Abstract
Developmental plasticity underlies widespread associations between early-life exposures and many components of adult phenotype, including the risk of chronic diseases. Humans take almost two decades to reach reproductive maturity, and yet the ‘critical windows’ of physiological sensitivity that confer developmental plasticity tend to close during fetal life or infancy. While several explanations for lengthy human maturation have been offered, the brevity of physiological plasticity has received less attention. I argue that offspring plasticity is only viable within the niche of maternal care, and that as this protection is withdrawn, the offspring is obliged to canalize many developmental traits in order to minimize environmental disruptions. The schedule of maternal care may therefore shape the duration of critical windows, and since the duration of this care is subject to parent–offspring conflict, the resolution of this conflict may shape the duration of critical windows. This perspective may help understand (i) why windows close at different times for different traits, and (ii) why the duration of critical windows may vary across human populations. The issue is explored in relation to population differences in the association between infant weight gain and later body composition. The occupation of more stable environments by western populations may have favoured earlier closure of the critical window during which growth in lean mass is sensitive to nutritional intake. This may paradoxically have elevated the risk of obesity following rapid infant weight gain in such populations.
Keywords: developmental plasticity, adaptation, critical window, parent–offspring conflict, growth, obesity
INTRODUCTION
Developmental plasticity is well established to play a central role in the aetiology of chronic degenerative diseases, an association now recognized through the ‘developmental origins of health and disease’ (DOHaD) hypothesis [1–5]. In this way, chronic disease risk is understood to be shaped by ecological stresses acting early in development, though adult lifestyle also remains important [6].
The paradox is that despite such implications for long-term disease risk, developmental plasticity is widely considered an adaptive process, often assumed to fine-tune the fit of the organism to its environment. For example, the ‘adaptive calibration’ model assumes that life history strategy is continually shaped by information relating to threats and opportunities during development [7, 8]. A key question therefore is how such an adaptive process can be central to the emergence of ill health.
For chronic diseases, the role of growth patterns in predicting outcome indicates an important developmental role for nutrition in early life. For placental mammals, fetal nutrition is obtained directly from maternal physiology, while postnatal nutrition commences with lactation. As with psychosocial stresses, it is generally accepted that the nutritional supply of the fetus and infant contains ‘ecological information’, and that associations of nutrition and developmental trajectory must in some or other way represent ‘information processing’ [9, 10]. It is more controversial as to what this information comprises, and how the offspring might be able to use it adaptively. In this article, I will focus on growth traits but the same concepts may apply to many other components of development.
The association of developmental plasticity with long-term disease risk can be attributed to the fact that plasticity is of finite duration. Classic studies on rats showed that the effect of undernutrition on growth is different according to when it occurs during development. Exposing rats to undernutrition in early infancy caused a permanent reduction in body size, whereas the same insult later in development only temporarily slowed growth [11, 12]. This work led to the concept of finite ‘critical windows’ of physiological sensitivity, during which many traits, and developmental trajectory in general, are influenced by environmental factors [13–15].
The same scenario applies to humans: for example, by the age of 2 years, height has little sensitivity to ecological conditions, and tends to track within individuals, a process known as canalization [16, 17]. In public health practice, the recognition of finite windows of plasticity has led to emphasis on the first 1000 days from conception [18], which in turn places great importance on information processing during fetal life and infancy. To the extent that the offspring ‘adapts’ its growth trajectory in early life, such adaptation is relatively irreversible.
The importance of the first 1000 days generates a key question: why is the primary period of human developmental plasticity so short, when the total duration of growth (18 years, or ∼6800 days from conception) is so long? Several evolutionary explanations have been offered for the lengthy duration of human maturation, and most of them assume that there are energetic benefits of reducing the rate of growth, thereby distributing its costs over a longer time period. For example, slowed growth has been assumed to allow increased investment in brain growth, and also to reduce the costs of maternal reproduction [19]. Less attention has been directed to the brief duration of critical windows of plasticity, but this also requires an evolutionary explanation.
PLASTICITY AND INFORMATION PROCESSING
An adaptive perspective considers the offspring to benefit from information by tailoring its life history strategy to local ecological conditions. The ability for life history strategy to respond to ecological signals is well established [20] and is undisputed, but this issue becomes complex once we take into account the origins of these signals in early life.
A crucial issue is that among placental mammals, the fetus does not receive information directly from the external environment, rather it is exposed to maternal phenotype [10]. It is now recognized that maternal physiology is capable of smoothing over short-term signals, such as peaks or troughs in energy availability [10, 21–23]. This smoothing can buffer against ecological stresses such as famine or drought during pregnancy, a process that undeniably benefits the vulnerable offspring, as demonstrated by the restricted falls in birth weight during famines [24, 25].
This maternal smoothing process thus brings the life history strategies of two generations into contact [10]. For over four decades, evolutionary biologists have considered maternal care through the lens of parent–offspring conflict theory, which assumes that nutrients and energy are scarce resources, and that the mother and each offspring are characterized by a conflict of interest regarding how much of her nutritional resources the mother should transfer to the offspring during the period of parental care [26, 27]. This conceptual approach has been applied to human developmental plasticity [10, 23, 28], but further elucidation is merited.
Beyond the physical resources themselves, it should not automatically be assumed that the offspring gains 100% of the pay-offs from the information it receives from its mother [10]. The information that shapes adaptation in growth trajectory can be considered as ‘signals’ transmitted from maternal metabolism to that of the offspring. As discussed by Haig [29], signals that generate no impact on the receiver could be said to contain no information; if they do generate an impact, then that impact derives ultimately from maternal life history strategy. Given such parent–offspring conflict, the transmission of signals through placental nutrition and lactation means that in the process of accessing information, the offspring is also open to maternal manipulation [10, 23].
To date, the majority of attention has been directed to the ‘quantity’ of resources that is transferred during the window of parental care. For example, an early analysis supported the concept of parent–offspring conflict theory, by showing that the most common birth weight is lower than the value that minimizes infant mortality [30]. This suggests that the average mother invests less in birth weight than would be optimal for the offspring. Maternal fitness-maximizing strategy is further evident in investment differences between sons and daughters, a scenario first proposed by Trivers and Willard [31]. In humans, the energy cost of pregnancy is higher for sons than for daughters [32, 33], while studies suggest that either the energy content or volume of milk transferred to male offspring may also be greater than that for females [34, 35]. However, these gender differences require confirmation, and studies of other species show heterogeneity of gender differences in milk energy transfer [36–38], suggesting more than one model of differential investment by gender, or lack of it, should be considered [36].
Beyond the magnitude of investment, maternal strategy is also evident in the ‘timing’ of investment decisions, such as the duration of pregnancy, or the initiation of weaning. These may likewise be considered subject to a conflict of interest: for example, continued gestation or breast-feeding of the current offspring reduces the ability of the mother to invest in the next offspring [39, 40]. Conflict over the timing of life history ‘decisions’ may be addressed at the level of behavior, e.g. conflict over weaning has been explored in the context of infant crying, or more recently night-time suckling [40, 41]. The consequences of how the conflict is resolved can then be considered in physical terms: e.g., the magnitude of growth attained by the offspring, versus the redirection of maternal nutritional reserves to the next offspring [41].
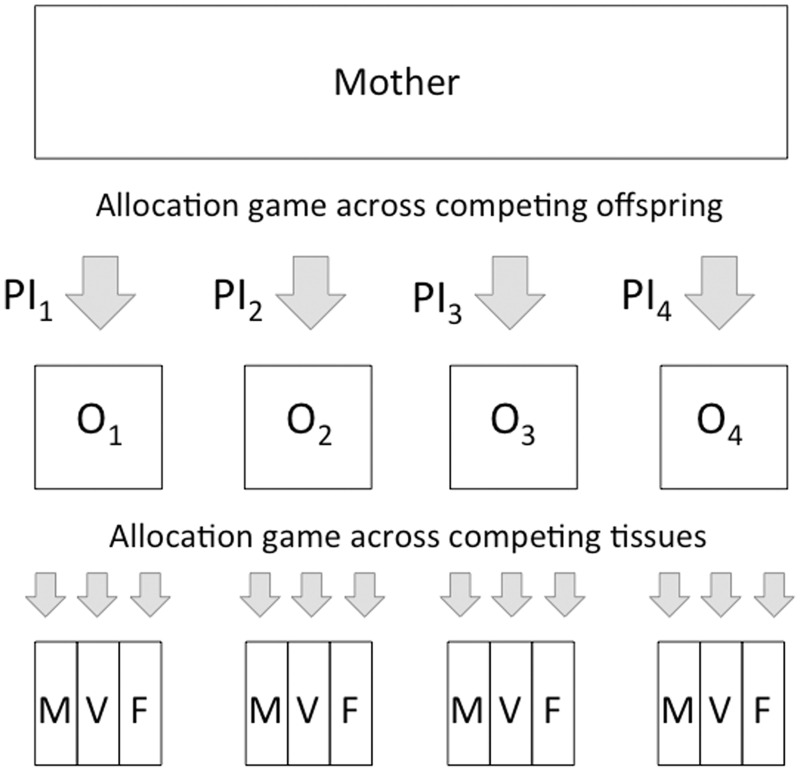
Seen through this lens, developmental plasticity cannot be seen simply as a process of the offspring fine-tuning its developmental trajectory to match prevailing ecological conditions; a counter-view is that the mother shapes the growth trajectory of each offspring according to the partitioning of investment that will maximize her own fitness [10]. We can think of two related ‘optimization games’ being solved: the mother must allocate her sum resources for reproduction across multiple offspring, while each offspring must allocate the resources it receives across competing traits (Fig. 1). At a superficial level, these traits may manifest as physical components of growth such as brain size versus energy reserves, but ultimately the offspring is allocating energy between competing life history functions—maintenance, growth, reproductive capacity and immune function. Both mother and offspring gain inclusive fitness benefits from the mother producing multiple offspring, but the optimal point at which the mother ceases to invest in one offspring and begins to invest in another is not equal for mother and offspring [42]. Thus, maternal and offspring strategies are characterized not by outright conflict, but by a ‘conflict of interest’ over the optimal pattern of maternal investment. It is also important to consider the paternal contribution to this conflict: while successive offspring of a given mother are equally genetically related to her, they may be conceived by different fathers. Variability in paternity across a mother’s offspring has the effect of increasing the conflict of interest between paternal genes expressed in the offspring and maternal genes expressed in the mother [43].
Figure 1.
Schematic diagram illustrating two different ‘allocation games’ in which each of the mother and the offspring optimize their inclusive fitness. In the first game, the mother optimizes her allocation of parental investment (PI) across competing offspring (O1–O4). In the second game, which is sensitive to the first game, each offspring optimizes its allocation of that investment between competing phenotypic traits, such as muscle mass (M), vital organ mass (V) and energy stores in fat (F). These traits also represent allocations across life history functions (maintenance, growth, reproduction and immune function)
Previous work has therefore addressed how parent–offspring conflict characterizes both the magnitude of resources transferred from mother to offspring, and the schedule of this investment. Information transfer occurs within the same context. However, there are also further implications, regarding how long ‘critical windows’ of physiological sensitivity should remain open, and this translates into variability in the ‘source’ of information received by the offspring. To address this issue, let us first consider in more detail how windows of plasticity are linked with maternal care in the first place.
MATERNAL BUFFERING MATCHES OFFSPRING CRITICAL WINDOWS
I have argued previously that the nesting of windows of plasticity within the period of maternal care is no coincidence [10]. This is because early growth comprises the process of hyperplasia, during which the cells increase in number and thereby shape the structure and long-term function of organs [44]. Environmental factors acting on this period therefore generate long-term effects on vital components of organ phenotype, whereas factors acting later in development affect only the hypertrophic component of growth, and therefore affect size rather than structure.
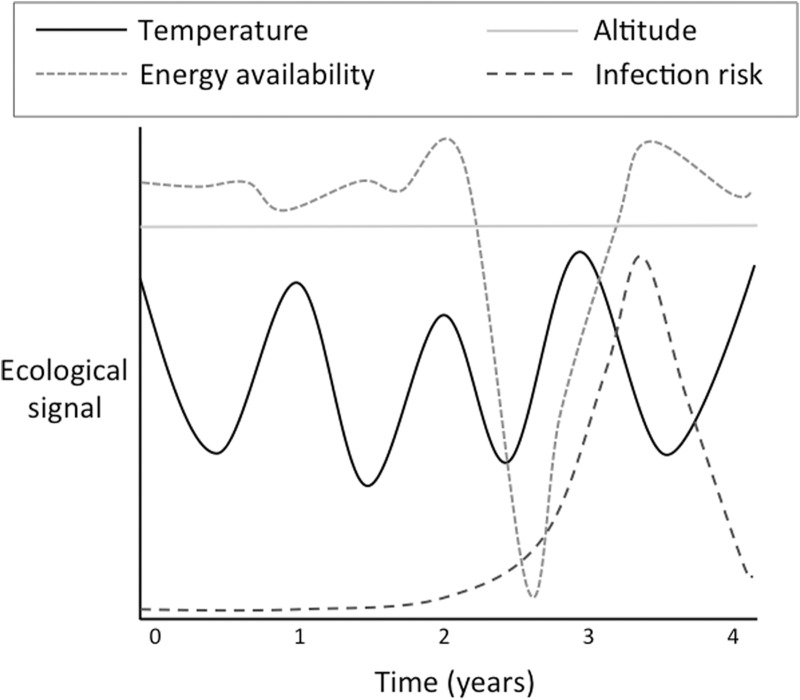
It might appear that the offspring would benefit from tailoring its metabolism, tissues and organs directly to prevailing conditions, but such a strategy is extremely challenging because a wide variety of signals is emitted by the environment. These signals include temperature, altitude, local infectious disease burden, energy availability, dietary macronutrient and micronutrient composition, and the psychosocial environment. There may also be transient but powerful stochastic stresses such as floods and droughts (such as those arising from climate cycles such as ‘El Niño’ Southern Oscillation cycles) and volcanic eruptions [45]. Importantly, each of these ecological signals may vary independently in its magnitude and periodicity (Fig. 2). For example, high altitude may represent a relatively consistent stress of hypoxia; temperature may represent a stress with a relatively consistent pattern of seasonal variability; rainfall may be prone to occasional extremes; and energy availability, infectious disease burden and the psychosocial environment may show irregular and random perturbations.
Figure 2.
Schematic diagram illustrating contrasting levels of stochasticity in four different ecological parameters. Some parameters such as altitude are relatively stable, others such as temperature may change relatively slowly, whereas others such as infection risk may alter suddenly
Sampling such a diverse range of signals represents a major challenge for the offspring, as it has not had sufficient time to develop sophisticated physiological sensing mechanisms capable of processing such multiple cues; and yet the period of hyperplasic growth is also the very period when it is most in need of metabolic stability. This dilemma can be resolved by sampling maternal phenotype instead of the external environment, enabling a coherent phenotype to be achieved [10, 22]. Maternal phenotype provides exactly the composite integrated signal of ecological quality that the developing offspring requires. This signal persists through pregnancy, and to a lesser extent through early lactation, when maternal phenotype remains the dominant influence on offspring nutrition, though not the only one (e.g. allo-mothering allows different women to breast-feed a given infant [46, 47]).
Since hyperplasic growth is so sensitive to environmental stresses, we can also assume, in the reverse direction, that maternal care has evolved to buffer the offspring during this period of development. This is achieved through powerful homeostatic physiological mechanisms. The duration of pregnancy is adequate to ensure that the amount of brain growth required for postnatal behavior is completed in utero [48], illustrated by increased risks of impaired cognitive function in offspring born preterm [49], hence the physiologies of maternal pregnancy and fetal development are closely aligned.
Since the mother is directly exposed to diverse ecological signals, what the offspring adapts to is a combination of maternal homeostatic systems that transduce ecological stresses, and elements of maternal phenotype that were shaped during her own development [10, 23, 50]. For example, the effect of hypoxia from high altitude on the developing offspring depends on the nature of maternal accommodation of this stress. A fetus exposed to an ‘adapted mother’ will experience a different in utero environment compared to a fetus exposed to an ‘maladapted mother’. The same concept is clearly illustrated by studies of the successive offspring of mothers who at some point develop type 2 diabetes. Offspring gestated after the onset of perturbed maternal metabolism show phenotypic differences to those gestated beforehand, with higher BMI and an elevated risk of themselves developing diabetes [51]. No such elevated risk is evident in relation to the timing of onset of paternal diabetes. Similar findings have emerged from studies of surgical intervention on maternal obesity, where the odds of producing a macrosomic infant decreased postsurgery, while those of producing a small-for gestational age infant increased [52]. Whatever the state of the external environment, it is the ‘mother’s metabolic adaptation to that environment’ that shapes the signals received by the offspring.
What then is the offspring exposed to, and what is it not exposed to? Short-term stresses are transduced and buffered (substantially rather than completely), so that maternal signals are strongly imprinted by ‘maternal capital’, which refers to more stable traits such as uterine volume and energy reserves. Maternal infection impacts the offspring in part by depleting maternal capital [53]. Long-term stresses such as hypoxia or heat/cold stresses are likewise transduced by maternal phenotype.
Many components of maternal metabolism are shaped during the mother’s own development, indicating grand–maternal effects [23, 54]. For example, studies of rodents show that epigenetic effects of maternal diet in the F0 generation may affect not only F1 offspring but also F2 grand–offspring, indicating epigenetic alterations in the germline [55]. In humans, the aggregation of type 2 diabetes in the matriline relative to the patriline is consistent with such a mechanism, although the transmission of mitochondrial DNA would be an alternative possible mechanism [56]. The ooctyes of F1 females are already present in fetal life and are therefore directly exposed to F0 metabolism during pregnancy [57], potentially allowing induced changes in mitochondrial DNA in F1 ooctyes to be transferred through cytoplasm to the F2 generation. In mice, for example maternal insulin resistance has been shown to disrupt oocyte mitochondrial function [58]. Further work is required to elucidate these mechanisms, but it is already clear that offspring accumulate exposure to stable components not only of maternal phenotype, but also longer-term matrilineal influence [10, 22, 23]. It is also becoming clear that through imprinting of the sperm, the offspring is exposed to factors that affected paternal development [59, 60], with paternal food supply during adolescence associated with mortality risk in the offspring [61, 62].
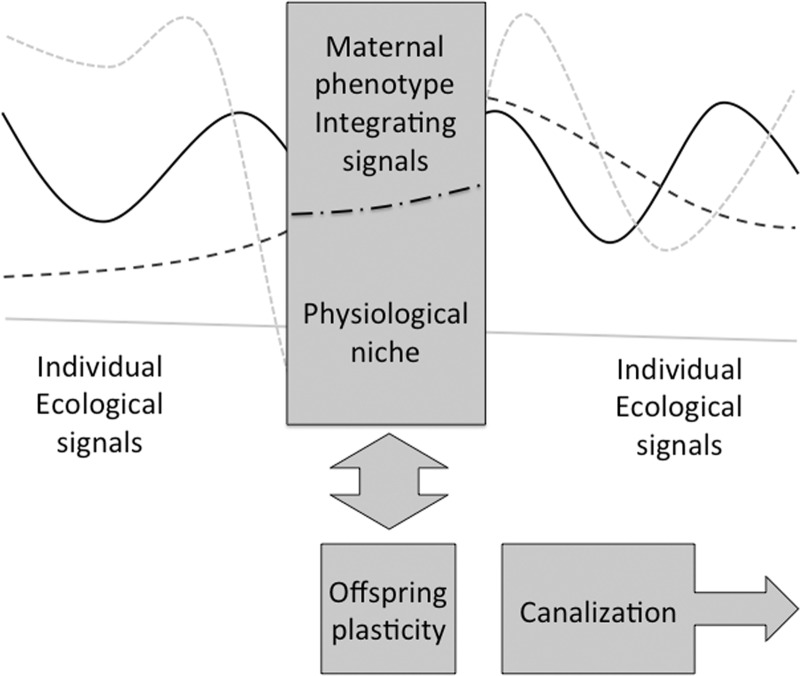
The exposure of the offspring to maternal phenotype is sharply enhanced at the start of pregnancy, and hence shows sensitivity to maternal diet and nutritional status around the time of conception [63, 64]. Once pregnancy is established, the offspring is exposed to stable components of maternal metabolism. Figure 3 shows how, by responding to signals transmitted by maternal phenotype during pregnancy and lactation, the offspring capitalizes on the mother’s multiple mature homeostatic processes that buffer diverse ecological stresses.
Figure 3.
Schematic diagram illustrating how the offspring can resolve the complexity of multiple ecological cues by sampling stable components of maternal metabolism, relying on maternal homeostatic systems to smooth over ecological disruptions. This means that when the offspring is weaned, it must either address all the ecological stochasticity, or develop its own buffering, which it can achieve through closing plasticity and canalizing traits
When the mother ceases to provide this stable developmental niche, however, the offspring will be exposed to whatever level of external ecological volatility is present. In order to resist such destabilizing influences, I suggested that the offspring shuts down information processing, and that this accounts for the canalization of many traits around the time of weaning [10].
Support for this model is given by a comparison of traits that are sensitive to maternal physiology during pregnancy and lactation, and those that are sensitive only during pregnancy. For example, offspring hemodynamics are in direct contact with maternal hemodynamics during the period of placental nutrition, however this link is lost at the time of birth. This may explain why the number of nephrons, related to blood pressure regulation, is ‘fixed’ at birth, and cannot change in response to nutritional signals in postnatal life [65]. In contrast, the offspring remains exposed to cues of energy availability through lactation, and the period during which nutritional cues shape development of the pancreas therefore extends into infancy [66, 67]. Infants exposed to poor maternal homeostasis, for example mothers with diabetes, develop long-term metabolic perturbations. This has been attributed to excess levels of sugar in the breast milk [68, 69].
This perspective provides a theoretical explanation for the variable duration of critical windows in physiology: they should persist during the umbrella of maternal care, and then close as this care is withdrawn [10]. A key question therefore is whether different levels of maternal care might be favoured in different ecological settings, and if so, whether this might further affect the duration of offspring plasticity. For example, in systematically poorer-quality environments mothers can be assumed to have fewer resources available for investment, necessitating increased thriftiness in the offspring [23]. This is demonstrated by shifts in the ratio of fat to lean tissue in low-birth weight Indian neonates [70]. However, environments also vary in their stability as well as their quality, so a related issue is whether variability in the stability of ecological conditions should impact the duration of critical windows.
Both mother and offspring share an interest in protecting the offspring during its most vulnerable stage of development, to ensure it develops a viable phenotype. On the basis of maximizing inclusive fitness [71, 72], both parties also benefit from the mother eventually switching investment to the next offspring. Three key points can now be made regarding this switch.
Since the offspring must canalize its phenotype when maternal investment ceases, the maternal strategy for making this switch should determine the duration of the critical window.
The optimal timing of this switch is predicted to vary between the two parties, and parent–offspring conflict during infancy is expected to be stronger than during pregnancy, as lactation is more costly to the mother in energetic terms than gestation [73].
The quality of the environment may affect the optimal schedule (for both mother and offspring, but potentially in different ways) regarding the switching of investment to the next offspring.
Bearing in mind these three points, we can now reconsider the conflict of interest between mother and offspring over the duration of maternal care, and its impact on offspring plasticity. Haig previously developed a model of the optimal duration of pregnancy, illustrating how the offspring favours a longer gestation than the mother, because, sharing only 50% of its genes with its mother, it discounts some of the fitness costs that are born by the mother if she extends the current pregnancy [39]. Haig [39] also noted that the shape of the fitness function would potentially influence the magnitude of the conflict of interest.
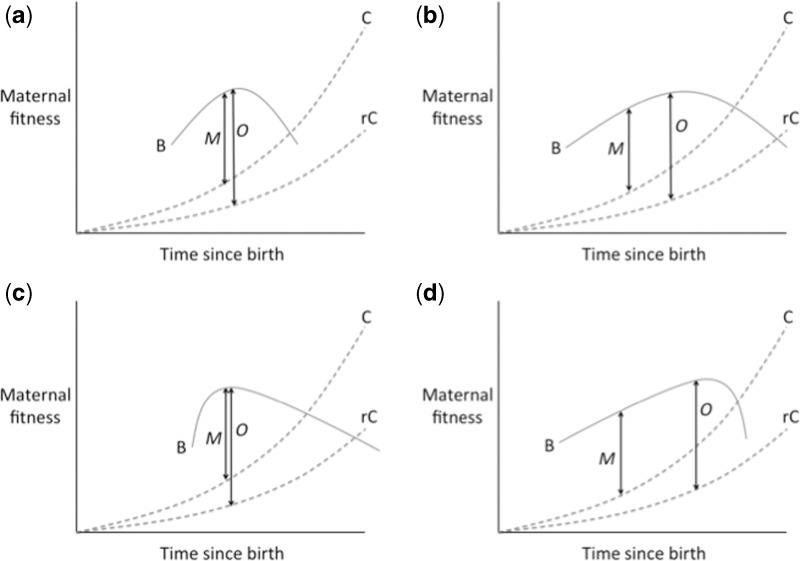
We can apply this approach to the optimal duration of maternal care during infancy. Figure 4 shows four different fitness functions, and their influence on the magnitude of the conflict of interest. If the fitness function has a sharp peak (Fig. 4a), then this forces the maternal and offspring optima to converge, thereby reducing the conflict of interest. Flattening the peak (Fig. 4b) allows the optimum of the offspring to diverge from that of the mother; this then predicts an increased duration of conflict between the two parties.
Figure 4.
Schematic diagram illustrating how the mother and the offspring have different optima for the duration of maternal care, according to the maternal fitness function (B), based on Haig’s model of optimal pregnancy duration [39]. The cost of continuing investment in the current offspring is given by C for the mother, but by rC for the offspring, which discounts some of these costs as it shares only 50% of its genes with its mother. Maternal fitness (M) is maximized by B–C, while offspring fitness (O) is maximized by B–rC. According to ecological conditions, the entire fitness function B may be shifted earlier or later, or the shape of the function may change, as shown in scenarios (a)–(d) and discussed in the article text
However, these scenarios assume that the peak is relatively symmetrical, whereas it is also possible for the peak to show a skewed shape. In Fig. 4c, the peak is right-skewed, indicating that it is very costly to maternal fitness if investment is withdrawn too early, but not if it is withdrawn much later. This scenario may occur when the offspring is very dependent on a maternal resource, or on buffering, up to a certain stage of development. In Fig. 4d, the reverse is shown. This scenario may occur if maternal investment or protection suddenly becomes of little value to the offspring, so that the benefits of switching to the next offspring rise rapidly.
This conceptual approach highlights how ecological conditions may affect the optimal duration of maternal care for each of the two parties, and hence influence the conflict of interest between them. Resolving this conflict in accordance with local ecological conditions may then shift the duration of the critical window backwards or forwards in terms of the stage of offspring development. What evidence is there that such variable duration of critical windows does exist?
EVOLUTIONARY TRENDS
At the broadest level, evolutionary trends in maternal care may have reshaped the profile of human developmental plasticity. While the total duration of growth lengthened in comparison with other apes during human evolution, the duration of pregnancy is very similar across these species, while the duration of lactation may have shortened in response to several stresses (Table 1) [48, 74]. The duration of critical windows may not map neatly onto the complete period before weaning, but these data do indicate a compression of the duration of maternal care in humans relative to other apes, complementary to the overall elongation of development.
Table 1.
Contributions of the components of maternal care (gestation and lactation) to total duration of development in apesa
| Species | Gestation (years) | Weaning age (years) | Total period of care (years) | Age at first birth (years) | Gestation/ development (%) | Total care/ development (%) |
|---|---|---|---|---|---|---|
| Orangutan | 0.71 | 7.0 | 7.7 | 15.6 | 4.4 | 47.3 |
| Gorilla | 0.70 | 2.8 | 3.5 | 10 | 6.5 | 32.7 |
| Chimpanzee | 0.62 | 4.5 | 5.1 | 13.3 | 4.5 | 38.6 |
| Bonobo | 0.65 | 4.5 | 5.1 | 13 | 4.8 | 37.7 |
| Humans | 0.73 | 2.8 | 3.5 | 19.5 | 3.6 | 17.4 |
aDuration of development taken as the sum of age at first birth and gestation. Total period of care taken as the sum of gestation and weaning age.
Several explanations have been proposed for reductions in the duration of lactation in the genus Homo, including the evolution of larger body size, and the emergence of cooperative breeding, each of which altered the fundamental energetics of reproduction [75, 76]. Within more recent human evolution, the emergence of agriculture may have further reduced lactation and shortened the average birth interval [77–80]. As discussed below, this issue could potentially be investigated by studying contemporary human populations with varying patterns of maternal care. Before considering this, let us consider what aspects of physiology might show such effects.
VARIABLE CRITICAL WINDOWS
One candidate for the variable duration of critical windows may be the tissues targeted by growth and nutrition during early infancy. Studies in multiple populations have shown relatively consistently that birth weight is strongly associated with later height and lean mass, whereas associations of birth weight with later adiposity are inconsistent, and appear dependent on patterns of postnatal growth [81]. The implications of this research are that the primary long-term target of fetal growth is lean mass, though there is also some indication that this is more the case for males than females [82–84]. This indicates that the two sexes have different optimal investment strategies, reflecting contrasting associations of fat and lean tissue with fitness in adulthood [85, 86].
In infancy, however, studies have shown contrasting results from industrialized populations and those from developing countries [81]. In industrialized populations, faster infant weight gain has been associated with later adiposity, and hence with obesity risk [87–93], whereas in developing countries, infant weight gain shows little or no association with later adiposity, and instead is associated with later lean mass [83, 84, 94–96]. In Brazilian children, for example an association between weight gain and subsequent fat mass was not evident in relation to birth weight or infant weight gain from 0 to 6 or 6 to 12 months, and only emerged from 1 year of age [94].
These contrasting findings suggest that the duration of plasticity during which lean mass is sensitive to cues of energy supply may vary across populations. Human growth has been characterized using an ‘Infancy, Childhood, Puberty’ model, which considers that there are successive stages, that do not however have clearly demarked starts and ends, but rather merge one with the next [97]. During the infant stage, growth has high sensitivity to the hormone insulin, and hence to nutritional intakes, whereas during the childhood period, this nutritional influence declines and growth becomes regulated by the growth hormone (GH)—IGF1 axis, which effectively canalizes growth. Variability in the transition between the infant and childhood stages might either reflect selection of different genotypes, or it might reflect regulation of hormonal axes on the basis of cues during development [98, 99]. Either way, ecological conditions would thereby shape the transition from one stage of growth to the next, and hence shape the schedule over which growth becomes canalized. In other words, this would introduce population variability into the duration of critical windows during which growth patterns are sensitive to nutrition.
Such a mechanism could potentially explain the population variability in the allocation of energy to lean versus fat tissue during early life, and long-term associations between early growth and later body composition. Hochberg and Albertsson-Wikland [98] speculated that a delayed transition to the childhood period of growth would be favoured by ecological stresses such as infectious disease and energy scarcity. While in poor environments this may render infants from such populations prone to long-term stunting, as reduced growth during infancy as well as fetal life would permanently reduce adult height, the counterpart is that faster weight gain during these periods would also benefit height and lean mass, as the data described above have shown. In contrast, infants from industrialized populations may have evolved an earlier transition to GH-regulated growth, as the overall lower burden of infectious disease may have favoured earlier weaning decisions by mothers, and hence earlier closure of the critical window comprising the infant period of growth. Such a strategy would then prevent increased infant energy supply from being directed to greater height and lean mass, and would instead promote excess fat accumulation. In other words, reshaping of the ‘programming of lean mass’ may exert influence on the ‘programming of adiposity’. In industrialized populations, increases in energy supply during childhood are associated with faster growth and greater weight and fatness, but not with greater final size [100].
OPPORTUNITIES TO TEST THE HYPOTHESIS
Experiments in non-human animals represent one opportunity for testing the hypothesis outlined above. Studies on cognitive development have already noted links between the schedule of brain development and the pattern of maternal care. For example, behavioral imprinting in birds and mammals can only occur after hatching or birth, respectively [101]. However, species might differ substantially as to whether adaptation in critical windows occurred through genetic versus non-genetic mechanisms, making it difficult to interpret results in the context of human physiology.
Amongst humans, several axes of behavioral variability are relevant. Contemporary foragers maintain substantially longer duration of lactation than farmers, though they may also have different infectious disease burdens. Amongst the Bofi of the Central African Republic, for example foraging populations wean their offspring at 36–53 months, whereas their farmer neighbours wean substantially earlier at 18–27 months [102]. Such behavioral differences might be associated with variable durations of critical windows.
A more widespread source of variability is infant feeding mode, where differences in breast-feeding duration, feeding style and milk composition might each generate contrasting signals received by the offspring. Breast-fed and formula-fed infants have been shown to differ in their accretion of fat and lean tissue in early life [103, 104], and to have different risk of obesity in the long term [105–107]. In addition, associations between breast-feeding and later obesity risk are heterogeneous across populations [108], which may relate to inconsistency between settings in either breast-feeding or formula-feeding practices. The possibility that infant feeding mode shapes the duration of critical windows is therefore another area meriting attention.
PUBLIC HEALTH IMPLICATIONS
As the duration of growth extended during the evolution of Homo and humans, the duration of maternal physiological care (placental nutrition and lactation) appears to have remained relatively brief, and to have shortened further since the origins of agriculture. If the arguments presented above are correct, this early cessation of maternal buffering is likely to have constrained the duration of early windows of plasticity, a key period of epigenetic adaptation. This would help explain why this brief period of development is so important for long-term health and function, as is increasingly demonstrated by epidemiological and trial evidence [4]. The short duration of early critical windows does not preclude phenotype responding to ecological stresses during subsequent periods such as adolescence, but the physiological effects are likely to be different to those that occur during early hyperplasic growth.
The notion that the ‘programming’ of obesity need not necessarily be constant across populations has important implications for public health policies. An evolutionary perspective emphasizes that biology may vary in relation to a variety of adaptive processes in response to local ecological conditions, and to intrinsic factors such as gender and age. Traditionally, metabolic differences between populations were explained using the ‘thrifty genotype’ hypothesis, which assumed that ancestral populations had varied in their exposure to ‘feast-famine’ cycles, and had evolved contrasting genotypes [109]. More recently, the thrifty phenotype hypothesis emphasized the role developmental experience in shaping subsequent metabolism, independent of genotype [6]. The mechanism described above may involve either genetic adaptation, or mechanisms of plasticity, and at this stage neither can be ruled out.
This perspective therefore highlights important gaps in knowledge regarding public health policies that target stunting and obesity risk in early life:
If population variability in the duration of critical windows were attributable to genetic factors, then different policies would be required for different populations.
If the variability emerged through plasticity, whereby different patterns of maternal care shape the developmental programming of hormonal axes, then a public health intervention might impact not only nutritional status per se, but also the fundamental physiology of growth.
The possibility that public health policies might impact the very regulatory systems that underlie the traits that they are seeking to influence is clearly challenging, but this is precisely the kind of insight that an evolutionary perspective using life history theory can contribute to mainstream medicine.
CONCLUSIONS
In this article, I have developed earlier work on the notion that developmental plasticity has been selected to match with the duration of maternal care by considering the additional influence of ecological conditions [10]. I have suggested that variability in ecological quality, represented by stresses such as the burden of infectious disease and the stability of energy supply, could shape patterns of maternal investment, and that this could then elicit population variability in the duration of critical windows of physiological sensitivity. In this model of dynamic interactions between mother and offspring, neither party should be assumed to have total control. Mothers have some ability to alter their patterns of parental care to benefit their own inclusive fitness, but only to the extent that their decisions do not fatally compromise offspring viability and survival. As elegantly expressed in the concept of the tug-of-war over maternal investment [27], mothers may shift their reproductive strategy if the returns on investment in the current offspring increase or decline in accordance with ecological conditions, and in this way may affect the duration of critical windows. This issue requires further work, but is important as it may have implications not only for what public health policies might achieve in terms of improving growth and nutritional status, but how they might influence more fundamental components of human biology in the process. This issue is relevant to the key challenge of reducing stunting and malnutrition without exacerbating longer term risks of obesity.
Conflict of interest: None declared.
REFERENCES
- 1.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Forsen TJ, et al. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–9. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 3.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 7.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–92. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis BJ, Del Giudice M. Beyond allostatic load: rethinking the role of stress in regulating human development. Dev Psychopathol. 2014;26:1–20. doi: 10.1017/S0954579413000849. [DOI] [PubMed] [Google Scholar]
- 9.Bateson P. Fetal experience and good adult design. Int J Epidemiol. 2001;30:928–34. doi: 10.1093/ije/30.5.928. [DOI] [PubMed] [Google Scholar]
- 10.Wells JC. The thrifty phenotype hypothesis: thrifty offspring or thrifty mother? J Theor Biol. 2003;221:143–61. doi: 10.1006/jtbi.2003.3183. [DOI] [PubMed] [Google Scholar]
- 11.McCance RA, Widdowson EM. The effects of chronic undernutrition and of total starvation on growing and adult rats. Br J Nutr. 1956;10:363–73. doi: 10.1079/bjn19560054. [DOI] [PubMed] [Google Scholar]
- 12.Widdowson EM, McCance RA. Some effects of accelerating growth. I. General somatic development. Proc R Soc Ser B. 1960;152:188–206. doi: 10.1098/rspb.1960.0032. [DOI] [PubMed] [Google Scholar]
- 13.Davison AN, Dobbing J. The developing brain. In: Davison AN, Dobbing J, editors. Applied Neurochemistry. Oxford: Blackwell; 1968. pp. 253–86. [Google Scholar]
- 14.Davison AN, Dobbing J. Myelination as a vulnerable period in brain development. Br Med Bull. 1966;22:40–4. doi: 10.1093/oxfordjournals.bmb.a070434. [DOI] [PubMed] [Google Scholar]
- 15.Lucas A. Programming by early nutrition in man. Ciba Found. Symp. 1991;156:38–50. [PubMed] [Google Scholar]
- 16.Smith DW, Truog W, Rogers JE, et al. Shifting linear growth during infancy: illustration of genetic factors in growth from fetal life through infancy. J Pediatr. 1976;89:225–30. doi: 10.1016/s0022-3476(76)80453-2. [DOI] [PubMed] [Google Scholar]
- 17.Mei Z, Grummer-Strawn LM, Thompson D, et al. Shifts in percentiles of growth during early childhood: analysis of longitudinal data from the California Child Health and Development Study. Pediatrics. 2004;113:e617–27. doi: 10.1542/peds.113.6.e617. [DOI] [PubMed] [Google Scholar]
- 18. Save the Children. Nutrition in the First 1,000 Days: State of the World’s Mothers 2012. London: Save the Children, 2012.
- 19.Gurven M, Walker R. Energetic demand of multiple dependents and the evolution of slow human growth. Proc Biol Sci. 2006;273:835–41. doi: 10.1098/rspb.2005.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- 21.Wells JC. Environmental quality, developmental plasticity and the thrifty phenotype: a review of evolutionary models. Evol.Bioinformat. 2007;3:109–20. [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzawa CW. Fetal origins of developmental plasticity: are fetal cues reliable predictors of future nutritional environments? Am J Hum Biol. 2005;17:5–21. doi: 10.1002/ajhb.20091. [DOI] [PubMed] [Google Scholar]
- 23.Wells JC. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22:1–17. doi: 10.1002/ajhb.20994. [DOI] [PubMed] [Google Scholar]
- 24.Stein AD, Zybert PA, van de Bor M, et al. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–6. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 25.Antonov AN. Children born during the siege of Leningrad in 1942. J Pediatr. 1947;30:250–9. doi: 10.1016/s0022-3476(47)80160-x. [DOI] [PubMed] [Google Scholar]
- 26.Trivers RL. Parent–offspring conflict. Am.Zool. 1974;14:249–64. [Google Scholar]
- 27.Moore T, Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 1991;7:45–9. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- 28.Haig D. Genetic conflicts in human pregnancy. Q Rev Biol. 1993;68:495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- 29.Haig D. Placental hormones, genomic imprinting, and maternal-fetal communication. J Evol Biol. 1996;9:357–80. [Google Scholar]
- 30.BlurtonJones N. Natural selection and birthweight. Ann Hum Biol. 1978;5:487–9. doi: 10.1080/03014467800003141. [DOI] [PubMed] [Google Scholar]
- 31.Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–2. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- 32.Tamimi RM, Lagiou P, Mucci LA, et al. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326:1245–6. doi: 10.1136/bmj.326.7401.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagiou P, Samoli E, Lipworth L, et al. Energy intake during pregnancy in relation to offspring gender by maternal height. Eur J Epidemiol. 2011;26:39–44. doi: 10.1007/s10654-010-9528-3. [DOI] [PubMed] [Google Scholar]
- 34.da Costa TH, Haisma H, Wells JC, et al. How much human milk do infants consume? Data from 12 countries using a standardized stable isotope methodology. J Nutr. 2010;140:2227–32. doi: 10.3945/jn.110.123489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. 2010;22:50–4. doi: 10.1002/ajhb.20941. [DOI] [PubMed] [Google Scholar]
- 36.Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21:512–9. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- 37.Hinde K, Carpenter AJ, Clay JS, et al. Holsteins favor heifers, not bulls: biased milk production programmed during pregnancy as a function of fetal sex. PLoS One. 2014;9:e86169. doi: 10.1371/journal.pone.0086169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koskela E, Mappes T, Niskanen T, et al. Maternal investment in relation to sex ratio and offspring number in a small mammal - a case for Trivers and Willard theory? J Anim Ecol. 2009;78:1007–14. doi: 10.1111/j.1365-2656.2009.01574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haig D. Genetic conflicts of pregnancy and childhood. In: Stearns SCs., editor. Evolution in Health and Disease. Oxford: Oxford University Press; 1999. pp. 77–90. [Google Scholar]
- 40.Haig D. Troubled sleep: Night waking, breastfeeding and parent–offspring conflict. Evol Med Public Health. 2014;2014:32–9. doi: 10.1093/emph/eou005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells JC. Parent–offspring conflict theory, signaling of need, and weight gain in early life. Q Rev Biol. 2003;78:169–202. doi: 10.1086/374952. [DOI] [PubMed] [Google Scholar]
- 42.Smith CC, Fretwell SD. The optimal balance between size and number of offspring. Am Nat. 1974;108:499–506. [Google Scholar]
- 43.Haig D. Parental antagonism, relatedness asymmetries, and genomic imprinting. Proc Biol Sci. 1997;264:1657–62. doi: 10.1098/rspb.1997.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bogin B. Patterns of Human Growth. 2nd edn. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 45.Wells JC. Ecological volatility and human evolution: a novel perspective on life history and reproductive strategy. Evol Anthropol. 2012;21:277–88. doi: 10.1002/evan.21334. [DOI] [PubMed] [Google Scholar]
- 46.Hrdy SB. Mothers and Others: the Evolutionary Origins of Mutual Understanding. Cambridge, MA: Belknap Press; 2009. [Google Scholar]
- 47.Hewlett BS, Winn S. Allomaternal nursing in humans. Curr Anthropol. 2014;55:200–29. [PubMed] [Google Scholar]
- 48.Wells JC, DeSilva JM, Stock JT. The obstetric dilemma: an ancient game of Russian roulette, or a variable dilemma sensitive to ecology? Am J Phys Anthropol. 2012;149:40–71. doi: 10.1002/ajpa.22160. [DOI] [PubMed] [Google Scholar]
- 49.MacKay DF, Smith GC, Dobbie R, et al. Gestational age at delivery and special educational need: retrospective cohort study of 407,503 schoolchildren. PLoS Med. 2010;7:e1000289. doi: 10.1371/journal.pmed.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells JC. The thrifty phenotype as an adaptive maternal effect. Biol Rev Camb Philos Soc. 2007;82:143–72. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 51.Dabelea D, Hanson RL, Lindsay RS, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–11. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 52.Roos N, Neovius M, Cnattingius S, et al. Perinatal outcomes after bariatric surgery: nationwide population based matched cohort study. BMJ. 2013;347:f6460. doi: 10.1136/bmj.f6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abrams ET, Meshnick SR. Malaria during pregnancy in endemic areas: a lens for examining maternal-fetal conflict. Am J Hum Biol. 2009;21:643–50. doi: 10.1002/ajhb.20919. [DOI] [PubMed] [Google Scholar]
- 54.Wells JC. A critical appraisal of the predictive adaptive response hypothesis. Int J Epidemiol. 2012;41:229–35. doi: 10.1093/ije/dyr239. [DOI] [PubMed] [Google Scholar]
- 55.Cropley JE, Suter CM, Beckman KB, et al. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci U S A. 2006;103:17308–12. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harder T, Franke K, Kohlhoff R, et al. Maternal and paternal family history of diabetes in women with gestational diabetes or insulin-dependent diabetes mellitus type I. Gynecol Obstet Invest. 2001;51:160–4. doi: 10.1159/000052916. [DOI] [PubMed] [Google Scholar]
- 57.Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–57. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- 58.Ou XH, Li S, Wang ZB, et al. Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum Reprod. 2012;27:2130–45. doi: 10.1093/humrep/des137. [DOI] [PubMed] [Google Scholar]
- 59.Kelsey G, Feil R. New insights into establishment and maintenance of DNA methylation imprints in mammals. Philos Trans R Soc Lond B Biol Sci. 2013;368:20110336. doi: 10.1098/rstb.2011.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pembrey ME, Bygren LO, Kaati G, et al. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 61.Kaati G, Bygren LO, Pembrey M, et al. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet. 2007;15:784–90. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 62.Pembrey ME. Male-line transgenerational responses in humans. Hum Fertil. 2010;13:268–71. doi: 10.3109/14647273.2010.524721. [DOI] [PubMed] [Google Scholar]
- 63.Waterland RA, Kellermayer R, Laritsky E, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominguez-Salas P, Moore SE, Baker MS, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manalich R, Reyes L, Herrera M, et al. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 2000;58:770–3. doi: 10.1046/j.1523-1755.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 66.Van Assche FA, De Prins F, Aerts L, et al. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–3. doi: 10.1111/j.1471-0528.1977.tb12486.x. [DOI] [PubMed] [Google Scholar]
- 67.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–70. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 68.Kerssen A, Evers IM, de Valk HW, et al. Effect of breast milk of diabetic mothers on bodyweight of the offspring in the first year of life. Eur J Clin Nutr. 2004;58:1429–31. doi: 10.1038/sj.ejcn.1601977. [DOI] [PubMed] [Google Scholar]
- 69.Plagemann A, Harder T, Franke K, et al. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diab Care. 2002;25:16–22. doi: 10.2337/diacare.25.1.16. [DOI] [PubMed] [Google Scholar]
- 70.Yajnik CS, Fall CH, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton WD. The genetical evolution of social behaviour. I. J Theor Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 72.Hamilton WD. The genetical evolution of social behaviour. II. J Theor Biol. 1964;7:17–52. doi: 10.1016/0022-5193(64)90039-6. [DOI] [PubMed] [Google Scholar]
- 73.Clutton-Brock TH, Albon SD, Guinness FE. Fitness costs of gestation and lactation in wild mammals. Nature. 1989;337:260–2. doi: 10.1038/337260a0. [DOI] [PubMed] [Google Scholar]
- 74.Robson SL, vanSchaik CP, Hawkes K. The derived features of human life history. In: Hawkes K, Paine RR, editors. Oxford: James Currie; 2006. pp. 17–44. [Google Scholar]
- 75.Aiello LC, Key C. Energetic consequences of being a Homo erectus female. Am J Hum Biol. 2002;14:551–65. doi: 10.1002/ajhb.10069. [DOI] [PubMed] [Google Scholar]
- 76.Isler K, van Shaik CP. How our ancestors broke through the grey ceiling: comparative evidence for cooperative breeding in early Homo. Curr Anthropol. 2012;53(Suppl. 6):S453–65. [Google Scholar]
- 77.Wickes IG. A history of infant feeding Part 1. Primitive peoples: ancient works: renaissance writers. Arch Dis Child. 1952;27:151–8. doi: 10.1136/adc.28.138.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dettwyler KA. A time to wean: the hominid blueprint for the natural age of weaning in modern human populations. In: Stuart MacAdam P, Dettwyler KA, editors. Breastfeeding: Biocultural Perspectives. New York: Aldine de Gruyter; 1995. pp. 39–73. [Google Scholar]
- 79.Galdikas BM, Wood JW. Birth spacing patterns in humans and apes. Am J Phys Anthropol. 1990;83:185–91. doi: 10.1002/ajpa.1330830207. [DOI] [PubMed] [Google Scholar]
- 80.Bentley GR, Goldberg T, Jasienska G. The fertility of agricultural and non-agricultural traditional societies. Popul Studies. 1993;47:269–81. [Google Scholar]
- 81.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–34. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 82.Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27:755–77. doi: 10.1038/sj.ijo.0802316. [DOI] [PubMed] [Google Scholar]
- 83.Li H, Stein AD, Barnhart HX, et al. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin.Nutr. 2003;77:1498–505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- 84.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82:456–66. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 85.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–30. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Wells JC. Sexual dimorphism in body composition across human populations: associations with climate and proxies for short- and long-term energy supply. Am J Hum Biol. 2012;24:411–9. doi: 10.1002/ajhb.22223. [DOI] [PubMed] [Google Scholar]
- 87.Chomtho S, Wells JC, Williams JE, et al. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–84. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 88.Ekelund U, Ong K, Linne Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–30. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 89.Stettler N, Kumanyika SK, Katz SH, et al. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77:1374–8. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 90.Stettler N, Stallings VA, Troxel AB, et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation. 2005;111:1897–903. doi: 10.1161/01.CIR.0000161797.67671.A7. [DOI] [PubMed] [Google Scholar]
- 91.Stettler N, Tershakovec AM, Zemel BS, et al. Early risk factors for increased adiposity: a cohort study of African American subjects followed from birth to young adulthood. Am J Clin.Nutr. 2000;72:378–83. doi: 10.1093/ajcn/72.2.378. [DOI] [PubMed] [Google Scholar]
- 92.Stettler N, Zemel BS, Kumanyika S, et al. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109:194–9. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 93.Karaolis-Danckert N, Buyken AE, Bolzenius K, et al. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84:1449–55. doi: 10.1093/ajcn/84.6.1449. [DOI] [PubMed] [Google Scholar]
- 94.Wells JC, Hallal PC, Wright A, et al. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes. 2005;29:1192–8. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 95.Victora CG, Sibbritt D, Horta BL, et al. Weight gain in childhood and body composition at 18 years of age in Brazilian males. Acta Paediatr. 2007;96:296–300. doi: 10.1111/j.1651-2227.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuzawa CW, Hallal PC, Adair L, et al. Birth weight, postnatal weight gain, and adult body composition in five low and middle income countries. Am J Hum Biol. 2012;24:5–13. doi: 10.1002/ajhb.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karlberg J. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatr Scand Suppl. 1989;350:70–94. doi: 10.1111/j.1651-2227.1989.tb11199.x. [DOI] [PubMed] [Google Scholar]
- 98.Hochberg Z, Albertsson-Wikland K. Evo-devo of infantile and childhood growth. Pediatr Res. 2008;64:2–7. doi: 10.1203/PDR.0b013e318177590f. [DOI] [PubMed] [Google Scholar]
- 99.Crespi B. The evolutionary biology of child health. Proc Biol Sci. 2011;278:1441–9. doi: 10.1098/rspb.2010.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cole TJ. Children grow and horses race: is the adiposity rebound a critical period for later obesity? BMC Pediatr. 2004;4:6. doi: 10.1186/1471-2431-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smart JL. Critical periods in brain development. Ciba Found. Symp. 1991;156:109–28. doi: 10.1002/9780470514047.ch8. [DOI] [PubMed] [Google Scholar]
- 102.Fouts HN, Hewlett BS, Lamb ME. Parent–offspring weaning conflicts among the Bofi farmers and foragers of central Africa. Curr Anthropol. 2005;46:29–50. [Google Scholar]
- 103.Dewey KG, Heinig MJ, Nommsen LA, et al. Breast-fed infants are leaner than formula-fed infants at 1 y of age: the DARLING study. Am J Clin Nutr. 1993;57:140–5. doi: 10.1093/ajcn/57.2.140. [DOI] [PubMed] [Google Scholar]
- 104.Gale C, Logan KM, Santhakumaran S, et al. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:656–69. doi: 10.3945/ajcn.111.027284. [DOI] [PubMed] [Google Scholar]
- 105.Arenz S, Ruckerl R, Koletzko B, et al. Breast-feeding and childhood obesity–a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247–56. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 106.Toschke AM, Martin RM, von KR, et al. Infant feeding method and obesity: body mass index and dual-energy X-ray absorptiometry measurements at 9–10 y of age from the Avon Longitudinal Study of Parents and Children (ALSPAC) Am J Clin Nutr. 2007;85:1578–85. doi: 10.1093/ajcn/85.6.1578. [DOI] [PubMed] [Google Scholar]
- 107.Owen CG, Martin RM, Whincup PH, et al. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82:1298–307. doi: 10.1093/ajcn/82.6.1298. [DOI] [PubMed] [Google Scholar]
- 108.Victora CG, Barros F, Lima RC, et al. Anthropometry and body composition of 18 year old men according to duration of breast feeding: birth cohort study from Brazil. BMJ. 2003;327:901. doi: 10.1136/bmj.327.7420.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neel V. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]