Abstract
Differences between microbial pathogenesis in male and female hosts are well characterized in disease conditions connected to sexual transmission. However, limited biological insight is available on variances attributed to sex specificity in host-microbe interactions, and it is most often a minimized variable outside these transmission events. In this work, we studied two gut microbes—a pathogen, Mycobacterium avium subsp. paratuberculosis, and a probiotic, Lactobacillus animalis NP-51—and the interaction between each agent and the male and female gastrointestinal systems. This trial was conducted in BALB/c mice (n = 5 per experimental group and per sex at a given time point), with analysis at four time points over 180 days. Host responses to M. avium subsp. paratuberculosis and L. animalis were sensitive to sex. Cytokines that were significantly different (P ≤ 0.05) between the sexes included interleukin-1α/β (IL-1α/β), IL-17, IL-6, IL-10, IL-12, and gamma interferon (IFN-γ) and were dependent on experimental conditions. However, granulocyte-macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), and IL-13/23 showed no sex specificity. A metabolomics study indicated a 0.5- to 2.0-fold (log2 scale) increase in short-chain fatty acids (butyrate and acetate) in males and greater increases in o-phosphocholine or histidine from female colon tissues; variances distinct to each sex were observed with age or long-term probiotic consumption. Two genera, Staphylococcus and Roseburia, were consistently overrepresented in females compared to males; other species were specific to one sex but fluctuated depending on experimental conditions. The differences observed suggest that male and female gut tissues and microbiota respond to newly introduced microorganisms differently and that gut-associated microorganisms with host immune system responses and metabolic activity are supported by biology distinct to the host sex.
INTRODUCTION
Microorganisms and their responses to sex-specific mediators were studied intensively for several decades leading into the 1970s (1, 2). Yotis and Stanke describe delayed doubling times and bacteriostatic properties for multiple strains of Staphylococcus aureus and the reactivation of some strains that were dependent on endocrine activity at infancy, puberty, pregnancy, and menopausal stages, providing evidence for the associations between commensal microbiota and sex-specific mediators (1). Mechanistic studies from the same era describe microbial endocrine associations by which staphylococci transform a derivative of 17-hydroxyprogesterone (4-androstene-3,17-dione) into testosterone, further demonstrating a long history of studies on interkingdom communication specific to sex.
Recent work again highlights the importance of medical studies and therapies related to sex (3). A specific study demonstrated a role for the regulation of type 1 diabetes mellitus through commensal microorganisms and sex hormones, establishing the ability of gut organisms to regulate testosterone and direct leukocyte development (3). Earlier studies outlined differences in innate and adaptive immunity due to lymphocyte or leukocyte response to sex hormones: estrogen, androgens, and progesterone (4–6). Several groups showed that estrogen not only influences the production of specific cytokines, like gamma interferon (IFN-γ), but that these cytokines in turn increase the expression of cell surface estrogen receptor alpha (ER-α), creating a positive-feedback loop that can lead to increases in transcriptional regulation (7–9). Male-associated hormones, such as testosterone, dihydrotestosterone, and other androgens, typically suppress immune functions through lower levels of cytokine and immunoglobulin synthesis (10, 11). Accordingly, it has long been understood that sex-associated compounds regulate host health (12).
In this study, we describe how the host sex elicits differences in commensal gut microbiota, metabolite synthesis, and immune system activity in response to pathogens (Mycobacterium avium subsp. paratuberculosis) or beneficial microbes (Lactobacillus animalis).
M. avium subsp. paratuberculosis is a zoonotic intracellular pathogen that causes the wasting Johne's disease (JD) in ruminant animals through progressive and chronic gastrointestinal tract (GIT) edema and inflammation (13–15). During infection, the organisms enter gut macrophages, and neighboring immune cells accumulate around the infected cell to form a calcified and rounded granuloma (16–18). Similar to most intracellular infections, M. avium subsp. paratuberculosis infection induces a type 1 cell-mediated immune (CMI) response that is reinforced through increases in interleukin-1α (IL-1α), IFN-γ, IL-6, and IL-12 family cytokines at the site of infection (17, 19). This response attracts more macrophages and Th1 cells to infected tissue until acute proinflammatory responses are suppressed by increases in transforming growth factor β (TGF-β) and IL-10, which are regulated through infected tissue by unknown mechanisms (13, 14, 19, 20). Unique to M. avium subsp. paratuberculosis disease etiology is the sustained production of IL-1α in infected tissue, which eventually induces tissue scarring by the production of reactive oxygen species (ROS) (16, 17). Similar inflammatory and immune responses are observed in humans. The most comparable conditions are observed in Crohn's disease (CD), where modifications to the gut microbiota are attributed to the disease (21, 22).
Similarities between JD and CD are not limited to disease symptoms; treatments for JD can be effective in some CD conditions. Studies by Motiwala et al. (20) have demonstrated that there may be specificities in M. avium subsp. paratuberculosis strains that infect different ruminants (including cattle versus sheep), in addition to those that may infect humans. Thus, there is controversy in the literature as to the contributions of M. avium subsp. paratuberculosis infection to CD. Many CD patients harbor M. avium subsp. paratuberculosis in their GIT tissues, and continued research may provide evidence to distinguish the two diseases (13–15, 23–25). However, the current controversy surrounding the contribution of M. avium subsp. paratuberculosis to disease provides an opportunity for new findings related to gut health, pathogen behavior, and the volatile relationship between host and microbe in the gut ecosystem (26).
Probiotics are beneficial microbes that have become commercial and medical resources for gut and systemic health in humans, especially those with CD and irritable bowel disease (IBD). Our laboratory has focused on the use of these beneficial microbes to reduce food-borne pathogens in livestock and the agricultural industry (27). Our recent data suggest that L. animalis NP-51 is able to modify inflammatory cytokines during M. avium subsp. paratuberculosis-induced acute inflammation (28). We therefore expanded upon these findings to determine if NP-51 revealed more traits associated with probiotics and important to deterring M. avium subsp. paratuberculosis infection, including modulating inflammatory mediators of T cells or metabolism (29–31). These lactic-acid-producing bacteria (LAB) are well known for synthesizing compounds like bacteriocins (peptides with antimicrobial properties) and peroxides and preventing food-borne pathogens from infecting the GIT (32). Thus, the ability of probiotics to manipulate the host immune system and metabolic activity strengthens the potential that these associations may also perpetuate and regulate sex-linked immune system activity.
MATERIALS AND METHODS
Ethics statement.
We evaluated BALB/c mice (Charles River Laboratories, Wilmington, MA) 23 to 28 days old that had been acclimated for 2 weeks in the Texas Tech University Animal Care and Use facilities and were later handled according to approved methods (Animal Care and Use Committee approval number 07060-12), including humane methods of minimizing pain or suffering and of euthanasia (28).
Experiment design.
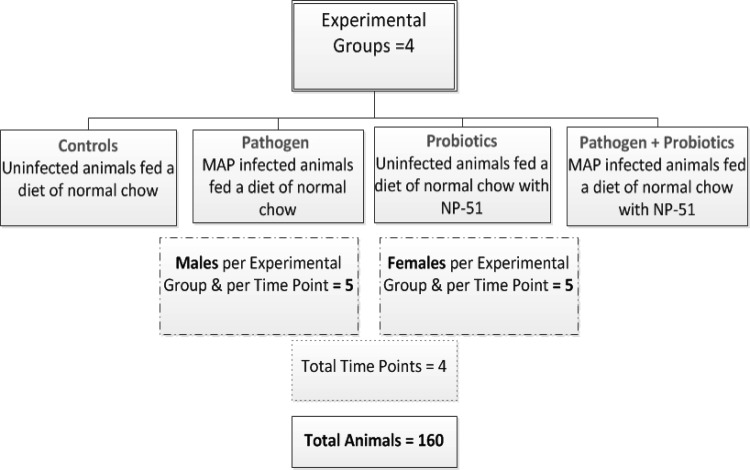
The experiment design for this study was established according to methods previously described by Karunasena et al. (28). Each experimental group included 5 animals per sex, as determined through a power test (power = 90%; control = 10; coefficient of variation [CV] = 5%; P ≤ 0.05) (28). For this study, we describe animals exposed to viable M. avium subsp. paratuberculosis and/or viable L. animalis NP-51 and compared to controls for a total of four experimental conditions (Fig. 1). At day 0, the animals ate a diet that included sterilized powdered chow (control diet; 7012 Teklad LM-485 Mouse/Rat Sterilizable Diet; Harlan Teklad Diets, Madison WI) or the same chow with 1 × 106 CFU/g L. animalis (probiotic diet). On day 46, a subpopulation of animals maintained on each diet were infected with M. avium subsp. paratuberculosis at 1 × 107 CFU using intraperitoneal injection by established and published methods (28). Each of the experimental groups was evaluated for up to 180 days (a recognized period to evaluate M. avium subsp. paratuberculosis infection in the described rodent model), and animals were euthanized every 45 days (days 45, 90, 135, and 180) for sample collection; these data describe the results for a total of 160 animals (80 per sex) (28). A detailed review of the experiment design is provided in Fig. 1.
FIG 1.
Experiment design. Shown are the four experimental conditions tested in this study, with equal numbers (n = 5) of male and female animals tested for each of the conditions and evaluated at 4 time points (days 45, 90, 135, and 180). In total, 160 animals were examined (80 of each sex) for the study. MAP, M. avium subsp. paratuberculosis.
At necropsy, blood was collected through cardiac puncture and pooled (SST Serum Separation Tubes; n = 5; 13 by 100 mm; Becton Dickinson, San Jose, CA); tissues (stomach, small and large intestine, spleen, and liver) were immediately frozen in liquid nitrogen and stored at −80°C for further experimentation. The tissues were processed for histopathology, RNA, DNA, and metabolite extractions according to methods described below and previously established (28).
M. avium subsp. paratuberculosis culture and growth conditions.
M. avium subsp. paratuberculosis cultures were originally harvested from cattle at the U.S. Department of Agriculture (USDA) National Animal Disease Center (NADC) and kindly provided by Judith Stabel (NADC, Ames, IA). The cultures were grown and harvested according to conditions described by Stabel et al. (16, 19). Frozen cultures (1-ml aliquots) were inoculated into 50 ml of sterile Middlebrook H79 (M7H9) broth with mycobactin J (Allied Monitor, Fayette, MO) and oleic acid-albumin-dextrose-catalase (OADC) in sterile 100-ml Erlenmeyer flasks. The cultures were incubated at 37°C without shaking for 3 to 4 weeks. Growth was monitored during the incubation period, and cultures with optical density readings at 540 nm of 0.2 to 0.4 were determined to be ready for harvest (the concentrations of cells were 1 × 107 to 1 × 109 CFU/ml) (16, 19).
Probiotic cultures and preparation.
Freeze-dried L. animalis NP-51 cultures with maltodextrin were provided through Culture Systems Inc. (Mishawaka, IN) in individual 20-g packets. NP-51 was mixed into powdered mouse chow using previously described methods to a concentration of 1 × 106 CFU/g, and cultures were prepared fresh daily in sterilized mouse chow and feeders (28).
DNA extractions from tissue.
Colon tissues were processed using mortar and pestle (stored at −80°C) in liquid nitrogen. From each sample, 0.2 g of tissue was used for RNA and DNA extractions. RNA was extracted using a TRIzol kit (Invitrogen, Carlsbad, CA), and the organic layer was used for DNA extractions with a DNeasy Blood and Tissue Kit according to protocols for total bacterial DNA extractions (Qiagen, Valencia, CA). The purified DNA was stored in 1× Tris-EDTA buffer, and concentrations of 40 to 50 ng/μl in 50 μl of water were provided for DNA sequencing and metagenomic evaluation (454 pyrosequencing technology; Roche Laboratories, Branford, CT) at Research and Testing Laboratories, LLC (Lubbock, TX) (28).
Cytokine analysis.
Two-hundred microliters of serum was provided to the Texas Tech University Health Sciences Center (TTUHSC) Core facilities at El Paso, TX, and analyzed on a mouse cytokine 20-plex panel (Invitrogen, Carlsbad, CA) with technical triplicates of serum samples for each of the experimental conditions and per sex evaluated on a Luminex 96-well platform; cytokine concentrations were determined relative to a standard curve developed for each of the 20 cytokines accompanying the described panel (28).
Sample preparation for metabolome analysis.
Approximately 100 mg of colon tissue from each animal and from all four of the experimental groups was analyzed for metabolite composition. Established extraction methods for nuclear magnetic resonance (NMR) analysis were used by Chenomx Inc. (Alberta, Canada). Samples and solutions were stored at −80°C when not in use. All samples were filtered using 3-kDa-molecular-mass-cutoff filters (Nanosep 3k Omega microcentrifuge filter tubes) to remove macromolecules (both lipids and proteins). The filters were washed 4 or 5 times with distilled water before use to remove glycerol preservatives. Samples that yielded less than 630 μl after filtration were diluted to ensure adequate volume for NMR acquisition. All samples were diluted slightly by adding an internal-standard solution that allowed quantification of detected compounds. The samples were vortexed for 30 s, and 600 μl of the mixed solution was transferred to an NMR tube for data acquisition. The samples were analyzed through proton NMR at 600 MHz (using a 600-MHz Varian Inova spectrometer; number of transients, 32; temperature, 298 K). Further details describing data processing and compound identification by Chenomx Inc. are provided in the supplemental material (http://tinyurl.com/modtg6k).
Computational methods and statistical analysis. (i) Cytokine expression from females versus males.
Signal intensities from the Luminex beads were converted to a tab-delimited format and were imported into the R statistical package (33). Intensities were determined using a standard curve for each bead and plotted in a box plot using a custom script in R. A difference between male and female distributions was determined for each bead–experimental-condition combination if the Student t test P value was <0.05.
(ii) Metabolomic profile of colon tissue.
Metabolite concentrations for each experimental condition and each metabolite were imported into R, and the fold differences between males and females were plotted using bar-plot in the graphics package of R. All values shown were relative to no difference between the sexes for that metabolite. The only metabolites that are shown are those with a significant difference between males and females based on a Student t test with a cutoff P value of <0.05.
(iii) Colon microbiota.
Sequencing, mapping, and identification were done as previously described by Karunasena et al., and normalized microbe levels were imported into R (28). The relative abundance of each organism under each experimental condition in females versus males was calculated using a customized R script. Only organisms that showed significant differences (t test P value of <0.05) between males and females were plotted.
Nucleotide sequence accession number.
The sequences obtained in this study were deposited in the Sequence Read Archive (SRA056455).
RESULTS
Differences in immune response by females versus males.
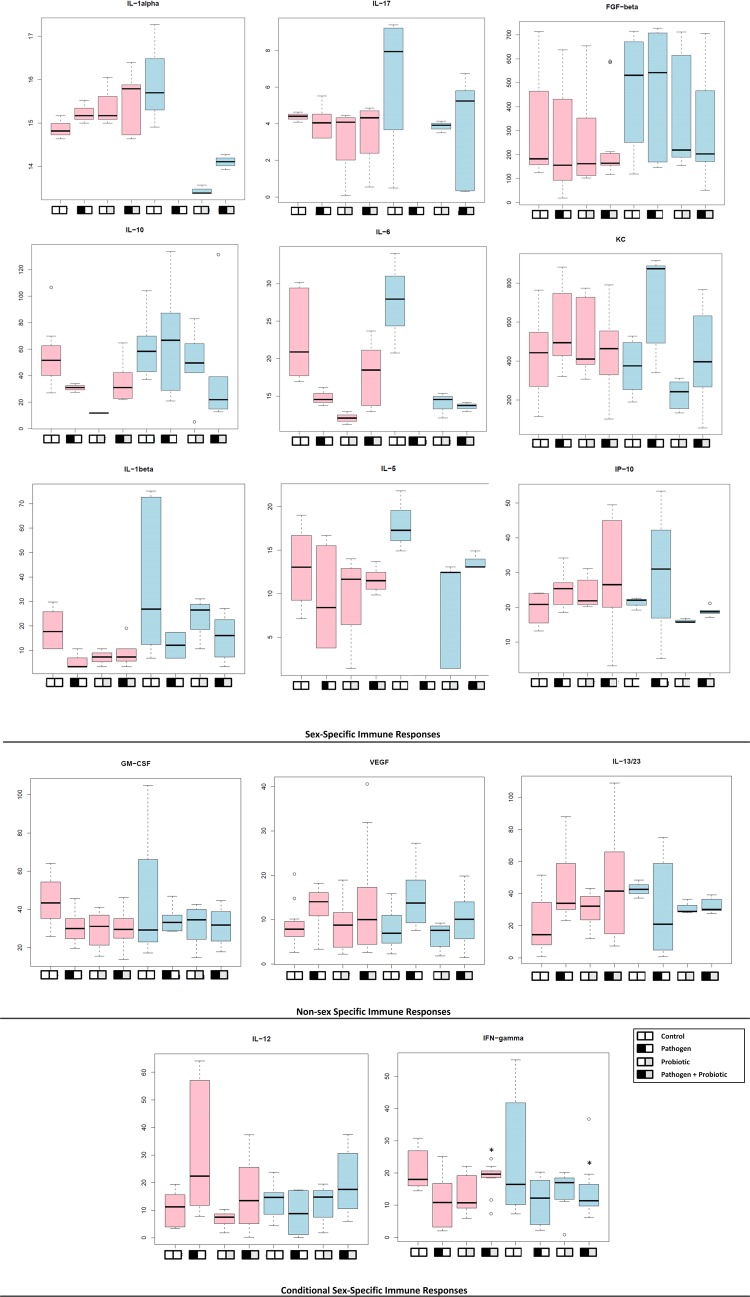
IL-1α, IL-1β, IL-17, IL-10, and IL-6 demonstrated different responses to a pathogen (M. avium subsp. paratuberculosis) and a probiotic (L. animalis) than controls for each sex (Fig. 2). IL-1α and IL-6 concentrations were greater in females than in males under all experimental conditions except controls. IL-10 production was minimal in females relative to males under all experimental conditions (Fig. 2), demonstrating suppression of anti-inflammatory mediators. Proinflammatory and Th1 mediators, including IL-12 and IFN-γ, were maintained in females exposed to the pathogen. Non-sex-linked patterns were observed in the production of growth factors (granulocyte-macrophage colony-stimulating factor [GM-CSF] and vascular endothelial growth factor [VEGF]); similarly, macrophage induced by IFN-γ (MIG) did not produce sex-specific differences (data not shown). However, the chemokine keratinocyte chemoattractant (KC) and the growth factor fibroblast growth factor β (FGF-β) both demonstrated sex specificity (Fig. 2). Compared to females, males infected with M. avium subsp. paratuberculosis showed increases in KC, while those fed probiotics demonstrated lower concentrations. These data suggest that in males relative to females, the activity of KC is influenced by the type of microbe (pathogen or beneficial). FGF-β is related in structure to IL-1β and contributes to cell differentiation, wound healing, and angiogenesis. In this case, decreased IL-1β and FGB-β were detected in females compared to males across all experimental conditions. Data collected on cytokines associated with the Th2 response (including IL-2 and IL-4) were incomplete and therefore could not be analyzed (data not shown). However, similar concentrations of IL-5 (12 to 13 ng/ml) were observed in all experimental groups except animals exposed to pathogens (8 ng/ml). Males showed higher levels of IL-5 (17 ng/ml) in controls than in other experimental groups (Fig. 2). IL-5 promotes eosinophil and Th2 responses by increasing the production of immunoglobulins. IL-13/23 levels were similar across experimental groups, except controls and animals exposed to pathogens; lower IL-13/23 production was detected in males infected with M. avium subsp. paratuberculosis. IL-13/23 promote the Th2 response and increase Th2 production of IL-4, IL-5, and IL-13; a decrease may suggest decreased Th2 immunity with chronic disease that was specific to males.
FIG 2.
Differences in cytokine expression for female versus male BALB/c mice with pathogen infection or fed probiotics. The y axis shows the concentration (ng/ml), and the x axis shows the experimental groups as described in the legend. Pink bars denote females, and blue bars describe males. The animals were infected once with 1 × 107 CFU M. avium subsp. paratuberculosis and fed daily 1 × 106 CFU/g of a probiotic, L. animalis NP-51 (Culture Systems Inc., Mishawaka, IN). We observed cytokine expression that was specific to sex, nonspecific, and related to experimental conditions. Those cytokines with sex-specific immune responses all showed statistically significant differences (P < 0.05) when each experimental group was compared to its counterpart of the opposite sex. Cytokines listed as non-sex specific did not demonstrate statistical differences (P ≥ 0.05) when female and male experimental groups were compared. Female mice infected with pathogens demonstrated significantly higher concentrations of IL-12 than males, and females infected with pathogens and fed probiotics demonstrated higher concentrations (P < 0.05) of IFN-γ; these data are highlighted with circles. For pathogen-derived IL-17, IL-6, IL-1α, and IL-5, the data are reported but were below the detection level for the assay. A box plot is used to visualize the distribution of the data set. Black horizontal bars represent the median values of the data set. The box encompasses the first and third quartiles, and the whiskers show the maximum and minimum values.
GIT microbial diversity in females versus males.
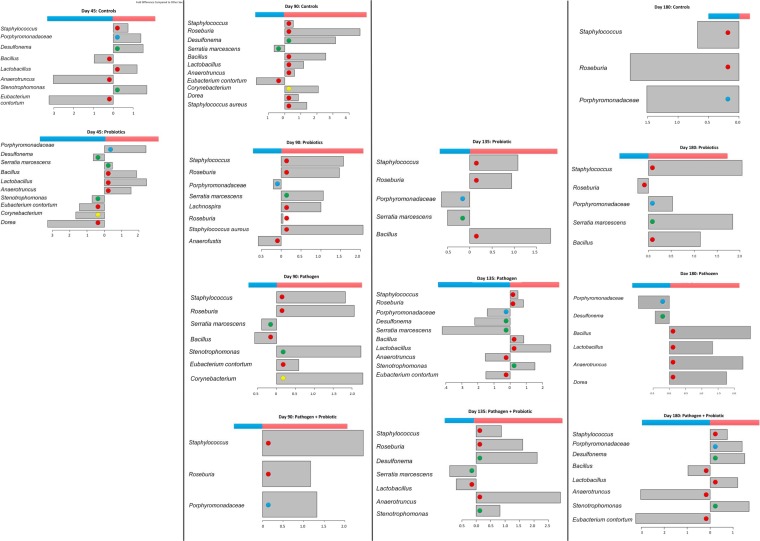
Staphylococcus and Roseburia species were overrepresented in females in all experimental groups except controls at day 180. Staphylococcal species, including S. aureus, are associated with mastitis in dairy cattle and breastfeeding women; Corynebacterium strains were >2.0-fold more numerous in females and are also associated with mastitis (Fig. 3) (34–36). Analysis of gut microbiota from pediatric Crohn's disease patients demonstrated that reductions in Roseburia were common in granulomatous disease; in our data, we observed a decrease in Roseburia with M. avium subsp. paratuberculosis infection over time, though Roseburia was nearly exclusively more abundant in females than in males (37).
FIG 3.
Differences in colon microbiota between female and male BALB/c mice. Colon tissues were collected from male and female animals (n = 5 per sex and experimental group). DNA was extracted and purified using methods described in Materials and Methods. Concentrations were measured spectrophotometrically using a NanoDrop 1000. Metagenomic analysis was conducted using the Roche 454 pyrosequencing platform (Laboratory and Testing Inc., Lubbock, TX). The x axis indicates the change on a log2 scale, and the y axis lists the organisms significantly overrepresented in females (red bars) or males (blue bars) under the described experimental conditions and at the indicated time points. The colored dots indicate the phyla that species belong to: red, Firmicutes; green, Proteobacteria; blue, Bacteroidetes; and yellow, Actinobacteria. The diversity of organisms that changed in concentration decreased with time in controls and animals fed probiotics; however, in animals exposed to pathogens or exposed to pathogens and fed probiotics, the opposite pattern was observed, with increasing species diversity at different concentrations in male and female colon tissue.
Stenotrophomonas sp. and Eubacterium contortum are gut microbes found in ulcerative colitis, IBD, Crohn's disease, and nonalcoholic fatty live disease (37–39). We identified Stenotrophomonas species at day 45 in control females with a >1.0-fold difference compared to males, and males revealed a <1.0-fold increase compared to females; at every time point, this species appeared to increase in females with M. avium subsp. paratuberculosis infection or those with M. avium subsp. paratuberculosis infection that were fed probiotics (Fig. 3). However, no differences were observed between the sexes maintained only on probiotics, suggesting sensitivity in the female colon to pathogen infection that may also support Stenotrophomonas species. E. contortum was identified mostly with males, including controls and animals infected with M. avium subsp. paratuberculosis or those with pathogen infection that were fed probiotics; in animals fed probiotics at days 90,135, and 180, biases between the sexes were not observed (Fig. 3).
Interestingly, animals maintained on a diet with probiotics demonstrated no difference in Lactobacillus species except at day 45, while in other experimental groups, Lactobacillus species were found in greater numbers in female colon tissues; this may suggest better compatibility between NP-51 and the female GIT specific to the feeding routine and concentrations described.
Desulfonema species are sulfate-reducing bacteria and were overrepresented in female control animals but demonstrated no difference at days 90 and 135 in animals fed probiotics. However, males with an M. avium subsp. paratuberculosis infection showed an increase of >2.0-fold, while pathogen-infected females maintained on a probiotic diet demonstrated a greater shift in population. This suggests that Desulfonema species may grow better in female GIT systems but are sensitive to changes induced by pathogens over time.
Serratia marcescens populations were less influenced by treatment group and were identified in males at days 90 and 135 under all conditions (Fig. 3). Interestingly, S. marcescens is able to metabolize zearalenone (a fungal metabolite that is similar to estrogen) (40).
Bacillus species could be identified at higher levels in females fed a diet including probiotics; in controls and the treatment groups exposed to pathogens, these populations were identified in greater numbers in males and females differently over time. These data are shown in Fig. 3.
Colon metabolite distinctions between females and males.
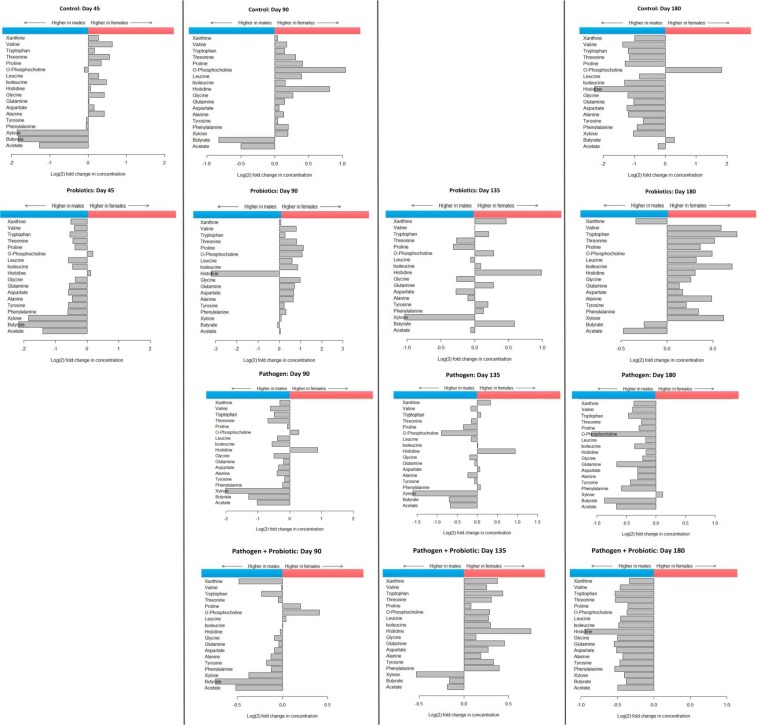
Introduction of probiotics to the gut appears to decrease the difference in concentrations between some metabolites in males and females, as shown for samples from day 90 and day 135; with age, females maintained on a diet with probiotics appeared to retain a more diversified metabolite profile than controls and pathogen-infected animals (Fig. 4). o-Phosphocholine (o-PC) was found at higher concentrations in female mice at days 45, 90, 135, and 180 than under experimental conditions; similar observations were made for histidine. Whereas in males, short-chain fatty acids (SCFAs) (butyrate and acetate) and xylose were observed at higher concentrations (0.5- to 2-fold) than in females except when fed probiotics and at day 90 (<0.5-fold) and day 135 (butyrate was >0.5-fold in females and acetate <0.5-fold in females). Consumption of probiotics decreased the differences in levels of SCFAs between males and females compared to controls or animals with pathogen infection. These data are profiled in Fig. 4.
FIG 4.
Differences in metabolomic profiles for female versus male BALB/c mouse colon tissue. In these experiments, colon tissues were processed for proton nuclear magnetic resonance (1H-NMR) spectroscopy analysis by Chenomx Inc. (Alberta, Canada). Mice were infected with M. avium subsp. paratuberculosis at day 45; day 90 is 45 days after M. avium subsp. paratuberculosis infection. The x axis describes the change in concentration on a log2 scale, and metabolites are listed on the y axis; the metabolites whose concentrations were significantly higher than those of controls are shown. Higher metabolite concentrations in males (blue) are represented by horizontal bars in the left sections, and females (red) are represented in the right sections.
DISCUSSION
IL-1α, IL-12, and IFN-γ are key regulatory cytokines in M. avium subsp. paratuberculosis immunopathogenesis; in this study, these cytokines were regulated differently in females than in males with pathogen infection. We also observed differences in IL-10, IL-17, IL-6, and IL-5 expression; thus, additional populations of immune cells, specifically regulatory T (Treg), Th17, Th1, and Th2 cells, could also be regulated through sex-linked factors. Analogous to other probiotic organisms, L. animalis NP-51 regulated proinflammatory cytokines, as shown in Fig. 2; however, these responses were related to the host sex. Based on these results, we also consider that female cattle may be more susceptible to immunopathogenesis associated with M. avium subsp. paratuberculosis infection, in part due to heightened CMI activity and sustained IL-1α production at the site of infection, than males who have decreased IL-1α and increased IL-10, IL-1β, FGF-β, and KC activities; further studies in cattle are needed to confirm these findings.
Higher levels of PC were observed in female colon tissues than in males under most experimental conditions and at most time points examined (Fig. 4). o-Phosphocholine (also, known as phosphorylcholine or choline phosphate) is a eukaryotic cell membrane component that is used by microbes to protect themselves from the host environment (41–43). PC is produced from cell membrane metabolism, when phosphatidylcholine is metabolized into glycerophospholipids and choline (43). Some Gram-positive bacteria can transport PC into the cell and incorporate PC with teichoic acid, and others express choline binding proteins; these mechanisms allow bacteria to evade the host immune system (43). Organisms that recruit PC into cells exploit phase variation (tandem-repeat loci or microsatellites) that are introduced through environmental queues, resulting in repeated DNA sequences introduced by DNA mismatch repair genes and other replication-associated machinery (43, 44). Microsatellites or phase variation in bacteria is associated with genes that regulate virulence factors or growth (44). Interestingly, PC-bound organisms, in addition to evading the immune response, are also able to suppress Th1 and CMI activity; parasitic organisms expressing PC decrease IL-12, TNF-α, and IFN-γ production and increase IL-4 (43). Similar patterns were observed in our study and previous studies, in which IL-6 and IL-5 activity increased in males while Th1 cytokines decreased in females (28, 43). This suggests that female-specific biology may contribute to increased production of o-phosphocholine that modifies microbial survival and population diversity, which could further influence the host immune response to M. avium subsp. paratuberculosis versus beneficial microbes. To determine if M. avium subsp. paratuberculosis or L. animalis produces homologs of proteins produced by organisms that incorporate PC, we used STRING 9.05 (http://string.embl.de/) (with a medium confidence score) to identify if known choline binding or -incorporating proteins could be identified in M. avium subsp. paratuberculosis or L. animalis. We were unable to identify matches to these organisms (L. animalis was not available in the database) (45, 46). However, we did find that Lactococcus lactis Il1403 PspB (glucosyltransferase-S) showed similarity to the choline binding proteins CbpA, LytC, and CbpD and L. lactis cell wall protein YcbG and Lactobacillus plantarum WCFS1 had similarity to LicD proteins associated with lipopolysaccharide (LPS) biosynthesis. This may suggest that phosphocholine-incorporating or binding proteins may occur in species of lactobacilli, which could contribute to their reception in the host GIT or by domestic host microbiota; however, extensive work is required before arriving at this conclusion.
The log2 increase in histidine (often accompanied by o-PC increases in females) suggests host and microbial contributions to its regulation. The precursor and end product, glutamate, can be further utilized by some GIT bacteria (including Bacteroides species) that synthesize new amino acids from SCFA and nitrates (NH3+). Histidine can be catabolized by Firmicutes as a carbon source, producing glutamate and NH3+ (47, 48). The histidine utilization (Hut) system is highly conserved in bacteria, and those with Hut pathways are able to use histidine as their main source of nitrogen (48). Synthesis of histidine is expensive to a cell, and therefore, catabolism is highly regulated and futile production and excessive catabolism are unwarranted (48). As such, high concentrations of histidine are able to inhibit bacterial growth and are especially toxic to organisms that do not have Hut systems, including some enteric food-borne pathogens, such as Escherichia coli and Salmonella, Enterobacter, Shigella, and Proteus species (48). Thus, if exogenous histidine levels are high (like those conditions identified at day 90 in mice fed probiotics) (Fig. 4), the microbes that are able to survive are organisms more tolerant of high histidine levels and with active Hut systems. Therefore, histidine regulation may also be a mechanism by which microbes are able to protect their ecosystems from nonnative organisms. However, if SCFA and histidine levels are high, amino acid synthesis and other anabolic activities may restore host metabolic homeostasis and promote growth by domestic organisms in the GIT ecosystems if probiotics are present (and are supported through some host sex-specific factors).
Similar to these results, metabolomics studies of human samples demonstrate similar correlations; o-phosphocholine was more abundant in females and less in males, and similar results were discovered for histidine (49, 50). Males also had higher levels of valine, leucine, and isoleucine and higher oxidation of amino acids than females (49). These differences were significant enough to differentiate each population and to produce identifiable biomarkers (49). Similar distinctions were noted in different murine strains, where C57BL/6 and 129S1/SvlmJ female mice produced lower levels of amino acids (including those previously described: serine, threonine, and carboxylic acid cycle metabolites) (51). In our study, we witnessed similar changes in these amino acids and SCFAs from males in all experimental groups (except for animals maintained on a diet of probiotics).
In our data, we frequently observed higher concentrations of butyrate, acetate, and xylose (a plant polysaccharide) in male colon tissues than in females, which suggested that these metabolites could contribute to increased anti-inflammatory mediators, specifically in males. Furthermore, we hypothesize that the smaller differences in SCFAs and other metabolites in both sexes consuming NP-51 may contribute to the regulation of proinflammatory mediators (importantly for M. avium subsp. paratuberculosis infections, IL-12 and IFN-γ). Most complex plant polysaccharides (including xylans) are not digestible by humans but are fermented by colon microbes into SCFAs, including butyrate, acetate, and propionate (47). The ratio of acetate to butyrate can optimize ATP production by microbes and, therefore, energy available for other cell functions (47). SCFAs like butyrate are utilized by host epithelial cells for energy, and acetate and propionate are used by peripheral host tissues (52). These compounds also affect host cell regulation; butyrate is a histone deacetylase inhibitor that regulates anti-inflammatory mediators (21, 53). Butyrate has antiadhesive properties in the presence of IL-1β and blocks IP-10 synthesis (54). In macrophages, butyrate is able to decrease the inflammatory compounds tumor necrosis factor alpha (TNF-α), IL-6, IL-12, and IFN-γ but increases the immune suppressor IL-10 (53). SCFAs also modulate leukocyte functions; butyrate inhibits T cell proliferation and Treg cells (53). Interestingly, the balance between these cytokines can also modify the GIT microbiota, further exacerbating conditions that contribute to chronic inflammation, like those observed in our study (21).
Conclusions.
Our research illustrates that female and male mice produce dissimilar responses to pathogens and probiotics using the same cytokines, demonstrating that (i) the regulation of these cytokines is directed through additional factors linked to sex and (ii) pathogens and commensal organisms are not recognized by certain parts of the immune system through similar mechanisms. These data also indicate that (i) o-phosphocholine and histidine concentrations in the colon are susceptible to female-specific biology, (ii) SCFAs are produced at higher concentrations in male colon tissue under most experimental conditions, and (iii) probiotic administration modifies metabolic activity, resulting in changes to SCFA synthesis. Additionally, we observed sex-specific associations between gut microbes, specifically, Firmicutes (especially Staphylococcus and Roseburia species), depending on the experimental conditions. Interestingly, the same select species fluctuated between male and female animals regardless of experimental conditions or time, and most of these organisms were Firmicutes, although with pathogen infection, Proteobacteria shifted more between the sexes. In addition, multiple species were associated with the female GIT compared to the male GIT, suggesting that the dynamic between metabolic and immune activities may influence the microbiota that perpetuate in the GIT of females compared with males.
M. avium subsp. paratuberculosis is a known pathogen in the livestock industry that contributes to over $1 billion in losses to the dairy industry alone (14). As demonstrated in this study, organisms associated with mastitis and gastrointestinal diseases were stimulated in the GIT of animals infected with M. avium subsp. paratuberculosis. As previous studies have also demonstrated, short-term changes to the gut microbiome, through antibiotics or probiotics, are only temporarily able to promote changes to the microbiota, resulting in a return to the native composition (47). For long-term modifications, like those observed in animals maintained on a diet with probiotics for 180 days, the modifiers may need to be consumed regularly and tolerated by the domestic microbial community and host to perpetuate significant change.
As this study and others have shown, microbes have evolved with their mammalian hosts, responding to environmental cues, like cytokines, hormones, and metabolites. This suggests that these mediators are multifunctional and could be recognized as important environmental signals by our cohabitants (3, 11, 55, 56). How microbes perceive these sex-specific regulatory mediators may prove to be relative. Women are 30% more likely to develop irritable bowel disease than men (57, 58). Studies have demonstrated estrogen to be contributive to GIT diseases, but the specific mechanisms associated with its action are largely understudied (57–61). Both females and males produce estrogen and estrogen receptors in GIT tissues, and learning more about these biological relationships could elucidate unexplored niches in disease (61–64). Equally, exploring testosterone and its properties could also lead to new understanding of the connections between the endocrine, immune, GIT, and nervous systems (61–64).
ACKNOWLEDGMENTS
We thank P. C. Kurkure, R. D. Lackey, and E. P. Kiernan for the original collection and processing of samples associated with this study (28). We also thank Judith Stabel (NADC), Mohamed Osman (ISU), and Don Beitz (ISU) for providing materials and insight into M. avium subsp. paratuberculosis animal models, as well as TTU and the TTUHSC Core laboratory facilities.
E. Karunasena conceived the immunoassays, metabolomics, and metagenomic experiments for the study; developed the experimental design; acquired and interpreted the data; and drafted the manuscript. K. W. McMahon developed computational and statistical analysis methods for all the experiments described, developed the figures, and drafted computational and statistical methods in the manuscript. D. Chang conducted the metabolomics experiments and the initial analysis of the data. M. M. Brashears contributed to the design of NP-51/probiotic use in the animal model.
Nutrition Physiology Inc. (NPC) and the Centers of Excellence support for the International Center for Food Industry Excellence (ICFIE) provided funding for this study, including some salary for M. M. Brashears, E. Karunasena, R. D. Lackey, and P. C. Kurkure. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
There are no financial, personal, or professional interests that could be construed to have influenced the paper.
Footnotes
Published ahead of print 9 May 2014
REFERENCES
- 1.Yotis W, Stanke R. 1966. Bacteriostatic action of progesterone on staphylococci and other microorganisms. J. Bacteriol. 92:1285–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yotis WW. 1967. In vivo and in vitro action of norethindrone on staphylococci. J. Bacteriol. 94:1353–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–1088. 10.1126/science.1233521 [DOI] [PubMed] [Google Scholar]
- 4.Oertelt-Prigione S. 2012. The influence of sex and gender on the immune response. Autoimmun. Rev. 11:A479–A485. 10.1016/j.autrev.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 5.Hughes GC, Clark EA. 2007. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity 40:470–481. 10.1080/08916930701464764 [DOI] [PubMed] [Google Scholar]
- 6.Bouman A, Heineman MJ, Faas MM. 2005. Sex hormones and the immune response in humans. Hum. Reprod. Update 11:411–423. 10.1093/humupd/dmi008 [DOI] [PubMed] [Google Scholar]
- 7.Panchanathan R, Duan X, Shen H, Rathinam VA, Erickson LD, Fitzgerald KA, Choubey D. 2010. Aim2 deficiency stimulates the expression of IFN-inducible Ifi202, a lupus susceptibility murine gene within the Nba2 autoimmune susceptibility locus. J. Immunol. 185:7385–7393. 10.4049/jimmunol.1002468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. 2010. Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity. PLoS One 5:e10868. 10.1371/journal.pone.0010868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox HS, Bond BL, Parslow TG. 1991. Estrogen regulates the IFN-gamma promoter. J. Immunol. 146:4362–4367 [PubMed] [Google Scholar]
- 10.Marriott I, Huet-Hudson YM. 2006. Sexual dimorphism in innate immune responses to infectious organisms. Immunol. Res. 34:177–192. 10.1385/IR:34:3:177 [DOI] [PubMed] [Google Scholar]
- 11.Marriott I, Bost KL, Huet-Hudson YM. 2006. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol. 71:12–27. 10.1016/j.jri.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Gomez E, Gonzalez-Pedrajo B, Camacho-Arroyo I. 2013. Role of sex steroid hormones in bacterial-host interactions. Biomed. Res. Int. 2013:928290. 10.1155/2013/928290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermon-Taylor J. 2009. Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut Pathog. 1:4757–4749. 10.1186/1757-4749-1-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris NB, Barletta RG. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489–512. 10.1128/CMR.14.3.489-512.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartor RB. 2005. Does Mycobacterium avium subspecies paratuberculosis cause Crohn's disease? Gut 54:896–898. 10.1136/gut.2004.055889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters WR, Miller JM, Palmer MV, Stabel JR, Jones DE, Koistinen KA, Steadham EM, Hamilton MJ, Davis WC, Bannantine JP. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130–5138. 10.1128/IAI.71.9.5130-5138.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo SR, Czuprynski CJ. 2008. Tactics of Mycobacterium avium subsp. paratuberculosis for intracellular survival in mononuclear phagocytes. J. Vet. Sci. 9:1–8. 10.4142/jvs.2008.9.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo SR, Sotos J, Hart AP, Barletta RG, Czuprynski CJ. 2006. Bovine monocytes and a macrophage cell line differ in their ability to phagocytose and support the intracellular survival of Mycobacterium avium subsp. paratuberculosis. Vet. Immunol. Immunopathol. 110:109–120. 10.1016/j.vetimm.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 19.Stabel JR, Ackermann MR. 2002. Temporal Mycobacterium paratuberculosis infection in T-cell receptor (TCR)-alpha and TCR-delta-deficient mice. Vet. Immunol. Immunopathol. 89:127–132. 10.1016/S0165-2427(02)00167-8 [DOI] [PubMed] [Google Scholar]
- 20.Motiwala AS, Janagama HK, Paustian ML, Zhu X, Bannantine JP, Kapur V, Sreevatsan S. 2006. Comparative transcriptional analysis of human macrophages exposed to animal and human isolates of Mycobacterium avium subspecies paratuberculosis with diverse genotypes. Infect. Immun. 74:6046–6056. 10.1128/IAI.00326-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tremaroli V, Backhed F. 2012. Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249. 10.1038/nature11552 [DOI] [PubMed] [Google Scholar]
- 22.Clemente JC, Ursell LK, Parfrey LW, Knight R. 2012. The impact of the gut microbiota on human health: an integrative view. Cell 148:1258–1270. 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumgart DC, Sandborn WJ. 2007. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657. 10.1016/S0140-6736(07)60751-X [DOI] [PubMed] [Google Scholar]
- 24.Jones JL, Loftus EV., Jr 2007. Lymphoma risk in inflammatory bowel disease: is it the disease or its treatment? Inflamm. Bowel Dis. 13:1299–1307. 10.1002/ibd.20211 [DOI] [PubMed] [Google Scholar]
- 25.Pierce ES. 2010. Ulcerative colitis and Crohn's disease: is Mycobacterium avium subspecies paratuberculosis the common villain? Gut Pathog. 2:21. 10.1186/1757-4749-2-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U. S. A. 104:13780–13785. 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brashears MM, Amezquita A, Jaroni D. 2005. Lactic acid bacteria and their uses in animal feeding to improve food safety. Adv. Food Nutr. Res. 50:1–31. 10.1016/S1043-4526(05)50001-9 [DOI] [PubMed] [Google Scholar]
- 28.Karunasena E, Kurkure PC, Lackey RD, McMahon KW, Kiernan EP, Graham S, Alabady MS, Campos DL, Tatum OL, Brashears MM. 2013. Effects of the probiotic Lactobacillus animalis in murine Mycobacterium avium subspecies paratuberculosis infection. BMC Microbiol. 13:1471–2180. 10.1186/1471-2180-13-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Baarlen P, Troost F, van der Meer C, Hooiveld G, Boekschoten M, Brummer RJ, Kleerebezem M. 2011. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl 1):4562–4569. 10.1073/pnas.1000079107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Baarlen P, Wells JM, Kleerebezem M. 2013. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 34:208–215. 10.1016/j.it.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 31.Konstantinov SR, Smidt H, de Vos WM, Bruijns SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. U. S. A. 105:19474–19479. 10.1073/pnas.0810305105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen HR, Frokiaer H, Pestka JJ. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171–178. 10.4049/jimmunol.168.1.171 [DOI] [PubMed] [Google Scholar]
- 33.R Development Core Team. 2014. R: a language and environment for statistical computing. R Development Core Team, Vienna, Austria [Google Scholar]
- 34.Kerro Dego O, van Dijk JE, Nederbragt H. 2002. Factors involved in the early pathogenesis of bovine Staphylococcus aureus mastitis with emphasis on bacterial adhesion and invasion. A review. Vet. Q. 24:181–198. 10.1080/01652176.2002.9695135 [DOI] [PubMed] [Google Scholar]
- 35.Crepinsek MA, Crowe L, Michener K, Smart NA. 2012. Interventions for preventing mastitis after childbirth. Cochrane Database Syst. Rev. 10:CD007239. 10.1002/14651858.CD007239.pub3 [DOI] [PubMed] [Google Scholar]
- 36.Reyher KK, Haine D, Dohoo IR, Revie CW. 2012. Examining the effect of intramammary infections with minor mastitis pathogens on the acquisition of new intramammary infections with major mastitis pathogens—a systematic review and meta-analysis. J. Dairy Sci. 95:6483–6502. 10.3168/jds.2012-5594 [DOI] [PubMed] [Google Scholar]
- 37.Kellermayer R, Mir SA, Nagy-Szakal D, Cox SB, Dowd SE, Kaplan JL, Sun Y, Reddy S, Bronsky J, Winter HS. 2012. Microbiota separation and C-reactive protein elevation in treatment-naive pediatric granulomatous Crohn disease. J. Pediatr. Gastroenterol. Nutr. 55:243–250. 10.1097/MPG.0b013e3182617c16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, Greenwood R, Sikaroodi M, Lam V, Crotty P, Bailey J, Myers RP, Rioux KP. 2013. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 11:868–875. 10.1016/j.cgh.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 39.Knosel T, Schewe C, Petersen N, Dietel M, Petersen I. 2009. Prevalence of infectious pathogens in Crohn's disease. Pathol. Res. Pract. 205:223–230. 10.1016/j.prp.2008.04.018 [DOI] [PubMed] [Google Scholar]
- 40.Aziz NH. 2002. The role of chitinese of Serratia marcescens in controlling the production of zearalenone by Fusarium graminearum. Acta Microbiol. Pol. 51:131–137 [PubMed] [Google Scholar]
- 41.Holmes E, Li JV, Marchesi JR, Nicholson JK. 2012. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 16:559–564. 10.1016/j.cmet.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 42.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. 2012. Host-gut microbiota metabolic interactions. Science 336:1262–1267. 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- 43.Clark SE, Weiser JN. 2013. Microbial modulation of host immunity with the small molecule phosphorylcholine. Infect. Immun. 81:392–401. 10.1128/IAI.01168-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gemayel R, Vinces MD, Legendre M, Verstrepen KJ. 2010. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu. Rev. Genet. 44:445–477. 10.1146/annurev-genet-072610-155046 [DOI] [PubMed] [Google Scholar]
- 45.Snel B, Lehmann G, Bork P, Huynen MA. 2000. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 28:3442–3444. 10.1093/nar/28.18.3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. 2013. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 41:D808–D815. 10.1093/nar/gks1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischbach MA, Sonnenburg JL. 2011. Eating for two: how metabolism establishes interspecies interactions in the gut. Cell Host Microbe 10:336–347. 10.1016/j.chom.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bender RA. 2012. Regulation of the histidine utilization (hut) system in bacteria. Microbiol. Mol. Biol. Rev. 76:565–584. 10.1128/MMBR.00014-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mittelstrass K, Ried JS, Yu Z, Krumsiek J, Gieger C, Prehn C, Roemisch-Margl W, Polonikov A, Peters A, Theis FJ, Meitinger T, Kronenberg F, Weidinger S, Wichmann HE, Suhre K, Wang-Sattler R, Adamski J, Illig T. 2011. Discovery of sexual dimorphisms in metabolic and genetic biomarkers. PLoS Genet. 7:e1002215. 10.1371/journal.pgen.1002215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertram HC, Duus JO, Petersen BO, Hoppe C, Larnkjaer A, Schack-Nielsen L, Molgaard C, Michaelsen KF. 2009. Nuclear magnetic resonance-based metabonomics reveals strong sex effect on plasma metabolism in 17-year-old Scandinavians and correlation to retrospective infant plasma parameters. Metabolism 58:1039–1045. 10.1016/j.metabol.2009.03.011 [DOI] [PubMed] [Google Scholar]
- 51.Qiao Q, Li T, Sun J, Liu X, Ren J, Fei J. 2011. Metabolomic analysis of normal (C57BL/6J, 129S1/SvImJ) mice by gas chromatography-mass spectrometry: detection of strain and gender differences. Talanta 85:718–724. 10.1016/j.talanta.2011.04.060 [DOI] [PubMed] [Google Scholar]
- 52.Sonnenburg ED, Sonnenburg JL, Manchester JK, Hansen EE, Chiang HC, Gordon JI. 2006. A hybrid two-component system protein of a prominent human gut symbiont couples glycan sensing in vivo to carbohydrate metabolism. Proc. Natl. Acad. Sci. U. S. A. 103:8834–8839. 10.1073/pnas.0603249103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. 2011. Regulation of inflammation by short chain fatty acids. Nutrients 3:858–876. 10.3390/nu3100858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmeliet P. 2005. VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10. 10.1159/000088478 [DOI] [PubMed] [Google Scholar]
- 55.Arnold AP. 2009. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J. Neuroendocrinol. 21:377–386. 10.1111/j.1365-2826.2009.01831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez A, Luckey D, Yeoman CJ, Marietta EV, Berg Miller ME, Murray JA, White BA, Taneja V. 2012. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS One 7:e36095. 10.1371/journal.pone.0036095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogan AM, Collins D, Baird AW, Winter DC. 2009. Estrogen and its role in gastrointestinal health and disease. Int. J. Colorectal Dis. 24:1367–1375. 10.1007/s00384-009-0785-0 [DOI] [PubMed] [Google Scholar]
- 58.Klein SL. 2012. Immune cells have sex and so should journal articles. Endocrinology 153:2544–2550. 10.1210/en.2011-2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Mahony F, Harvey BJ. 2008. Sex and estrous cycle-dependent rapid protein kinase signaling actions of estrogen in distal colonic cells. Steroids 73:889–894. 10.1016/j.steroids.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 60.Kahl S, Elsasser TH, Li CJ. 2011. Modeling the effects of estradiol and progesterone on the acute phase proinflammatory axis: variability in tumor necrosis factor-alpha, nitric oxide, and xanthine oxidase responses to endotoxin challenge in steers. Domest. Anim. Endocrinol. 40:213–221. 10.1016/j.domaniend.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 61.Kawano N, Koji T, Hishikawa Y, Murase K, Murata I, Kohno S. 2004. Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem. Cell Biol. 121:399–405. 10.1007/s00418-004-0644-6 [DOI] [PubMed] [Google Scholar]
- 62.Araujo AB, Wittert GA. 2011. Endocrinology of the aging male. Best Pract. Res. Clin. Endocrinol. Metab. 25:303–319. 10.1016/j.beem.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bested AC, Logan AC, Selhub EM. 2013. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part I—autointoxication revisited. Gut Pathog. 5:5. 10.1186/1757-4749-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bested AC, Logan AC, Selhub EM. 2013. Intestinal microbiota, probiotics and mental health: from Metchnikoff to modern advances: part II—contemporary contextual research. Gut Pathog. 5:3. 10.1186/1757-4749-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]