Abstract
Environmental surveillance is an effective approach in investigating the circulation of polioviruses (PVs) and other human enteroviruses (EVs) in the population. The present report describes the results of environmental surveillance conducted in Shandong Province, China, from 2008 to 2012. A total of 129 sewage samples were collected, and 168 PVs and 1,007 nonpolio enteroviruses (NPEVs) were isolated. VP1 sequencing and typing were performed on all isolates. All PV strains were Sabin-like, with the numbers of VP1 substitutions ranging from 0 to 7. The NPEVs belonged to 19 serotypes, and echovirus 6 (E6), E11, coxsackievirus B3 (CVB3), E3, E12, and E7 were the six main serotypes, which accounted for 18.3%, 14.8%, 14.5%, 12.9%, 9.0%, and 5.7% of NPEVs isolated, respectively. Typical summer-fall peaks of NPEV were observed in the monthly distribution of isolation, and an epidemic pattern of annual circulation was revealed for the common serotypes. Phylogenetic analysis was performed on environmental CVB3 and E3 strains with global reference strains and local strains from aseptic meningitis patients. Shandong strains formed distinct clusters, and a close relationship was observed between local environmental and clinical strains. As an EV-specific case surveillance system is absent in China and many other countries, continuous environmental surveillance should be encouraged to investigate the temporal circulation and phylogeny of EVs in the population.
INTRODUCTION
Human enteroviruses (EVs) belong to the genus Enterovirus, family Picornaviridae. Human EVs comprise more than 100 serotypes which are classified into 4 species, EV-A to EV-D (1). EVs usually cause silent infection, but sometimes they are associated with serious diseases, such as acute flaccid paralysis (AFP), aseptic meningitis and encephalitis, acute myocarditis, acute hemorrhagic conjunctivitis (AHC), and hand, foot, and mouth disease (HFMD).
Polioviruses (PVs) belong to EV-C and have three serotypes. Infection with PVs is known to be associated with acute paralytic poliomyelitis. The global incidence of poliomyelitis has dropped a lot since the Global Polio Eradication Initiative (GPEI), and no cases due to indigenous wild poliovirus (WPV) have been identified in Shandong Province since 1991. However, WPV importation from countries where it is endemic maintains a threat to the polio-free status of China, and several incidents of WPV importation have been reported, including the importation of WPV1 in Xinjiang in 2011. The standard approach recommended by the WHO for polio surveillance is the detection and investigation of AFP cases, and environmental surveillance offers a supplementary method which has been demonstrated to play an important role in early warning of WPV importation and vaccine-derived poliovirus (VDPV) circulation (2). In several countries, WPVs and VDPVs have been detected in sewage in the absence of reported AFP cases (3–6).
Although environmental surveillance has served primarily as part of PV surveillance in many parts of the world, gradually more focus has been put on the circulation and molecular characterization of environmental nonpolio enteroviruses (NPEVs) (7–12). In China, information on the circulating NPEVs is limited due to the absence of a specific enterovirus surveillance system. Surveillance based on human specimens is limited and mainly includes testing of specimens collected through AFP and hand, foot, and HFMD surveillance and occasional testing of patients with meningitis or encephalitis. So, despite the increasingly detailed information on temporal/geographical circulation and molecular epidemiology of EVs from various parts of the world, substantial geographical gaps remain in mainland China.
Environmental surveillance has been conducted in Shandong Province, China, since 2008. Previously we reported the isolation of a rare recombinant poliovirus with chimeric capsid VP1 protein from sewage in 2009 (13) and molecular epidemiology and intercity spread of echovirus 6 (E6) in 2008 to 2011 (10, 11), demonstrating the high sensitivity of the surveillance. In this report, we present an overview of serotype distribution and temporal dynamics of PVs and NPEVs from environmental surveillance from 2008 to 2012 and phylogenetic comparison of the relationship of predominant serotypes between environmental and clinical strains, to reflect the circulation and molecular characterization of EVs in Shandong, China.
MATERIALS AND METHODS
Sampling sites, frequency, and method.
Sewage samples were collected in the cities of Jinan and Linyi, with metropolitan populations of 2.6 million and 1.9 million, respectively. The sampling sites were the inlet collector canals of the sewage treatment plants of each city—Jinan Everbright Water (JNEW) and Linyi Shouchuang (LYSC).
Generally, sewage samples were collected monthly in Jinan between February 2008 and July 2010 and semimonthly in Jinan between August 2010 to December 2012 and in Linyi from April 2010 to April 2012. Approximately 1 liter of sewage was collected by the grab sampling method in the afternoon between 2:00 and 3:00 p.m. A cold temperature (approximately 4°C) was maintained during sample transport to the laboratory, storage (<24 h), and processing.
Concentration and virus isolation.
Sewage samples were concentrated approximately 40-fold by using membrane absorption/elution as described previously (8, 11). Briefly, 800 ml of the sewage was centrifuged at 3,000 × g for 30 min at 4°C. MgCl2 (2.5 M) was added to the supernatant to a final concentration of 0.05 M. The pH was adjusted to 3.5 with 0.5 M hydrochloric acid. Then the solution was filtered through a 0.45-μm mixed-cellulose ester membrane filter (ADVANTEC, Tokyo, Japan). Absorbents on the filter were then eluted with three additions of 10 ml of 3% beef extract solution, followed by ultrasonication for 1 min each time (for sewage specimens collected from 2008 to June 2011) or twice for 1.5 min each time (samples collected from July 2011 to December 2012). After centrifugation at 3,000 × g for 30 min, the supernatant was filtered through a 0.22-μm filter and was ready for cell inoculation. L20B, RD, and HEp-2 cell lines were used for virus isolation. Cells were seeded in each tube at 1 × 105. For each cell line and each sewage sample, 18 parallel cell vials with standard monolayer cell cultures were inoculated with 200 μl of concentrated solution for each vial.
Comparison of elutions at different pHs.
During the 5-year surveillance, the pH of elution fluid (3% beef extract solution) had been changed from 7.0 to 9.0 since July 2011. Its influence on virus elution was examined by a laboratory-based recovery experiment. Briefly, a sewage specimen was collected from the Jinan treatment plant. After inactivation at 56°C for 2 h and centrifugation at 3,000 × g for 30 min, the PV type 1 (PV1) Sabin strain was seeded into the supernatant (400 ml) to a final titer of 256 to 512 50% tissue culture infective doses (TCID50)/100 μl. After absorption and sonication, elution was performed with beef extract at pH 7.0 and 9.0. The microtiter assay on prefiltration fluid, filtrated fluid, and elution fluid (20 ml) was performed. Serial 4-fold dilutions were prepared with minimal essential medium (MEM), and 100 μl of each was transferred to the RD cell monolayer in a microplate. The titer (TCID50/100 μl) was determined by reading cytopathic effect (CPE) microscopically after 5 days. The assays were performed in triplicate under both pH conditions.
Clinical isolates.
The EVs isolated from cerebrospinal fluid (CSF) specimens from local acute meningitis and encephalitis patients were used for VP1 sequencing and phylogenetic analysis. A total of 8 coxsackievirus B3 (CVB3) strains and 1 E3 strain were used in this study, and they were isolated according to the WHO's Polio Laboratory Manual (14).
Serotyping.
According to standard protocols recommended by the WHO (14), PV serotyping was carried out via microneutralization assays in 96-well tissue culture plates using polyclonal antisera against PV types 1, 2, and 3 (National Institute for Public Health and the Environment, RIVM, the Netherlands). NPEV serotyping was performed by using RIVM antiserum pools A to G (15).
VP1 amplification, sequencing, and molecular typing.
VP1 sequencing was performed on all the NPEV isolates. Total RNA was extracted from 140 μl of the viral isolates using a QIAamp viral RNA minikit (Qiagen, Valencia, CA) according to the manufacturer's recommended procedure. Reverse transcription-PCR (RT-PCR) was performed using the Access RT-PCR system (Promega, USA). Primer pair UG1/UC11 (16) was used to amplify the entire VP1 coding region of PV isolates. Primer pair 187/011 (17), which corresponds to the 3′ end of VP1 and 5′ end of 2A, was used for amplification of an ∼750-nucleotide (nt) sequence of NPEVs. In order to detect cross contamination, an RT-PCR using the RNA extracted from normal RD cells served as a blank control, and a negative control containing all the components of the reaction except for the template was also included. PCR-positive products were purified and sequenced bidirectionally with the BigDye Terminator v3.0 cycle sequencing kit (Applied Biosystems, Foster City, CA), and sequences were analyzed with an ABI 3130 genetic analyzer (Applied Biosystems, Hitachi, Japan). Molecular typing based on VP1 sequences was performed using the online Enterovirus Genotyping Tool, version 0.1 (18).
Homologous comparison and phylogenetic analysis.
Nucleotide sequence alignments were carried out by BioEdit 7.0.5.3 software (19). Phylogenetic trees were constructed by Mega 4.0 (20) using the neighbor-joining method after estimation of genetic distance using the Kimura two-parameter method (21). A bootstrapping test was performed with 1,000 duplicates, and the transition/transversion rate was set at 10 (22). In order to reduce excessive computational load, the 100% identical sequences collected at the same location and time were manually removed.
Nucleotide sequence accession numbers.
The VP1 nucleotide sequences of environmental and clinical isolates described in this report were deposited in the GenBank database under the accession numbers GU272011 to GU272015, JQ364885, KF747380 to KF747467, KF747491 to KF747578, KF246751, KF246758, GQ329744, GQ329767, GQ246518, FJ919564, FJ919566, JQ364849, and JQ364850.
RESULTS
Comparison of elution at different pH conditions.
Virus recovery in laboratory-based experiments showed that the recovery rates for the PV1 Sabin strain were 7.1% to 10.0% at pH 7.0 and 56.6% to 67.2% at pH 9.0 (Table 1). The titer of the filtrated fluid is 0 TCID50/100 μl, reflecting efficient absorption of the virus to the membrane.
TABLE 1.
Rate of recovery of PV1 Sabin strain by elution solution under different pHsa
| Expt no. | pH | Prefiltration TCID50/100 μl | Elution TCID50/100 μl | Recovery rate (%) | Avg (%) |
|---|---|---|---|---|---|
| 1 | 7.0 | 362 | 256 | 7.1 | |
| 2 | 7.0 | 304 | 256 | 8.4 | 8.5 |
| 3 | 7.0 | 512 | 512 | 10.0 | |
| 1 | 9.0 | 362 | 2,048 | 56.6 | |
| 2 | 9.0 | 512 | 3,444 | 67.2 | 63.7 |
| 3 | 9.0 | 431 | 2,896 | 67.2 |
The volume for prefiltration was 400 ml in all experiments, and the volume for elution was 40 ml in all experiments.
Virus isolation.
A total of 129 sewage samples (80 in Jinan and 49 in Linyi) were collected from February 2008 to December 2012. EVs were detected from 99 samples (63 in Jinan and 36 in Linyi), for a positivity rate of 76.7%.
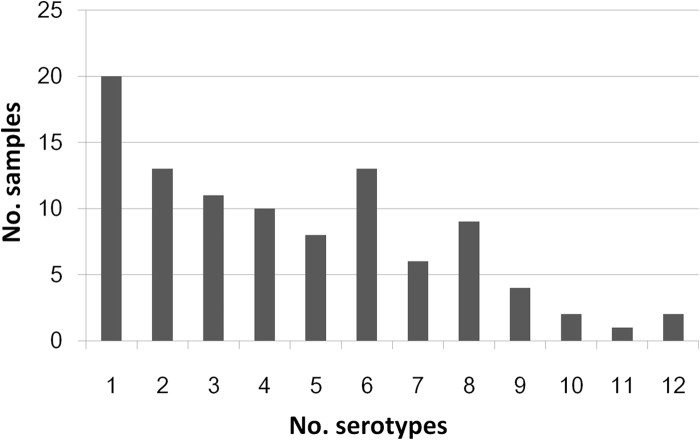
A total of 168 PVs and 1,007 NPEVs were isolated during the 5-year surveillance (Table 2). Multiple serotypes could be detected simultaneously from a single sewage sample. For all the 99 EV-positive samples, the numbers of serotypes in a single sewage sample ranged from 1 to 12, although most samples (n = 90) contained no more than 8 serotypes (Fig. 1). Of the 1,007 NPEV strains isolated, 677 (67.2%) were isolated from the RD cell line and the other 330 isolates were from the HEp-2 cell line (Table 3).
TABLE 2.
Number of isolates of different serotypes from environmental surveillance conducted in the two cities of Shandong from 2008 to 2012
| Serotype | No. of isolates in city |
Total no. of isolates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jinan |
Linyi |
||||||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | Sum | 2010 | 2011 | 2012 | Sum | ||
| NPEV | |||||||||||
| E1 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 4 |
| E3 | 6 | 3 | 0 | 19 | 78 | 106 | 0 | 9 | 15 | 24 | 130 |
| E6 | 1 | 0 | 29 | 66 | 10 | 106 | 3 | 70 | 5 | 78 | 184 |
| E7 | 1 | 0 | 11 | 17 | 12 | 41 | 1 | 14 | 1 | 16 | 57 |
| E11 | 0 | 0 | 22 | 32 | 59 | 113 | 32 | 4 | 0 | 36 | 149 |
| E12 | 0 | 0 | 0 | 24 | 8 | 32 | 0 | 59 | 0 | 59 | 91 |
| E13 | 0 | 0 | 9 | 2 | 0 | 11 | 6 | 1 | 1 | 8 | 19 |
| E14 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 |
| E19 | 0 | 4 | 0 | 3 | 3 | 10 | 5 | 0 | 0 | 5 | 15 |
| E24 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 |
| E25 | 0 | 0 | 1 | 3 | 5 | 9 | 0 | 9 | 8 | 17 | 26 |
| E29 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 3 | 0 | 3 | 4 |
| E30 | 0 | 0 | 0 | 3 | 14 | 17 | 4 | 2 | 2 | 8 | 25 |
| CVA21 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 2 |
| CVB1 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 8 | 0 | 10 | 12 |
| CVB2 | 1 | 0 | 4 | 1 | 0 | 6 | 2 | 0 | 0 | 2 | 8 |
| CVB3 | 7 | 1 | 1 | 40 | 49 | 98 | 0 | 8 | 40 | 48 | 146 |
| CVB4 | 0 | 0 | 1 | 0 | 0 | 1 | 4 | 0 | 0 | 4 | 5 |
| CVB5 | 0 | 4 | 3 | 8 | 14 | 29 | 0 | 0 | 0 | 0 | 29 |
| Mixa | 0 | 7 | 4 | 12 | 44 | 67 | 4 | 23 | 3 | 30 | 97 |
| Total | 17 | 19 | 87 | 233 | 301 | 657 | 63 | 212 | 75 | 350 | 1,007 |
| PV type | |||||||||||
| 1 | 0 | 0 | 3 | 7 | 26 | 36 | 1 | 2 | 2 | 5 | 41 |
| 2 | 0 | 0 | 4 | 13 | 44 | 61 | 4 | 3 | 5 | 12 | 73 |
| 3 | 0 | 1 | 3 | 8 | 31 | 43 | 3 | 6 | 2 | 11 | 54 |
| Total | 0 | 1 | 10 | 28 | 101 | 140 | 8 | 11 | 9 | 28 | 168 |
| Total EV | 17 | 20 | 97 | 261 | 402 | 797 | 71 | 223 | 84 | 378 | 1,175 |
“Mix” indicates NPEV mixtures. They could not be serotyped by RIVM antibody pools, and VP1 sequencing revealed mixed peaks.
FIG 1.
Distribution of numbers of EV serotypes identified in EV-positive sewage samples. Most samples contained fewer than 8 serotypes, while up to 12 EV serotypes could be detected in a single sample.
TABLE 3.
Number of NPEV isolates from RD and HEp-2 cell lines
| Serotype | No. of isolates detected in cell line |
Total no. of isolates | |
|---|---|---|---|
| RD | HEp-2 | ||
| E1 | 1 | 3 | 4 |
| E3 | 111 | 19 | 130 |
| E6 | 93 | 91 | 184 |
| E7 | 52 | 5 | 57 |
| E11 | 132 | 17 | 149 |
| E12 | 84 | 7 | 91 |
| E13 | 16 | 3 | 19 |
| E14 | 2 | 0 | 2 |
| E19 | 11 | 4 | 15 |
| E24 | 2 | 0 | 2 |
| E25 | 21 | 5 | 26 |
| E29 | 4 | 0 | 4 |
| E30 | 23 | 2 | 25 |
| CVA21 | 2 | 0 | 2 |
| CVB1 | 0 | 12 | 12 |
| CVB2 | 5 | 3 | 8 |
| CVB3 | 35 | 111 | 146 |
| CVB4 | 1 | 4 | 5 |
| CVB5 | 8 | 21 | 29 |
| Mix | 74 | 23 | 97 |
| Total | 677 | 330 | 1,007 |
In concentration of the 53 sewage samples collected from 2008 to 2010, the absorbents on the filter were eluted with 10 ml of 3% beef extract solution by ultrasonication three times (1 min each time). Among the 205 EV isolates from this period, 101 (49.3%), 64 (31.2%), and 40 (19.5%) were recovered from the first, second, and third elutions by ultrasonication, revealing that the greater the degree of sonication, the lower the titer in the eluted fluid. Hence, the sonication protocol for the sewage collected from July 2011 was changed to two sonications of 1.5 min each.
Polioviruses.
Of the 129 sewage samples during the 5-year surveillance, 50 were positive for PV (positivity rate, 38.8%), and altogether, 168 PVs were isolated. Numbers of PV1, PV2, and PV3 isolates were 41 (24.4%), 73 (43.5%), and 54 (32.1%), respectively. All these PVs were Sabin strains, with no WPV. The numbers of VP1 substitutions ranged from 0 to 7 (Fig. 2). Except for one VDPV2 isolate with 7 substitutions in VP1 coding region, no VDPV strains were identified. The monthly PV isolation in the two cities is illustrated in Fig. 3F and 4F. PV detection peaked in winter and spring, while in NPEV active seasons of summer and early autumn, PV detection decreased dramatically.
FIG 2.
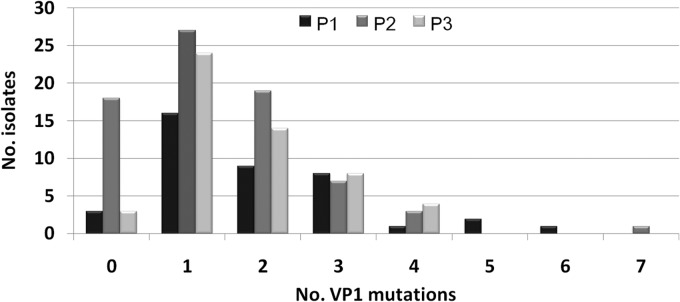
Numbers of substitutions in the VP1 coding regions of all the 168 PV isolates from environmental surveillance in Shandong, 2008 to 2012.
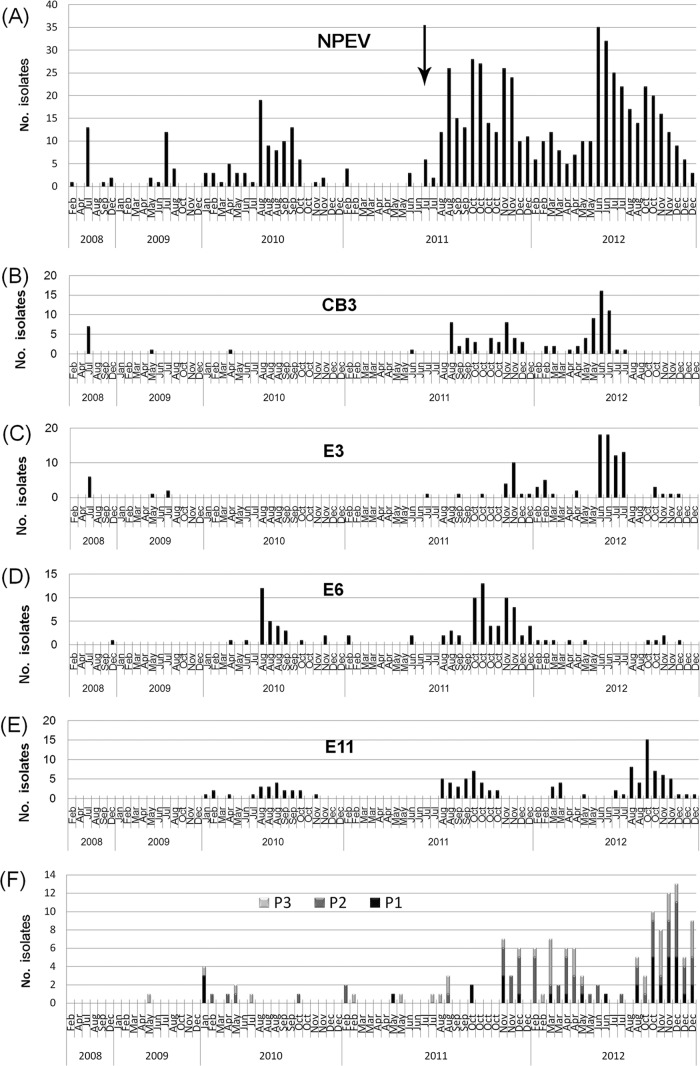
FIG 3.
Temporal distribution of EVs in different samples collected in Jinan, 2008 to 2012. Monthly distributions of total NPEV, CVB3, E3, E6, E11, and PVs are illustrated in panels A to F, respectively. The black arrow in panel A indicates the time when the modified method was used. (Panels A and D are adapted from reference 11.)
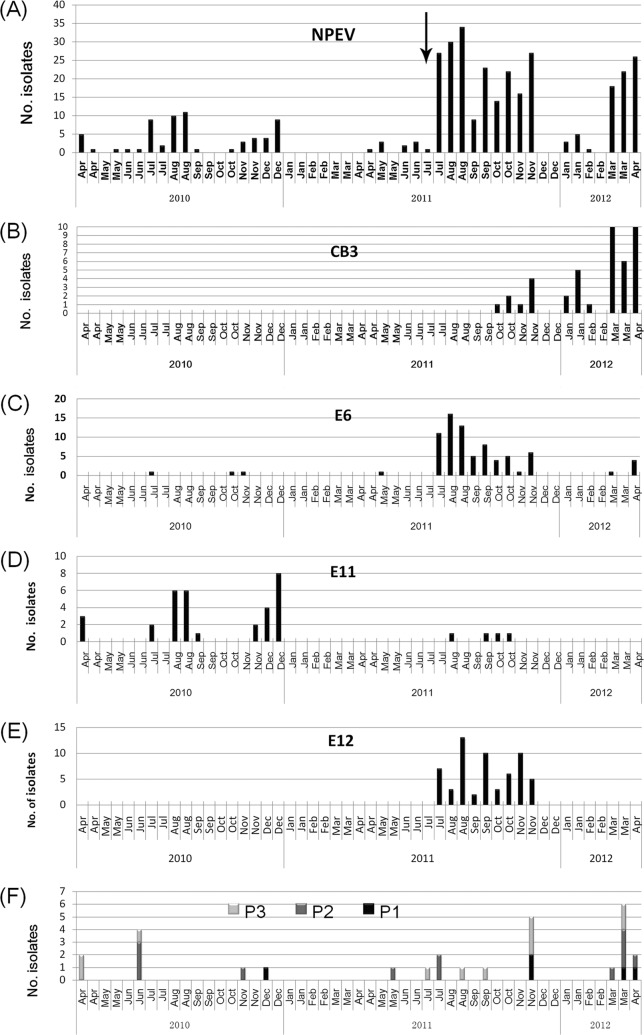
FIG 4.
Temporal distribution of EVs in different samples collected in Linyi, 2010 to 2012. Monthly distributions of total NPEV, CVB3, E6, E11, E12, and PV are illustrated in panels A to F, respectively. The black arrow in panel A indicates the time when the modified method was used. (Panels A and C are adapted from reference 11.)
NPEVs.
Of the 129 sewage samples during the 5-year surveillance, 96 were positive for NPEV (positivity rate, 74.4%), and altogether, 1,007 NPEVs were isolated. Serotyping and molecular typing were performed on all isolates, and 19 serotypes were identified (Table 2). Except for two coxsackievirus A21 (CVA21) isolates of species EV-C, all isolates belonged to EV-B. E6, E11, CVB3, E3, E12, and E7 were the six main serotypes and accounted for 18.3%, 14.8%, 14.5%, 12.9%, 9.0%, and 5.7% of the NPEVs isolated, respectively. There were still 97 isolates that could not be typed by serological methods, and VP1 sequencing revealed mixed peaks, demonstrating the existence of multiple NPEV serotypes in the isolates. Generally, the serotype constitutions of the two cities are similar, except for four serotypes (E14, E24, CVA21, and CVB5) that were detected only in Jinan.
Typical summer-fall peak of detection of NPEV was observed in the monthly distribution of isolation (Fig. 3 and 4). In different years, the constitutions of most common serotypes were different. In Jinan, the annual most common serotypes were E3 plus CVB3, E3 plus E19, E6 plus E11, E6 plus E11 plus CVB3, and E3 plus E11 plus CVB3 from 2008 to 2012, respectively. In Linyi, the annual most common serotypes were E11, E6 plus E12, and CVB3 plus E3 from 2010 to 2012, respectively.
An epidemic pattern of annual circulation was revealed for these common serotypes by environmental surveillance (Fig. 3 and 4). CVB3 had low detection rates in 2009 and 2010 in Jinan. Then, an abrupt occurrence in sewage in August 2011 initiated a period of high CVB3 activity for about 12 months. However, in August 2012, CVB3 could not been detected from sewage, and since then, it has come into quiescence. E6 had low detection rates in 2008 and 2009, and after the high activity in 2010 and 2011, it came into quiescence in 2012 again. Similar temporal fluctuation was also observed for other common serotypes such as E3 and E11.
E30 is an important serotype associated with aseptic meningitis throughout the world (23). In the 5-year surveillance, E30 was present at a relatively higher frequency but lower number of isolates for each sewage sample, implying a low titer. From October 2011 to December 2012, a total of 13 sewage samples in Jinan were positive for E30, but the number of isolates for each sewage specimen was less than 3. Similar results were observed in Linyi. CVB5 is also associated with aseptic meningitis in Shandong Province (24). Its isolation had a characterization similar to that of E30. A total of 18 sewage samples in Jinan were positive for CVB5, and the number of CVB5 isolates for each sewage sample was less than 4 in all cases.
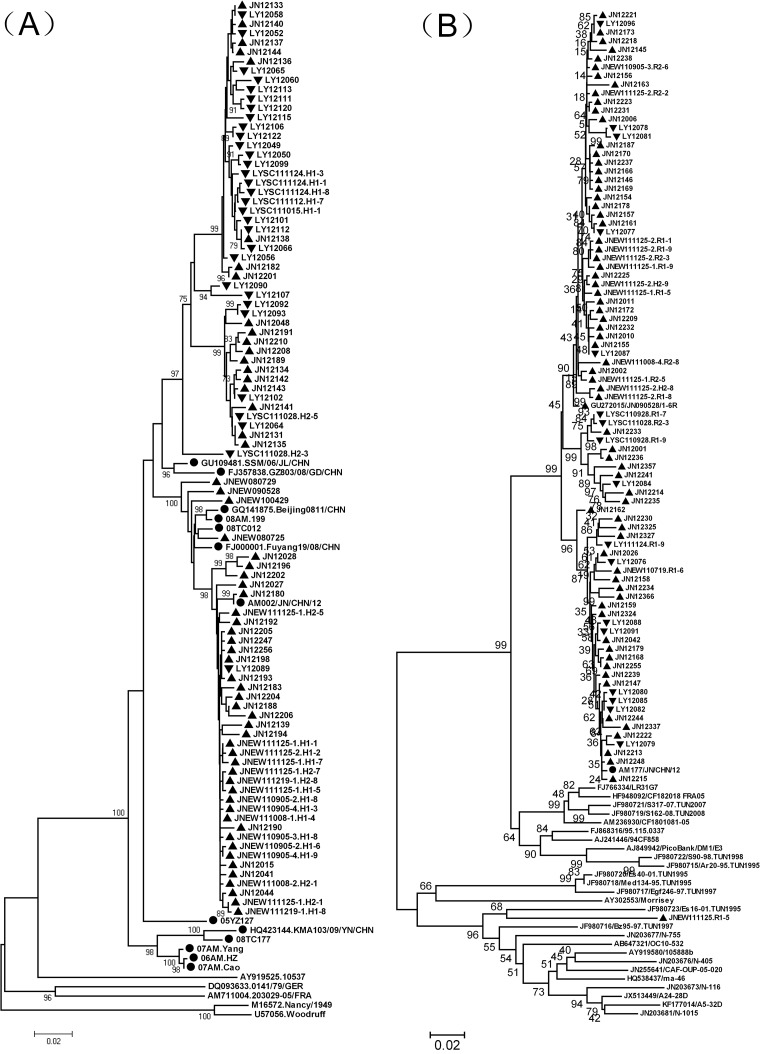
Homologous comparison and phylogenetic analysis.
Phylogenetic analysis was performed on partial VP1 sequences of CVB3 and E3 strains from the environment, local aseptic meningitis patients, and global reference strains. Both serotypes had all been previously demonstrated to be associated with aseptic meningitis in Shandong Province and were present at a high frequency of isolation in sewage.
Environmental CVB3 segregated into two lineages (Fig. 5A). Both lineages contained isolates from Jinan and Linyi, and a close relationship was observed for the CVB3 isolates from the two cities, suggesting the occurrence of frequent intercity spread. Strain AM002/JN/CHN/12, isolated from an aseptic meningitis patient in Jinan in 2012, had a high similarity (99.8%) to environmental strain JN12180, isolated in Jinan in 2012. Phylogenetic analysis also revealed a close relationship between them.
FIG 5.
Phylogenetic relationships of CVB3 (A) and E3 (B) isolates from sewage and clinical specimens in Shandong, 2008 to 2012, and strains from other regions. The phylogenetic trees were constructed using Mega, version 4.0, using the neighbor-joining method based on 704-nt (positions 2601 to 3304 of strain Nancy) and 716-nt (positions 2608 to 3323 of strain Morrisey) partial VP1 sequences of CVB3 and E3, respectively. ▲ and ▼, isolates from sewage in Jinan and Linyi, respectively. ●, isolates from patients of aseptic meningitis. The isolates before 2012 are identified by a code that consists of JNEW or LYSC, followed by the sample date (presented as YYMMDD [i.e., year, month, and day]) and the tube number. The isolates from 2012 are identified by JN or LY, followed by a 3-code serial number.
Most Shandong environmental E3 isolates formed an exclusive cluster with no foreign strains (Fig. 5B). However, an E3 isolate (JNEW111125.R1-5) had a remote relationship with other Shandong strains and segregated into a cluster together with other foreign strains. Strain AM177/JN/CHN/12 was isolated from an aseptic meningitis patient in Jinan in 2012. It had a close relationship with strain JN12213 (similarity, 99.7%), isolated from sewage in Jinan in 2012.
DISCUSSION
Environmental surveillance has been demonstrated to be a sensitive method for monitoring PV and NPEV and estimating the extent and duration of EV circulation in a population (25, 26). In 2008, it was initiated in two provincial poliovirus laboratories (Shandong and Guangdong) in China as a supplementary method of poliovirus surveillance. This report presents an overview of serotypes distribution, temporal dynamics, and molecular epidemiology of EVs from environmental surveillance conducted in Shandong Province from 2008 to 2012.
A dramatic increase of PV and NPEV isolation was observed for sewage samples collected since July 2011 (Fig. 3 and 4). This is supposedly due to the optimization of the concentration method at that time. In the membrane absorption/elution method used for sewage concentration, beef extract solution was used to elute EVs from the membrane. Since July 2011, the pH of beef extract solution has been adjusted from neutral to alkaline (∼9). The subsequent increased isolation from July 2011 to December 2012 demonstrated the importance of the alkaline condition for eluting EVs from the membrane. Moreover, the laboratory-based recovery test on PV1 further demonstrated the importance of the alkaline condition for EV elution (Table 1).
In this study, the sensitivity of the environmental surveillance was reinforced in two ways. First, besides the L20B and RD cell lines routinely used in poliovirus laboratory network, the HEp-2 cell line was used in this study for the isolation of group B coxsackieviruses. The frequent detection of CVB3 and CVB5 demonstrated the necessity of including the HEp-2 cell line in the environmental surveillance. Second, the number of parallel vials inoculated (18 vials of each cell line for each sewage specimen) was increased compared with that in other studies (7–9). This may increase the laboratory workload. However, our study suggested that by increasing the number of parallel vials inoculated, more serotypes can be detected from a single specimen (especially for the minor circulating serotypes), more sequences can be obtained for further molecular epidemiological study, and temporal dynamics can be manifested more clearly.
In Shandong Province, the last WPV-associated paralytic poliomyelitis patient was identified in 1991. Since then, no WPV has been detected, and VPDVs were detected in Shandong AFP cases in 2007, 2009, and 2011. In the 5-year environmental surveillance, all the 168 PVs isolated were Sabin strains, and only one VDPV2 strain with 7 substitutions in VP1 coding region was isolated. No further circulation of VPDV was detected. Previously, we reported the isolation of a recombinant poliovirus with chimeric capsid VP1 protein from sewage in 2009 (13). No such virus was identified from the local AFP surveillance system at that time. In this study, the environmental VDPV also had no related cases via AFP surveillance. So, our study demonstrates that AFP surveillance combined with continuous environmental surveillance is of great importance in improving the sensitivity of poliovirus detection.
An epidemic pattern is the characterization of temporal circulation of many EV serotypes (27). It is characterized by substantial fluctuations in circulation levels over time, including large peaks when the serotype was among the most prevalent serotypes in a given year. In this study, the monthly isolation of the common serotypes such as E3, E6, E11, E12, and CVB3 also reflected a distinct epidemic pattern. An interesting observation was made for the monthly detection of E30 and CVB5. They both are important pathogens of aseptic meningitis. During the surveillance period, their detection in sewage was frequent. However, the numbers of isolates were low. Case-based enterovirus surveillance in the United States in 1970 to 2005 had demonstrated an epidemic pattern for both serotypes (23, 27). So, the low isolate number of E30 and CVB5 might due to the low activity in the surveillance period or is just the result of low copy numbers in the excreta of infected individuals. Further investigation and surveillance are needed for clarification.
In this study, a total of 19 NPEV serotypes were identified from the sewage. Except for two isolates of CVA21 of EV-C, the serotypes belonged to EV-B. The predominance of EV-B in sewage is similar to findings described in previous reports (8, 9, 12, 26). As EV-A71- and CVA16-associated HFMD has become an emerging concern in mainland China since 2007 (28, 29), the absence of detection of these two serotypes from sewage is not correlated to the clinical situation. One reason may be the cell culture method used in this study. Because most echoviruses propagate more quickly in RD cells, if echoviruses and EV-A71 are inoculated into the same RD cell tube, the growth of EV-A71 can easily be suppressed by echovirus 6. Another reason may be that the viral copy numbers in the excreta of EV-A71-infected individuals are possibly lower than those from HEV-B-infected individuals, which will result in low titers in sewage. These explanations may partly account for the absence of detection.
The phylogenetic analysis revealed that Shandong environmental strains had a high degree of genetic diversity from foreign strains. CVB3 had been demonstrated to be the causative agents of aseptic meningitis outbreaks in Shandong in recent years (30), and CVB3 and E3 were also isolated from sporadic aseptic meningitis patients in 2012. A close relationship was found between environmental stains and clinical strains, demonstrating the sensitivity and significance of environmental surveillance, especially in regions with limited case surveillance. Moreover, the increase in CVB3 and E3 isolation from sewage initiated in the latter half of 2011 (Fig. 3) and the sporadic clinical cases present since the first half of 2012 reflect the importance of environmental surveillance in early warning of associated diseases.
ACKNOWLEDGMENTS
This study was supported by a grant from the Health Department of Shandong Province (2011HZ058), a grant from the National Natural Science Foundation of China (81302481), the Bill & Melinda Gates Foundation (grant OPP1039272), and a grant for Research on Emerging and Re-Emerging Infectious Diseases from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print 16 May 2014
REFERENCES
- 1.Knowles NJ, Hovi T, Hyypiä T, King AMQ, Lindberg AM, Pallansch MA, Palmenberg AC, Simmonds P, Skern T, Stanway G, Yamashita T, Zell R. 2011. Picornaviridae, p 855–880 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses; Elsevier, San Diego, CA [Google Scholar]
- 2.WHO. 2003. Guidelines for environmental surveillance of poliovirus circulation. WHO, Geneva, Switzerland [Google Scholar]
- 3.Blomqvist S, Savolainen C, Laine P, Hirttiö P, Lamminsalo E, Penttilä E, Jöks Roivainen SM, Hovi T. 2004. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J. Virol. 78:4876–4883. 10.1128/JVI.78.9.4876-4883.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Bassioni L, Barakat I, Nasr E, de Gourville EM, Hovi T, Blomqvist S, Burns C, Stenvik M, Gary H, Kew OM, Pallansch MA, Wahdan MH. 2003. Prolonged detection of indigenous wild polioviruses in sewage from communities in Egypt. Am. J. Epidemiol. 158:807–815. 10.1093/aje/kwg202 [DOI] [PubMed] [Google Scholar]
- 5.Shulman LM, Manor Y, Handsher R, Delpeyroux F, McDonough MJ, Halmut T, Silberstein I, Alfandari J, Quay J, Fisher T, Robinov J, Kew OM, Crainic R, Mendelson E. 2000. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J. Clin. Microbiol. 38:3729–3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida H, Horie H, Matsuura K, Kitamura T, Hashizume S, Miyamura T. 2002. Prevalence of vaccine-derived polioviruses in the environment. J. Gen. Virol. 83:1107–1111 [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Liu Q, Wang D, Chen Y, Feng B, Li G, Yao W, Shu B, He Y. 2013. Surveillance and analysis of enteroviruses in water environments in Shenzhen from 2010 to 2011. Arch. Virol. 158:1343–1347. 10.1007/s00705-013-1611-0 [DOI] [PubMed] [Google Scholar]
- 8.Iwai M, Yoshida H, Matsuura K, Fujimoto T, Shimizu H, Takizawa T, Nagai Y. 2006. Molecular epidemiology of echoviruses 11 and 13, based on an environmental surveillance conducted in Toyama Prefecture, 2002–2003. Appl. Environ. Microbiol. 72:6381–6387. 10.1128/AEM.02621-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khetsuriani N, Kutateladze T, Zangaladze E, Shutkova T, Peñaranda S, Nix WA, Pallansch MA, Oberste MS. 2010. High degree of genetic diversity of non-polio enteroviruses identified in Georgia by environmental and clinical surveillance, 2002–2005. J. Med. Microbiol. 59:1340–1347. 10.1099/jmm.0.023028-0 [DOI] [PubMed] [Google Scholar]
- 10.Tao Z, Wang H, Li Y, Xu A, Zhang Y, Song L, Yoshida H, Xu Q, Yang J, Zhang Y, Liu Y, Feng L, Xu W. 2011. Cocirculation of two transmission lineages of echovirus 6 in Jinan, China, as revealed by environmental surveillance and sequence analysis. Appl. Environ. Microbiol. 77:3786–3792. 10.1128/AEM.03044-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Z, Song Y, Wang H, Zhang Y, Yoshida H, Ji S, Xu A, Song L, Liu Y, Cui N, Ji F, Li Y, Chen P, Xu W. 2012. Intercity spread of echovirus 6 in Shandong Province, China: application of environmental surveillance in tracing circulating enteroviruses. Appl. Environ. Microbiol. 78:6946–6953. 10.1128/AEM.01861-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng H, Lu J, Zhang Y, Yoshida H, Guo X, Liu L, Li H, Zeng H, Fang L, Mo Y, Yi L, Chosa T, Xu W, Ke C. 2013. Prevalence of non-polio enteroviruses in the sewage of Guangzhou City, China, from 2009 to 2012. Appl. Environ. Microbiol. 79:7679–7693. 10.1128/AEM.02058-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Z, Wang H, Xu A, Zhang Y, Song L, Zhu S, Li Y, Yan D, Liu G, Yoshida H, Liu Y, Feng L, Chosa T, Xu W. 2010. Isolation of a recombinant type 3/type 2 poliovirus with a chimeric capsid VP1 from sewage in Shandong, China. Virus Res. 150:56–60. 10.1016/j.virusres.2010.02.014 [DOI] [PubMed] [Google Scholar]
- 14.WHO. 2004. Polio laboratory manual, 4th ed. WHO, Geneva, Switzerland [Google Scholar]
- 15.Melnick JL, Rennick V, Hampil B, Schmidt NJ, Ho HH. 1973. Lyophilized combination pools of enterovirus equine antisera: preparation and test procedures for the identification of field strains of 42 enteroviruses. Bull. World Health Organ. 48:263–268 [PMC free article] [PubMed] [Google Scholar]
- 16.Balanant J, Guillot S, Candrea A, Delpeyroux F, Crainic R. 1991. The natural genomic variability of poliovirus analyzed by a restriction fragment length polymorphism assay. Virology 184:645–654. 10.1016/0042-6822(91)90434-D [DOI] [PubMed] [Google Scholar]
- 17.Oberste MS, Maher K, Flemister MR, Marchetti G, Kilpatrick DR, Pallansch MA. 2000. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J. Clin. Microbiol. 38:1170–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroneman A, Vennema H, Deforche K, Avoort HVD, Peñaranda S, Oberste MS, Vinjé J, Koopmans M. 2011. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 51:121–125. 10.1016/j.jcv.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 19.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95–98 [Google Scholar]
- 20.Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- 21.Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111–120. 10.1007/BF01731581 [DOI] [PubMed] [Google Scholar]
- 22.Jorba J, Campagnoli R, De L, Kew O. 2008. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J. Virol. 82:4429–4440. 10.1128/JVI.02354-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberste MS, Maher K, Kennett ML, Campbell JJ, Carpenter MS, Schnurr D, Pallansch MA. 1999. Molecular epidemiology and genetic diversity of echovirus type 30 (E30): genotypes correlate with temporal dynamics of E30 isolation. J. Clin. Microbiol. 37:3928–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen P, Tao Z, Song Y, Liu G, Wang H, Liu Y, Song L, Li Y, Lin X, Cui N, Xu A. 2013. A coxsackievirus B5 associated aseptic meningitis outbreak in Shandong province, China in 2009. J. Med. Virol. 85:483–489. 10.1002/jmv.23478 [DOI] [PubMed] [Google Scholar]
- 25.Hovi T, Shulman LM, van der Avoort H, Deshpande J, Roivainen M, Gourville DEEM. 2012. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 140:1–13. 10.1017/S095026881000316X [DOI] [PubMed] [Google Scholar]
- 26.Sedmak G, Bina D, MacDonald J. 2003. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from Milwaukee, Wisconsin, collected August 1994 to December 2002. Appl. Environ. Microbiol. 69:7181–7187. 10.1128/AEM.69.12.7181-7187.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease Control and Prevention 2006. Enterovirus surveillance—United States, 1970–2005. MMWR Surveill. Summ. 55:1–20 [PubMed] [Google Scholar]
- 28.Zhang Y, Tan XJ, Wang HY, Yan DM, Zhu SL, Wang DY, Ji F, Wang XJ, Gao YJ, Chen L, An HQ, Li DX, Wang SW, Xu AQ, Wang ZJ, Xu WB. 2009. An outbreak of hand, foot, and mouth disease associated with subgenotype C4 of human enterovirus 71 in Shandong, China. J. Clin. Virol. 44:262–267. 10.1016/j.jcv.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Wang D, Yan D, Zhu S, Liu J, Wang H, Zhao S, Yu D, Nan L, An J, Chen L, An H, Xu A, Xu W. 2010. Molecular evidence of persistent epidemic and evolution of subgenotype B1 coxsackievirus A16-associated hand, foot, and mouth disease in China. J. Clin. Microbiol. 48:619–622. 10.1128/JCM.02338-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao Z, Song Y, Li Y, Liu Y, Jiang P, Lin X, Liu G, Song L, Wang H, Xu A. 2012. Coxsackievirus B3, Shandong Province, China, 1990–2010. Emerg. Infect. Dis. 18:1865–1867. 10.3201/eid1811.120090 [DOI] [PMC free article] [PubMed] [Google Scholar]