Abstract
The microenvironment within the bone marrow that maintains hematopoietic stem cell (HSC) quiescence is the subject of intense study. In a recent Nature paper, Kunisaki et al combine imaging techniques and computational modeling to define a novel arteriolar niche for quiescent HSCs within the bone marrow.
The hematopoietic stem cell (HSC) niche is a spatially confined unit within the bone marrow cavity, which uniquely regulates the choice between HSC quiescence and proliferation (1). In such microenvironments, HSCs receive regulatory signals from neighboring or adjacent cells, extracellular matrix, andsoluble factors. During the past decade, the existence of at least two subcompartments of the HSC niche has been considered. The endosteal niche, hallmarked by osteoblasts lining the bone marrow cavity, has been proposed as a source of factors important for HSC quiescence, while the vascular sinusoidal niche has been implicated in HSC maintenance and regeneration (2,3). Accumulating evidence however, argues that considerable overlap exists between HSC microenvironments. Also, recent studies suggest that the HSC vascular niche may not be limited to sinusoids, since hematopoietic stem and progenitor cells also lie adjacent to diverse non-sinusoidal vascular structures including arteries and arterioles (4). Direct visualization of the HSC niche has proven challenging due to technical limitations in imaging of skeletal bones, the need for multi-parameter marker combinations to detect rare HSC populations, and the ability to analyze large cell populations at the single cell level in situ.
In a recent article in Nature, Kunisaki et al. (5) describe a sophisticated, novel three-dimensional imaging approach to analyze the spatial relationship between endogenous HSCs and vascular structures. The studies were carried out in long bones and also in the sternum. The latter offers the advantage that all HSCs can be enumerated, allowing for accurate quantitation in 3D images. They find that the phenotypically rare CD150+, CD48−, CD41−, Lineage – HSCs preferentially localize in the periphery of the bone marrow, in close proximity to the endosteum. This region, referred to as the endosteal zone, contains both sinusoids as well as thin-walled arterioles. The majority of HSC were associated with sinusoids (~67% were within 20 μm from sinusoids), while a smaller fraction (~37%) resided close or adjacent to Sca-1hi, VEGFR2+, VEGFR3− arterioles. The association with sinusoids, however, is statistically indistinguishable from a random distribution, since the bone marrow contains a very dense sinusoidal network with a regular spacing of ~46 μm. Thus, any randomly placed cell in the bone marrow will be <23 μm (half the inter-vessel spacing) away from the nearest sinusoidal vessel. Using computational simulations, Kunisaki et al. showed that, in contrast to the random association of HSCs with sinusoids, the association of HSCs with arterioles is specific and statistically nonrandom since these endosteal arterioles comprised a much smaller volumetric fraction of the bone marrow.
The authors then utilized a combination of novel in situ imaging and a series of transgenic mouse models to define the distinctive features of the arteriolar and sinusoidal vascular niches. First, the vascular niches contain phenotypically and molecularly unique mesenchymal stromal cells. The arteriolar stromal niche cells are defined as Nestin-GFP bright, NG2+, LepR− pericytes, whereas the sinusoidal stromal niche cells are described as Nestin-dim, NG2-,LepR+ cells with reticular morphology. Second, the arteriolar niche cells are quiescent while their counterparts in the sinusoidal niche are more actively cycling cells. Remarkably, quiescent HSCs are also distributed near the quiescent arteriolar niche cells, and mobilization of HSCs following treatment with G-CSF or alteration of their cycling patterns via pharmacologic or genetic manipulations led to a re-distribution of HSCs away from the arteriolar niche. Depletion of NG2+ pericytes similarly results in the loss of HSC quiescence, change in HSC localization, and overall reduction in HSC pool size. It is unclear however, how the quiescent state is regulated.
One prevailing model involving stem cells in general, i.e. pluripotent cells and tumor stem cells, as well as adult stem cells, is the influence of a hypoxic environment on cell quiescence and metabolism (6). The bone marrow cavity is regarded to be a physiologically hypoxic tissue (estimated range between 1% and 6% O2) and it has been proposed that quiescent HSCs reside in zones with the deepest hypoxia (7), which are commonly thought to be in the endosteal region away from vascular structures. The findings of Kunasaki et al. differ from this existing view in significant ways. The authors show that the endosteal region is not only endowed with sinusoidal blood vessels (3), but also with arteriolar vessels that carry oxygenated blood to the bone marrow. Several independent lines of evidence are consistent with this data. In vivo 3D imaging of mouse calvaria has shown the endosteal zone to be perivascular (8). Studies in long bones (4) have clearly demonstrated the close association between hematopoietic stem and progenitor cells with small arterioles, which transition into the venous system and likely represent the major site of oxygen exchange, as occurs in other tissues. Moreover, hematopoietic stem and progenitor cells display a characteristic hypoxic profile independent of their localization in bone marrow, adjacency to vascular structures or cell cycle status (4).
The quiescent state and self renewal potential of HSCs have also been tightly linked to their metabolic state. It is conceivable that extrinsic factors, both cellular and soluble, in the HSC niche could influence the balance between glycolysis and oxidative metabolism (6), and hence HSC activation. Gaining deeper insight into the metabolic profile of HSCs, ideally in the context of the oxygen availability in the bone marrow microenvironment, will be fundamental to understanding HSC fate decisions in vivo. Recently, the role of the sympathetic nervous system has also received considerable attention (10). Interestingly, the periarteriolar mesenchymal stromal niche cells are highly innervated with sympathetic nerves and Schwann cells, which play a crucial role in steady state HSC homeostasis as well G-CSF induced mobilization (5).
These data highlight increasing appreciation of the importance of the HSC niche. Future development of imaging approaches complemented by computational methods for automated, objective data acquisition will certainly advance the field. Moreover, transgenic mouse models will continue to provide excellent tools to manipulate the niche and assess the in vivo function of individual niche components. Combining these approaches, as highlighted by Kunisaki et al., will yield further insights, and our knowledge of the HSC niche under physiologic conditions is rapidly evolving. Future studies will undoubtedly build on this work, providing valuable insights into questions such as how how the niche is affected during aging and by inflammatory and/or malignant conditions.
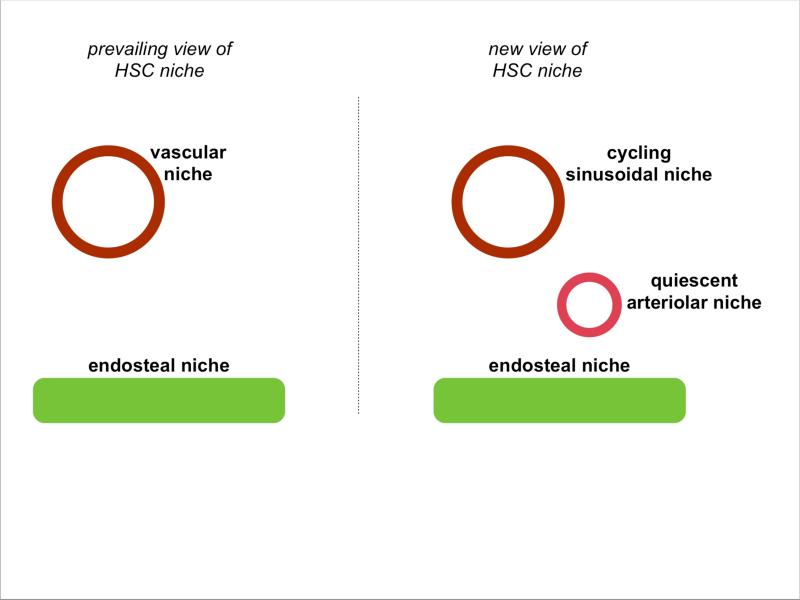
Figure 1.
The prevailing view of the hematopoietic stem cell niche in the bone marrow, with HSCs residing close to either the vasculature or the endosteal surface (left).. Kunisaki et al. demonstrate the co-existence of multiple vasculature niches in the bone marrow, with the arteriolar niche maintaning quiescent HSCs while the sinusoidal niche supports cuycling HSCs (right).
References
- 1.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 2.Adams GB, Scadden DT. The hematopoietic stem cell in its place. Nat. Immunol. 2006;7:333–337. doi: 10.1038/ni1331. [DOI] [PubMed] [Google Scholar]
- 3.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nature Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013 doi: 10.1038/nature12612. (advanced online publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011;9:298–310. doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl Acad. Sci. USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, Côté D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–6. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazo IB, Massberg S, von Andrian UH. Hematopoietic stem and progenitor cell trafficking. Trends Immunol. 2011;32:493–503. doi: 10.1016/j.it.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]