Abstract
Tumors of the central nervous system, the most common solid tumors of childhood, are a major source of cancer-related morbidity and mortality in children. Survival rates have improved significantly following treatment for childhood brain tumors, with this growing cohort of survivors at high risk of adverse medical and late effects. Endocrine morbidities are the most prominent disorder among the spectrum of long-term conditions, with growth hormone deficiency the most common endocrinopathy noted, either from tumor location or after cranial irradiation and treatment effects on the hypothalamic/pituitary unit. Deficiency of other anterior pituitary hormones can contribute to negative effects on growth, body image and composition, sexual function, skeletal health, and quality of life. Pediatric and adult endocrinologists often provide medical care to this increasing population. Therefore, a thorough understanding of the epidemiology and pathophysiology of growth failure as a consequence of childhood brain tumor, both during and after treatment, is necessary and the main focus of this review.
Keywords: Pediatric Brain Tumors, Brain Tumor Treatment, Growth after Tumor Treatment, Irradiation, Chemotherapy, Growth Hormone, Pituitary, Late Sequelae
Introduction
Tumors of the central nervous system (CNS) are the most common solid tumors of childhood and the primary source of cancer-related morbidity and mortality in children (1). Over the last two decades, survival rates have improved significantly, predominantly due to improvements in neuroimaging, neurosurgical techniques, radiation therapy (RT), chemotherapy and supportive care. Currently, the relative 5-year survival probability for children with all brain malignancies combined is greater than 65% (2–4). Although newer treatment strategies have substantially decreased mortality rates, improvement in survival has been achieved at a serious cost of late effects. These survivors are at high risk of adverse medical, neurocognitive and psychosocial late effects with endocrine morbidities the most prominent among the spectrum of long-term conditions that are directly attributable to previous cancer treatment (5–8). Growth hormone (GH) deficiency is the most common endocrinopathy found in survivors of brain tumors, either directly from tumor location or more commonly, after irradiation and treatment effects on the hypothalamic-pituitary (HP) unit (9,10). In addition, other anterior pituitary hormone deficiencies can exert negative effects on growth, body image and composition, sexual function, skeletal health, and quality of life. As pediatric and adult endocrinologists often provide care for this growing number of survivors, an understanding of the epidemiology and pathophysiology of growth failure as a consequence of childhood brain tumor is essential. This review focuses on growth disorders seen in children during and after treatment for childhood brain malignancy.
Brain Tumor Prevalence, Treatment, and Survival in Pediatrics
CNS tumors represent approximately 20% of all childhood cancers, with 2.5–4 cases diagnosed per 100,000 children per year. There is a slight male predominance (male:female = 1.29) at all ages less than 20 years. With the exception of infants under 1 year of age, the majority of brain tumors in children occur Infratentorially in the brain stem and cerebellum (1,11,12). While the etiology of most childhood brain tumors is unknown, specific genetic syndromes, such as neurofibromatosis (NF1 and NF2), tuberous sclerosis, Li-Fraumeni and Turcot syndromes are associated with a higher incidence of tumors; however, this group represents fewer than 10% of pediatric brain tumors (3).
The distinction between benign and malignant is often less useful for brain tumors since histologically “benign” tumors may behave in a clinically “malignant” fashion due to an unfavorable location in the brain. The extent of surgical resection may be limited by unacceptably high mortality or morbidity associated with surgery in certain areas of the brain. The most common pediatric malignant brain tumor is medulloblastoma, accounting for 10–20% of CNS neoplasms and about 40% of all tumors within the posterior fossa (13,14). While traditionally malignant tumors are thought of as aggressive and likely to disseminate, certain types, such as completely resected, localized medulloblastoma in children over 3 years of age, have cure rates approaching 90% (15). In contrast, benign (low grade) astrocytomas may continue to grow and become life-threatening despite treatment.
While the reported incidence of pediatric primary malignant brain tumors in the U.S. increased dramatically during the last few decades (4), careful analysis suggests that this change is primarily due to increased detection by magnetic resonance imaging (MRI) and improved techniques for surgical biopsy of previously unapproachable lesions, rather than a true increase in disease frequency (16).
The management of brain tumors depends on histology, tumor location and extent, and patient age, but typically involves surgery, chemotherapy and radiation therapy (Table 1) (17). Surgery in most cases by an experienced pediatric neurosurgeon is required to determine histology and to attempt maximal tumor debulking. Treatment is tailored to specific tumor type and patient age. In general, regardless of tumor type, radiation therapy is avoided in infants and very young children as they are especially vulnerable to irradiation associated toxicities and neurocognitive deficits. Alternative therapies for this population include intensified chemotherapy followed by autologous stem cell transplantation. However, for some brain tumors, such as low-grade gliomas, complete surgical resection may be the only therapy indicated.
Table 1.
Common Pediatric Brain Tumors
| Tumor Type | Relative Incidence | Treatment |
|---|---|---|
| Low Grade Glioma | 35–50% | Maximal surgical removal observation |
| Medulloblastoma/PNET | 16–20% | Complete surgical resection cranial/spinal radiation (RT)* and chemotherapy |
| Brain Stem Glioma | 10–20% | Surgical debulking (if possible) and observation, or RT* ± chemotherapy |
| Ependymomas | 8–10% | Complete surgical resection + RT* (chemotherapy in children) |
| Malignant Glioma | 10% | Diagnosis by MRI RT* ± chemotherapy |
| Germ Cell Tumors | 4–7% | Mature teratoma: complete surgical resection Pure germinoma: surgical resection + RT* Non-germinomatous: surgical resection + chemotherapy +RT* |
| Craniopharyngioma | 3% | Complete surgical resection, Reoperation for recurrence/residual, or RT* |
| Aggressive Infantile Embryonal Tumors (ie. Atypical Teratoid Rhabdoid Tumor) | 3% | Complete surgical resection RT* and chemotherapy |
RT – Radiation Therapy
Delay radiation therapy in children younger than 5 years of age
Adapted from: Sievert A, Minturn J. Brian Tumors. In: Florin T, Ludwig S. eds. Netter’s Pediatrics. Philadelphia, PA: Elsevier, June 2011 (in press)
Medulloblastoma is the most frequent malignant brain tumor of childhood with extensive studies demonstrating poor growth after therapy. Current treatment regimens for children with metastatic medulloblastoma consist of primary operative debulking followed by craniospinal irradiation, with the dose ranging from 23 to 39 Gy, dependent upon local or national protocols as well as additional radiation doses to the posterior fossa for up to 53 Gy. Adjuvant chemotherapy with various agents including vincristine, cisplatinum and lomustine is necessary to improve treatment outcome. However, in the last few decades, recent protocols have attempted to achieve acceptable cure rates with reduced radiation exposure in the hopes of decreasing late effects in children with local disease (no metastasis). Such individualized therapies targeted to specific patient populations, tumor types, and risk groups are being studied with the goal of minimizing treatment-related toxicities while improving long-term survival.
Issues during Acute Treatment
Pediatric patients diagnosed with brain tumors typically exhibit early growth failure with loss of up to 1 SD of height prior to initiating GH therapy (19). Reasons for inadequate growth during therapy are multi-factorial with chemotherapy-induced nausea, cachexia and poor nutritional intake as significant contributing factors. In a small study by Meacham et al, five children with diagnosis of brain tumor were followed quarterly for 2 years with close surveillance of auxological parameters, nutritional indices, and endocrine measurements including GH stimulation testing every 6 months, to identify the onset of GH deficiency. Patients showed a nadir for height velocity 6 months after tumor diagnosis with poor gains in height significantly correlating with decreased caloric intake, poor weight gain, decreased BMI and lowered leptin levels, despite normal secretion of GH during this period. Based on their study results, these investigators proposed a triphasic pattern of growth failure in children diagnosed and treated for brain tumors: initial growth failure occurring from cachexia, followed by a transient phase of normal growth and a subsequent decline in growth velocity from treatment-related GH deficiency (19). No studies have demonstrated catch up growth in childhood survivors of brain tumor after therapy despite adequate hormonal replacement (20–24). Stunted growth of the spine from craniospinal radiation therapy is also a contributing factor to lack of catch-up growth in these patients. Although aggressive nutritional rehabilitation of patients during therapy may mitigate early growth failure during treatment, additional studies with larger cohorts of patients are necessary to further elucidate this issue.
Sequelae of Craniospinal Irradiation
Radiation-induced neurotoxicity depends on total radiation dose, fraction size, and the time allowed between fractions for tissue repair (25–27). Actively dividing tissues such as tumors are highly radiosensitive since the radiosensitivity of any cell is directly proportional to its mitotic activity (28). On the other hand, quiescent cells such as neurons are relatively radioresistant yet more susceptible to the cumulative effects of radiation dose, ultimately leading to progressive damage of the cell’s ability to repair itself and late effect sequelae. Therefore, administering radiation in fractionated doses enhances the therapeutic ratio between tumor control and reduced damage to other cells (29,30). In general, radiation schedules do not deliver more than 2 Gy per fraction with no more than 5 fractions per week, as increasing the fraction size above 2 Gy per fraction for the same total dose can induce more injury to neuronal cells than the tumor tissues (25,27,29,30). Mathematical models such as the linear quadratic model are used to calculate the biological effective dose of irradiation (BED) in a systematic way in order to quantify the biological effects of different radiation schedules, and thus, provide a similar method of comparing radiation impact on hypothalamic and/or pituitary function (26,31,32).
Radiation-induced HP dysfunction also depends on the duration of follow-up after treatment (33). Both incidence and severity of hormonal deficits increase with longer time following cranial irradiation. This progressive nature may be due to delayed effects of the radiation directly and/or to loss of hypothalamic releasing hormones or other trophic factors that lead to a secondary atrophy of the pituitary. Thus, long-term surveillance of pediatric brain tumor survivors with serial testing is required to properly diagnose deficiencies as they evolve. Importantly, childhood cranial RT can affect the HP-adrenal axis particularly after exposure to higher radiation doses (>50 Gy), resulting in secondary adrenal insufficiency (25,27). Therefore, life-long surveillance of the HP-adrenal axis is also recommended in survivors of childhood brain tumor, as failure to mount a biologically sufficient cortisol response may lead to deleterious and life-threatening consequences (80). This topic will not be further reviewed as it does not have a major impact on growth.
Growth Hormone Deficiency
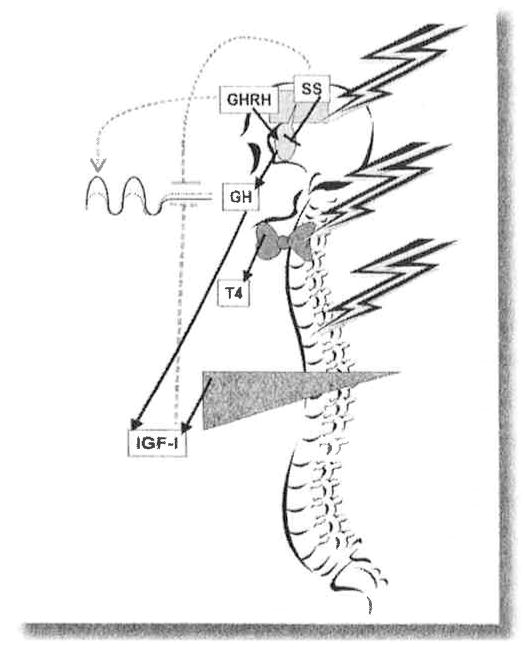
Radiation is a potent cause of dysfunction in the HP unit (Figure 1). Remarkable differences observed in the incidence of anterior pituitary hormone deficiencies suggest different radiosensitivities of the various HP cell lines. In addition, younger age at radiation is usually more frequently associated with multiple pituitary hormone deficiencies suggesting a higher vulnerability of the HP axis to radiation damage in children (25,27,34–36).
Figure 1. GH system effects of craniospinal irradiation that decrease growth.
Levels of all hormones are reduced. Normal growth hormone (GH) secretion is shown by the solid line; following irradiation by the dashed line. Decreased growth hormone releasing hormone (GHRH) secretion diminishes the growth hormone (GH) pulse amplitude and with time, leads to atrophy of the pituitary somatotrophs. Decreased somatostatin (ss) and insulin-like growth factor-1 (IGF-I) inhibition lead to enhanced tonic GH secretion by the remaining functional somatotrophs. Direct radiation injury to the thyroid and reduced thyroid stimulating hormone (TSH) secretion decrease thyroid hormone (T4) production, which further reduces GH secretion centrally and reduces growth plate responsiveness. Likewise, direct radiation injury to the spine decreases its growth response to GH.
The most radiosensitive of the HP systems is GH, with GH deficiency (GHD) the first and most frequent manifestation of HP injury following cranial irradiation (34,37). The severity and time to onset of radiation-induced GHD are dose-dependent, and the incidence increases with time elapsed after cranial irradiation. Current evidence suggests that almost 100% of children treated with radiation doses above 30 Gy will have blunted GH responses to insulin tolerance test within two to five years after radiation therapy (38). Importantly, children show greater vulnerability to developing GHD than adults, as isolated GHD can be seen more frequently in children treated with cranial irradiation doses as low as 18–24 Gy (39–42) or after total body irradiation (TBI) with doses as low as 10 Gy (35,36,42–48).
Growth hormone neurosecretory dysfunction (GHNSD) is a specific form of GHD well described following radiation damage to the HP unit (49–53). GHNSD is typically characterized by diminished endogenous GH secretion yet preserved peak GH responses to provocative testing (54), suggesting intact somatotroph synthetic function and a primary defect in the up-stream signals regulating GH secretion. Importantly, the impaired physiological GH secretion in patients with GHNSD becomes more apparent during puberty with the failed expected increase in GH secretion, accompanied by attenuated pubertal growth velocity (25,27,55,56). Radiation-induced GHNSD usually is dose dependent and often encountered in children with leukemia who require prophylactic cranial irradiation with their treatment (51) and/or TBI after bone marrow transplantation (46,48).
Radiation-induced GHD is a quantitative phenomenon. Recent studies in adult survivors of brain tumor revealed preservation of the diurnal variation and pulsatile character of GH secretion, with marked dampening of the pulse amplitude and relative preservation of tonic (nonpulsatile) GH secretion (57). This preserved basal GH secretion is due to reduction in IGF-1 dependent negative feedback as well as radiation-induced attenuation in somatostatin release, resulting in enhanced tonic GH release from the residual functioning somatotrophs (58). At the same time, reduced hypothalamic GHRH release in conjunction with diminished somatotroph mass is responsible for the decreased amplitude of the GH secretory pulses (58,59). Howeyer, the poorly understood mechanisms controlling GH pulse generation and sleep-entrained diurnal variation remain unaffected by irradiation, even when cranial irradiation is delivered during childhood (57). Therefore, even though the integrity of HP unit and GH neuroregulation appears fundamentally preserved in radiation-induced GH-deficient patients, the overall secretory process is distorted and can manifest as inadequate growth during childhood (25,27,57).
Effects on Puberty and Gonadotropin Secretion
Clinically significant gonadotropin deficiency is usually a late complication after HP irradiation, making it the second most common anterior pituitary hormone deficit recognized in 20–50% of children treated with radiation doses above 40 Gy (60–62). However, this deficiency presents in a wide spectrum of clinical severity from subtle abnormalities of the axis only detected by gonadotropin releasing hormone (GnRH)-stimutated testing to severe impairment with reduced circulating sex hormone levels (25,27,62). Typically, these patients have normal basal levels of LH and FSH with accompanied reduced to low-normal sex hormone concentrations. Concomitantly, GnRH testing reveals a delayed peak gonadotropin response with a subsequent delay in gonadotropin decline, indicating pituitary damage (25,27).
In contrast, lower doses of cranial irradiation in childhood paradoxically can result in early or precocious puberty (63–68) through disinhibition of cortical influences on the hypothalamus and reduction in the inhibitory GABAnergic tone (68,69). There is a sexual dichotomy; precocious puberty predominantly occurs in females after exposure to lower radiation doses (18–24 Gy), such as those previously used for prophylactic cranial irradiation in patients with acute lymphoblastic leukemia (ALL), while the frequency of precocious puberty is no different between male ALL survivors and that seen in the normal population (66,67). This disparity has been attributed to fundamental gender differences in the interaction between higher centers in the CNS and hypothalamic function, with the restraint on puberty more easily disrupted in females by any insult, including cranial irradiation (65,70).
Higher cranial irradiation doses (25–50 Gy), on the other hand, can cause precocious puberty equally in both sexes with a linear association between the age at irradiation and age at onset of puberty (65). In a study of 46 GH-deficient children after cranial irradiation (25–47.5 Gy) for treatment of brain tumor, an early onset of pubertal development was noted in both sexes, with mean chronological age of puberty occurring at 8.5 years in girls and 9.2 years in boys (65). The occurrence of precocious puberty in the context of GHD is common in children after cranial irradiation, while children with non-radiation associated GHD often exhibit pubertal delay due to concomitant gonadotropin deficiency.
Hypothyroidism
The HP-thyroid axis is the least vulnerable axis to radiation damage with damage occurring only after exposure to radiation doses in excess of 50 Gy (62,71–73). Secondary hypothyroidism has not been noted following low dose prophylactic cranial irradiation or TBI (36,74), with frequency of TSH deficiency as low as 3–6% in survivors of brain tumor not involving the pituitary gland (75,76). Subtle abnormalities in the dynamics of TSH secretion, such as elevated basal and stimulated TSH levels in the presence of normal free T4 levels, are common after cranial irradiation (72,77,78). However, patients treated with craniospinal irradiation are also at risk for radiation-induced primary hypothyroidism with the possibility of developing mixed (primary and secondary) hypothyroidism evident with declining free T4 levels over time. In addition, GH therapy can reduce free T4 levels, unmasking central hypothyroidism in patients with hypopituitarism who exhibit normal free T4 levels at baseline (70,79). Given the importance of thyroid hormone for growth, annual monitoring of thyroid function tests before as well as after starting GH therapy, is essential in these patients.
Spine Growth
Disproportionate growth after craniospinal radiation therapy (CSRT) is well recognized with notable high risk for short adult height. The probability of attaining adult height below the third percentile is increased 6-fold after CSRT (81) with sitting height z-scores of −3 to −3.4 SD (21,82–85). Spinal irradiation most likely causes damage to the growth plates, as patients treated with spinal RT are consistently shorter than patients treated with cranial RT despite treatment with GH (22,76,86–89). Although GH therapy improves linear growth, adult height remains significantly less than expected when compared with mid-parental height and especially when CSRT is combined with chemotherapy (22,90,91). Additional studies addressing this issue have consistently shown a decreased upper to lower segment ratio in patients after spinal RT compared to cranial RT and better improvement in both final and sitting heights in children treated with GH after cranial RT alone (20,76,81,83,91–94). The impact of spinal irradiation on vertebral growth depends on both radiation dosage and patient age, with greater impairment noted in children irradiated at a young age (24,89). Even at a young age (less than 6 years of age), Xu et al reported significantly greater adult and sitting heights in children with medulloblastoma treated with lower doses of CSRT (18 Gy) combined with chemotherapy compared with a similar group of patients treated with higher doses of CSRT (23–25 Gy) and chemotherapy (24).
Spinal RT has a larger negative effect for boys compared to girls (85). This may reflect gender differences in spinal growth potential, as growth curves of sitting height show greater remaining percentage in boys than girls at every age until final height (85,95,96). Although studies reporting these findings have considerable heterogeneity including different methods of radiation treatment, they highlight the notable sex-dependent growth differences in sitting height despite GH treatment after spinal RT (85,97). Analyses with larger cohorts of similar patients are necessary to verify some of these reported findings.
Chemotherapy Effects on Growth
Data on the effects of intensive chemotherapy on growth are controversial, as the majority of studies assessing growth outcome in pediatric patients after cancer therapy include intensive chemotherapy in addition to irradiation. Olshan et al reported overall significantly worse growth in prepubertal children with medulloblastoma treated with adjuvant chemotherapy and CSRT compared to CSRT alone, with little or no improvement in growth velocity during the 4 years of post-treatment observation, particularly in patients who were treated with adjuvant chemotherapy (98). Several other studies have shown that despite GH therapy, adult height in children treated with CSRT combined with chemotherapy is significantly less than expected, especially when compared with mid-parental height (20–24). In 2004 Gleeson et al reported deleterious effects of adjuvant chemotherapy on growth in addition to negative effects of cranial RT or CSRT with loss of up to 7 cm in adult height in these patients (99), suggesting that cytotoxic drugs potentiate the irradiation damage to the HP unit (50) or directly affect the production of IGF-1 with additional impairment of IGF-1 actions on the growth plate (100). Rose et al reported GHD in 15 of 31 identified children in a retrospective chart review of cancer survivors who had received chemotherapy but no cranial or total body RT and no CNS tumor. These findings were not reported as a study of prevalence but rather an evaluation of cancer survivors referred to an endocrinology clinic for abnormal growth following cancer therapy (101). Of note, these authors also reported central hypothyroidism (TSH deficiency) in 16 of 31 (52%) of their small patient cohort, felt due to hypothalamic dysfunction (101), which is much higher than most reported study results on central thyroid dysfunction after cancer treatment (102,103). On the other hand, patients with severe aplastic anemia requiring bone marrow transplantation who are conditioned with only chemotherapy (cyclophosphamide alone or with busulfan) have been reported to grow normally (46,104,105). Thus, while adjuvant chemotherapy may exacerbate poor growth following CSRT, the etiology is multi-factorial and additional well-designed multicenter studies in large cohort of children are necessary to further elucidate if chemotherapy alone can cause GHD.
Clinical Management of Growth Hormone Deficiency in Survivors of Pediatric Brain Tumors
Diagnostic Challenges
The hallmark features of pediatric GHD are abnormally slow growth velocity with progressive decline in height z-score, often associated with delayed skeletal maturation, low levels of circulating IGF-I and/or IGFBP-3, and inadequate GH secretory response to pharmacologic secretagogues (106). The same holds true for GHD in survivors of pediatric brain tumors, though certain situations can lead to diagnostic challenges. For example, children who clearly have GHD from resection of craniopharyngioma may develop hypothalamic obesity and maintain normal or even accelerated statural growth. This phenomenon, called growth without GH, has been attributed to obesity-related increases in leptin, insulin, and sex hormone production (107).
Discordant responses on testing of IGF-I levels, stimulated GH peaks, and sampled endogenous GH secretion can be confusing and have raised questions regarding which test(s) are best for patients following cranial RT (33,108). Numerous limitations have been identified with GH stimulation tests to diagnose GHD in general (109), and are further compounded in pediatric brain tumor survivors by potential HP disruptions that can lead to a result on pharmacologic secretagogue testing that does not reflect the functional status of the endogenous GH system (33). Although abnormally low IGF-I concentrations (<−2 SD) generally indicate GHD in patients following cranial RT (after exclusion of other factors like undernutrition that can independently lower IGF-I levels), IGF-I concentrations above −2 SD are not sensitive enough to exclude abnormal peak GH responses to stimulation testing (33,110,111). Methodological issues may be playing a contributory role to this finding. For example, in a study of 28 children and adolescents following cranial or CSRT, only 7 of the 15 patients with GHD (identified as peak GH level <7.5 ng/ml after a stimulation test) had circulating IGF-I concentrations below −2 SD for age and gender (110). The IGF-I concentrations in this study were measured by radioimmunoassay following acid-ethanol extraction, a method that can be influenced by IGFBP competition with the radiolabelled IGF-I trace (112). Circulating IGFBP-2 levels are elevated in some patients with brain tumors (113,114), and the increased circulating IGFBP-2 may falsely elevate the IGF-I values measured by this technique. In another study of 48 children following bone marrow transplantation or treatment of solid brain tumor, only one of the 22 children with GHD (identified as peak GH level <8 on Arginine/L-DOPA testing) had an IGF-I concentration <−2 SD. However, IGF-I and IGFBP-3 levels correlated with individual height changes while GH peak did not (111). Thus, the diagnosis of GHD in patients following cranial irradiation remains challenging, and requires the integration of multiple clinical clues rather than absolute reliance on a single test.
Adult Height in Treated GH Deficient Survivors
Suboptimal growth in brain tumor patients is multi-factorial and related to poor nutrition, tumor recurrence, impaired spinal growth from spinal irradiation, chemotherapy, radiation-induced GHD, and precocious puberty (Table 2). As noted previously, the majority of children treated with radiation doses in excess of 27 Gy to the HP unit will become GH deficient (34). In addition, cranial RT doses in excess of 18 Gy can induce early or precious puberty, particularly in females with the age of onset of puberty related to the age at irradiation (64,65). In 1995, Ogilvy-Stuart et al demonstrated detrimental effects of spinal irradiation and the additive adverse effect of chemotherapy in a group of 29 children treated with GH for radiation-induced GHD (21). Subsequently, Adan et al reported improvements in adult height (AH) in brain tumor survivors treated with GH compared with a previous study 10 years earlier from the same group of investigators, and attributed the improvement to changes in GH regimen as well as the use of GnRH analog (GnRHa) therapy (82). Other factors identified with improvements in AH over time include earlier testing for GHD and a reduction in lag time from completion of RT to start of GH replacement, with GnRHa significantly improving AH outcome in patients treated with cranial RT (86). Although no definitive comparison studies are available to assess the true benefit of GnRHa therapy in AH outcome in children with radiation-induced GHD and precocious puberty, the use of combined GnRHa and GH may contribute to better auxological outcome in these patients.
Table 2.
Factors that improve growth outcome following brain tumor treatment
| Older age at treatment |
| Optimized nutrition during treatment |
Radiation exposure:
|
Timely diagnosis and replacement of hormone deficiencies
|
| GnRH agonist treatment of precocious puberty, if applicable |
Unfortunately, the youngest children have the worst prognosis, as they are more sensitive to the damaging effects of cranial RT to the HP unit and becoming severely GH deficient at a younger age. In addition, younger age at irradiation results in a longer period of potential growth affected by spinal RT resulting in greater sitting height deficits compared to AH (89,115). As spinal growth exceeds lower leg growth during puberty, an exaggerated disproportion at AH is noted in children treated with CSRT (116).
Comparison of AH in a group of children treated for GHD after treatment of medulloblastoma to the subset of GH-treated individuals with idiopathic GHD (iGHD) from the Pfizer International Growth Study (KIGS) demonstrated significantly lower height velocity, gain in height as well as responsiveness to GH among medulloblastoma patients than patients with iGHD (117). Following treatment with GH, children with iGHD were significantly taller, gained more height, weighed relatively less in relation to their height and had a greater sitting height even though parental height, birth weight, height and weight at initiation of GH treatment were higher in medulloblastoma patients (117). Similarly, AH correlated positively with both age at the time of tumor diagnosis and height at the start of GH therapy (117), although the “index of responsiveness” was much lower in children with medulloblastoma (118). These studies demonstrate that despite GH therapy, final height gain in GH deficient children after treatment for brain tumor, although improved, is still less than predictive height. Promising changes in future treatment modalities may possibly mitigate the degree of growth impairment in these children.
Risk of Cancer Recurrence and Second Neoplasms with GH Therapy
GHD is the most common endocrinopathy noted in childhood cancer survivors (38), with GH replacement an accepted and beneficial form of therapy for growth failure secondary to GHD induced by cranial RT or direct destruction of the HP region by tumor (119). Because GH has mitogenic properties and induces IGF-I, safety concerns have been raised that treating cancer survivors with GH may possibly result in an increased risk of disease recurrence or the development of secondary neoplasms (SN). These concerns were rooted in earlier yet still controversial clinical studies demonstrating an increased risk of colon cancer in subjects with acromegaly (120), as well as early reports of increased incidence of leukemias occurring in pediatric patients treated with GH (121,122). However, the reports of increased leukemia were subsequently disproven by other studies, particularly as the original report published in the Lancet included children with other cancer risk factors, thus confounding their interpretation. More recently, epidemiological studies in adults have reported associations between higher risk for common adulthood malignancies and circulating IGF-1 levels in the highest quartile of the normal population, and less consistently, with lower concentrations of circulating IGFBP-3 (123–127).
The effects of IGFs on normal and cancer cells are mediated via the type 1 IGF receptor (128,129). In a study of pediatric leukemia risk, circulating IGF-1 concentration was not found to be a risk factor, but low levels of IGFBP-3 were (130). As GH induces both IGF-1 and IGFBP-3, its role in driving the association between IGF-1 and cancer risk seems less likely, particularly since the co-elevation in IGFBP-3 may counteract the mitogenic effects of IGF-1 (129,131,132). A number of reported studies have failed to demonstrate an increased incidence of cancer among adult GH recipients who were treated for GHD (133,134), nor among pediatric subjects who received GH treatment for various indications (127,131,135). Specifically, careful follow-up of large cohorts of pediatric cancer survivors treated with GH have not indicated an increased risk of solid tumor recurrence, CNS tumor relapse or development of leukemia (134,136–143). Three large series reporting on survivors of pediatric brain tumors treated with GH found a reduced risk of primary disease recurrence (140,142,143), though this reduced relative risk (RR) may represent an inherent selection bias favoring GH treatment in survivors with better prognosis (143). These large series highlight the importance of not withholding GH therapy in survivors with treatment-associated GHD based on fear of primary tumor recurrence. Nonetheless, convention has developed to defer GH therapy until the patient is at least one year tumor-free due to early fears of increased recurrence, which usually is the highest in the first year following cancer treatment even without GH therapy.
Survivors of childhood cancer are known to be at increased risk of developing SN as a consequence of exposure to specific therapies such as irradiation, alkylating agents, and topoisomerase II inhibitors (144–149). Genetic factors play an important role in a small subset of patients with underlying genetic predisposition to cancer; however, only a few studies have assessed the risk of secondary cancer and/or leukemia in cancer survivors treated with GH. Sklar et al first reported a possible increased RR (3.21) of developing SN in a cohort of GH-treated cancer survivors from the Childhood Cancer Survivor Study (CCSS) (143). Although the increased risk of secondary leukemia was not increased with GH replacement therapy, these investigators found an increase in the number of secondary solid tumors, specifically osteogenic sarcoma and meningioma (143). The absolute number of excess solid tumors resulting from GH therapy was small (3–4/1000 person year at 15 years from diagnosis), inferring that the small risk of SN in cancer survivors would need to be weighed against the substantial established benefits of GH therapy (143).
In an updated analysis of the CCSS cohort after an additional 32 months of follow-up, even though an elevated risk of developing SN in cancer survivors treated with GH was noted once again, the RR decreased from 3.21 to 2.15 just with the longer duration of follow-up (150). Meningiomas were the most common type of SN diagnosed, and all of the GH-treated survivors who developed a meningioma had received cranial RT as part of their primary cancer treatment (150). Importantly, a shorter latency period between irradiation and the diagnosis of meningioma in the GH-treated group was evident in comparison with survivors not treated with GH after cranial RT (150), raising the possibility of a true biological effect of GH on the development and progression of these meningiomas (123), Meningiomas are known to develop after irradiation to the head for benign and malignant conditions (144,151,152), and tend to remain asymptomatic for prolonged periods of time (153,154). Thus, the possibility of detection bias due to more consistent and frequent medical surveillance with MRI of the head in GH-treated survivors compared with the group not receiving GH treatment cannot be excluded as a plausible explanation for these findings (135,150). Once again, even though these analyses raise concern for an increased risk of developing SN with GH therapy in survivors of childhood cancer, additional studies are necessary to confirm whether these elevated risks decrease with increasing lengths of follow-up, particularly as the overall risks remain small and must be weighed against the potential benefits of GH therapy in survivors (135).
Future Directions: Proton Therapy
Children cured of their CNS tumors live to experience the long-term sequelae of radiation treatment including developmental, neurocognitive, neuroendocrine, and hearing late effects. Newer RT techniques such as proton therapy are being developed to decrease the inadvertent radiation dose to normal tissues and thereby reduce long-term sequelae. As proton radiation eliminates exit dose and exposes only normal tissue proximal to the tumor, it eliminates over 50% of unnecessary irradiation to normal tissues (155). Thus, proton therapy is the most promising form of external beam RT to date with hopes of reduced treatment-related late effects, particularly among the pediatric population with malignancies requiring RT. As the number of proton facilities in the U.S. and worldwide increases in the near future, more children will receive proton therapy in anticipation of this treatment modality evolving into standard of care for treating curable pediatric CNS tumors requiring RT. As a result, the long term benefits of proton therapy, particularly a substantial reduction of treatment-related deleterious effects on the neuroendocrine axis, will soon become available clinically and reported in the literature.
Footnotes
Disclosure
Dr. Grimberg is funded by grant 5R01-HD057037 from the National Institutes of Health/NICHD. In the past three years, the Children’s Hospital of Philadelphia Endocrine Division has been participating in the growth hormone registries of all the major growth hormone manufacturers (Eli Lilly, Genentech, Novo Nordisk, Pfizer, and Serono), and Dr. Grimberg received honoraria and reimbursed travel expenses for presenting her research at growth hormone meetings sponsored by Pfizer and Novo Nordisk (though has never served on speaker bureaus). Dr. Mostoufi-Moab is funded by the St. Baldrick’s Foundation and does not have any financial disclosures.
References
- 1.Packer RJ, MacDonald T, Vezina G. Central nervous system tumors. Pediatr Clin North Am. 2008;55(1):121–145. xi. doi: 10.1016/j.pcl.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Bleyer WA. Epidemiologic impact of children with brain tumors. Childs Nerv Syst. 1999;15(11–12):758–763. doi: 10.1007/s003810050467. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ. Childhood brain tumors: accomplishments and ongoing challenges. J Child Neurol. 2008;23(10):1122–1127. doi: 10.1177/0883073808320758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weir HK, Thun MJ, Hankey BF, Ries LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst. 2003;95(17):1276–1299. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 5.Sklar CA. Overview of the effects of cancer therapies: the nature, scale and breadth of the problem. Acta Paediatr. 1999;88(suppl 433):1–4. doi: 10.1111/j.1651-2227.1999.tb14395.x. [DOI] [PubMed] [Google Scholar]
- 6.Anderson DM, Rennie KM, Ziegler RS, Neglia JP, Robison LR, Gurney JG. Medical and neurocognitive late effects among survivors of childhood central nervous system tumors. Cancer. 2001;92(10):2709–2719. doi: 10.1002/1097-0142(20011115)92:10<2709::aid-cncr1625>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, Stovall M, Yasui Y, Nicholson HS, Wolden S, McNeil DE, Mertens AC, Robison LL. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: childhood cancer survivor study. Cancer. 2003;97(3):663–673. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 8.Sklar CA. Childhood brain tumors. J Pediatr Endocrinol Metab. 2002;15(suppl 2):669–673. doi: 10.1515/jpem.2002.15.s2.669. [DOI] [PubMed] [Google Scholar]
- 9.Shalet SM. Irradiation-induced growth failure. Clin Endocrinol Metab. 1986;15(3):591–606. doi: 10.1016/s0300-595x(86)80011-1. [DOI] [PubMed] [Google Scholar]
- 10.Shalet SM, Beardwell CG, Aarons BM, Pearson D, Jones PH. Growth impairment in children treated for brain tumours. Arch Dis Child. 1978;53(6):491–494. doi: 10.1136/adc.53.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17(9):503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 12.Babcock MA, Kostova FV, Guha A, Packer RJ, Pollack IF, Maria BL. Tumors of the central nervous system: clinical aspects, molecular mechanisms, unanswered questions, and future research directions. J Child Neurol. 2008;23(10):1103–1121. doi: 10.1177/0883073808321767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Packer RJ, Cogen P, Vezina G, Rorke LB. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–250. doi: 10.1215/15228517-1-3-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birch JM, Marsden HB. A classification scheme for childhood cancer. Int J Cancer. 1987;40(5):620–624. doi: 10.1002/ijc.2910400508. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol. 2008;65(11):1419–1424. doi: 10.1001/archneur.65.11.1419. [DOI] [PubMed] [Google Scholar]
- 16.Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90(17):1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 17.Sievert A, Minturn J. Brain Tumors. In: Florin T, Ludwig S, editors. Netter’ Ediatrics. Philadelphia, PA: Elsevier; Jun, 2011. in press. [Google Scholar]
- 18.Jane JA., Jr Management of pediatric sellar tumors. Pediatr Endocrinol Rev. 2008;5(suppl 2):720–726. [PubMed] [Google Scholar]
- 19.Meacham LR, Mason PW, Sullivan KM. Auxologic and biochemical characterization of the three phases of growth failure in pediatric patients with brain tumors. J Pediatr Endocrinol Metab. 2004;17(5):711–717. doi: 10.1515/jpem.2004.17.5.711. [DOI] [PubMed] [Google Scholar]
- 20.Clayton PE, Shalet SM, Price DA. Growth response to growth hormone therapy following craniospinal irradiation. Eur J Pediatr. 1988;147(6):597–601. doi: 10.1007/BF00442471. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvy-Stuart AL, Shalet SM. Growth and puberty after growth hormone treatment after irradiation for brain tumours. Arch Dis Child. 1995;73(2):141–146. doi: 10.1136/adc.73.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sulmont V, Brauner R, Fontoura M, Rappaport R. Response to growth hormone treatment and final height after cranial or craniospinal irradiation. Acta Paediatr Scand. 1990;79(5):542–549. doi: 10.1111/j.1651-2227.1990.tb11509.x. [DOI] [PubMed] [Google Scholar]
- 23.Darendeliler F, Livesey EA, Hindmarsh PC, Brook CG. Growth and growth hormone secretion in children following treatment of brain tumours with radiotherapy. Acta Paediatr Scand. 1990;79(10):950–956. doi: 10.1111/j.1651-2227.1990.tb11357.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Janss A, Packer RJ, Phillips P, Goldwein J, Moshang T., Jr Endocrine outcome in children with medulloblastoma treated with 18 Gy of craniospinal radiation therapy. Neuro Oncol. 2004;6(2):113–118. doi: 10.1215/S1152851703000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darzy KH, Shalet SM. Hypopituitarism as a consequence of brain tumours and radiotherapy. Pituitary. 2005;8(3–4):203–211. doi: 10.1007/s11102-006-6042-4. [DOI] [PubMed] [Google Scholar]
- 26.Thames H, Hendry J. Fractionation in Radiotherapy. Taylor and Francis; London: 1987. Response of tissues to fractionated irradiaiton: effect of repair; pp. 53–99. [Google Scholar]
- 27.Darzy KH, Shalet SM. Hypopituitarism following Radiotherapy Revisited. Endocr Dev. 2009;15:1–24. doi: 10.1159/000207607. [DOI] [PubMed] [Google Scholar]
- 28.Coggle J. The effect of radiation at the tissue level biological effects of radiation. Taylor and Francis; London: 1983. pp. 89–109. [Google Scholar]
- 29.Withers H. Biology of radiation oncology. In: Tobias J, PRM T, editors. Current Radiation Oncology. Edward Arnold; London: 1994. pp. 5–23. [Google Scholar]
- 30.Hopewell JW. Radiation injury to the central nervous system. Med Pediatr Oncol. 1998;(suppl 1):1–9. doi: 10.1002/(sici)1096-911x(1998)30:1+<1::aid-mpo1>3.0.co;2-y. [DOI] [PubMed]
- 31.Schmiegelow M, Lassen S, Poulsen HS, Feldt-Rasmussen U, Schmiegelow K, Hertz H, Muller J. Cranial radiotherapy of childhood brain tumours: growth hormone deficiency and its relation to the biological effective dose of irradiation in a large population based study. Clin Endocrinol (Oxf) 2000;53(2):191–197. doi: 10.1046/j.1365-2265.2000.01079.x. [DOI] [PubMed] [Google Scholar]
- 32.Jones L, Hoban P, Metcalfe P. The use of the linear quadratic model in radiotherapy: a review. Australas Phys Eng Sci Med. 2001;24(3):132–146. doi: 10.1007/BF03178355. [DOI] [PubMed] [Google Scholar]
- 33.Darzy KH. Radiation-induced hypopituitarism after cancer therapy: who, how and when to test. Nat Clin Pract Endocrinol Metab. 2009;5(2):88–99. doi: 10.1038/ncpendmet1051. [DOI] [PubMed] [Google Scholar]
- 34.Shalet SM, Beardwell CG, Morris-Jones P, Bamford FN, Ribeiro GG, Pearson D. Growth hormone deficiency in children with brain tumors. Cancer. 1976;37(2 suppl):1144–1148. doi: 10.1002/1097-0142(197602)37:2+<1144::aid-cncr2820370824>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 35.Brauner R, Czernichow P, Rappaport R. Greater susceptibility to hypothalamopituitary irradiation in younger children with acute lymphoblastic leukemia. J Pediatr. 1986;108(2):332. doi: 10.1016/s0022-3476(86)81027-7. [DOI] [PubMed] [Google Scholar]
- 36.Ogilvy-Stuart AL, Clark DJ, Wallace WH, Gibson BE, Stevens RF, Shalet SM, Donaldson MD. Endocrine deficit after fractionated total body irradiation. Arch Dis Child. 1992;67(9):1107–1110. doi: 10.1136/adc.67.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalet SM, Beardwell CG, Morris-Jones PH, Pearson D. Pituitary function after treatment of intracranial tumours in children. Lancet. 1975;2(7925):104–107. doi: 10.1016/s0140-6736(75)90006-9. [DOI] [PubMed] [Google Scholar]
- 38.Clayton PE, Shalet SM. Dose dependency of time of onset of radiation-induced growth hormone deficiency. J Pediatr. 1991;118(2):226–228. doi: 10.1016/s0022-3476(05)80487-1. [DOI] [PubMed] [Google Scholar]
- 39.Costin G. Effects of low-dose cranial radiation on growth hormone secretory dynamics and hypothalamic-pituitary function. Am J Dis Child. 1988;142(8):847–852. doi: 10.1001/archpedi.1988.02150080053022. [DOI] [PubMed] [Google Scholar]
- 40.Shalet SM, Beardwell CG, Jones PH, Pearson D. Growth hormone deficiency after treatment of acute leukaemia in children. Arch Dis Child. 1976;51(7):489–493. doi: 10.1136/adc.51.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirk JA, Raghupathy P, Stevens MM, Cowell CT, Menser MA, Bergin M, Tink A, Vines RH, Silink M. Growth failure and growth-hormone deficiency after treatment for acute lymphoblastic leukaemia. Lancet. 1987;1(8526):190–193. doi: 10.1016/s0140-6736(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 42.Brennan BM, Rahim A, Mackie EM, Eden OB, Shalet SM. Growth hormone status in adults treated for acute lymphoblastic leukaemia in childhood. Clin Endocrinol (Oxf) 1998;48(6):777–783. doi: 10.1046/j.1365-2265.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 43.Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Applegate G, Sutton ML. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989;70(262):145–160. [PubMed] [Google Scholar]
- 44.Sanders JE, Buckner CD, Sullivan KM, Doney K, Appelbaum F, Witherspoon R, Storb R, Thomas ED. Growth and development in children after bone marrow transplantation. Horm Res. 1988;30(2–3):92–97. doi: 10.1159/000181036. [DOI] [PubMed] [Google Scholar]
- 45.Papadimitriou A, Urena M, Hamill G, Stanhope R, Leiper AD. Growth hormone treatment of growth failure secondary to total body irradiation and bone marrow transplantation. Arch Dis Child. 1991;66(6):689–692. doi: 10.1136/adc.66.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borgstrom B, Bolme P. Growth and growth hormone in children after bone marrow transplantation. Horm Res. 1988;30(2–3):98–100. doi: 10.1159/000181037. [DOI] [PubMed] [Google Scholar]
- 47.Olshan JS, Willi SM, Gruccio D, Moshang T., Jr Growth hormone function and treatment following bone marrow transplant for neuroblastoma. Bone Marrow Transplant. 1993;12(4):381–385. [PubMed] [Google Scholar]
- 48.Hovi L, Saarinen UM, Siimes MA. Growth failure in children after total body irradiation preparative for bone marrow transplantation. Bone Marrow Transplant. 1991;8 (suppl 1):10–13. [PubMed] [Google Scholar]
- 49.Chrousos GP, Poplack D, Brown T, O’Neill D, Schwade J, Bercu BB. Effects of cranial radiation on hypothalamic-adenohypophyseal function: abnormal growth hormone secretory dynamics. J Clin Endocrinol Metab. 1982;54(6):1135–1139. doi: 10.1210/jcem-54-6-1135. [DOI] [PubMed] [Google Scholar]
- 50.Spoudeas HA, Hindmarsh PC, Matthews DR, Brook CG. Evolution of growth hormone neurosecretory disturbance after cranial irradiation for childhood brain tumours: a prospective study. J Endocrinol. 1996;150(2):329–342. doi: 10.1677/joe.0.1500329. [DOI] [PubMed] [Google Scholar]
- 51.Blatt J, Bercu BB, Gillin JC, Mendelson WB, Poplack DG. Reduced pulsatile growth hormone secretion in children after therapy for acute lymphoblastic leukemia. J Pediatr. 1984;104(2):182–186. doi: 10.1016/s0022-3476(84)80989-0. [DOI] [PubMed] [Google Scholar]
- 52.Darzy KH, Pezzoli SS, Thorner MO, Shalet SM. Cranial irradiation and growth hormone neurosecretory dysfunction: a critical appraisal. J Clin Endocrinol Metab. 2007;92(5):1666–1672. doi: 10.1210/jc.2006-2599. [DOI] [PubMed] [Google Scholar]
- 53.Bercu BB, Root AW, Shulman DI. Preservation of dopaminergic and alpha-adrenergic function in children with growth hormone neurosecretory dysfunction. J Clin Endocrinol Metab. 1986;63(4):968–973. doi: 10.1210/jcem-63-4-968. [DOI] [PubMed] [Google Scholar]
- 54.Bercu BB, Shulman D, Root AW, Spiliotis BE. Growth hormone (GH) provocative testing frequently does not reflect endogenous GH secretion. J Clin Endocrinol Metab. 1986;63(3):709–716. doi: 10.1210/jcem-63-3-709. [DOI] [PubMed] [Google Scholar]
- 55.Crowne EC, Moore C, Wallace WH, Ogilvy-Stuart AL, Addison GM, Morris-Jones PH, Shalet SM. A novel variant of growth hormone (GH) insufficiency following low dose cranial irradiation. Clin Endocrinol (Oxf) 1992;36(1):59–68. doi: 10.1111/j.1365-2265.1992.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 56.Moell C, Garwicz S, Westgren U, Wiebe T, Albertsson-Wikland K. Suppressed spontaneous secretion of growth hormone in girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1989;64(2):252–258. doi: 10.1136/adc.64.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Darzy KH, Pezzoli SS, Thorner MO, Shalet SM. The dynamics of growth hormone (GH) secretion in adult cancer survivors with severe GH deficiency acquired after brain irradiation in childhood for nonpituitary brain tumors: evidence for preserved pulsatility and diurnal variation with increased secretory disorderliness. J Clin Endocrinol Metab. 2005;90(5):2794–2803. doi: 10.1210/jc.2004-2002. [DOI] [PubMed] [Google Scholar]
- 58.Ogilvy-Stuart AL, Wallace WH, Shalet SM. Radiation and neuroregulatory control of growth hormone secretion. Clin Endocrinol (Oxf) 1994;41(2):163–168. doi: 10.1111/j.1365-2265.1994.tb02525.x. [DOI] [PubMed] [Google Scholar]
- 59.Jorgensen EV, Schwartz ID, Hvizdala E, Barbosa J, Phuphanich S, Shulman DI, Root AW, Estrada J, Hu CS, Bercu BB. Neurotransmitter control of growth hormone secretion in children after cranial radiation therapy. J Pediatr Endocrinol. 1993;6(2):131–142. [PubMed] [Google Scholar]
- 60.Pasqualini T, Escobar ME, Domene H, Muriel FS, Pavlovsky S, Rivarola MA. Evaluation of gonadal function following long-term treatment for acute lymphoblastic leukemia in girls. Am J Pediatr Hematol Oncol. 1987;9(1):15–22. doi: 10.1097/00043426-198721000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Rappaport R, Brauner R, Czernichow P, Thibaud E, Renier D, Zucker JM, Lemerle J. Effect of hypothalamic and pituitary irradiation on pubertal development in children with cranial tumors. J Clin Endocrinol Metab. 1982;54(6):1164–1168. doi: 10.1210/jcem-54-6-1164. [DOI] [PubMed] [Google Scholar]
- 62.Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, Rubin P. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328(2):87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 63.Brauner R, Rappaport R. Precocious puberty secondary to cranial irradiation for tumors distant from the hypothalamo-pituitary area. Horm Res. 1985;22(1–2):78–82. doi: 10.1159/000180076. [DOI] [PubMed] [Google Scholar]
- 64.Brauner R, Czernichow P, Rappaport R. Precocious puberty after hypothalamic and pituitary irradiation in young children. N Engl J Med. 1984;311(14):920. doi: 10.1056/NEJM198410043111414. [DOI] [PubMed] [Google Scholar]
- 65.Ogilvy-Stuart AL, Clayton PE, Shalet SM. Cranial irradiation and early puberty. J Clin Endocrinol Metab. 1994;78(6):1282–1286. doi: 10.1210/jcem.78.6.8200926. [DOI] [PubMed] [Google Scholar]
- 66.Leiper AD, Stanhope R, Kitching P, Chessells JM. Precocious and premature puberty associated with treatment of acute lymphoblastic leukaemia. Arch Dis Child. 1987;62(11):1107–1112. doi: 10.1136/adc.62.11.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quigley C, Cowell C, Jimenez M, Burger H, Kirk J, Bergin M, Stevens M, Simpson J, Silink M. Normal or early development of puberty despite gonadal damage in children treated for acute lymphoblastic leukemia. N Engl J Med. 1989;321(3):143–151. doi: 10.1056/NEJM198907203210303. [DOI] [PubMed] [Google Scholar]
- 68.Roth C, Schmidberger H, Schaper O, Leonhardt S, Lakomek M, Wuttke W, Jarry H. Cranial irradiation of female rats causes dose-dependent and age-dependent activation or inhibition of pubertal development. Pediatr Res. 2000;47(5):586–591. doi: 10.1203/00006450-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Roth C, Schmidberger H, Lakomek M, Witt O, Wuttke W, Jarry H. Reduction of gamma-aminobutyric acid-ergic neurotransmission as a putative mechanism of radiation induced activation of the gonadotropin releasing-hormone-pulse generator leading to precocious puberty in female rats. Neurosci Lett. 2001;297(1):45–48. doi: 10.1016/s0304-3940(00)01663-3. [DOI] [PubMed] [Google Scholar]
- 70.Darzy KH, Shalet SM. Hypopituitarism after cranial irradiation. J Endocrinol Invest. 2005;28(suppl 5):78–87. [PubMed] [Google Scholar]
- 71.Littley MD, Shalet SM, Beardwell CG, Robinson EL, Sutton ML. Radiation-induced hypopituitarism is dose-dependent. Clin Endocrinol (Oxf) 1989;31(3):363–373. doi: 10.1111/j.1365-2265.1989.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 72.Lam KS, Tse VK, Wang C, Yeung RT, Ho JH. Effects of cranial irradiation on hypothalamic-pituitary function - a 5-year longitudinal study in patients with nasopharyngeal carcinoma. Q J Med. 1991;78(286):165–176. [PubMed] [Google Scholar]
- 73.Samaan NA, Vieto R, Schultz PN, Maor M, Meoz RT, Sampiere VA, Cangir A, Ried HL, Jesse RH., Jr Hypothalamic, pituitary and thyroid dysfunction after radiotherapy to the head and neck. Int J Radiat Oncol Biol Phys. 1982;8(11):1857–1867. doi: 10.1016/0360-3016(82)90442-4. [DOI] [PubMed] [Google Scholar]
- 74.Littley MD, Shalet SM, Morgenstern GR, Deakin DP. Endocrine and reproductive dysfunction following fractionated total body irradiation in adults. Q J Med. 1991;78(287):265–274. [PubMed] [Google Scholar]
- 75.Livesey EA, Hindmarsh PC, Brook CG, Whitton AC, Bloom HJ, Tobias JS, Godlee JN, Britton J. Endocrine disorders following treatment of childhood brain tumours. Br J Cancer. 1990;61(4):622–625. doi: 10.1038/bjc.1990.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oberfield SE, Sklar CA. Endocrine sequelae in survivors of childhood cancer. Adolesc Med. 2002;13(1):161–169. viii. [PubMed] [Google Scholar]
- 77.Darzy KH, Shalet SM. Circadian and stimulated thyrotropin secretion in cranially irradiated adult cancer survivors. J Clin Endocrinol Metab. 2005;90(12):6490–6497. doi: 10.1210/jc.2005-1593. [DOI] [PubMed] [Google Scholar]
- 78.Rose SR, Lustig RH, Pitukcheewanont P, Broome DC, Burghen GA, Li H, Hudson MM, Kun LE, Heideman RL. Diagnosis of hidden central hypothyroidism in survivors of childhood cancer. J Clin Endocrinol Metab. 1999;84(12):4472–4479. doi: 10.1210/jcem.84.12.6097. [DOI] [PubMed] [Google Scholar]
- 79.Agha A, Walker D, Perry L, Drake WM, Chew SL, Jenkins PJ, Grossman AB, Monson JP. Unmasking of central hypothyroidism following growth hormone replacement in adult hypopituitary patients. Clin Endocrinol (Oxf) 2007;66(1):72–77. doi: 10.1111/j.1365-2265.2006.02688.x. [DOI] [PubMed] [Google Scholar]
- 80.Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, Lange M, Poulsen HS, Muller J. Assessment of the hypothalamo-pituitary-adrenal axis in patients treated with radiotherapy and chemotherapy for childhood brain tumor. J Clin Endocrinol Metab. 2003;88(7):3149–3154. doi: 10.1210/jc.2002-021994. [DOI] [PubMed] [Google Scholar]
- 81.Noorda EM, Somers R, van Leeuwen FE, Vulsma T, Behrendt H. Adult height and age at menarche in childhood cancer survivors. Eur J Cancer. 2001;37(5):605–612. doi: 10.1016/s0959-8049(00)00438-x. [DOI] [PubMed] [Google Scholar]
- 82.Adan L, Sainte-Rose C, Souberbielle JC, Zucker JM, Kalifa C, Brauner R. Adult height after growth hormone (GH) treatment for GH deficiency due to cranial irradiation. Med Pediatr Oncol. 2000;34(1):14–19. doi: 10.1002/(sici)1096-911x(200001)34:1<14::aid-mpo3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 83.Burns EC, Tanner JM, Preece MA, Cameron N. Growth hormone treatment in children with craniopharyngioma: final growth status. Clin Endocrinol (Oxf) 1981;14(6):587–595. doi: 10.1111/j.1365-2265.1981.tb02969.x. [DOI] [PubMed] [Google Scholar]
- 84.Kiltie AE, Lashford LS, Gattamaneni HR. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28(5):348–354. doi: 10.1002/(sici)1096-911x(199705)28:5<348::aid-mpo4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 85.Lerner SE, Huang GJ, McMahon D, Sklar CA, Oberfield SE. Growth hormone therapy in children after cranial/craniospinal radiation therapy: sexually dimorphic outcomes. J Clin Endocrinol Metab. 2004;89(12):6100–6104. doi: 10.1210/jc.2004-1515. [DOI] [PubMed] [Google Scholar]
- 86.Gleeson HK, Stoeter R, Ogilvy-Stuart AL, Gattamaneni HR, Brennan BM, Shalet SM. Improvements in final height over 25 years in growth hormone (GH)-deficient childhood survivors of brain tumors receiving GH replacement. J Clin Endocrinol Metab. 2003;88(8):3682–3689. doi: 10.1210/jc.2003-030366. [DOI] [PubMed] [Google Scholar]
- 87.Brauner R, Rappaport R, Prevot C, Czernichow P, Zucker JM, Bataini P, Lemerle J, Sarrazin D, Guyda HJ. A prospective study of the development of growth hormone deficiency in children given cranial irradiation, and its relation to statural growth. J Clin Endocrinol Metab. 1989;68(2):346–351. doi: 10.1210/jcem-68-2-346. [DOI] [PubMed] [Google Scholar]
- 88.Schriock EA, Schell MJ, Carter M, Hustu O, Ochs JJ. Abnormal growth patterns and adult short stature in 115 long-term survivors of childhood leukemia. J Clin Oncol. 1991;9(3):400–405. doi: 10.1200/JCO.1991.9.3.400. [DOI] [PubMed] [Google Scholar]
- 89.Probert JC, Parker BR, Kaplan HS. Growth retardation in children after megavoltage irradiation of the spine. Cancer. 1973;32(3):634–639. doi: 10.1002/1097-0142(197309)32:3<634::aid-cncr2820320316>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 90.Ogilvy-Stuart AL, Shalet SM. Growth hormone and puberty. J Endocrinol. 1992;135(3):405–406. doi: 10.1677/joe.0.1350405. [DOI] [PubMed] [Google Scholar]
- 91.Xu W, Janss A, Moshang T. Adult height and adult sitting height in childhood medulloblastoma survivors. J Clin Endocrinol Metab. 2003;88(10):4677–4681. doi: 10.1210/jc.2003-030619. [DOI] [PubMed] [Google Scholar]
- 92.Shalet SM, Gibson B, Swindell R, Pearson D. Effect of spinal irradiation on growth. Arch Dis Child. 1987;62:461–464. doi: 10.1136/adc.62.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Clayton PE, Shalet SM. The evolution of spinal growth after irradiation. Clin Oncol. 1991;3:220–222. doi: 10.1016/s0936-6555(05)80744-7. [DOI] [PubMed] [Google Scholar]
- 94.Chin HW, Maruyama Y. Age at treatment and long-term performance results in medulloblastoma. Cancer. 1984;53:1952–1958. doi: 10.1002/1097-0142(19840501)53:9<1952::aid-cncr2820530925>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 95.Dangour AD, Schilg S, Hulse JA, Cole TJ. Sitting height and subischial leg length centile curves for boys and girls from southeast England. Ann Hum Biol. 2002;29:290–305. doi: 10.1080/03014460110085331. [DOI] [PubMed] [Google Scholar]
- 96.Katz JR, Bareille P, Levitt G, Stanhope R. Growth hormone and segmental growth in survivors of head and neck embryonal rhabdomyosarcoma. Arch Dis Child. 2001;84:436–439. doi: 10.1136/adc.84.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sibler JH, Littman PS, Meadows AT. Stature loss following skeletal irradiation for childhood cancer. J Clin Oncol. 1990;8:304–312. doi: 10.1200/JCO.1990.8.2.304. [DOI] [PubMed] [Google Scholar]
- 98.Olshan JS, Gubernick J, Packer RJ, D’Angio GJ, Goldwein JW, Willi SM, Moshang T., Jr The effects of adjuvant chemotherapy on growth in children with medulloblastoma. Cancer. 1992;70(7):2013–2017. doi: 10.1002/1097-0142(19921001)70:7<2013::aid-cncr2820700734>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 99.Gleeson HK, Gattamaneni HR, Smethurst L, Brennan BM, Shalet SM. Reassessment of growth hormone status is required at final height in children treated with growth hormone replacement after radiation therapy. J Clin Endocrinol Metab. 2004;89(2):662–666. doi: 10.1210/jc.2003-031224. [DOI] [PubMed] [Google Scholar]
- 100.Nivot S, Benelli C, Clot JP, Saucet C, Adan I, Souberbielle JC, Zucker JM, Rappaport R, Brauner R. Nonparallel changes of growth hormone (GH) and insulin-like growth factor-I, insulin-like growth factor binding protein-3, and GH-binding protein, after craniospinal irradiation and chemotherapy. J Clin Endocrinol Metab. 1994;78(3):597–601. doi: 10.1210/jcem.78.3.7510304. [DOI] [PubMed] [Google Scholar]
- 101.Rose SR, Schreiber RE, Kearney NS, Lustig RH, Danish RK, Burghen GA, Hudson MM. Hypothalamic dysfunction after chemotherapy. J Pediatr Endocrinol Metab. 2004;17(1):55–66. doi: 10.1515/jpem.2004.17.1.55. [DOI] [PubMed] [Google Scholar]
- 102.Oberfield SE, Sklar C, Allen J, Walker R, Macelwain M, Papadakis V, Maenza J. Thyroid and gonadal function and growth of long-term survivors of medulloblastoma/PNET. In: Green DM, D’Angio GJ, editors. Late effects of treatment for childhood cancer. Wiley-Liss Inc; New York: 1992. [Google Scholar]
- 103.Darzy KH, Shalet SM. Circadian an stimulated thyrotropin secretion in cranially irradiated adult cancer survivors. J Clin Endocrinol Metab. 2005;90:6490–6497. doi: 10.1210/jc.2005-1593. [DOI] [PubMed] [Google Scholar]
- 104.Freidlaender GE, Tross RB, Doganis AC, Kirkwood JM. Effects of chemotherapeutic agents on bone. J Bone Joint Surg. 1984;66:602. [PubMed] [Google Scholar]
- 105.Wingard JR, Plotnick LP, Freemer CS, Zahurak M, Piantadosi S, Miller DF, Vriesendorp HM, Yeager AM, Santos GW. Growth in children after bone marrow transplantation: busulfan plus cyclophosphamide versus cyclophosphamide plus total body irradiation. Blood. 1992;79(4):1068–1073. [PubMed] [Google Scholar]
- 106.Rosen CJ. IGF-I and osteoporosis. Clin Lab Med. 2000;20(3):591–602. [PubMed] [Google Scholar]
- 107.Phillip M, Moran O, Lazar L. Growth without growth hormone. J Pediatr Endocrinol Metab. 2002;15 (suppl 5):1267–1272. [PubMed] [Google Scholar]
- 108.Darzy KH, Thorner MO, Shalet SM. Cranially irradiated adult cancer survivors may have normal spontaneous GH secretion in the presence of discordant peak GH responses to stimulation tests (compensated GH deficiency) Clin Endocrinol (Oxf) 2009;70(2):287–293. doi: 10.1111/j.1365-2265.2008.03359.x. [DOI] [PubMed] [Google Scholar]
- 109.Gandrud LM, Wilson DM. Is growth hormone stimulation testing in children still appropriate? Growth Horm IGF Res. 2004;14(3):185–194. doi: 10.1016/j.ghir.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 110.Tillmann V, Shalet SM, Price DA, Wales JK, Pennells L, Soden J, Gill MS, Whatmore AJ, Clayton PE. Serum insulin-like growth factor-I, IGF binding protein-3 and IGFBP-3 protease activity after cranial irradiation. Horm Res. 1998;50(2):71–77. doi: 10.1159/000023237. [DOI] [PubMed] [Google Scholar]
- 111.Cicognani A, Cacciari E, Pession A, Pasini A, De lasio R, Gennari M, Alvisi P, Pirazzoli P. Insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3) concentrations compared to stimulated growth hormone (GH) in the evaluation of children treated for malignancy. J Pediatr Endocrinol Metab. 1999;12(5):629–638. doi: 10.1515/jpem.1999.12.5.629. [DOI] [PubMed] [Google Scholar]
- 112.Rosenfeld RG, Gargosky SE. Assays for insulin-like growth factos and their binding proteins: practices and pitfalls. J Pediatr. 1996;128:S52–S57. doi: 10.1016/s0022-3476(96)70012-4. [DOI] [PubMed] [Google Scholar]
- 113.Lin Y, Jiang T, Zhou K, Xu L, Chen B, Li G, Qiu X, Jiang T, Zhang W, Song SW. Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neuro Oncol. 2009;11(5):468–476. doi: 10.1215/15228517-2008-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de Bont JM, van Doorn J, Reddingius RE, Graat GH, Passier MM, den Boer ML, Pieters R. Various components of the insulin-like growth factor system in tumor tissue, cerebrospinal fluid and peripheral blood of pediatric medulloblastoma and ependymoma patients. Int J Cancer. 2008;123(3):594–600. doi: 10.1002/ijc.23558. [DOI] [PubMed] [Google Scholar]
- 115.Shalet SM, Gibson B, Swindell R, Pearson D. Effect of spinal irradiation on growth. Arch Dis Child. 1987;62(5):461–464. doi: 10.1136/adc.62.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clayton PE, Shalet SM. The evolution of spinal growth after irradiation. Clin Oncol (R Coll Radiol) 1991;3(4):220–222. doi: 10.1016/s0936-6555(05)80744-7. [DOI] [PubMed] [Google Scholar]
- 117.Ranke MB, Price DA, Lindberg A, Wilton P, Darendeliler F, Reiter EO. Final height in children with medulloblastoma treated with growth hormone. Horm Res. 2005;64(1):28–34. doi: 10.1159/000087325. [DOI] [PubMed] [Google Scholar]
- 118.Ranke MB, Lindberg A, Martin DD, Bakker B, Wilton P, Albertsson-Wikland K, Cowell CT, Price DA, Reiter EO. The mathematical model for total pubertal growth in idiopathic growth hormone (GH) deficiency suggests a moderate role of GH dose. J Clin Endocrinol Metab. 2003;88(10):4748–4753. doi: 10.1210/jc.2003-030600. [DOI] [PubMed] [Google Scholar]
- 119.Shalet SM, Whitehead E, Chapman AJ, Beardwell CG. The effects of growth hormone therapy in children with radiation-induced growth hormone deficiency. Acta Paediatr Scand. 1981;70(1):81–86. doi: 10.1111/j.1651-2227.1981.tb07177.x. [DOI] [PubMed] [Google Scholar]
- 120.Orme SM, McNally RJ, Cartwright RA, Belchetz PE. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab. 1998;83(8):2730–2734. doi: 10.1210/jcem.83.8.5007. [DOI] [PubMed] [Google Scholar]
- 121.Watanabe S, Tsuenematsu Y, Fujimoto J, Komiyama A. Leukemia in patients treated with growth hormone. Lancet. 1988;1:1159. [Google Scholar]
- 122.Fradkin JE, Mills JL, Schonberger LB, Wysowski DK, Thomson R, Durako SJ, Robison LL. Risk of leukemia after treatment with pituitary growth hormone. Jama. 1993;270(23):2829–2832. [PubMed] [Google Scholar]
- 123.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21(3):215–244. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 124.Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36(10):1224–1228. doi: 10.1016/s0959-8049(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 125.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 126.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91 (7):620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 127.Grimberg A. Cancer. Adv Exp Med Biol. 2005;567:305–339. doi: 10.1007/0-387-26274-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253:1–6. doi: 10.1006/excr.1999.4667. [DOI] [PubMed] [Google Scholar]
- 129.Grimberg A. Mechanisms by which IGF-I may promote cancer. Cancer BiolTher. 2003;2(6):630–635. [PMC free article] [PubMed] [Google Scholar]
- 130.Petridou E, Dessypris N, Spanos E, Mantzoros C, Skalkidou A, Kalmanti M, Koliouskas D, Kosmidis H, Panagiotou JP, Piperopoulou F, Tzortzatou F, Trichopoulos D. Insulin-like growth factor-I and binding protein-3 in relation to childhood leukaemia. Int J Cancer. 1999;80(4):494–496. doi: 10.1002/(sici)1097-0215(19990209)80:4<494::aid-ijc2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 131.Cohen P, Clemmons DR, Rosenfeld RG. Does the GH-IGF axis play a role in cancer pathogenesis? Growth Horm IGF Res. 2000;10(6):297–305. doi: 10.1054/ghir.2000.0171. [DOI] [PubMed] [Google Scholar]
- 132.Pollak M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. 2008;22(4):625–638. doi: 10.1016/j.beem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 133.Abs R, Bengtsson BA, Hernberg-Stahl E, Monson JP, Tauber JP, Wilton P, Wuster C. GH replacement in 1034 growth hormone deficient hypopituitary adults: demographic and clinical characteristics, dosing and safety. Clin Endocrinol (Oxf) 1999;50(6):703–713. doi: 10.1046/j.1365-2265.1999.00695.x. [DOI] [PubMed] [Google Scholar]
- 134.Banerjee I, Clayton PE. Growth hormone treatment and cancer risk. Endocrinol Metab Clin North Am. 2007;36(1):247–263. doi: 10.1016/j.ecl.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 135.Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167–177. doi: 10.1210/jc.2009-0178. [DOI] [PubMed] [Google Scholar]
- 136.Ogilvy-Stuart AL, Ryder WD, Gattamaneni HR, Clayton PE, Shalet SM. Growth hormone and tumour recurrence. Bmj. 1992;304(6842):1601–1605. doi: 10.1136/bmj.304.6842.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nishi Y, Tanaka T, Takano K, Fujieda K, Igarashi Y, Hanew K, Hirano T, Yokoya S, Tachibana K, Saito T, Watanabe S. Recent status in the occurrence of leukemia in growth hormone-treated patients in Japan. GH Treatment Study Committee of the Foundation for Growth Science, Japan. J Clin Endocrinol Metab. 1999;84(6):1961–1965. doi: 10.1210/jcem.84.6.5716. [DOI] [PubMed] [Google Scholar]
- 138.Arslanian SA, Becker DJ, Lee PA, Drash AL, Foley TP., Jr Growth hormone therapy and tumor recurrence. Findings in children with brain neoplasms and hypopituitarism. Am J Dis Child. 1985;139(4):347–350. doi: 10.1001/archpedi.1985.02140060029020. [DOI] [PubMed] [Google Scholar]
- 139.Moshang T, Jr, Rundle AC, Graves DA, Nickas J, Johanson A, Meadows A. Brain tumor recurrence in children treated with growth hormone: the National Cooperative Growth Study experience. J Pediatr. 1996;128(5 Pt 2):S4–S7. doi: 10.1016/s0022-3476(96)70002-1. [DOI] [PubMed] [Google Scholar]
- 140.Swerdlow AJ, Reddingius RE, Higgins CD, Spoudeas HA, Phipps K, Qiao Z, Ryder WD, Brada M, Hayward RD, Brook CG, Hindmarsh PC, Shalet SM. Growth hormone treatment of children with brain tumors and risk of tumor recurrence. J Clin Endocrinol Metab. 2000;85(12):4444–4449. doi: 10.1210/jcem.85.12.7044. [DOI] [PubMed] [Google Scholar]
- 141.Clayton PE, Shalet SM, Gattamaneni HR, Price DA. Does growth hormone cause relapse of brain tumours? Lancet. 1987;1(8535):711–713. doi: 10.1016/s0140-6736(87)90355-2. [DOI] [PubMed] [Google Scholar]
- 142.Packer RJ, Boyett JM, Janss AJ, Stavrou T, Kun L, Wisoff J, Russo C, Geyer R, Phillips P, Kieran M, Greenberg M, Goldman S, Hyder D, Heideman R, Jones-Wallace D, August GP, Smith SH, Moshang T. Growth hormone replacement therapy in children with medulloblastoma: use and effect on tumor control. J Clin Oncol. 2001;19(2):480–487. doi: 10.1200/JCO.2001.19.2.480. [DOI] [PubMed] [Google Scholar]
- 143.Sklar CA, Mertens AC, Mitby P, Occhiogrosso G, Qin J, Heller G, Yasui Y, Robison LL. Risk of disease recurrence and second neoplasms in survivors of childhood cancer treated with growth hormone: a report from the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2002;87(7):31363–41. doi: 10.1210/jcem.87.7.8606. [DOI] [PubMed] [Google Scholar]
- 144.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93(8):618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 145.Tucker MA, D’Angio GJ, Boice JD, Jr, Strong LC, Li FP, Stovall M, Stone BJ, Green DM, Lombardi F, Newton W, Hoover RN, Fraumeni JF, Jr The Late Effects Study Group. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317:588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 146.Meadows AT, Baum E, Fossati-Bellani F, Green D, Jenkin RDT, Marsden B, Nesbit M, Newton W, Oberlin O, Sallan SG, Siegel S, Strong LC, Voute PA. Second malignant neoplasms in children: an update from The Late Effects Study Group. J Clin Oncol. 1985;3:532–538. doi: 10.1200/JCO.1985.3.4.532. [DOI] [PubMed] [Google Scholar]
- 147.Meadows AT, Silber J. Delayed consequences of therapy for childhood cancer. CA Cancer J Clin. 1985;35(5):271–286. doi: 10.3322/canjclin.35.5.271. [DOI] [PubMed] [Google Scholar]
- 148.Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows AT. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334(12):745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 149.Socie G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, Sullivan KM, Rowlings PA, Kingma DW, Banks PM, Travis WD, Witherspoon RP, Sanders J, Jaffe ES, Horowitz MM. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18(2):348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 150.Ergun-Longmire B, Mertens AC, Mitby P, Qin J, Heller G, Shi W, Yasui Y, Robison LL, Sklar CA. Growth hormone treatment and risk of second neoplasms in the childhood cancer survivor. J Clin Endocrinol Metab. 2006;91(9):3494–3498. doi: 10.1210/jc.2006-0656. [DOI] [PubMed] [Google Scholar]
- 151.Mann I, Yates PC, Ainslie JP. Unusual case of double primary orbital tumour. Br J Ophthalmol. 1953;37(12):758–762. doi: 10.1136/bjo.37.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pollak L, Walach N, Gur R, Schiffer J. Meningiomas after radiotherapy for tinea capitis - still no history. Tumori. 1998;84(1):65–68. doi: 10.1177/030089169808400114. [DOI] [PubMed] [Google Scholar]
- 153.Kamiguchi H, Shiobara R, Toya S. Accidentally detected brain tumors: clinical analysis of a series of 110 patients. Clin Neurol Neurosurg. 1996;98(2):171–175. doi: 10.1016/0303-8467(96)00016-9. [DOI] [PubMed] [Google Scholar]
- 154.Kuratsu J, Kochi M, Ushio Y. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. 2000;92(5):766–770. doi: 10.3171/jns.2000.92.5.0766. [DOI] [PubMed] [Google Scholar]
- 155.Hoffman KE, Yock TI. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24(11):1387–1396. doi: 10.1177/0883073809342275. [DOI] [PubMed] [Google Scholar]