Abstract
Direct interspecies electron transfer (DIET) is potentially an effective form of syntrophy in methanogenic communities, but little is known about the diversity of methanogens capable of DIET. The ability of Methanosarcina barkeri to participate in DIET was evaluated in coculture with Geobacter metallireducens. Cocultures formed aggregates that shared electrons via DIET during the stoichiometric conversion of ethanol to methane. Cocultures could not be initiated with a pilin-deficient G. metallireducens strain, suggesting that long-range electron transfer along pili was important for DIET. Amendments of granular activated carbon permitted the pilin-deficient G. metallireducens isolates to share electrons with M. barkeri, demonstrating that this conductive material could substitute for pili in promoting DIET. When M. barkeri was grown in coculture with the H2-producing Pelobacter carbinolicus, incapable of DIET, M. barkeri utilized H2 as an electron donor but metabolized little of the acetate that P. carbinolicus produced. This suggested that H2, but not electrons derived from DIET, inhibited acetate metabolism. P. carbinolicus-M. barkeri cocultures did not aggregate, demonstrating that, unlike DIET, close physical contact was not necessary for interspecies H2 transfer. M. barkeri is the second methanogen found to accept electrons via DIET and the first methanogen known to be capable of using either H2 or electrons derived from DIET for CO2 reduction. Furthermore, M. barkeri is genetically tractable, making it a model organism for elucidating mechanisms by which methanogens make biological electrical connections with other cells.
INTRODUCTION
Effective interspecies electron transfer is key to the efficient functioning of methanogenic communities (1–4). Promoting interspecies electron transfer to methanogens enhances the anaerobic digestion of wastes, and appropriate models of the pathways for interspecies electron transfer are necessary in order to predictively model the response of methanogenic communities to environmental change.
The best-known strategy for interspecies electron transfer is H2 interspecies transfer (HIT), in which electron-donating microorganisms reduce protons to H2 and methanogens oxidize the H2 with the reduction of carbon dioxide to methane (1). In some instances, formate substitutes for H2 as the electron carrier (2–6). The abundance of H2/formate-consuming methanogens in anaerobic soils (7, 8) and sediments (9, 10) as well as some anaerobic digesters (11) suggests that HIT plays an important role in methane production in those environments. HIT has been documented in studies with defined cocultures of H2-donating microorganisms and H2-consuming methanogens, and the physiology and biochemistry of both H2 production and H2 consumption are relatively well understood (1, 12, 13).
Direct interspecies electron transfer (DIET) is an alternative to HIT. Methanosaeta (Methanotrix) harundinacea, which is representative of the Methanosaeta species, which are responsible for a substantial portion of the global methane production (14), directly accepted electrons from Geobacter metallireducens for the reduction of carbon dioxide to methane in defined cocultures (15). DIET in these cocultures required that G. metallireducens produce pili (15), that have a metal-like conductivity (16, 17), but further details of the interspecies electrical connections are as yet unknown. Multiple lines of evidence suggested that Methanosaeta species were also participating in DIET in anaerobic digesters treating brewery wastes (15, 18). Geobacter species were abundant in the digester granules, which possessed a metal-like conductivity (18) similar to the metal-like conductivity of Geobacter pili (16–18). The potential importance of conductive pili in electron transfer to Methanosaeta species is consistent with the clear importance of conductive pili in DIET in cocultures of G. metallireducens and G. sulfurreducens, cooperating to oxidize ethanol with the reduction of fumarate (19–22). The electrical connections that M. harundinacea employs for DIET are unknown, and elucidating them may be technically difficult because a system for genetic manipulation of this organism has not yet been identified.
Another genus of acetoclastic methanogens, Methanosarcina, is often abundant in methanogenic soils and sediments (23–25), coal mines (26, 27), landfills (28), and anaerobic digesters (29, 30). Several studies suggested that Methanosarcina species could accept electrons from nonbiological extracellular surfaces (31, 32). For example, M. barkeri attached onto electrically conductive granular activated carbon (GAC), which served as a mediator for electron transfer between M. barkeri and G. metallireducens (32). Conductive iron minerals were proposed to function as mediators between Geobacter and Methanosarcina species in a similar manner, based on the abundance of these genera in mineral-amended enrichment cultures (31). Here we provide evidence that M. barkeri does not require a conductive mediator for DIET because it is capable of forging direct biological electrical connections to receive electrons from G. metallireducens.
MATERIALS AND METHODS
Strains and conditions for cultivation.
Methanosarcina barkeri (DSM 800) and Pelobacter carbinolicus (DSM 2380) were purchased from the DSMZ culture collection. Geobacter metallireducens strain ATCC 53774 as well as gene deletion strains in which either the gene for PilA, the structural pilin protein (Gmet 1399) gene (33), or the Gmet 1868 gene, encoding a putative outer surface c-type cytochrome (34), was deleted were obtained from our laboratory culture collection. Strict anaerobic culturing procedures with either pressure tubes or serum bottles sealed with thick butyl rubber stoppers were used throughout.
M. barkeri was grown on 30 mM acetate in a modification of DSM 120 medium, which was determined to also support good growth of G. metallireducens. The DSM 120 modifications were as follows: 0.5 mM sulfide, 1 mM cysteine, 0.002 g/liter CaCl2·2H2O, 1 g/liter NaCl, no yeast extract, no Casitone, no resazurin, and 2 g/liter NaHCO3.
G. metallireducens wild-type and mutant strains were routinely cultured on a ferric citrate medium amended with 10 mM acetate (33). However, prior to initiating cocultures with M. barkeri, the G. metallireducens strains were adapted for at least three transfers (5 to 10% inoculum) in ferric citrate medium in which the acetate was replaced with 20 mM ethanol.
P. carbinolicus was routinely grown on 10 mM acetoin, as previously described (20).
The coculture inocula were obtained from cultures that were in late exponential or early stationary phase, as determined by monitoring methane production (M. barkeri), Fe(II) production (G. metallireducens), or the optical density at 600 nm (OD600) (P. carbinolicus).
The G. metallireducens-M. barkeri cocultures were initiated with a 0.5-ml inoculum of the ethanol-adapted G. metallireducens and a 0.5-ml inoculum of an acetate-grown M. barkeri culture added to 9 ml of the modified DSM 120 medium described above, but with the acetate replaced with 20 mM ethanol as the electron donor. CO2 or HCO3− was the only potential electron acceptor. After the coculture was established, it was routinely transferred into 45 ml of medium with a 10% inoculum. Coculture growth was also tested in the absence of sulfide and cysteine, but for long-term incubations, both sulfide and cysteine are required as sulfur sources for M. barkeri (35–37) and were therefore added consistently to the coculture medium. When noted, GAC was added as previously described (32). Similar to GAC-free cocultures, 9 ml medium with GAC was inoculated with 0.5 ml of a G. metallireducens strain culture and 0.5 ml of M. barkeri culture.
M. barkeri grew in the same medium as P. carbinolicus, but P. carbinolicus could not grow in DSM 120 medium or the modified version of it. Therefore, the P. carbinolicus-M. barkeri cocultures were initiated with a 5% inoculum of acetoin-grown P. carbinolicus and a 5% inoculum of acetate-grown M. barkeri in the previously described P. carbinolicus medium (20). After establishing the coculture, P. carbinolicus and M. barkeri were routinely transferred together using 10% inocula.
Analytics.
Headspace methane (0.5 ml) was sampled at regular intervals using strict anaerobic sampling techniques (38), injected on a Shimadzu 8A gas chromatograph (GC) equipped with an 80/100 Hayasep Q column heated at 110°C. The injector port and flame ionization detector (FID) were both set at 200°C (15). To determine the concentration of organic acids and ethanol, 200 μl of culture medium was sampled, diluted, and placed in tightly sealed vials specially designed for high-performance liquid chromatography (HPLC) analysis (for fatty acids) and GC analysis (for ethanol). Organic acid samples were analyzed immediately or stored at 4°C for a maximum of 1 week. HPLC analyses were performed as previously described (39) by separating the organic acids on an Aminex NPX-87H column using 8 mM H2SO4 as the eluent. Organic acids were detected at 210 nm by a UV detector. Under these conditions, the following organic acids could be monitored: acetate, formate, succinate, fumarate, lactate, propionate, and butyrate. The detection limit for all was ca. 5 μM. In all our samples, only acetate was detected. Gas chromatography analyses of ethanol were performed on a PerkinElmer Clarus600 GC equipped with a headspace automatic sampler and an FID. Separation of ethanol was obtained on an Elite 5 (PerkinElmer) column (30-m length, 0.25-mm inner diameter) using He as the carrier gas and the following separation parameters: 50°C for 1 min, a ramp of 12°C per minute to reach 200°C, and 1.5 min at 200°C. The injector and detector temperatures were set at 200°C and 300°C, respectively. The chromatography data from GC and HPLC analyses were analyzed with an integrated TotalChrom data analysis system.
The increase in aggregate biomass over time was determined as previously described (40) by harvesting (15 min, 3,600 × g) 50 ml of three M. barkeri-G. metallireducens cocultures at different growth stages: during the initial growth phase (day 5) and in stationary phase (day 28). Aggregates were washed in isotonic buffer and freeze-dried for 48 h on a Labconco lyophilizer at −50°C. The weighted dry biomass was solubilized in 0.5% SDS by steaming. The total protein in the steamed biomass was determined using the Pierce bicinchoninic acid (BCA) protein assay kit according to the manufacturer's instructions. Bovine serum albumin was used as a protein standard.
Microscopy.
To evaluate cell-to-cell contact, we checked unfixed cells immediately after removing them from a culture tube and visualized the cultures with phase-contrast microscopy on a Leica Axioplann microscope. The presence of the methanogens was verified by fluorescence at 420 nm and identification of their specific coccus shape. To better visualize how cells were distributed within the aggregates, we performed fluorescence in situ hybridization. Cells were sampled in mid-log phase, fixed in premixed glutaraldehyde (0.5%) and paraformaldehyde (1%), rinsed in 50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] buffer, dehydrated (30 min at 4°C in an ethanol series at 30%, 50%, 70%, 80%, and 100%), and preembedded in a 50:50 mix of ethanol (100%) and butyl-methyl-methacrylate (BMM). Ultimately, aggregates were embedded in BMM resin and polymerized on a Spectorlinker cross-link XL-1000 instrument at room temperature overnight. Sections of the aggregates were obtained by microtome and stained with Cy3 probes (red) targeting Methanosarcina species (MS1414, 5′-CTCACCCATACCTCACTCGGG-3′) (41) and Cy5 probes (green) specific for the Geobacter cluster (Geo3ABC, 5′-CCGCAACACCTRGTWCTCATC-3′) (42) or Cy5 (green)-labeled probes specific for P. carbinolicus (PCARB1, 5′-GCCTATTCGACCACGATA-3′) (42) depending on the coculture. The labeled cocultures were visualized using a confocal laser microscope as previously described (20).
RESULTS AND DISCUSSION
Growth of M. barkeri via DIET with G. metallireducens.
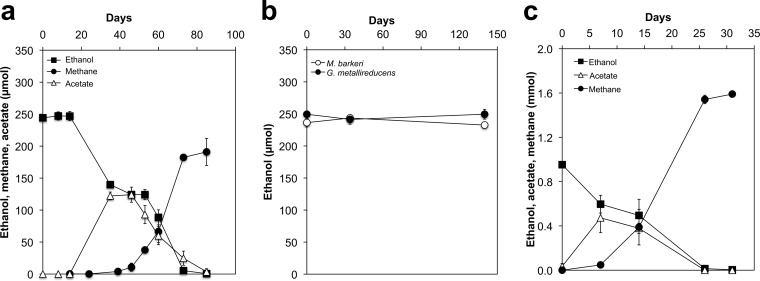
Methanosarcina barkeri was cocultured with Geobacter metallireducens in a medium with ethanol as the sole electron donor in order to determine whether it was capable of DIET. Multiple lines of evidence have indicated that G. metallireducens is unable to release the electrons derived from the oxidation of ethanol to acetate as either H2 or formate but can directly transfer these electrons to microorganisms such as G. sulfurreducens or Methanosaeta harundinacea, which are capable of DIET (15, 19–21). After a lag of ca. 39 days, cocultures of G. metallireducens and M. barkeri started metabolizing ethanol to methane (Fig. 1a). Neither G. metallireducens nor M. barkeri metabolized ethanol in pure culture (Fig. 1b). With continued transfer of the coculture, the initial long lag in ethanol metabolism was eliminated, and ethanol began to be metabolized to methane in less than 7 days (Fig. 1c).
FIG 1.
Syntrophic growth of M. barkeri with G. metallireducens. (a) Metabolism of ethanol when the cocultures were initiated with a 5% (vol/vol) inoculum of each species. (b) Lack of ethanol metabolism with single species. (c) Decreased lag after 25 transfers (10% inoculum) of the cocultures. All cultures were grown in triplicate (n = 3). A difference in the y axis scale can be noted between panels a and b and panel c. This is because of differences in the total incubation volumes: 10 ml to initiate the cocultures (a, b) and 50 ml for later incubations (c).
Acetate only transiently accumulated in the cocultures (Fig. 1a and c). The total ethanol consumed (mean ± standard deviation, 0.95 ± 0.01 mmol; n = 4) resulted in 1.59 ± 0.04 mmol methane, consistent with the expected production of 1.5 mol of methane for each mole of ethanol consumed according to the following equation: 2 C2H6O + H2O → 3 CH4 + CO2.
This stoichiometric conversion of ethanol to methane requires that M. barkeri not only metabolize the acetate that G. metallireducens produces from ethanol (2 C2H6O + 2 H2O → 2 C2H4O2 + 8 H+ + 8 e− via the reaction 2 C2H4O2 → 2 CH4 + 2 CO2) but also use the electrons released from ethanol metabolism to reduce carbon dioxide to methane: 8 H+ + 8 e− + CO2 → CH4 + 2 H2O. Thus, the high electron recovery of electrons available from ethanol in methane and the fact that G. metallireducens is incapable of producing H2 during ethanol metabolism (22) strongly suggested that M. barkeri was accepting electrons for carbon dioxide reduction via DIET.
The fact that the coculture could be continually transferred (10% inoculum) indicated that a small fraction of the carbon and electrons derived from ethanol must also be consumed for biomass production. Biomass was examined by sacrificing triplicate cocultures for protein analysis at day 5, when methane started increasing, and at day 28, when methane production was complete. Protein increased from 233 ± 62 μg ml−1 (mean ± standard deviation) to 861 ± 115 μg ml−1, verifying coculture growth.
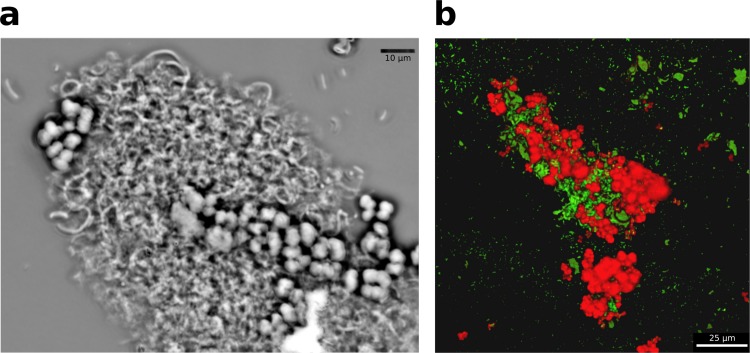
Cells involved in DIET need physical connections. G. metallireducens and M. barkeri cocultures formed aggregates of ca. 100- to 200-μm diameter (Fig. 2a) in which Methanosarcina and Geobacter were intertwined (Fig. 2b).
FIG 2.
Coculture composition. (a) Phase-contrast micrograph of an aggregate of M. barkeri (cocci) and G. metallireducens (rods). (b) Scanning laser confocal microscope image of coculture aggregate after in situ hybridization, which targeted Methanosarcina cells with a red probe (Cy3) and G. metallireducens cells with a green probe (Cy5). Images are representative of triplicate samples taken during the mid-exponential growth phase of the cocultures.
Electrical connections via outer surface proteins and granular activated carbon.
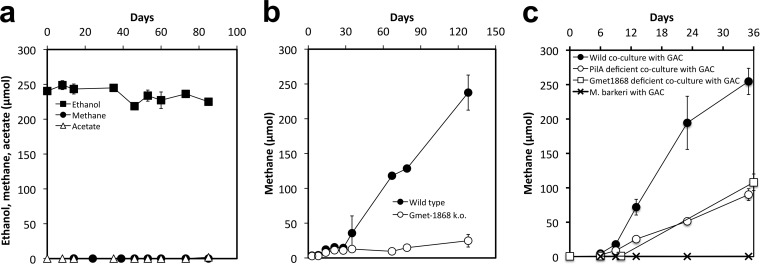
Previous studies have demonstrated that deleting pilA, which encodes the structural protein of the conductive pili of Geobacter species, eliminates the ability of G. metallireducens to participate in DIET (15, 21). Cocultures initiated with a pilA-deficient strain (33) of G. metallireducens failed to metabolize ethanol or produce methane (Fig. 3a). Furthermore, cocultures initiated with a strain of G. metallireducens in which a gene (Gmet 1868 gene) for an outer surface cytochrome required for Fe(III) oxide reduction was deleted (34) also failed to metabolize ethanol to methane (Fig. 3b).
FIG 3.
Mutant studies. (a) Lack of ethanol metabolism and methane production in a coculture initiated with a pilA-deficient G. metallireducens strain. (b) Methane production in cocultures initiated with wild-type G. metallireducens or a strain deficient in the Gmet 1868 gene, which encodes a predicted extracellular cytochrome. (c) GAC stimulation of methane production in cocultures initiated with wild-type G. metallireducens and enabling methane production in cocultures initiated with either the PilA- or cytochrome (Gmet 1868)-deficient strains of G. metallireducens. All incubations were set up with a 5% (vol/vol) inoculum of each coculture partner.
However, cocultures initiated with either the pilA-deficient or Gmet 1868-deficient strains of G. metallireducens did effectively produce methane in the presence of granular activated carbon (GAC) (Fig. 3c). This is consistent with previous studies that demonstrated that GAC, which is electrically conductive, served as an electrical conduit for DIET between G. metallireducens and G. sulfurreducens, permitting the two species to share electrons even when the coculture was initiated with strains of G. sulfurreducens that could not produce pili or the pilin-associated c-type cytochrome OmcS (32). As previously noted (32), GAC amendments also stimulated the conversion of ethanol to methane in cocultures initiated with wild-type G. metallireducens and M. barkeri (Fig. 3c), substantially reducing the 39-day lag phase observed in the absence of GAC (Fig. 1a). Methane production of the cocultures initiated with the pilA-deficient or Gmet 1868-deficient strains of G. metallireducens was slower than that of the wild-type cocultures amended with GAC, but even in the absence of pili or the outer surface c-type cytochrome, methane production rates in the presence of GAC required a <6-day lag period to initiate ethanol metabolism (Fig. 3b). Previous studies demonstrated that in cocultures of G. metallireducens with either M. barkeri or G. sulfurreducens, both syntrophic partners attached to the GAC surface, but the individual cells were too far separated for biological electrical connections between the species (32). These results suggest that GAC can substitute for pili and/or outer surface cytochromes as the electrical conduit between the electron-donating G. metallireducens and the electron-accepting M. barkeri.
Growth of M. barkeri via HIT with P. carbinolicus.
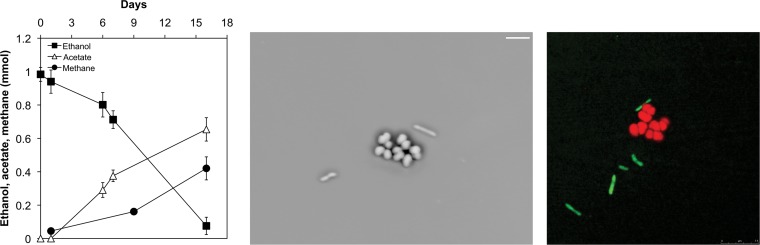
Like G. metallireducens, P. carbinolicus can grow on ethanol in the presence of a syntrophic partner, but it relies on HIT rather than DIET (20, 43). P. carbinolicus and M. barkeri grew in coculture in medium with ethanol as the sole electron donor, but unlike G. metallireducens-M. barkeri cocultures, there was a steady accumulation of acetate (Fig. 4 left panel). This was probably due to the fact that acetate metabolism in M. barkeri is repressed when H2 is available (44–46).
FIG 4.
Syntrophic growth in cocultures of P. carbinolicus and M. barkeri. (Left) Syntrophic growth in the third transfer of the coculture. (Center) Phase-contrast image illustrating the lack of aggregation of P. carbinolicus (rods) and M. barkeri (cocci). (Right) Scanning laser confocal microscope image of coculture after in situ hybridization, which targeted Methanosarcina cells with a red probe (Cy3) and P. carbinolicus cells with a green probe (Cy5). Images are representative of triplicate samples taken during the mid-exponential growth phase of the cocultures.
P. carbinolicus metabolizes ethanol to acetate and H2 according to the following equation (20): 2 C2H6O + 2 H2O → 2 C2H4O2 + 4 H2. The metabolism of ethanol to acetate and H2 is exergonic only if M. barkeri can oxidize H2 with the reduction of carbon dioxide as follows: 4 H2 + CO2 → CH4 + H2O.
The stoichiometry of ethanol consumed (mean ± standard deviation, 0.91 ± 0.07 mmol; n = 4), methane produced (0.41 ± 0.07 mmol), and acetate accumulated (0.66 ± 0.07 mmol) was consistent with methane being produced almost exclusively from H2 oxidation with the reduction of carbon dioxide, assuming that some of the acetate produced was diverted to biomass production. A similar partial oxidation of ethanol with the accumulation of acetate was observed in the ethanol-metabolizing cocultures of P. carbinolicus and G. sulfurreducens, which were dependent on HIT (20), whereas acetate did not accumulate in G. metallireducens-G. sulfurreducens cocultures that relied on DIET (20).
In contrast to the G. metallireducens-M. barkeri cocultures (Fig. 2a and b), the P. carbinolicus-M. barkeri cocultures did not form multispecies aggregates (Fig. 4b and c). In a similar manner, P. carbinolicus-G. sulfurreducens cocultures did not aggregate (20), whereas G. metallireducens-G. sulfurreducens cocultures did (19). These results are consistent with the need for direct cell-to-cell contact for DIET, but not for HIT.
Implications.
The results demonstrate that M. barkeri is able to participate in DIET. It is only the second methanogen found to have this capability. Methanosarcina species are important contributors to methane production in some anaerobic digesters (29) as well as soils and sediments (7–10). Thus, the potential for DIET should be considered when attempting to promote the growth of Methanosarcina in digesters or when modeling their activity in soils and sediments.
In previous pure-culture studies, Methanosaeta harundinacea, which is incapable of utilizing H2 as an electron donor, was found to be capable of DIET, but the two known H2-utilizing methanogens tested, Methanospirillum hungatei and Methanobacterium formicicum, were not (15). Thus, M. barkeri is more versatile than other methanogens that have been evaluated for DIET, with the capacity to either accept electrons in the form of H2 from syntropic partners like Desulfovibrio (46) and Pelobacter (this study) or to participate in DIET.
In the studies on DIET with M. harundinacea, acetate was available as an additional substrate for methane production (15), as it was in the M. barkeri cocultures reported here. However, studies with cocultures of G. metallireducens and a strain of G. sulfurreducens unable to oxidize acetate demonstrated that electrons derived from DIET could support fumarate reduction and growth of G. sulfurreducens in the absence of acetate as an additional electron donor (22). Similar studies should be feasible with an M. barkeri mutant unable to metabolize acetate (47) to determine whether carbon dioxide reduction to methane with electrons derived from DIET provides sufficient energy to support cell maintenance and growth. Other than the obvious importance of DIET in some anaerobic digesters treating brewery wastes (15, 18), the prevalence of DIET in other methanogenic communities is unknown. The fact that at least two major genera of methanogens have evolved the capacity for DIET suggests that there are conditions in some soils and sediments in which DIET confers a selective advantage.
The ability of GAC to promote methanogenic DIET has important implications for the design of anaerobic digesters. Previous studies that have reported stimulation in methane production when carbon cloth was introduced into digesters have attributed this response to the cloth retaining methanogens (48–50). However, the possibility that these conductive materials might promote DIET should also be considered.
Although the importance of electrically conductive pili and outer surface cytochromes in extracellular electron exchange, including DIET, is becoming increasingly apparent for Geobacter species (51, 52), the potential extracellular electron contacts that might permit methanogens to accept electrons via DIET are less clear. Methanosaeta and Methanosarcina are the only genera of methanogens with membrane-bound cytochromes (53, 54) that could potentially play a role in extracellular electron exchange. The availability of tools for genetic manipulation of M. barkeri (55) suggests that it may be the ideal candidate for functional analysis of DIET mechanisms in methanogens.
ACKNOWLEDGMENTS
We thank Pier-Luc Tremblay and Jessica Smith for providing G. metallireducens deletion mutant strains. We acknowledge the laboratory assistance of Dan Carlo Flores, Trevor Woodard, and Joy Ward.
This work was supported by the Office of Science, U.S. Department of Energy, award number DE-SC0004485.
Footnotes
Published ahead of print 16 May 2014
REFERENCES
- 1.Sieber JR, McInerney MJ, Gunsalus RP. 2012. Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu. Rev. Microbiol. 66:429–452. 10.1146/annurev-micro-090110-102844 [DOI] [PubMed] [Google Scholar]
- 2.Boone DR, Johnson RL, Liu Y. 1989. Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl. Environ. Microbiol. 55:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Bok FA, Luijten ML, Stams AJ. 2002. Biochemical evidence for formate transfer in syntrophic propionate-oxidizing cocultures of Syntrophobacter fumaroxidans and Methanospirillum hungatei. Appl. Environ. Microbiol. 68:4247–4252. 10.1128/AEM.68.9.4247-4252.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bok FAM, Plugge CM, Stams AJM. 2004. Interspecies electron transfer in methanogenic propionate degrading consortia. Water Res. 38:1368–1375. 10.1016/j.watres.2003.11.028 [DOI] [PubMed] [Google Scholar]
- 5.Dolfing J, Jiang B, Henstra AM, Stams AJ, Plugge CM. 2008. Syntrophic growth on formate: a new microbial niche in anoxic environments. Appl. Environ. Microbiol. 74:6126–6131. 10.1128/AEM.01428-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiele JH, Zeikus JG. 1988. Control of interspecies electron flow during anaerobic digestion: significance of formate transfer versus hydrogen transfer during syntrophic methanogenesis in flocs. Appl. Environ. Microbiol. 54:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosskopf R, Janssen PH, Liesack W. 1998. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl. Environ. Microbiol. 64:960–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrad R. 1999. Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiol. Ecol. 28:193–202. 10.1111/j.1574-6941.1999.tb00575.x [DOI] [Google Scholar]
- 9.Conrad R, Klose M, Claus P, Enrich-Prast A. 2010. Methanogenic pathway, 13C isotope fractionation, and archaeal community composition in the sediment of two clear-water lakes of Amazonia. Limnol. Oceanogr. 55:689–702. 10.4319/lo.2009.55.2.0689 [DOI] [Google Scholar]
- 10.Chan OC, Claus P, Casper P, Ulrich A, Lueders T, Conrad R. 2005. Vertical distribution of structure and function of the methanogenic archaeal community in Lake Dagow sediment. Environ. Microbiol. 7:1139–1149. 10.1111/j.1462-2920.2005.00790.x [DOI] [PubMed] [Google Scholar]
- 11.Demirel B, Scherer P. 2008. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: a review. Rev. Environ. Sci. Biotechnol. 7:173–190. 10.1007/s11157-008-9131-1 [DOI] [Google Scholar]
- 12.McInerney MJ, Struchtemeyer CG, Sieber J, Mouttaki H, Stams AJ, Schink B, Rohlin L, Gunsalus RP. 2008. Physiology, ecology, phylogeny, and genomics of microorganisms capable of syntrophic metabolism. Ann. N. Y. Acad. Sci. 1125:58–72. 10.1196/annals.1419.005 [DOI] [PubMed] [Google Scholar]
- 13.Hedderich R, Whitman W. 2013. Physiology and biochemistry of the methane-producing Archaea, p 635–662 In Rosenberg E, DeLong E, Lory S, Stackebrandt E, Thompson F. (ed), The prokaryotes. Springer, Berlin, Germany [Google Scholar]
- 14.Smith KS, Ingram-Smith C. 2007. Methanosaeta, the forgotten methanogen. Trends Microbiol. 15:150–155. 10.1016/j.tim.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Rotaru A-E, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, Zengler K, Nevin K, Lovley D. 2014. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 7:408–415. 10.1039/c3ee42189a [DOI] [Google Scholar]
- 16.Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen MT, Lovley DR. 2011. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 6:573–579. 10.1038/nnano.2011.119 [DOI] [PubMed] [Google Scholar]
- 17.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. 10.1038/nature03661 [DOI] [PubMed] [Google Scholar]
- 18.Morita M, Malvankar NS, Franks A-E, Summers ZM, Giloteaux L, Rotaru A-E, Rotaru C, Lovley DR. 2011. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2:e00159–11. 10.1128/mBio.00159-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. 10.1126/science.1196526 [DOI] [PubMed] [Google Scholar]
- 20.Rotaru A-E, Shrestha PM, Liu F, Ueki T, Summers ZM, Lovley DR. 2012. Interspecies electron transfer via hydrogen and formate rather than direct electrical connections in cocultures of Pelobacter carbinolicus and Geobacter sulfurreducens. Appl. Environ. Microbiol. 78:7645–7651. 10.1128/AEM.01946-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrestha PM, Rotaru A-E, Summers ZM, Shrestha M, Liu F, Lovley DR. 2013. Transcriptomic and genetic analysis of direct interspecies electron transfer. Appl. Environ. Microbiol. 79:2397–2404. 10.1128/AEM.03837-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrestha PM, Rotaru A-E, Aklujkar M, Liu F, Shrestha M, Summers ZM, Malvankar N, Flores DC, Lovley DR. 2013. Syntrophic growth with direct interspecies electron transfer as the primary mechanism for energy exchange. Environ. Microbiol. Rep. 5:904–910. 10.1111/1758-2229.12093 [DOI] [PubMed] [Google Scholar]
- 23.Zepp Falz K, Holliger C, Grosskopf R, Liesack W, Nozhevnikova A, Muller B, Wehrli B, Hahn D. 1999. Vertical distribution of methanogens in the anoxic sediment of Rotsee (Switzerland). Appl. Environ. Microbiol. 65:2402–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H-W, Zhang L-M, Yuan C-L, He J-Z. 2013. Contrasting Euryarchaeota communities between upland and paddy soils exhibited similar pH-impacted biogeographic patterns. Soil Biol. Biochem. 64:18–27. 10.1016/j.soilbio.2013.04.003 [DOI] [Google Scholar]
- 25.Kudo Y, Nakajima T, Miyaki T, Oyaizu H. 1997. Methanogen flora of paddy soils in Japan. FEMS Microbiol. Ecol. 22:39–48. 10.1111/j.1574-6941.1997.tb00354.x [DOI] [Google Scholar]
- 26.Wei M, Yu Z, Zhang H. 2013. Microbial diversity and abundance in a representative small-production coal mine of central China. Energy Fuels 27:3821–3829. 10.1021/ef400529f [DOI] [Google Scholar]
- 27.Beckmann S, Lueders T, Kruger M, von Netzer F, Engelen B, Cypionka H. 2011. Acetogens and acetoclastic Methanosarcinales govern methane formation in abandoned coal mines. Appl. Environ. Microbiol. 77:3749–3756. 10.1128/AEM.02818-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staley BF, Francis L, Barlaz MA. 2011. Effect of spatial differences in microbial activity, pH, and substrate levels on methanogenesis initiation in refuse. Appl. Environ. Microbiol. 77:2381–2391. 10.1128/AEM.02349-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Vrieze J, Hennebel T, Boon N, Verstraete W. 2012. Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 112:1–9. 10.1016/j.biortech.2012.02.079 [DOI] [PubMed] [Google Scholar]
- 30.Goberna M, Gadermaier M, Garcia C, Wett B, Insam H. 2010. Adaptation of methanogenic communities to the cofermentation of cattle excreta and olive mill wastes at 37 degrees C and 55 degrees C. Appl. Environ. Microbiol. 76:6564–6571. 10.1128/AEM.00961-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato S, Hashimoto K, Watanabe K. 2012. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 14:1646–1654. 10.1111/j.1462-2920.2011.02611.x [DOI] [PubMed] [Google Scholar]
- 32.Liu F, Rotaru A-E, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. 2012. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 5:8982–8989. 10.1039/c2ee22459c [DOI] [Google Scholar]
- 33.Tremblay P-L, Aklujkar M, Leang C, Nevin KP, Lovley D. 2012. A genetic system for Geobacter metallireducens: role of the flagellin and pilin in the reduction of Fe(III) oxide. Environ. Microbiol. Rep. 4:82–88. 10.1111/j.1758-2229.2011.00305.x [DOI] [PubMed] [Google Scholar]
- 34.Smith J, Lovley D, Tremblay PL. 2013. Outer cell surface components essential for Fe(III) oxide reduction in Geobacter metallireducens. Appl. Environ. Microbiol. 79:901–907. 10.1128/AEM.02954-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mountfort D, Asher R. 1979. Effect of inorganic sulfide on the growth and metabolism of Methanosarcina barkeri strain DM. Appl. Environ. Microbiol. 37:670–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazumder TK, Nishio N, Fukuzaki S, Nagai S. 1986. Effect of sulfur-containing compounds on growth of Methanosarcina barkeri in defined medium. Appl. Environ. Microbiol. 52:617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherer P, Sahm H. 1981. Influence of sulphur-containing compounds on the growth of Methanosarcina barkeri in a defined medium. Eur. J. Appl. Microbiol. Biotechnol. 12:28–35. 10.1007/BF00508115 [DOI] [Google Scholar]
- 38.Balch WE, Wolfe RS. 1976. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl. Environ. Microbiol. 32:781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nevin KP, Hensley SA, Franks AE, Summers ZM, Ou J, Woodard TL, Snoeyenbos-West OL, Lovley DR. 2011. Electrosynthesis of organic compounds from carbon dioxide is catalyzed by a diversity of acetogenic microorganisms. Appl. Environ. Microbiol. 77:2882–2886. 10.1128/AEM.02642-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagarajan H, Embree M, Rotaru A-E, Shrestha PM, Feist AM, Palsson BØ, Lovley DR, Zengler K. 2013. Characterization and modelling of interspecies electron transfer mechanisms and microbial community dynamics of a syntrophic association. Nat. Commun. 4:2809. 10.1038/ncomms3809 [DOI] [PubMed] [Google Scholar]
- 41.Raskin L, Zheng D, Griffin ME, Stroot PG, Misra P. 1995. Characterization of microbial communities in anaerobic bioreactors using molecular probes. Antonie Van Leeuwenhoek J. Microbiol. 68:297–308. 10.1007/BF00874140 [DOI] [PubMed] [Google Scholar]
- 42.Richter H, Lanthier M, Nevin KP, Lovley DR. 2007. Lack of electricity production by Pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl. Environ. Microbiol. 73:5347–5353. 10.1128/AEM.00804-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schink B. 2006. The genus Pelobacter, p 5–11 In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E. (ed), The prokaryotes, vol 7 Springer, New York, NY [Google Scholar]
- 44.Boone DR, Mah RA. 1987. Effect of molecular hydrogen on acetate degradation by Methanosarcina barkeri 227, p 111–121 In Cardoso Duarte JM, Archer LJ, Bull AT, Holt G. (ed), Perspectives in biotechnology, vol 128 Springer-Verlag, New York, NY [Google Scholar]
- 45.Ferguson TJ, Mah RA. 1983. Effect of H2-CO2 on methanogenesis from acetate or methanol in Methanosarcina spp. Appl. Environ. Microbiol. 46:348–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McInerney MJ, Bryant MP. 1981. Anaerobic degradation of lactate by syntrophic associations of Methanosarcina barkeri and Desulfovibrio species and effect of H2 on acetate degradation. Appl. Environ. Microbiol. 41:346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MR, Lequerica JL. 1985. Methanosarcina mutant unable to produce methane or assimilate carbon from acetate. J. Bacteriol. 164:618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki K, Morita M, Sasaki D, Hirano S-i, Matsumoto N, Watanabe A, Ohmura N, Igarashi Y. 2011. A bioelectrochemical reactor containing carbon fiber textiles enables efficient methane fermentation from garbage slurry. Bioresour. Technol. 102:6837–6842. 10.1016/j.biortech.2011.04.022 [DOI] [PubMed] [Google Scholar]
- 49.Sasaki K, Sasaki D, Morita M, Hirano S-i, Matsumoto N, Ohmura N, Igarashi Y. 2010. Bioelectrochemical system stabilizes methane fermentation from garbage slurry. Bioresour. Technol. 101:3415–3422. 10.1016/j.biortech.2009.12.076 [DOI] [PubMed] [Google Scholar]
- 50.De Vrieze J, Gildemyn S, Arends JBA, Vanwonterghem I, Verbeken K, Boon N, Verstraete W, Tyson GW, Hennebel T, Rabaey K. 2014. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 54:211–221. 10.1016/j.watres.2014.01.044 [DOI] [PubMed] [Google Scholar]
- 51.Lovley DR. 2012. Electromicrobiology. Annu. Rev. Microbiol. 66:391–409. 10.1146/annurev-micro-092611-150104 [DOI] [PubMed] [Google Scholar]
- 52.Lovley DR, Ueki T, Zhang T, Malvankar NS, Shrestha PM, Flanagan KA, Aklujkar M, Butler JE, Giloteaux L, Rotaru A-E, Holmes DE, Franks AE, Orellana R, Risso C, Nevin KP. 2011. Geobacter: the microbe electric's physiology, ecology, and practical applications. Adv. Microb. Physiol. 59:1–100. 10.1016/B978-0-12-387661-4.00004-5 [DOI] [PubMed] [Google Scholar]
- 53.Kühn W, Fiebig K, Hippe H, Mah RA, Huser BA, Gottschalk G. 1983. Distribution of cytochromes in methanogenic bacteria. FEMS Microbiol. Lett. 20:407–410. 10.1111/j.1574-6968.1983.tb00157.x [DOI] [Google Scholar]
- 54.Thauer RK, Kaster A-K, Seedorf H, Buckel W, Hedderich R. 2008. Methanogenic Archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6:579–591. 10.1038/nrmicro1931 [DOI] [PubMed] [Google Scholar]
- 55.Kohler PR, Metcalf WW. 2012. Genetic manipulation of Methanosarcina spp. Front. Microbiol. 3:259. 10.3389/fmicb.2012.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]