, the phenotypic data available on this cohort did not include detailed information on humoral alloimmune responses, such as the development of de novo DSA posttransplant, the presence of transplant glomerulopathy on biopsy, or the presence of proteinuria as a surrogate marker of the latter pathology. Therefore, we cannot exclude that FCGR2B genotype might affect more specific aspects of antibody-mediated alloimmunity. However, because many studies have demonstrated a clear association between acute and chronic AMR and DSA and reduced allograft survival (1-3), one would expect this outcome measure to reflect the presence of clinically significant antibody-mediated pathology. In addition, it should be noted that this was a retrospective analysis of CTS data, which includes self-reported data from multiple centers and is therefore subject to the inherent limitations of such a study.
In conclusion, in two cohorts of Caucasian and African renal transplant recipients, we found no effect of FCGR2B genotype on pretransplant PRA nor on 10-year transplant or patient survival.
MATERIALS AND METHODS
Subjects
Transplant Recipients
DNA samples and data on patients were collected between 1988 and 2010 by the CTS facility in Heidelberg, Germany. The CTS collates information and samples from voluntarily participating transplantation centers around the world with the goal of expanding scientific knowledge in the area of transplantation. Written informed consent for the study was obtained from patients at the individual participating centers, and approval for the study was granted by the University of Heidelberg ethics committee (application no. 083/2005).
Three patient cohorts were studied (see Table 1 for details):
Cohort A: n=2851 Caucasian transplant recipients. The DNA used for this cohort was randomly chosen from a bank at the CTS.
Cohort B: n=570 Afro-Caribbean subjects.
Cohort C: n=236 patients with a primary diagnosis of SLE.
When considering overall allograft survival, assuming that the difference in graft survival between a zero HLA mismatch graft, and a six HLA-mismatched graft (80% 5-year survival vs. 60% 5 year survival) represents the potential frequency of graft loss related to immunologic mechanisms, then with a sample size of 3000, and an FcγRIIBT/T232 homozygosity rate of 1.5%, the study would have 87% power to detect such a difference. We had rejection data available on 1407 Caucasians, providing 85% power to detect an OR of 3.9, when comparing patients with rejection against controls, and assuming a one year acute rejection rate of 20%.
Clinical Data
Details of the donors and recipients are stored in the CTS database. This information includes age; sex; continent of residence; transplant year; number of previous transplants; cold ischemia time; presence of HLA-A, HLA-B, and HLA-DR mismatches; pretransplant PRA level; disease leading to renal failure; means of initial immunosuppression; serum creatinine level; number of rejection episodes; timing and cause of death; and details about allograft loss and transplantation outcome. Subjects were not routinely screened for the development of de novo HLA antibodies posttransplant. Information on rejection episodes is gathered as follows: if a patient has a functioning graft at the end of a follow-up year (e.g., years 1, 2, 3, 5, 10), an additional follow-up request is made asking whether a rejection episode has been treated during the preceding year. Some questionnaires are not returned, thus information on rejection is not available on all patients in the study.
Genotyping
Patients were genotyped for rs1050501 as described previously using genomic DNA (18). All DNAs were diluted to a concentration of 4 ng/μL before using as a template in the genotyping assay. Polymerase chain reaction was performed using the following primers: sense 5′-CCT-TTA-GAC-CCT-GCT-GGA-AAG-AAG-3′ and antisense 5′CAC-TAC-ACT-GCT-CTC-CCC-AAG-AC-3′ (Applied Biosystems). Polymerase chain reaction products were purified using exonuclease I and shrimp alkaline phosphatase (Exosap; GE Healthcare, Hertfordshire, UK) and then genotyped using an Applied Biosystems Custom TaqMan Human SNP Genotyping Assay (FCGR2BEX5-232T) in accordance with the manufacturer’s protocol.
Genotype was undetermined in 4.8% of samples from cohort A, 3.2% of cohort B, and 3.0% of samples from cohort C, an acceptable fail-rate for this assay (18).
Statistical Analyses
Statistical analysis was performed with the use of SPSS software, version 20, and R software, version 2.15. Categorical variables were compared by means of chi-square analysis and continuous variables by means of the Kruskall-Wallis test. The Kaplan-Meier algorithm was used to compare death censored graft survival among genotype groups using the Mantel-Cox log rank test. Death censored graft survival was calculated from the date of transplantation to the date of return to dialysis (or retransplantation) or the date of last follow-up with a functioning graft. In the event of death with functioning graft, the follow-up period was censored at the date of death. Survival results were adjusted for potential confounders using multivariable Cox regression including the following variables: geographic region (divided into three categories Europe, North America and Other), year of transplant, transplant number (first or retransplant), recipient age and sex, donor type (deceased or living), donor age and sex, original disease leading to end-stage renal disease, general evaluation of patient as candidate for transplantation (based on the question “Your general evaluation of this patient as candidate for transplantation: good, moderate, poor” on the initial questionnaire of CTS), time on dialysis, pretransplant panel reactive antibodies, HLA-A+HLA-B+HLA-DR mismatches, cold ischemia time, immunosuppressive therapy (calcineurin inhibitors, steroids). All confounders were suitably categorized. We included all known relevant confounders and excluded confounders with P greater than 0.2 through a backstep algorithm.
Supplementary Material
Acknowledgments
This work was supported by a Wellcome Trust Intermediate Fellowship (WT081020) to MRC, by a grant from the Roche Organ Transplant Research Fund, and by the National Institute for Health Research Cambridge Biomedical Research Centre. K.G.C.S. was funded by a Wellcome Trust (programme grant number 083650/Z/07/Z).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Stegall MD, Raghavaiah S, Gloor JM. The (re)emergence of B cells in organ transplantation. Curr Opin Organ Transplant. 2010;15:451. doi: 10.1097/MOT.0b013e32833b9c11. [DOI] [PubMed] [Google Scholar]
- 2.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348. doi: 10.1038/nrneph.2012.81. [DOI] [PubMed] [Google Scholar]
- 3.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76. doi: 10.1111/j.1600-6143.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 4.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 5.Titus JA, Perez P, Kaubisch A, et al. Human K/natural killer cells targeted with hetero-cross-linked antibodies specifically lyse tumor cells in vitro and prevent tumor growth in vivo. J Immunol. 1987;139:3153. [PubMed] [Google Scholar]
- 6.Metes D, Ernst LK, Chambers WH, et al. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcgammaRIIC gene. Blood. 1998;91:2369–2380. [PubMed] [Google Scholar]
- 7.Smith KGC, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baerenwaldt A, Lux A, Danzer H, et al. Fcgamma receptor IIB (FcgammaRIIB) maintains humoral tolerance in the human immune system in vivo. Proc Natl Acad Sci U S A. 2011;108:18772. doi: 10.1073/pnas.1111810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Z, Cutler AJ, Brownlie RJ, et al. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 10.Takai T, Ono M, Hikida M, et al. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 11.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 12.Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 13.Clatworthy MR, Smith KG. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199:717. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clatworthy MR, Willcocks L, Urban B, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A. 2007;104:7169. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callaghan CJ, Win TS, Motallebzadeh R, et al. Regulation of allograft survival by inhibitory FcgammaRIIb signaling. J Immunol. 2012;189:5694. doi: 10.4049/jimmunol.1202084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floto RA, Clatworthy MR, Heilbronn KR, et al. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 17.Kono H, Kyogoku C, Suzuki T, et al. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 18.Willcocks LC, Carr EJ, Niederer HA, et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107:7881. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Wu J, Carter RH, et al. A novel polymorphism in the Fcgamma receptor IIB (CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. 2003;48:3242. doi: 10.1002/art.11313. [DOI] [PubMed] [Google Scholar]
- 20.Haas M. Pathology of C4d-negative antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant. 2013;18:319. doi: 10.1097/MOT.0b013e32835d4daf. [DOI] [PubMed] [Google Scholar]
- 21.Sellares J, Reeve J, Loupy A, et al. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant. 2013;13:971. doi: 10.1111/ajt.12150. [DOI] [PubMed] [Google Scholar]
- 22.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 23.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 24.Hirohashi T, Chase CM, Della Pelle P, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randhawa PS, Schonder K, Shapiro R, et al. Polyomavirus BK neutralizing activity in human immunoglobulin preparations. Transplantation. 2010;89:1462. doi: 10.1097/tp.0b013e3181daaaf1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gjertsson I, Kleinau S, Tarkowski A. The impact of Fcgamma receptors on Staphylococcus aureus infection. Microb Pathog. 2002;33:145. [PubMed] [Google Scholar]
- 27.Maglione PJ, Xu J, Casadevall A, et al. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 28.Da Silva DM, Fausch SC, Verbeek JS, et al. Uptake of human papillomavirus virus-like particles by dendritic cells is mediated by Fcgamma receptors and contributes to acquisition of T cell immunity. J Immunol. 2007;178:7587. doi: 10.4049/jimmunol.178.12.7587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.