Abstract
Background
Familial hypercholesterolemia (FH) is a congenital disorder of lipid metabolism characterized by a marked elevation of the plasma concentration of LDL (low-density lipoprotein) cholesterol beginning in childhood and by the early onset of coronary heart disease. It is among the commonest genetic disorders, with an estimated prevalence in Germany of at least 1 per 500 persons.
Method
Review of pertinent literature retrieved by a selective search.
Results
FH is underdiagnosed and undertreated in Germany. It is clinically diagnosed on the basis of an elevated LDL cholesterol concentration (>190 mg/dL [4.9 mmol/L]), a family history of hypercholesterolemia, and early coronary heart disease, or the demonstration of xanthomas. The gold standard of diagnosis is the identification of the underlying genetic defect, which is possible in 80% of cases and enables the identification of affected relatives of the index patient. The recommended goals of treatment, based on the results of observational studies, are to lower the LDL cholesterol concentration by at least 50% or to less than 100 mg/dL (2.6 mmol/L) (for children: <135 mg/dL [3.5 mmol/L]). The target value is lower for patients with clinically overt atherosclerosis (<70 mg/dL [1.8 mmol/L]). Statins, combined with a health-promoting lifestyle, are the treatment of choice. Lipoprotein apheresis is used in very severe cases; its therapeutic effects on clinical endpoints and its side effect profile have not yet been documented in randomized controlled trials.
Conclusion
Familial hypercholesterolemia is a common disease that can be diagnosed simply and reliably on clinical grounds and by molecular genetic testing. Timely diagnosis and appropriate treatment can lower the risk of atherosclerosis in heterozygous patients to that of the general population.
Familial hypercholesterolemia (FH) is characterized by congenital abnormalities in low-density lipoprotein (LDL) catabolism. Its main clinical consequences are premature atherosclerotic diseases. FH is an illustration of the causal relationship between elevated LDL cholesterol concentrations and vascular disease (1, 2). The extent, timing, and frequency of clinical complications vary and are related to the severity of the molecular defect and to additional risk factors (3). Research into FH led to the discovery of the LDL receptor (LDLR) and paved the way for the development of HMG-CoA reductase inhibitors (statins) (4– 6).
In most European countries, and probably also in Germany, FH is diagnosed in only 15% of cases, typically not until after a heart attack has occurred at an early age or following multiple myocardial infarctions in a family (7, 8). There are no studies investigating the prevalence rate in a representative German sample. A frequency of 1 in 500 is assumed for heterozygous FH (HeFH) in the population of Germany (Table 1). Danish and Norwegian data suggest an even higher prevalence rate of between 1 in 200 and 1 in 300 (9, 10). In some populations HeFH prevalence is extremely high as a result of founder effects (increased frequency of a genetic abnormality in an isolated population) (11– 15).
Table 1. Forms of manifestation, prevalence rates, and clinical characteristics of familial hypercholesterolemia (autosomal dominant hypercholesterolemia*1).
| Manifestation | Prevalence*2 | Typical LDL cholesterol concentrations | Clinical characteristics |
|---|---|---|---|
| Heterozygous | 1 in 500 | 190 to 450 mg/dL(4.9 to 11.6 mmol/L) | Familial clustering, tendon xanthomas of the extensor tendons of the joints of the fingers and Achilles tendons, xanthelasmata, arcus corneae, arthritis, manifestations of cardiovascular disease from early adulthood onwards possible |
| Homozygous or combined heterozygous | 1 in 1000000 | 400 to >1000 mg/dL (10 to >26 mmol/L) and above possible | Familial clustering, interdigital planar xanthomas, cutaneous xanthomas, tendon xanthomas of the extensor tendons of the joints of the fingers and Achilles tendons, xanthelasmata, arcus corneae, arthritis, severe cardiovascular atherosclerosis, and atherosclerosis of the aortic valve in early childhood possible |
*1Umbrella term for hypercholesterolemia caused by LDL receptor mutations (85 to 90%), apolipoprotein B-100 mutations (2 to 7%), or PSCK9 (proprotein convertase subtilisin/kexin type 9) gain-of-function mutations (less than 3%). Mutations of LDL receptor adapter protein 1 (LDLRAP1) are extremely rare; these cause autosomal recessive hypercholesterolemia (ARH) and lead to a phenotype corresponding to familial hypercholesterolemia.
*2Prevalence figures are based on assumptions made for Western populations. The prevalence rate of 1 in 500 is probably also an underestimate for Germany. Higher prevalence rates are found in some populations, e.g. 1 in 270 for HeFH in specific Canadian populations (11); a particularly high prevalence rate of 1 in 72 is found in South Africa (12). A HoFH prevalence rate of 1 in 860 000 is reported for Germany (e48), 1 in 640 000 for the Netherlands (13), 1 in 275 000 for Canada (11), and as high as 1 in 30 000 for South Africa (12).
Lowering LDL cholesterol concentrations can effectively reduce the particularly high risk that results from elevated LDL cholesterol concentrations and exposure from childhood onwards. This makes it essential to provide diagnosis and treatment as early as possible (16– 19). An update is needed as a result of new findings concerning FH diagnosis and treatment, because primary, i.e. congenital, lipid metabolism disorders such as FH are not covered thoroughly even by recent guidelines (20– 22). The current US guideline on cholesterol-lowering therapy makes no mention of genetic lipid metabolism disorders (23).
Pathogenesis
FH is an autosomal dominant disorder of lipid metabolism. In 85% to 90% of cases it is caused by a mutation in the LDL receptor (LDLR) gene (1, 2). Reduced LDLR number or LDLR dysfunction inhibits the uptake and breakdown of LDL and subsequently increases hepatic cholesterol synthesis (4, 5, 25). This leads to considerable increases in plasma LDL cholesterol concentrations and to extra-plasmatic deposits. There are currently more than 1700 LDLR mutations listed in databases of mutations (e.g. LDLR@www.ucl.ac.uk/ldlr/LOVDv.1.1.0/).
In addition to LDLR mutations, genetic defects in apolipoprotein B-100 and protease PCSK9 (proprotein convertase subtilisin/kexin type 9) can also cause elevated LDL cholesterol (25).
It is not possible to distinguish reliably between the three known causes of FH on a phenotypic, i.e. clinical, basis. The term “autosomal dominant hypercholesterolemia (ADH)” has therefore been coined (1, 2, 26). This review article uses the conventional, synonymous term “FH.” This should not be confused with the rare condition autosomal recessive hypercholesterolemia, which is caused by two defective alleles of LDLR adapter protein 1 (LDLRAP1) (26).
Clinical presentation
Heterozygous FH patients present elevated LDL cholesterol concentrations ranging from 190 mg/dL to over 450 mg/dL (4.9 mmol/L to over 10.3 mmol/L) (Table 1) (27). Homozygous carriers may have an LDL cholesterol concentration of between 400 mg/dL (10.3 mmol/L) and more than 1000 mg/dL (>26 mmol/L). Dichotomous classification of FH as either heterozygous (HeFH) or homozygous (HoFH) is often inaccurate in molecular genetic terms, since usually two different mutations underlie a phenotypic 'homozygote'; these individuals are therefore heterozygous carriers.
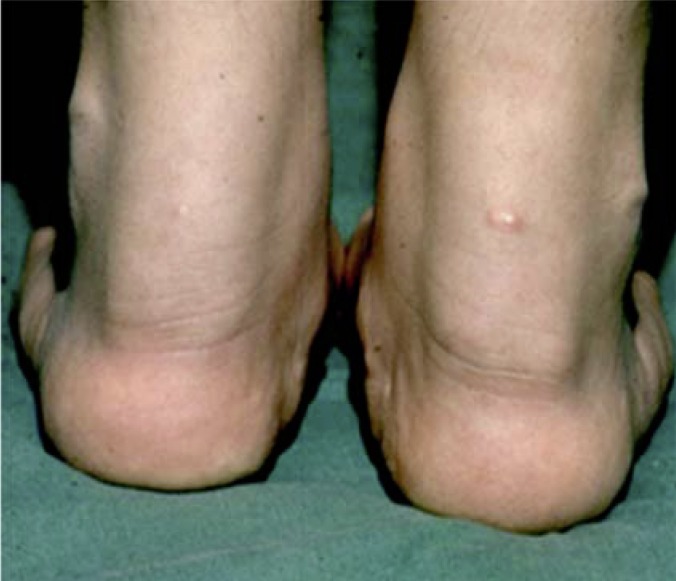
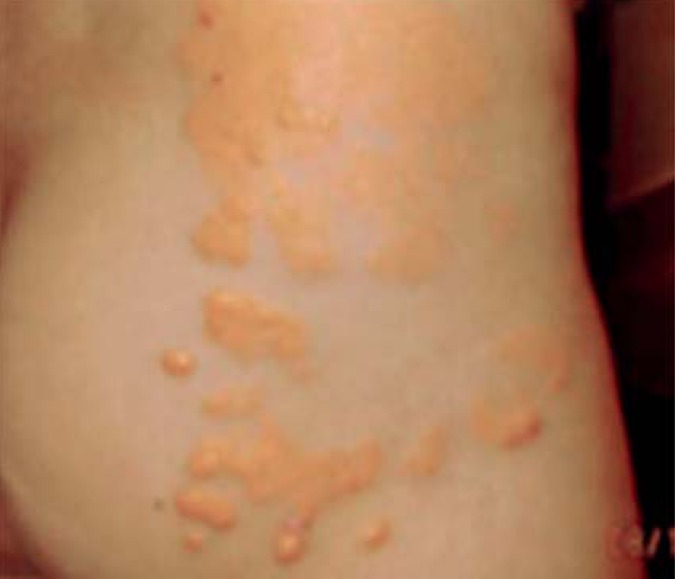
A patient's prognosis is worsened by early manifestations, particularly coronary manifestations, of atherosclerotic plaques. In HoFH in particular, supravalvular or valvular aortic stenosis with possible involvement of the coronary ostia can give rise to an unfavorable prognosis (28- 30). Tendon and cutaneous xanthomas are typical outward signs of abnormal cholesterol storage (Figure 1). The most common locations of tendon xanthomas are the Achilles tendon and extensor tendons of the joints of the fingers; xanthomas of the patellar and triceps tendons are less common. In homozygous individuals, cutaneous xanthomas in the gluteal (Figure 2), elbow, interdigital, or prepatellar regions are also typical.
Figure 1.
Broadening of the Achilles tendon in familial hypercholesterolemia, caused by tendon xanthomas
Figure 2.
Cutaneous xanthomas in a child with homozygous familial hypercholesterolemia
More frequent but less specific are cholesterol deposits in the form of xanthelasmata and the development of arcus corneae before the age of 45 (31). Arthropathies caused by precipitation of cholesterol crystals in the synovial fluid have been described (32- 34).
Prognosis
In homozygous FH, fatal myocardial infarction is possible even in early childhood.
The age at which cardiovascular complications manifest in heteroygous FH can vary greatly. Early manifestation is possible from the age of 30, or even earlier, if other risk factors such as elevated lipoprotein(a) (Lp(a)) or low HDL cholesterol are present. However, they may not manifest until the age of 50 or above, or they may remain clinically inapparent (35, 36).
Genetic mutations in FH patients mean that they have a risk of coronary heart disease events eight times the risk of unaffected relatives (RR 8.54; 95% CI 5.29 to 13.8) (37). FH patients with xanthomas have three times the risk of coronary heart disease of those without xanthomas (38). It has been estimated that approximately 20% of FH patients do not develop symptomatic coronary heart disease at any point during their lives, possibly thanks to healthy lifestyles and/or genetically determined protective factors and due to other, competing causes of death (16).
Diagnosis
In the past, data on the prevalence and prognosis of FH was often based on algorithms with clinical criteria such as the Simon Broome Register or the Dutch Lipid Clinic Network (27).
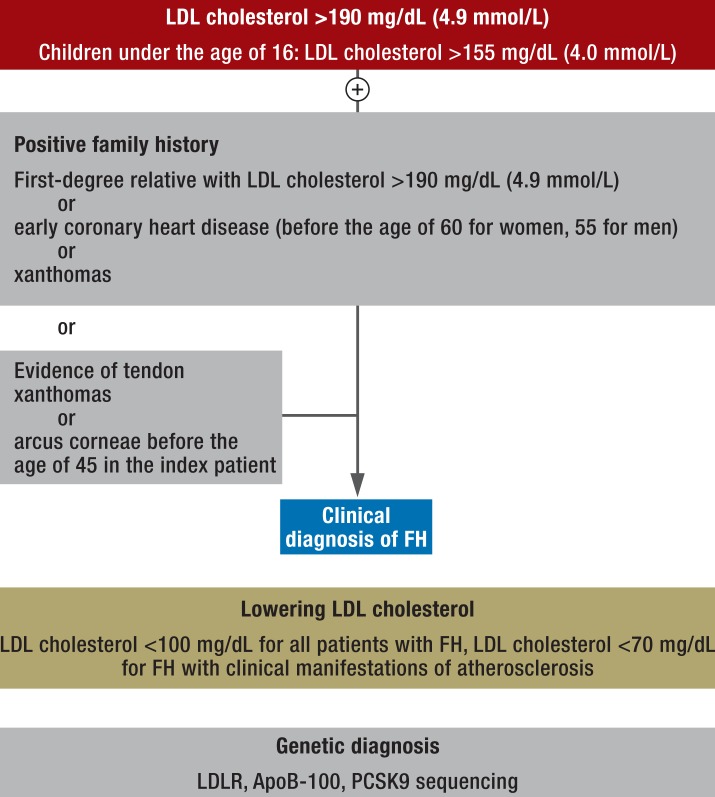
On the basis of these criteria, the following procedure for clinical practice has been tried and tested (Figure 3):
Figure 3.
Procedure for identifying and treating patients with familial hypercholesterolemia in clinical practice
Diagnosis is based on LDL cholesterol concentrations. FH should be suspected if LDL cholesterol is above 190 mg/dL (4.9 mmol/L), or above 155 mg/dL (4.0 mmol/L) in children under the age of 16. The additional presence of tendon xanthomas or arcus corneae before the age of 45 years makes FH even more likely.
The second step is family history. If first-degree relatives with LDL cholesterol above 190 mg/dL (4.9 mmol/L) or early coronary heart disease (before the age of 60 for women or 55 for men) are identified or if first-degree relatives have xanthomas, the index patient can also be clinically diagnosed with FH, with no further clinical manifestation required.
Cascade screening can be used as an effective strategy to identify as many affected individuals as possible. Cascade screening is the testing of relatives of the index patient. This procedure is particularly effective for FH, because the dominant mode of inheritance means that half the index patient's relatives are affected. This strategy is also cost-effective if combined with genetic diagnostics (39, 40).
Abnormal cholesterol metabolism can be masked by concurrent statin treatment, which currently also seems to be reducing the frequency of xanthomas. This makes it even more important not to overlook familial clustering of cardiovascular events.
Because of the genetic heterogeneity of the clinical phenotype, it is advisable to include LDLR, the receptor-binding domain of apolipoprotein B-100, PCSK9, or in individual cases LDLRAP1 in molecular genetic diagnosis.
If FH is defined as the presence of at least one functionally significant mutation at one of these gene loci, the analytical sensitivity and specificity of genetic diagnosis are virtually 100% (e1). The positive predictive value of evidence of familial hypercholesterolemia for a manifestation of cardiovascular disease is age-dependent; the lifelong figure is approximately 60%.
With good clinical preselection based on the severity of the LDL cholesterol abnormality, familial manifestation, and potential stigmata, the pathobiochemically relevant mutations can be identified in up to 80% of suspected cases (7, 36, e2).
In other cases, either mutation occurs in genes that have not yet been identified as being relevant to FH or the cause of hypercholesterolemia is polygenic (e2). If there is no evidence of a disease-causing mutation, diagnosis is based on clinical characteristics (20- 22). The costs of genetic diagnosis are only incurred in full once, for the index patient, because after a relevant mutation has been identified it can be the subject of a targeted search in relatives.
Legal issues, regarding insurance or employment behavior for example, are covered by the German Genetic Diagnostics Act (e3). Whether relatives benefit if significant risk factors for disease are discovered in a patient is the subject of ethical discussion (e4). The elderly and those already affected by atherosclerotic complications do not benefit from genetic diagnosis unless there are younger family members, as intensive statin therapy is indicated in any case. FH must be diagnosed according to guideline-based identification of clinical and subclinical manifestations of atherosclerosis (7, 21, 22).
In summary, molecular genetic diagnostics appears to be worthwhile in the following cases:
More precise prognostic evaluation and justification of early, intensive treatment in younger hypercholesterolemia patients.
Simpler cascade screening in the patient's relatives, including children and mutation carriers with a less marked phenotype, once one or more disease-causing mutations have been identified in an index patient (37, 40, e1).
Because earlier detection in children of affected adults is possible, prompt treatment can be started, beginning with an appropriate lifestyle.
Standard risk tables (SCORE, Framingham, PROCAM) underestimate the coronary heart disease risk of FH patients, as they do not address lifetime risk (21); the need for treatment for elevated LDL cholesterol concentrations can be underestimated at younger ages (under 50) in particular.
Specific diagnosis will improve compliance: both the thoroughness of treatment and patients' own treatment compliance (e4, 40).
Differential diagnosis
Differential diagnosis must distinguish between familial hypercholesterolemia and polygenic hypercholesterolemia, familial combined hyperlipidemia, type 3 hyperlipoproteinemia, and severe forms of secondary hypercholesterolemia (hypothyroidism, cholestasis). In cases of massive hypertriglyceridemia, VLDL (very low density lipoproteins) and remnant particles as a proportion of cholesterol can contribute to a considerable increase in total cholesterol concentrations which is otherwise seen only in HoFH (29, e6). If cutaneous xanthomas occur in early childhood to adolescence, phytosterolemia (ABCG5/8 membrane transporter mutations) should be suspected; if there is a combination of xanthomas and neurological symptoms, cerebrotendinous xanthomatosis (sterol 27-hydroxylase mutations) should be suspected. Both diseases are detected by determining plasma non-cholesterol sterol concentrations (e7, e8).
Treatment
There are no randomized controlled trials to enable evidence-based treatment recommendations for FH to be made; such trials do not seem to be ethically justifiable. Retrospective analyses and cohort studies show improved prognosis for FH patients who receive cholesterol-lowering therapy (e9, e10). For HoFH, relative risk is reduced to 0.34 for death and 0.49 for cardiovascular events, according to a retrospective comparison of lipid-modifying therapy available in the past and that available now (e9).
In a Dutch HeFH cohort (n = 2146), the risk of myocardial infarction, 6.7 per 1000 patient years, of FH patients given statin treatment fell to close to the figure for the general population of Rotterdam, 4.1 per 1000 patient years (e10). The absolute risk for untreated individuals was 60.5 per 1000 patient years. Guidelines recommend a target LDL cholesterol concentration of less than 100 mg/dL (2.6 mmol/L) for adults with FH (7, 21). For FH patients with clinical manifestations of atherosclerosis, the recommendation is to lower LDL cholesterol concentrations to less than 70 mg/dL (1.8 mmol/L). If these targets cannot be achieved, LDL cholesterol must be reduced by at least 50% using the strongest statin at the highest tolerated and authorized dose (7, 21, 22, 39, e12).
The findings of a systematic review and meta-analysis of seven studies indicate that there is insufficient evidence to support the target of 135 mg/dL (3.5 mmol/L) proposed for children over the age of 10 (e13). Before the age of 10 treatment is based on nonpharmacological measures, particularly diet.
Lifestyle measures
Lifestyle measures focus on those that have been shown to be effective in reducing cardiovascular risk in general, particularly regular physical exercise and strict abstention from smoking. Cholesterol intake is also reduced by replacing animal fats, which are mostly saturated fatty acids, with plant oils, most of which are unsaturated fatty acids (e14). However, elevated LDL cholesterol in patients with FH cannot be sufficiently reduced through diet; statin treatment is always required. Given that there are numerous dietary recommendations insufficiently supported by evidence, it is possible that ineffective dietary recommendations may significantly compromise a patient's quality of life.
Pharmacological treatment
The most important component of HeFH therapy is statin treatment. Meta-analyses of numerous randomized controlled trials are available on the general benefits of statin treatment (6).
Data on FH patients is limited to retrospective analyses and cohort comparisons (e9, e10). Even homozygous receptor-negative (mean 15% lower LDL cholesterol) and receptor-defective (mean 26% lower LDL cholesterol) FH patients have some response to statins (e15). The most common adverse effects of statins, which are not known to differ between FH patients and others, are muscular symptoms, increased liver enzyme activities, and a higher incidence of diabetes mellitus (OR: 1.09; 95% CI: 1.12 to 1.17).
For children, the severity of clinical manifestation, including vascular findings such as intima-media thickness (IMT) and the age at which relatives develop cardiovascular complications, are taken into account on an individual basis when deciding when to begin pharmacological treatment (e13).
A Cochrane analysis of nine placebo-controlled trials of statin treatment in childhood found no evidence of increased adverse drug effects for trial durations of up to 48 weeks (e16). The following statins have been approved in Germany:
Pravastatin from the age of eight
Fluvastatin from the age of nine
Atorvastatin and rosuvastatin from the age of 10.
Lipid concentrations typically increase in pregnant women both with and without FH. No fetal malformations were observed in a study of statin exposure during pregnancy (e17- e19), but the terms of statin authorization themselves require treatment to be interrupted for the duration of pregnancy. Contraception must be used during statin treatment in fertile women.
If an HeFH patient's LDL cholesterol concentration is not sufficiently lowered using the highest tolerated dose of a powerful statin, combined therapy with the cholesterol resorption inhibitor ezetimibe and/or an anion exchanger (colesevelam, which has better tolerability than cholestyramine) is used (Table 2) (e20- e22).
Table 2. Lipid-modifying treatment for familial hypercholesterolemia.
| Manifestation of heterozygous FH | Manifestation of homozygous FH |
|---|---|
| Statins*1 | Statins*1 |
| Cholesterol resorption inhibitor (ezetimibe)*2 | Cholesterol resorptioan inhibitor (ezetimibe)*2 |
| Anion exchanger*3 | Anion exchanger*3 |
| MTP inhibition (lomitapide)*4 | |
| LDL apheresis | LDL apheresis |
| In selected cases: liver transplant (historically: portocaval shunt) | |
Undergoing clinical development:
|
Undergoing clinical development:
|
*1Although statins are the first-line therapy for HeFH, HoFH patients have less or no response to this treatment because statins reduce cholesterol by increasing expression of LDL receptors that are absent in HoFH. Guidelines recommend use of the strongest statins at the highest authorized dose tolerated by the patient.
*2Restriction of enteral resorption of both dietary and biliary cholesterol by using ezetimibe to inhibit an intestinal cholesterol transporter, Niemann-Pick C1-like protein 1 (NPC1L1), increases the LDL-lowering effects of statins. This mechanism of action explains a therapeutic effect beyond that of stain treatment in HoFH (e46, e47).
*3Anion exchangers lead to lowered LDL cholesterol by inhibiting enterohepatic bile acid circulation. Use is restricted due to relatively poor tolerability, but this can be improved using the newer bile acid sequestrant colesevelam (e22).
*4Inhibition of synthesis of very-low-density lipoprotein (VLDL), the precursor of low-density lipoprotein (LDL)
Lipid apheresis
Initial positive results of plasma exchange in HoFH patients led to the development of extracorporeal treatment methods for selective LDL elimination. As clinical experience increased, the treatment indication was extended to include HeFH patients and high-risk patients who had failed to achieve their target LDL cholesterol (e23- e29). There is no evidence from randomized controlled trials on the benefits of treatment. The plausible premise for the effectiveness of lipid apheresis is based on clinical observation and retrospective comparison of event rates before and after the beginning of regular apheresis therapy.
The requirement for cost reimbursement is more than 12 months of documented maximum dietary and pharmacological treatment without sufficient reduction of LDL cholesterol. Lipid apheresis is performed at least every two weeks, usually every week, and leads to a more than 50% decrease in LDL cholesterol and Lp(a) compared to baseline. Acute side effects can include a drop in blood pressure, headache, fatigue, edema, and dizziness; frequencies of up t o 3% are reported for these (e26). Complications at shunt sites and long-term iron-deficiency anemia are also possible. More serious adverse effects reported include allergic reactions, hemolysis, and shock. There is no systematically collated information on the frequency or severity of adverse events for these procedures; such information is expected from registry data (e29).
Surgery
Before current treatment measures became available, ileal bypass was sometimes performed. This worked by interrupting enterohepatic bile acid circulation (e30). The evidence of the clinical benefit of this as found in the POSCH study is an early demonstration of the effectiveness of lowering LDL cholesterol (e31). Historically, a portocaval shunt was fitted (e32). Liver transplantation to restore LDLR function in otherwise treatment-refractory HoFH has been described as successful in one patient (e33).
New developments
Further treatment options are being sought as a result of failure to achieve target LDL cholesterol concentrations, lack of tolerability, and limited access to treatment (e34- e45). Such further options include lomitapide, a microsomal triglyceride transfer protein (MTP) inhibitor. Lomitapide is authorized by the EMA for HoFH only, among other reasons because it increases hepatocellular fat and transaminase activities (e35).
The antisense oligonucleotide mipomersen, which has been approved by the FDA but not the EMA, lowers LDL cholesterol by blocking messenger RNA for apolipoprotein B-100. This effect lowers LDL-cholesterol even in HoFH patients by 49% (e36- e38).
There are particularly high expectations for serine protease PCSK9 inhibition (e39, e41- e45). This is attractive because it has been observed that carriers of loss-of-function PCSK9 mutations have lower LDL cholesterol from birth onward and a significantly lower risk of cardiovascular events than the normal population (e40). The good tolerability and lipid modification of monoclonal antibodies specific for PCSK9 in patients with FH described in phase II trials are also being confirmed in ongoing phase III trials (e44, e45).
Key Messages.
FH patients are at significantly increased risk of atherosclerosis as a result of both elevated LDL cholesterol concentrations and exposure from childhood onwards.
Effective treatment for LDL cholesterol can reduce the risk to the same level as in the general population.
Clinical diagnosis is based on LDL cholesterol concentrations above 190 mg/dL (4.9 mmol/L) together with a family history of hypercholesterolemia or early atherosclerosis, or evidence of xanthomas.
Molecular genetic methods contribute to higher specificity of diagnosis, justification of intensity of treatment, and simpler screening of the patient's relatives.
New treatment options to lower LDL cholesterol levels are being tested in randomized clinical trials.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. März is a partner of Synlab Holding GmbH. He has received consultancy fees; reimbursement of conference fees, travel expenses, and accommodation expenses; and fees for preparing continuing medical education events from Amgen Deutschland, Aegerion Pharma, Merck Sharp, Dohme Deutschland, Roche, Sanofi-Aventis, and Pfizer.
Prof. Klose has received consultancy fees from Amgen, Sanofi, MSD, Genzyme, and BMS. He has received fees for conference participation and reimbursement of travel and accommodation expenses from Sanofi and MSD. He has received fees for preparing continuing medical education events from MSD and BMS.
Prof. Laufs has received consultancy fees from Amgen, Sanofi, MSD, and Roche. He has received fees for conference participation and reimbursement of travel and accommodation expenses from MSD.
Prof. Windler declares that no conflict of interests exists.
References
- 1.Raal FJ, Santos RDl. Homozygous familial hypercholesterolemia: current perspectives on diagnosis and treatment. Atherosclerosis. 2012;223:262–268. doi: 10.1016/j.atherosclerosis.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Hovingh GK, Davidson MH, Kastelein JJP, O'Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013;34:962–971. doi: 10.1093/eurheartj/eht015. [DOI] [PubMed] [Google Scholar]
- 3.Besseling J, Kindt I, Hof M, Kastelein JJP, Hutten BA, Hovingh GK. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: A study of a cohort of 14,000 mutation carriers. Atherosclerosis. 2014;233:219–223. doi: 10.1016/j.atherosclerosis.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci. 1973;70:2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 6.Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 7.Nordestgaard BG, Chapman MJ, Humphries SE, et al. for the European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brice P, Burton H, Edwards CW, Humphries SE, Aitman TJ. Familial hypercholesterolaemia: A pressing issue for European health care. Atherosclerosis. 2013;231:223–226. doi: 10.1016/j.atherosclerosis.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Benn M, Watts GF, Tybjaerg-Hansen A, Nordestgaard BG. Familial hypercholesterolemia in the Danish general population: prevalence, coronary artery disease, and cholesterol-lowering medication. J Clin Endocrinol Metab. 2012;97:3956–3964. doi: 10.1210/jc.2012-1563. [DOI] [PubMed] [Google Scholar]
- 10.Heiberg A, Berg K. The inheritance of hyperlipoproteinaemia with xanthomatosis. A study of 132 kindreds. Clin Genet. 1976;9:203–233. doi: 10.1111/j.1399-0004.1976.tb01569.x. [DOI] [PubMed] [Google Scholar]
- 11.Moorjani S, Roy M, Gagné C, et al. Homozygous familial hypercholesterolemia among French Canadians in Québec Province. Arteriosclerosis. 1989;9:211–216. doi: 10.1161/01.atv.9.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Steyn K, Goldberg JP, Kotze MJ, et al. Estimation of the prevalence of familial hypercholesterolaemia in a rural Afrikaner community by direct screening for three Afrikaner founder low density lipoprotein receptor gene mutations. Hum Genet. 1996;98:479–484. doi: 10.1007/s004390050243. [DOI] [PubMed] [Google Scholar]
- 13.Kusters DM, Huijgen R, Defesche JC, et al. Founder mutations in the Netherlands: geographical distribution of the most prevalent mutations in the low-density lipoprotein receptor and apolipoprotein B genes. Neth Heart J. 2011;19:175–182. doi: 10.1007/s12471-011-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahed AC, Safa RM, Haddad FF, et al. Homozygous familial hypercholesterolemia in Lebanon: a genotype/phenotype correlation. Mol Genet Metab. 2011;102:181–188. doi: 10.1016/j.ymgme.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Vergopoulos A, Bajari T, Jouma M, et al. A xanthomatosis-susceptibility gene may exist in a Syrian family with familial hypercholesterolemia. Eur J Hum Genet. 1997;5:315–323. [PubMed] [Google Scholar]
- 16.Neil A, Cooper J, Betteridge J, Capps N, et al. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29:2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso R, Mata P, Zambón D, Mata N, Fuentes-Jiménez F. Early diagnosis and treatment of familial hypercholesterolemia: improving patient outcomes. Expert Rev Cardiovasc Ther. 2013;11:327–342. doi: 10.1586/erc.13.7. [DOI] [PubMed] [Google Scholar]
- 18.Harada-Shiba M, Sugisawa T, Makino H, et al. Impact of statin treatment on the clinical fate of heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2010;17:667–674. doi: 10.5551/jat.4143. [DOI] [PubMed] [Google Scholar]
- 19.Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202–2207. doi: 10.1161/CIRCULATIONAHA.111.042523. [DOI] [PubMed] [Google Scholar]
- 20.Therapieempfehlungen der Arzneimittelkomission der Deutschen Ärzteschaft. Arzneiverordnung in der Praxis. 2012. Empfehlungen zur Therapie von Fettstoffwechselstörungen. [Google Scholar]
- 21.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 22.Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 23.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association task force on Practice Guidelines. Circulation. 2013 Nov 12; [Epub ahead of print] [Google Scholar]
- 24.Brown MS, Dana SE, Goldstein JL. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974;249:789–796. [PubMed] [Google Scholar]
- 25.Abifadel M, Varret M, Rabès JP, Allard D, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 26.Fahed AC, Nemer GM. Familial hypercholesterolemia: the lipids or the genes? Nutr Metab. 2011;22 doi: 10.1186/1743-7075-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks D, Thorogood M, Andrew H, Neil W, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 28.Keller C, v. Eckardstein A, Hersberger M. Primäre Dyslipoproteinämien, Störungen des LDL - Cholesterin-Stoffwechsels. In: Schwandt P, Parhofer KG, editors. Handbuch der Fettstoffwechselstörungen. Stuttgart: Schattauer Verlag; 2007. pp. 80–112. [Google Scholar]
- 29.Parhofer KG. Klassifikation von Dyslipoproteinämien. In: Schwandt P, Parhofer KG, editors. Handbuch der Fettstoffwechselstörungen. Stuttgart: Schattauer Verlag; 2007. pp. 74–80. [Google Scholar]
- 30.Rallidis L, Naoumova RP, Thompson GR, Nihoyannopoulos P. Extent and severity of atherosclerotic involvement of the aortic valve and root in familial hypercholesterolaemia. Heart. 1998;80:583–590. doi: 10.1136/hrt.80.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christoffersen M, Frikke-Schmidt R, Schnor P, et al. Xanthelasmata, arcus corneae, and ischaemic vascular disease and death in general population: prospective cohort study. BMJ. 2011;343 doi: 10.1136/bmj.d5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glueck CJ, Levy RI, Fredrickson DS. Acute tendinitis and arthritis. A presenting symptom of familial type II hyperlipoproteinemia. JAMA. 1968;206:2895–2897. doi: 10.1001/jama.206.13.2895. [DOI] [PubMed] [Google Scholar]
- 33.Li SG. Images in clinical medicine familial hypercholesterolemia. N Engl J Med. 2009;360 doi: 10.1056/NEJMicm0707976. [DOI] [PubMed] [Google Scholar]
- 34.Alfadhli El. Cholesterol deposition around small joints of the hands in familial hypercholesterolemia mimicking „Bouchard's and Heberden's Nodes“ of Osteoarthritis. Intern Med. 2010;49:1675–1676. doi: 10.2169/internalmedicine.49.2849. [DOI] [PubMed] [Google Scholar]
- 35.Seed M, Hoppichler F, Reaveley D, et al. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N Engl J Med. 1990;322:1494–1499. doi: 10.1056/NEJM199005243222104. [DOI] [PubMed] [Google Scholar]
- 36.Hill JS, Hayden MR, Frohlich J, Pritchard PH. Genetic and environmental factors affecting the incidence of coronary artery disease in heterozygous familial hypercholesterolemia. Arterioscler Thromb. 1991;11:290–297. doi: 10.1161/01.atv.11.2.290. [DOI] [PubMed] [Google Scholar]
- 37.Umans-Eckenhausen MA, Sijbrands EJ, Kastelein JJ, Defesche JC. Low-density lipoprotein receptor gene mutations and cardiovascular risk in a large genetic cascade screening population. Circulation. 2002;106:3031–3036. doi: 10.1161/01.cir.0000041253.61683.08. [DOI] [PubMed] [Google Scholar]
- 38.Oosterveer DM, Versmissen J, Yazdanpanah M, Hamza TH, Sijbrands EJ. Differences in characteristics and risk of cardiovascular disease in familial hypercholesterolemia patients with and without tendon xanthomas: a systematic review and meta-analysis. Atherosclerosis. 2009;207:311–317. doi: 10.1016/j.atherosclerosis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 39.NICE. Clinical guidelines, CG71 - Issued: 2008 (CG71) http://guidance.nice.org.uk/CG71. Familial hypercholesterolaemia, Identification and management of familial hypercholesterolaemia. Last accessed on 22 May 2014. [Google Scholar]
- 40.Alonso R, Defesche JC, Tejedor D, et al. Genetic diagnosis of familial hypercholesterolemia using a DNA-array based platform. Clin Biochem. 2009;42:899–903. doi: 10.1016/j.clinbiochem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- e1.Kassner U, Wühle-Demuth M, Missala I, et al. Clinical utility gene card for: Hyperlipoproteinemia, Type II. Eur J Hum Genet. 2013 Nov 20. doi; doi: 10.1038/ejhg.2013.271. 10.1038/ejhg.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Talmud PJ, Shah S, Wittall R, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: a case-control study. Lancet. 2013;381:1293–1301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- e3.Gendiagnostikgesetz. www.bmg.bund.de/glossarbegriffe/g/gendiagnostikgesetz.html. Last accessed on 22 May 2014. [Google Scholar]
- e4.Stollorz V. Erbkrankheiten, mehr als nur ein Familiengeheimnis. www.faz.net/aktuell/wissen/mensch-gene/erbkrankheiten-mehr-als-nur-ein-familiengeheimnis1623128.html. Last accessed on 22 May 2014. [Google Scholar]
- e5.Umans-Eckenhausen MAW, Defesche JC, van Dam MJ, Kastelein JJ. Longterm compliance with lipid-lowering medication after genetic screening for familial hypercholesterolemia. Arch Intern Med. 2003;163:65–68. doi: 10.1001/archinte.163.1.65. [DOI] [PubMed] [Google Scholar]
- e6.Steinmetz A, Kaffarnik H. Familiäre Dysbetalipoproteinämie. In: Schwandt P, Parhofer KG, editors. Handbuch der Fettstoffwechselstörungen. Stuttgart: Schattauer Verlag; 2007. pp. 185–198. [Google Scholar]
- e7.Björkhem I, Leitersdorf E. Sterol 27-hydroxylase deficiency: a rare cause of xanthomas in normocholesterolemic humans. Trends Endocrinol Metab. 2000;11:180–183. doi: 10.1016/s1043-2760(00)00255-1. [DOI] [PubMed] [Google Scholar]
- e8.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33:945–955. [PubMed] [Google Scholar]
- e9.Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124:2202–2207. doi: 10.1161/CIRCULATIONAHA.111.042523. [DOI] [PubMed] [Google Scholar]
- e10.Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ. 2008;337 doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Stein EA, Strutt K, Southworth H, Diggle PJ, Miller E heFH Study Group. Comparison of rosuvastatin versus atorvastatin in patients with heterozygous familial hypercholesterolemia. Am J Cardiol. 2003;92:1287–1293. doi: 10.1016/j.amjcard.2003.08.009. [DOI] [PubMed] [Google Scholar]
- e12.Watts G, Gidding S, Wierzbicki AS, et al. Integrated guidance on the care of familial hypercholesterolemia from the International FH Foundation. J Clin Lipidol. 2014;I2:148–172. doi: 10.1016/j.jacl.2014.01.002. [DOI] [PubMed] [Google Scholar]
- e13.Lebenthal Y, Horvath A, Dziechciarz P, Szajewska H, Shamir R. Are treatment targets for hypercholesterolemia evidence based? Systematic review and meta-analysis of randomised controlled trials. Arch Dis Child. 2010;95:673–680. doi: 10.1136/adc.2008.157024. [DOI] [PubMed] [Google Scholar]
- e14.Hopkins PN, Toth PP, et al. Goldberg AC. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients: Clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. Journal of Clinical Lipidology. 2011;5:133–140. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- e15.Marais AD, Firth JC, Blom DJ. Homozygous familial hypercholesterolemia and its management. Semin Vasc Med. 2004;4:43–50. doi: 10.1055/s-2004-822985. [DOI] [PubMed] [Google Scholar]
- e16.Vuorio A, Kuoppala J, Kovanen PT, et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst Rev 7 ( 2010 Jul 7); doi: 10.1002/14651858.CD006401.pub2. CD006401. [DOI] [PubMed] [Google Scholar]
- e17.Lecarpentier E, Morel O, Fournier T, Elefant E, Chavatte-Palmer P, Tsatsaris V. Statins and pregnancy: between supposed risks and theoretical benefits. Drugs. 2012;72:773–788. doi: 10.2165/11632010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- e18.Winterfeld U, Allignol A, Panchaud A, et al. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. BJOG. 2013;120:463–471. doi: 10.1111/1471-0528.12066. [DOI] [PubMed] [Google Scholar]
- e19.Kusters DM, Lahsinoui HH, van de Post JA, et al. Statin use during pregnancy: a systematic review and metaanalysis. Expert Rev Cardiovasc Ther. 2012;10:363–378. doi: 10.1586/erc.11.196. [DOI] [PubMed] [Google Scholar]
- e20.Yamamoto A, Harada-Shiba M, Endo M, et al. The effect of ezetimibe on serum lipids and lipoproteins in patients with homozygous familial hypercholesterolemia undergoing LDL-apheresis therapy. Atherosclerosis. 2006;86:126–131. doi: 10.1016/j.atherosclerosis.2005.06.039. [DOI] [PubMed] [Google Scholar]
- e21.Kastelein JJ, Akdim F, Stroes EG, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- e22.Davidson M. The efficacy of colesevelam HCl in the treatment of heterozygous familial hypercholesterolemia in pediatric and adult patients. Clin Ther. 2013;35:1247–1252. doi: 10.1016/j.clinthera.2013.06.014. [DOI] [PubMed] [Google Scholar]
- e23.Thompson GR, Miller JP, Breslow JL. Improved survival of patients with homozygous familial hypercholesterolaemia treated with plasma exchange. BMJ. 1985;291:1671–1673. doi: 10.1136/bmj.291.6510.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e24.Keller C. LDL-apheresis in homozygous LDL-receptor-defective familial hypercholesterolemia: the Munich experience. Atheroscler Suppl. 2009;10:21–26. doi: 10.1016/S1567-5688(09)71805-7. [DOI] [PubMed] [Google Scholar]
- e25.Hemphill LC. Familial hypercholesterolemia: current treatment options and patient selection for low-density lipoprotein apheresis. J Clin Lipidol. 2010;4:346–349. doi: 10.1016/j.jacl.2010.08.013. [DOI] [PubMed] [Google Scholar]
- e26.Stefanutti C, Julius U. Lipoprotein apheresis: state of the art and novelties. Atheroscler Suppl. 2013;14:19–27. doi: 10.1016/j.atherosclerosissup.2012.10.021. [DOI] [PubMed] [Google Scholar]
- e27.Thompson GR. The evidence-base for the efficacy of lipoprotein apheresis in combating cardiovascular disease. Atheroscler Suppl. 2013;14:67–70. doi: 10.1016/j.atherosclerosissup.2012.10.001. [DOI] [PubMed] [Google Scholar]
- e28.McGowan MP. Emerging low-density lipoprotein (LDL) therapies: Management of severely elevated LDL cholesterol—The role of LDL-apheresis. J Clin Lipidol. 2013;7(Suppl 3):21–26. doi: 10.1016/j.jacl.2013.03.002. [DOI] [PubMed] [Google Scholar]
- e29.Schettler VN, Neumann CL, Hulpe-Wette M, et al. Current view: indications for extracorporeal lipid apheresis treatment. Clin Res Cardiol Suppl 2012. 7(Suppl 1):15–19. doi: 10.1007/s11789-012-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e30.Buchwald H, Moore RB, Lee GB. Partial ileal bypass for hypercholesterolemia and atherosclerosis. Surg Gynecol Obstet. 1967;124:1231–1238. [PubMed] [Google Scholar]
- e31.Buchwald H, Varco RL, Matts JP, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. Report of the Program on the Surgical Control of the Hyperlipidemias (POSCH) N Engl J Med. 1990;323:946–955. doi: 10.1056/NEJM199010043231404. [DOI] [PubMed] [Google Scholar]
- e32.Starzl TE, Putnam CW, Koep LJ. Portocaval shunt in hyperlipoproteinaemia. Lancet. 1973;2:940–944. doi: 10.1016/s0140-6736(73)92599-3. [DOI] [PubMed] [Google Scholar]
- e33.Ibrahim M, El-Hamamsy I, Barbir M, Yacoub MH. Translational lessons from a case of combined heart and liver transplantation for familial hypercholesterolemia 20 years post-operatively. J Cardiovasc Transl Res. 2012;5:351–358. doi: 10.1007/s12265-011-9311-1. [DOI] [PubMed] [Google Scholar]
- e34.Rader DJ, Kastelein JJ. Lomitapide and Mipomersen: Two First-in-class drugs for reducing Low-Density Lipoprotein Cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–1032. doi: 10.1161/CIRCULATIONAHA.113.001292. [DOI] [PubMed] [Google Scholar]
- e35.Cuchel M, Meagher EA, du Toit Theron H, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–46. doi: 10.1016/S0140-6736(12)61731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e36.Visser ME, Witztum JL, Stroess ES, Kastelein JJ. Antisense oligonucleotides for the treatment of dyslipidaemia. Eur Heart J. 2012;33:1451–1458. doi: 10.1093/eurheartj/ehs084. [DOI] [PubMed] [Google Scholar]
- e37.Vogt A, Parhofer KG. The potential of mipomersen, an ApoB synthesis inhibitor, to reduce necessity for LDL-apheresis in patients with heterozygous familial hypercholesterolemia and coronary artery disease. Expert Opin Pharmacother. 2013;14:691–697. doi: 10.1517/14656566.2013.779253. [DOI] [PubMed] [Google Scholar]
- e38.Gelsinger C, Steinhagen-Thiessen E, Kassner U. Therapeutic potential of mipomersen in the management of familial hypercholesterolaemia. Drugs. 2012;72:1445–1455. doi: 10.2165/11635060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- e39.Horton JD, Cohen JC, Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50:172–177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e40.Cohen JC, Boerwinkle E, Mosley TH, jr, Hobbs HH. Sequence variations in PCSK9, Low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- e41.Stein EA, Swergold GD. Potential of proprotein convertase subtilisin/kexin type 9 based therapeutics. Curr Atheroscler Rep. 2013;15 doi: 10.1007/s11883-013-0310-3. [DOI] [PubMed] [Google Scholar]
- e42.Raal F, Scott R, Somaratne, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 142, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;20:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- e43.Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- e44.Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of Evolocumab in hyperlipidemia. NEJM. 2014 doi: 10.1056/NEJMoa1316222. online 2014 doi: 10.1056/NEJMoa1316222ia. [DOI] [PubMed] [Google Scholar]
- e45.Koren MJ, Giugliano RP, Raal FJ, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the open-label study of long-term evaluation against LDL-C (OSLER) randomized trial. Circulation. 2014;129:234–243. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- e46.Lind S, Olsson AG, Erikkson M, Rudling M, Eggertsen G, Angelin B. Autosomal recessive hypercholesterolaemia: normalization of plasma LDL cholesterol by ezetimibe in combination with statin treatment. J Intern Med. 2004;256:406–412. doi: 10.1111/j.1365-2796.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- e47.Gagné C, Gaudet D, Bruckert E. Safety of Ezetimibe coadministered with Atorvastatin or Simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- e48.Walzer S, Travers K, Rieder S, Erazo-Fischer E, Matusiewicz D. Homozygous familial hypercholesterolemia (HoFH) in Germany: an epidemiological survey. Clinicoecon Outcomes Res. 2013;5:189–192. doi: 10.2147/CEOR.S43087. [DOI] [PMC free article] [PubMed] [Google Scholar]