Abstract
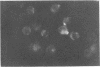
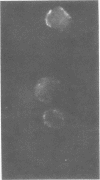
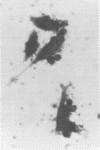
Indirect immunofluorescence and lymphocyte cytotoxicity experiments demonstrated the presence of a tumor-specific antigen(s) on the surface of cells from an equine sarcoid cell line (Mc1). Autologous serum (taken from the horse from which the Mc1 cells were derived) and sera from three other sarcoid-bearing horses revealed a similar membrane immunofluorescence when reacted with Mc1 cells, indicating the existence of cross-reacting antibodies. Results of serum colony inhibition experiments indicate that these antibodies are not cytotoxic. Incubation of Mc1 cells with autologous lymphocytes resulted in a toxicity to Mc1 cells, thereby demonstrating a cell-mediated immune reaction to autologous tumor cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankerst J., Sjögren H. O. Cross-reacting tumor-specific transplantation of antigens in tumors induced by adenoviruses, 3, 14, and 12. Cancer Res. 1970 May;30(5):1499–1505. [PubMed] [Google Scholar]
- Ankerst J., Sjögren H. O. Demonstration of two group-specific TSTAs in adenovirus-induced tumors. Int J Cancer. 1970 Jul 15;6(1):84–94. doi: 10.1002/ijc.2910060113. [DOI] [PubMed] [Google Scholar]
- Fleissner E. Virus-specific antigens in hamster cells transformed by Rous sarcoma virus. J Virol. 1970 Jan;5(1):14–21. doi: 10.1128/jvi.5.1.14-21.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Ginsberg H. S. Characterization of the tumorlike (T) antigen induced by type 12 adenovirus. II. Physical and chemical properties. J Virol. 1968 Jan;2(1):15–20. doi: 10.1128/jvi.2.1.15-20.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Habel K. Antigens of virus-induced tumors. Adv Immunol. 1969;10:229–250. doi: 10.1016/s0065-2776(08)60418-6. [DOI] [PubMed] [Google Scholar]
- Hellström I., Hellström K. E., Sjögren H. O. Serum mediated inhibition of cellular immunity to methylcholanthrene-induced murine sarcomas. Cell Immunol. 1970 May;1(1):18–30. doi: 10.1016/0008-8749(70)90058-4. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Hollinshead A., Bunnag B., Alford T., Cusumano C. Purification and analysis of adenovirus group-specific 'T' antigen. J Gen Virol. 1969 Apr;4(3):433–435. doi: 10.1099/0022-1317-4-3-433. [DOI] [PubMed] [Google Scholar]
- Joel D. D., Adamik E. R., Chanana A. D., Cronkite E. P., Schiffer L. M., Sipe C. R. Separation of lymphocytes from blood of calves and goats. Am J Vet Res. 1969 Jul;30(7):1099–1104. [PubMed] [Google Scholar]
- Kurth R., Bauer H. Cell-surface antigens induced by avian RNA tumor viruses: detection by a cytotoxic microassay. Virology. 1972 Feb;47(2):426–433. doi: 10.1016/0042-6822(72)90278-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis M. G., Phillips T. M. The specificity of surface membrane immunofluorescence in human malignant melanoma. Int J Cancer. 1972 Jul 15;10(1):105–111. doi: 10.1002/ijc.2910100114. [DOI] [PubMed] [Google Scholar]
- Pearson G., Orr T., Redmon L., Bergs V. Membrane immunofluorescence studies on cells producing rat C-type virus particles. Int J Cancer. 1972 Jul 15;10(1):14–19. doi: 10.1002/ijc.2910100104. [DOI] [PubMed] [Google Scholar]
- Potter C. W., Oxford J. S., McLaughlin B. C. A comparison of adenovirus 12 induced T and tumour antigens by rate-zonal centrifugation. J Gen Virol. 1970 Jan;6(1):105–116. doi: 10.1099/0022-1317-6-1-105. [DOI] [PubMed] [Google Scholar]
- Sinkovics J. G., Cabiness J. R., Shullenberger C. C. Disappearance after chemotherapy of blocking serum factors as measured in vitro with lymphocytes cytotoxic to tumor cells. Cancer. 1972 Dec;30(6):1428–1437. doi: 10.1002/1097-0142(197212)30:6<1428::aid-cncr2820300603>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Spendlove R. S. Optimal labeling of antibody with fluorescein isothiocyanate. Proc Soc Exp Biol Med. 1966 Jun;122(2):580–583. doi: 10.3181/00379727-122-31196. [DOI] [PubMed] [Google Scholar]
- Tacker J. R., Hyde R. M. Partial characterization of an antigen in spontaneous murine mammary tumors. Int J Cancer. 1969 Jan 15;4(1):21–30. doi: 10.1002/ijc.2910040104. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Crouch N. A., Melnick J. L., Rapp F. Detection of specific surface antigens by colony inhibition in cells transformed by papovavirus SV40. Int J Cancer. 1970 Mar 15;5(2):176–184. doi: 10.1002/ijc.2910050203. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S., Diamandopoulos G. T., Rapp F., Enders J. F. Lack of relationship between virus-specific surface and transplantation antigens in hamster cells transformed by simian papovavirus SV40. J Immunol. 1968 Dec;101(6):1192–1198. [PubMed] [Google Scholar]
- Watson R. E., Jr, England J. J., Larson K. A. Cultural characteristics of a cell line derived from an equine sarcoid. Appl Microbiol. 1972 Nov;24(5):727–731. doi: 10.1128/am.24.5.727-731.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiblin C. N., MacPherson I. A. The transformation of BHK 21 hamster cells by simian virus 40. Int J Cancer. 1972 Sep 15;10(2):296–309. doi: 10.1002/ijc.2910100210. [DOI] [PubMed] [Google Scholar]