Abstract
The anatomy of the LASIK interface allows for a variety of potential complications to arise, unique etiologies with overlapping clinical presentations. Primary interface complications include infectious keratitis, diffuse lamellar keratitis (DLK), central toxic keratopathy (CTK), pressure-induced stromal keratopathy (PISK), and epithelial ingrowth. Infectious keratitis is most commonly caused by Methicill in resistant S. aureus (early onset) or atypical Mycobacterium (late onset) postoperatively, and immediate treatment includes flap lift and irrigation, cultures, and initiation of broad-spectrum topical antibiotics, with possible flap amputation for recalcitrant cases. DLK is a white blood cell infiltrate that appears within the first 5 days postoperatively and is acutely responsive to aggressive topical and oral steroid use in the early stages but may require flap lift and irrigation to prevent flap necrosis if inflammation worsens. In contrast, PISK is caused by acute steroid response and resolves only with cessation of steroid use and IOP lowering. Without appropriate therapy PISK can result in severe optic nerve damage. CTK mimics stage 4 DLK but occurs early in the postoperative period is non-inflammatory. Observation is the only effective treatment, and flap lift is usually not warranted. Epithelial ingrowth is easily distinguishable from other interface complications and may be self-limited or require flap lift to treat irregular astigmatism and prevent flap melt. Differentiating between interface entities is critical to rapid appropriate diagnosis, treatment, and ultimate visual outcome. While initial presentations may overlap significantly, the conditions can be readily distinguished with close follow-up and most complications can resolve without significant visual sequelae when treated appropriately.
With the advent of keratomileusis procedures, primarily laser in situ keratomileusis (LASIK), a new anatomic region in the cornea came into existence: the potential space between anterior and posterior corneal lamellae commonly referred to as the LASIK interface. Within this region a number of biochemical processes occur after creation of the corneal flap, including limited wound healing and intercellular reorganization.1 The anatomy of the LASIK interface allows for a variety of potential unique complications to arise from different etiologies with often overlapping clinical presentations.
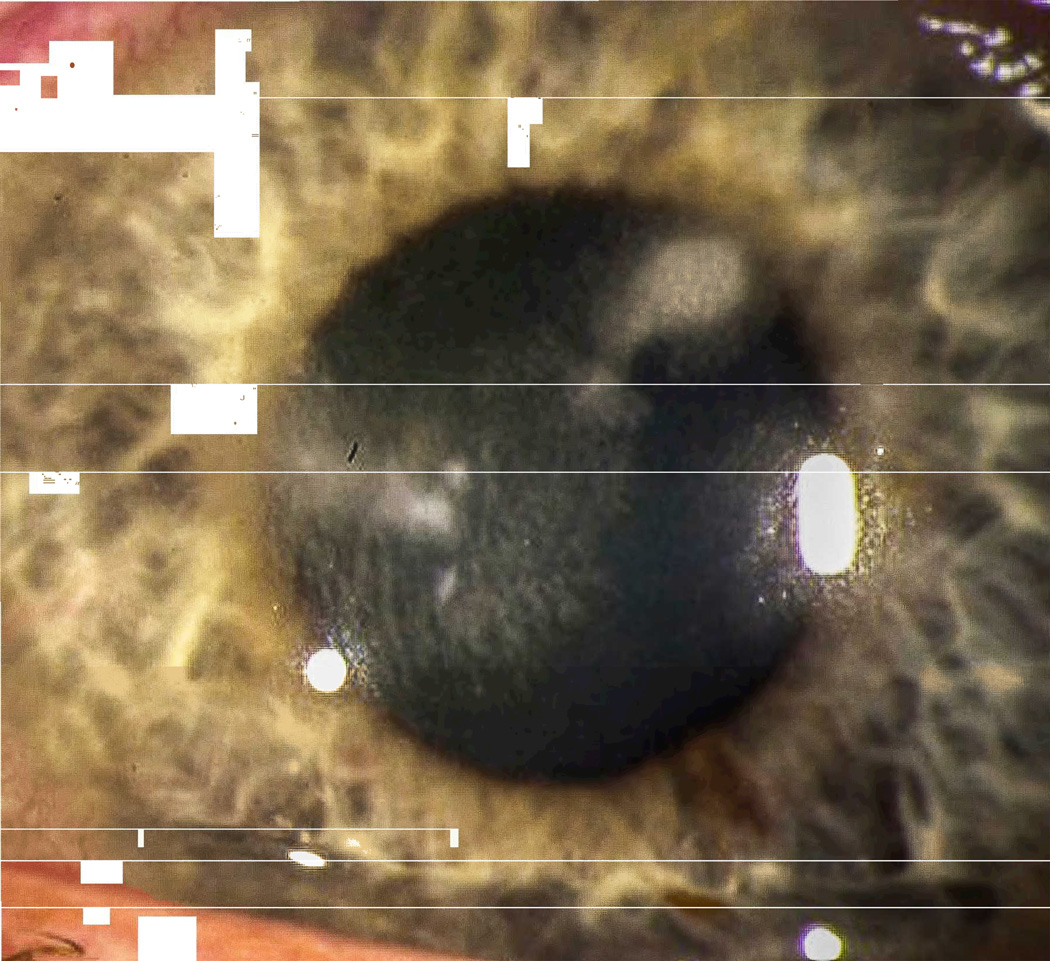
Primary interface complications include infectious keratitis, diffuse lamellar keratitis (DLK), pressure-induced stromal keratopathy (PISK), central toxic keratopathy (CTK), and epithelial ingrowth. Clarifying the nomenclature used for each entity is critical to enhancing future communication, and we propose the preceding terms be used. Benign interface debris may also be present in the interface, and while this does not routinely cause any problems, it may be confused with more serious disorders, resulting in inappropriate diagnoses and treatments. While some of these entities originate specifically within the interface, others may originate elsewhere but ultimately coalesce in this potential space.
Differentiating between interface entities rapidly is critical to appropriate diagnosis, treatment, and ultimate visual outcome. While initial similarities exist, taking a careful history of symptoms, timing, and onset, combined with a focused clinical exam, significantly improves the potential for accurate initial diagnosis and treatment protocol at presentation. In cases with significant overlap, continued close and frequent follow-up in the early postoperative period will allow for accurate diagnosis and potential modifications in treatment protocol when necessary.
Among interface complications, infectious keratitis is usually the most sight threatening, while DLK is both the most common to present and the most common etiology mistaken for other conditions, including infections, CTK, and PISK. Epithelial ingrowth is generally the easiest entity to identify and treat, although it may occasionally be associated with other interface disorders.
Each of these entities is relatively rare, occurring approximately in less than 1/500–1/1000 eyes in aggregate in the absence of outbreaks.2, 3 This rarity makes the initial diagnosis increasingly challenging for less experienced surgeons and clinicians. The goal of this review is to define the nomenclature and describe the etiology, diagnostic criteria, and optimal management strategies for these primary LASIK interface complications.
Literature Search Methodology
The search engine PubMed (www.pubmed.org) was utilized for literature search. The following search terms were used both in isolation and a variety of combinations: LASIK Interface, LASIK Infection, DLK & “diffuse lamellar keratitis”, LASIK keratitis, PISK and “pressure induced stromal keratitis”, CTK and “central toxic keratopathy”, and epithelial ingrowth. The articles reviewed were restricted to studies published in English, and animal studies were only included if they elucidated specific mechanisms for the complications reviewed. The searches were then reviewed by title for relevance. Among included citations, the reference list for each was also examined for relevant articles. While, at a minimum, the abstracts of all related articles were reviewed, only the articles deemed most relevant and current to this review topic were included. The references included in this review are therefore not all-inclusive; rather, they represent the majority of major citations, regardless of publication date, and the majority of the most recent references on the topics covered in this review.
LASIK Interface Complications: Overview
While each of these entities may belong in the initial differential of patients presenting with symptoms and interface findings, most can be quickly differentiated based on key historical elements, such as time of onset after surgery, level of discomfort (or lack thereof), as well as a thorough physical examination, including intraocular pressure (IOP) measurements [Table 1]. Interface complications can have a focal or diffuse appearance, present with or without conjunctival erythema, with or without foreign body sensation, and may present from 1 day to 3–4 weeks postoperatively [Table 1]. It is important to determine the specific etiology rapidly, because appropriate interventions are quite different depending on etiology, and in some instances may be exactly opposite [Table 2]. Interface complications evolve in appearance over time [Table 3]. A photographic array provides the classic appearance for each condition (Figures 1–6). Unfortunately, many of these entities may overlap in initial clinical appearance.
Table 1.
LASIK Interface Disorders: Characteristics
| Clinical parameters |
DLK | CTK | PISK | Infection | Epithelia l Ingrowth |
|---|---|---|---|---|---|
| Etiology | Inflammatory | Toxic | Fluid (steroid response) | Bacteria Mycobacterium Fungi |
Epithelial cells in interface |
| Typical Onset | 1–5 days | 2–7 days | 10–21 days | 3–21 days | 14 days or later |
| Initial effect on visual acuity | Minimal | Pronounced | Pronounced | Variable | Minimal |
| Pain | Foreign Body sensation | None | Minimal | Moderate | None |
| Location | Diffuse | Focal, well defined | Diffuse | Focal, less defined | At flap edge |
| Corneal Haze | Granular until stage 4 | Dense focally, clear surrounding | Diffuse haze | Dense at infiltrate, variable surrounding | Focal at cells |
| Conjunctival erythema | None | None | None | Moderate to severe | None |
| IOP (measured) | Normal | Normal | Normal or low | Normal | Normal |
| IOP (real) | Normal | Normal | High | Normal | Normal |
| Interface Fluid | None | None | Yes (may not be apparent) | None | None |
| Response to Topical Steroids | Minimal/good | Minimal/none | Poor with worsening | Variable | None |
| Response to Oral Steroids | Good/excellent | Minimal/none | None/mild worsening | Limited | None |
| Response to Flap lift | Good | Poor | None | Good, depending on flap integrity | Good, may require flap sutures |
DLK = Diffuse lamellar keratitis
CTK = Central Toxic Keratopathy
PISK = Pressure-induced stromal keratopathy
Table 2.
LASIK Interface Disorders Management
| Etiology | Primary Intervention | Secondary Intervention |
Contraindicated Management Strategies |
|---|---|---|---|
| Infectious Keratitis | Topical Antibiotics, Flap lift/Irrigate/Culture | Flap Amputation if unresponsive | Steroid use without antibiotics |
| DLK | Steroids (Topical & Oral) | Flap Lift & irrigate | Failure to use steroids |
| PISK | Steroid Cessation | IOP lowering Medications | Continued Steroid use |
| CTK | Observation | Observation | Flap lift |
| Epithelial Ingrowth | Observation if only at flap edge | Flap lift/cell removal/flap suture | N/A |
DLK = Diffuse lamellar keratitis
PISK = Pressure-induced stromal keratopathy
CTK = Central Toxic Keratopathy
Table 3.
Signs & Symptoms of Interface Disorders Over Time
| Day | DLK | CTK | PISK | Infectious |
|---|---|---|---|---|
| 1 | FBS ± peripheral infiltrate (I) | None | None | Extremely Rare |
| 2–3 | Increased infiltrate (II) | Dense central opacification | None | Rarely |
| 4–7 | More dense infiltrate, coalescing (II–III) | Stable opacification | None | FBS, conjunctival hyperemia, Infiltrate (bacterial) |
| 8–14 | Coalescence to focal opacity ± scarring (III–IV) | Stable | None | Worsening of symptoms (bacterial) Onset of symptoms (fungal) |
| 15–21 | Final coalescence to stage IV or resolution | Stable | Onset of haze | Worsening (bacterial, fungal) Onset (mycobacterial) |
| 22–35 | Final outcome | Slow resolution | Worsening haze ± fluid cleft | Variable depending on organism and treatment |
DLK = Diffuse lamellar keratitis
CTK = Central Toxic Keratopathy
PISK = Pressure-induced stromal keratopathy
WBC = white blood cells
FBS = foreign body sensation
I–IV = stages of DLK
Figure 1.
Benign interface debris in the LASIK interface. (Reprinted from Randleman JB. Chapter 8: Etiology and clinical presentations of irregular astigmatism after keratorefractive surgery. In: Wang M, ed. Irregular Astigmatism: Diagnosis and Treatment. SLACK Inc., Thorofare, NJ 2007: 79–90)
Figure 6.
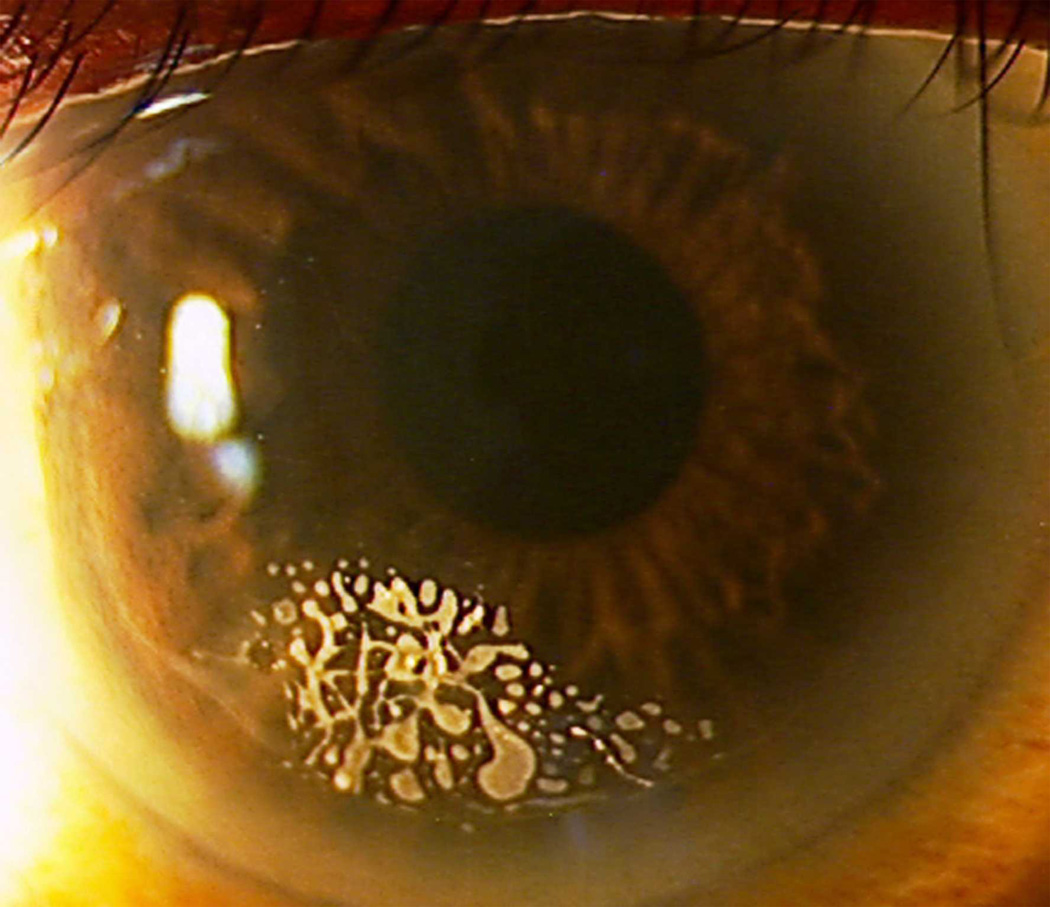
Epithelial ingrowth, with a nest of epithelial cells notable extending in from the 7 o’clock meridian.
Infectious Keratitis
Etiology
Infectious keratitis is a rare but potentially devastating complication after LASIK.2, 4–13 Table 4 provides an overview from the largest studies to date on infectious keratitis after LASIK. A variety of organisms are implicated, including viruses (esp. Adenovirus and HSV), bacteria (esp. Staphylococcus and Pseudomonas species), atypical mycobacteria, Fungi, and acanthamoeba.2, 4, 8, 9 Adenovirus usually has a relatively benign course with ultimately good acuity, while HSV frequently leads to loss of best acuity after PRK or LASIK and is a relative contraindication for excimer laser surgery.8
Table 4.
Overview of Infectious Keratitis after LASIK
| Study | Year | Total eyes |
Total Infections (eyes) |
Incidence | # Culture positive |
Primary Organism |
Final CDVA > 20/20 |
Final CDVA > 20/40 |
|---|---|---|---|---|---|---|---|---|
| Karp5 | 2003 | N/A | 15 | N/A | 15 |
Mycobacterium (6) S. aureus (4) Pseudomonas (1) Fungal (3) Acanthamoeba (1) |
5/15 (33%) | 10/15(66%) NLP: 2 (13%) |
| Freitis4 | 2003 | N/A | 10 | N/A | N/A | Mycobacterium (10) | 0 (0%) | 4 (40%) |
| Solomon8 | 2003 | 338,500* | 116 | 0.03%* | 46/69 (66%) |
Mycobacterium (33) Staph sp. (23) Strep sp. (2) Gram negative (2) Fungal (7) Nocardia (2) |
^37/69 (53%) | ^58 (84%) |
| Moshifar7 | 2007 | 10,477 | 33 | 0.31% | 23 cases (viral) 10/33 cases (bacterial) |
Adenovirus (18) HSV (5) Mycobacterium (4) Staph sp. (3) Pseudomonas (1) Polymicrobial (2) |
20/33 (59%) | 26/33 (77%) |
| Solomon9 | 2007 | N/A | 10 (LASIK) | N/A | N/A | MRSA (100%) | 1 (10%) | 8 (80%) |
| Llovet6 | 2010 | 204,586 | 72 | 0.035% | 21/54 (39%) |
S. epidermidis (9) S. pneumoniae (8) S. viridans (2) S. pyogenes (1) S. aureus (1) |
38/72 (53%) | 67/72 (93%) |
| Garg10 | 2010 | N/A | 17 | N/A | N/A | Nocardia (5) Mycobacterium (4) Fungal (4) Acanthamoeba (2) Corynebacterium (1) S. epidermidis (1) |
1 (6%) | 4 (24%) CF or worse: 8 (47%) |
| Yamaguchi11 | 2011 | N/A | 39 | N/A | 11/30 (36%) |
Mycobacterium (9) Corynebacterium (1) Gram + (1) |
22 (56%) | 33 (85%) |
: the total number of cases in So lomon et al. was estimated based on survey responses
: %20/20 and 20/40 were derived from culture-positive cases only
The most common non-viral cause of infectious keratitis has evolved over time: prior to 2005, atypical Mycobacterium was the most common infectious etiology after LASIK,4, 6, 7 while recently gram-positive organisms, particularly Methicillin resistant S. aureus (MRSA), have become more common in the early postoperative period.2, 8, 10 Strep sp., pseudomonas sp., fungal infections, and acanthamoeba are less common organisms.2, 4, 8, 9 The reduced incidence of atypical Mycobacterium has been attributed to the routine use of fourth generation fluoroquinolones, which are more effective against these species.8, 14, 15 Concurrently, MRSA incidence may be increasing due to the potential for these species to develop concurrent resistance to fluoroquinolones.16
Risk factors for the development of infectious keratitis include blepharitis, dry eye, intraoperative epithelial defects, intraoperative contamination, delayed postoperative reepithelialization of the cornea, use of topical corticosteroids, and patients in the health profession.2, 4, 7 Preoperatively, the lids and lacrimal apparatus should be examined thoroughly, and appropriate treatment of lid disease should be undertaken.5 Furthermore, several epidemics of atypical mycobacteria have been associated with the use of non-sterile water to clean instruments or the use of ice during LASIK.6
Infectious keratitis after LASIK has been divided into infections occurring within the first 2 weeks (early onset) and after 2 week to 3 months (late onset).5 The organisms responsible for early onset infections include staphylococcal and streptococcal species, whereas organisms more commonly seen in late onset infections include atypical mycobacteria, and fungi.
Management
In the initial phase of treatment, LASIK flaps should be lifted, cultures taken, the flap bed irrigated with fortified antibiotics, and broad-spectrum topical antibiotics started [Table 2]. For infections with a delayed onset, the use of amikacin may be beneficial in treating atypical mycobacteria.5 In no n-responsive LASIK infections, flap amputation may be necessary to facilitate antibiotic penetration.5
Outcomes
Most infections resolve with mild to moderate loss of best visual acuity,13 but rarely therapeutic penetrating keratoplasty is necessary [Figure 7].
Figure 7.
Infectious keratitis. The infection that began in the interface has now extended through the flap anteriorly and posteriorly to become a full-thickness infiltrate with a hypopyon. This cornea perforated the following day, requiring penetrating keratoplasty.
Diffuse Lamellar Keratitis (DLK)
Etiology
Diffuse lamellar keratitis (DLK) is a white blood cell infiltrate that coalesces between the flap and stromal bed that appears within a few days (1–5) after laser in-situ keratomileusis (Figure 2).3, 17, 18 Confocal microscopy has confirmed the presence of inflammatory cells in the corneal stroma and interface.19 This non-specific interface inflammation is certainly associated with intraoperative epithelial defects,20 and has been linked to multiple rare potential inciting factors.21 However, the majority of cases, especially DLK epidemics, can be linked to bacterial endotoxins, especially in sterilization reservoirs3, 22–26 or issues during the instrument sterilization process.27, 28 Isolated idiopathic DLK cases occur sporadically,29 however, if more than one case occurs in a short time, steps should be undertaken to evaluate the sterilization process.3, 22, 23 Late onset DLK has also been described in literature30, 31; however, most of these cases are likely due to some specific causative factor.
Figure 2.
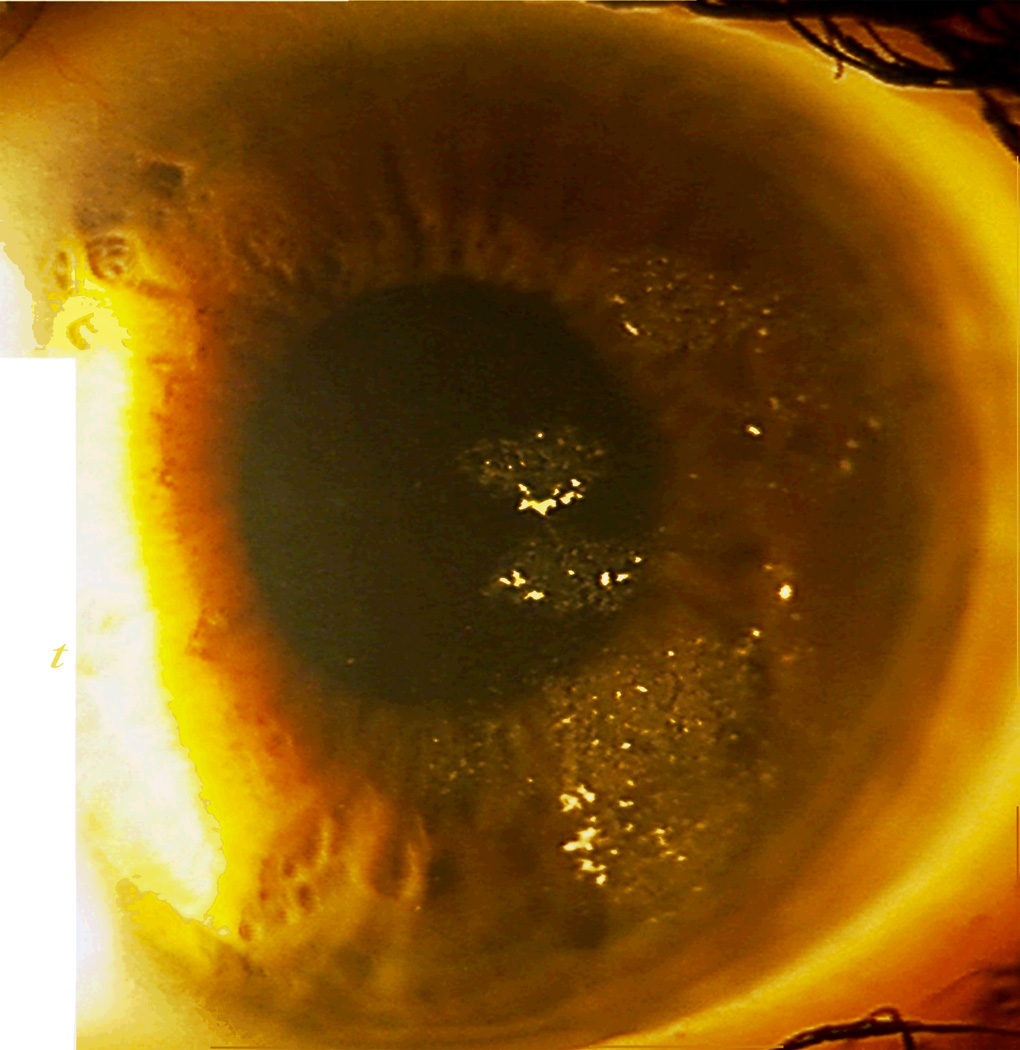
Infectious keratitis. Note the focal infiltrates interspersed within the somewhat diffuse inflammatory reaction. The causative organism was Mycobacteria.
DLK is typically classified clinically in to 4 stages as described by Linebarger and colleagues.32 Stage 1 has inflammatory cells in the far periphery only, which are first present in the corneal stroma and then coalesce in the LASIK interface. Stage 2 has a diffuse infiltrate frequently involving the paracentral and peripheral flap margins but sparing the central axis. Stage 3 has a denser infiltrate within the flap interface, which involves the visual axis and is frequently associated with decreased visual acuity. Stage 4 has a focal, coalesced dense haze with scarring, signifying flap necrosis and usually results in permanent corneal scarring, with visual acuity loss of varying severity (Figure 8). DLK appears to be slightly more common when the femtosecond laser is used for flap creation33–35; however, most of these cases appear to be mild and resolve with minimal treatment.
Figure 8.
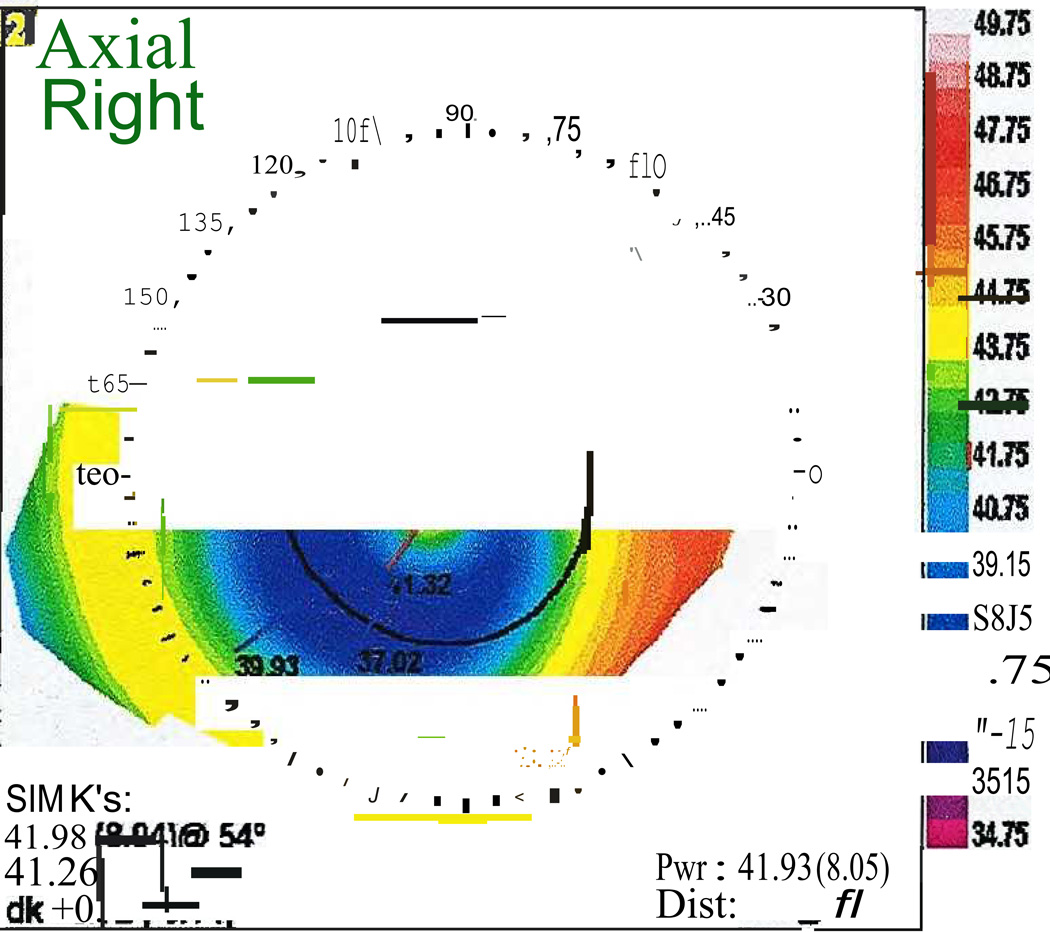
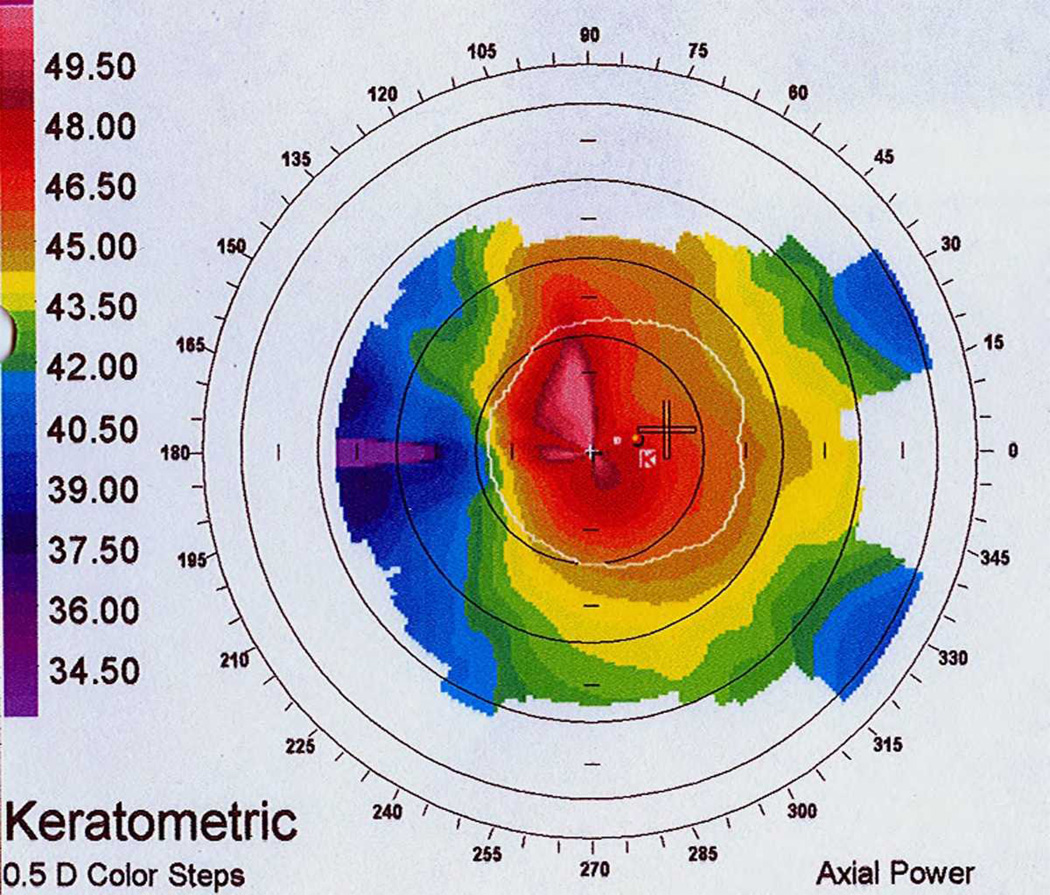
DLK, Stage 4. A) Note the focal corneal haze with a striate appearance within the center of the haze similar to the appearance of CTK. B) Corresponding topography with notable focal flattening corresponding to the focal haze. (Reprinted from Randleman JB. Chapter 8: Etiology and clinical presentations of irregular astigmatism after keratorefractive surgery. In: Wang M, ed. Irregular Astigmatism: Diagnosis and Treatment. SLACK Inc., Thorofare, NJ 2007: 79–90)
Management
DLK is highly sensitive to steroids and responsive to aggressive topical steroids for mild cases, frequently up to every hour administration, and a combination of topical and oral steroids in moderate cases.3, 36, 37 Many surgeons advocate using oral steroids for stage 2 and 3 DLK, with flap lift and irrigation for stage 2 cases that are not responding rapidly to treatment and all stage 3 cases.3, 36, 37 For Stage 4, aggressive steroids to minimize scar density are warranted and may be partially effective.32, 36 Flap lift for stage 4 is more controversial, with some surgeons advocating cautious flap lift and irrigation to remove inciting toxins, but extreme care must be taken to prevent tissue loss; therefore some advocate no flap lift for stage 4 cases.32
Outcomes
Reported uncorrected and corrected visual acuity after DLK resolves is generally quite good [Table 5].3, 18, 22, 29, 38 However, this may be misleading, as DLK appears to affect visual quality and contrast sensitivity more than absolute Snellen acuity measurements.38 DLK frequently induces a mild to moderate hyperopic shift after resolution, with potentially both regular and irregular astigmatism, especially in stage 4 cases.18, 38 Enhancement rates are higher in patients that develop DLK.3, 29, 38 If significant ametropia persists, patients may undergo retreatment without increased risk of developing DLK a second time as long as the inciting issue has been resolved, such as elimination of endotoxin in sterilization units. For stage 4 cases, retreatment may still be warranted if the refractive error is not irregular, but in these cases surface ablation is often a better option than flap lift for the aforementioned reasons.
Table 5.
Diffuse lamellar Keratitis (DLK) Incidence, Outcomes, and Associations
| Study | Year | # Eyes Studied |
Incidence | CDVA Outcomes |
UDVA Outcomes |
Association |
|---|---|---|---|---|---|---|
| Holland19 | 2000 | 983 | 52 (5.3%) | 98.1%≥20/30 | Not reported | Endotoxins released from gram-negative biofilms in sterilizer reservoirs |
| Johnson16 | 2001 | 2,711 | 36 (1.3%) | 66.6%≥20/20 88.8%≥20/25 |
27.7%≥20/20 47.2%≥20/25 |
Overlying epithelial defects |
| Wilson26 | 2002 | 1,352 primary 217 enhancements |
17 (0.9%) 3 (1.4%) |
100%≥20/30 (primary) 100%≥20/30 (enhancement) |
Not reported | Multifactorial endogenous factors |
| Stulting15 | 2004 | 15,119 | 61 (0.40%) | 81.3% ≥ 20/20 96.6% ≥ 20/25 |
55.9% ≥ 20/20 72.8% ≥ 20/25 |
Endotoxin (Cassette autoclave with reservoir) (47 eyes- 0.94%) |
| Javaloy20, 29 | 2011 | 1,161 | 209 (18%) | 76.5% ≥20/20 91.4% ≥20/25 |
54.5% ≥ 20/20 75.2% ≥ 20/25 |
*Endotoxin (Cassette autoclave with reservoir) *Multifactorial Causes |
Pressure-Induced Stromal Keratopathy (PISK)
Etiology
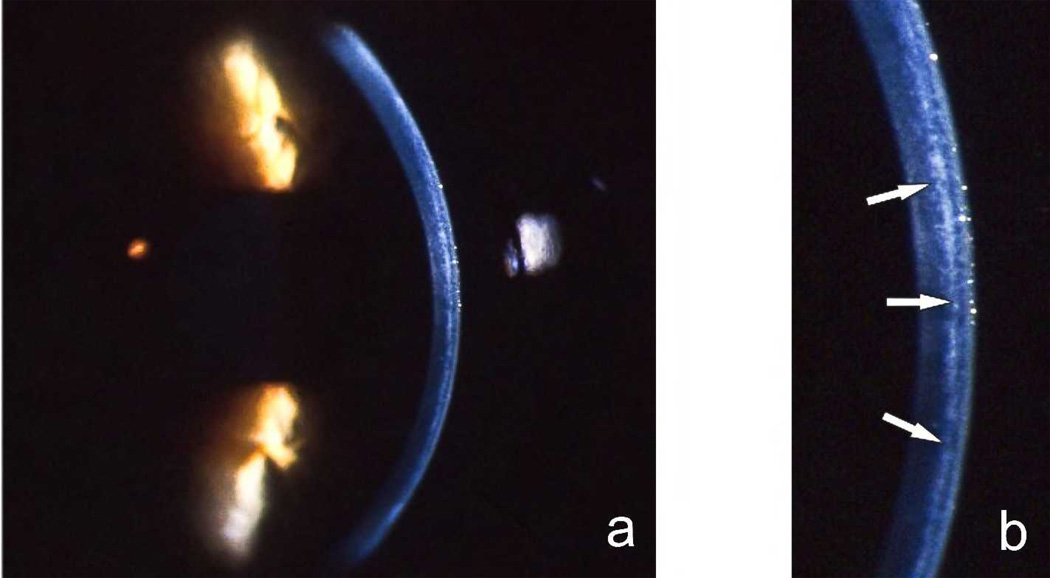
In the setting of LASIK, PISK is a relatively rapid steroid response resulting in high intraocular pressure with resulting fluid accumulation in the interface. The amount of fluid present may be relatively small, resulting in diffuse haziness in the interface and overlying stroma without an obvious fluid layer39 [Figure 4], or it may be pronounced [Figure 9], resulting in a visible fluid cleft separating the anterior flap from the posterior residual bed.40 PISK appears to be a more complex entity to identify than other interface complications due to confusing nomenclature in the literature and to the wide variety of appearances and findings on presentation.
Figure 4.
Pressure induced stromal keratopathy (PISK) with a diffuse, hazy interface but without an obvious fluid layer. (Image courtesy of Theofilos Tourtas, M.D.)
Figure 9.
PISK with an obvious fluid cleft. (Image courtesy of Marc Michelson, M.D. and Matthew J. Fabrizio, M.D.)
Nomenclature
Multiple names have been given to the various manifestations of this entity, potentially resulting in confusion and misleading diagnostic criteria. Among the most common terms are pressure-induced stromal keratitis (PISK),39 pressure-induced interface keratitis,41 and interface fluid syndrome (IFS).42, 43 “Keratitis” is a misnomer in this condition, as confocal evaluations have demonstrated that no actual keratocyte inflammation is present.44, 45 Interface fluid syndrome (IFS) is technically correct; however, IFS may occur from a variety of different mechanisms unrelated to acute steroid response in the early postoperative period, and to many clinicians the term implies that interface fluid that is readily visible. Therefore, while correct, we recommend against the routine use of IFS to communicate information about this specific entity occurring in the early postoperative period after LASIK associated with steroid use and presenting with or without obvious interface fluid. Recently, Tourtas and Cursiefen46 coined the term “pressure induced stromal keratopathy (PISK),” which maintains the most common abbreviated term “PISK” while also accurately and most effectively communicating the etiology of the condition. We therefore recommend this term be adopted moving forward and will use it throughout this review.
Management
While PISK has been described in detail,39–45, 47–50 the significant variability in clinical presentation, from diffuse haze to demonstrable interface fluid, requires added diligence in establishing the diagnosis, differentiating this condition from diffuse lamellar keratitis (DLK), and initiating an appropriate treatment strategy. The degree of interface fluid accumulation masks true IOP in various ways when measured using standard approaches. In all cases, actual IOP is greater than IOP measured centrally, and peripheral measurements generate a more accurate IOP. With small amounts of fluid, IOP measurements may be elevated but still falsely low, while with larger fluid clefts IOP will measure extremely low due to the cushioning effect of the interface fluid.
For any patient on chronic steroids after LASIK (two weeks or longer), it is critical to routinely measure IOP, even in the early postoperative period. IOP measurement, especially when obtained centrally, is artifactually reduced in a predictable way after routine LASIK51; therefore, any increased IOP postoperatively warrants further investigation. Whenever IOP measurements are suspect, alternative means should be attempted to determine IOP, including peripheral IOP measurements with Goldmann applanation or tonopen.52–54 Dynamic contour tonometry may also be employed to assess true IOP, as it has been shown to be relatively immune to changes in corneal biomechanics and pachymetry after LASIK.55 Appropriate management includes cessation of steroid use and initiating topical anti-glaucoma medications until the fluid resolves.39–41, 43, 47, 48, 50
Outcomes
Severe glaucomatous field loss and decreased central visual acuity can occur if PSIK is not recognized early and managed appropriately.40, 41, 47, 49 If identified and appropriately managed early, typically patients do well without loss of BCVA.39, 50
Central Toxic Keratopathy (CTK)
Etiology
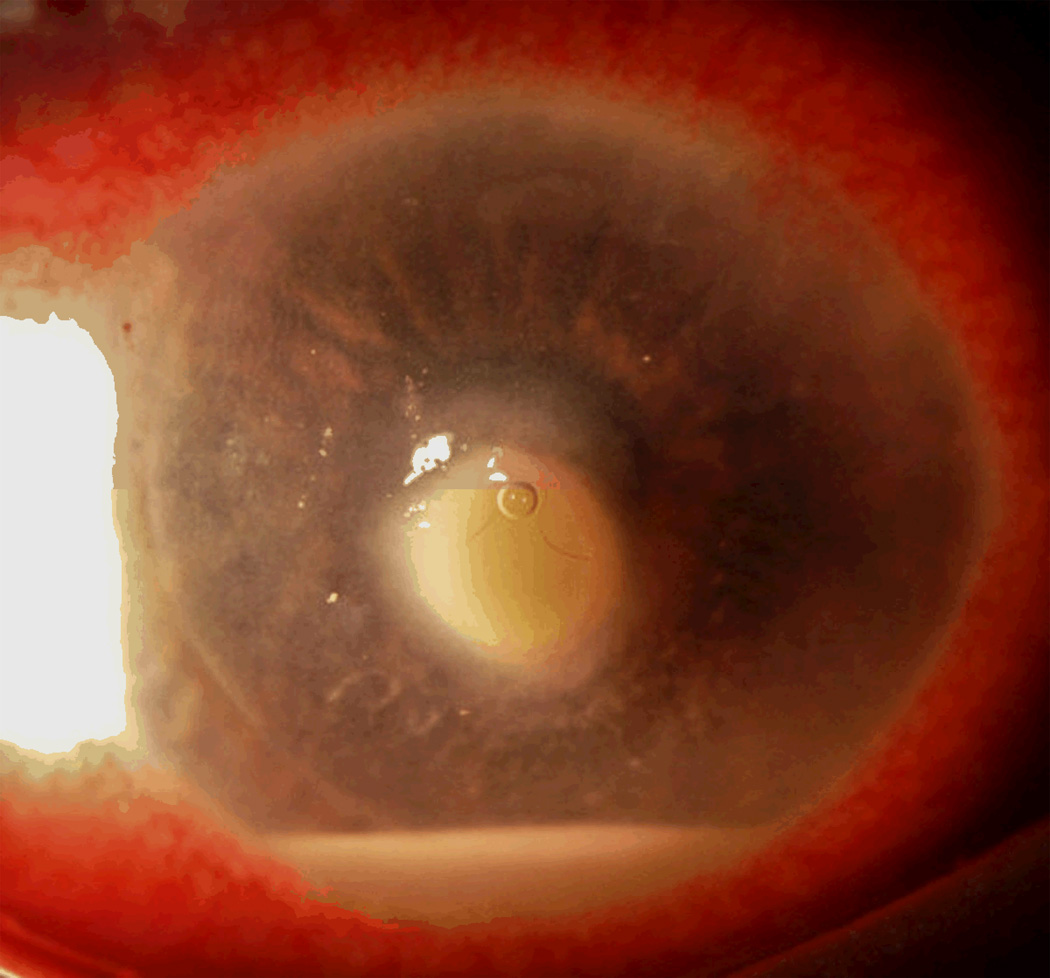
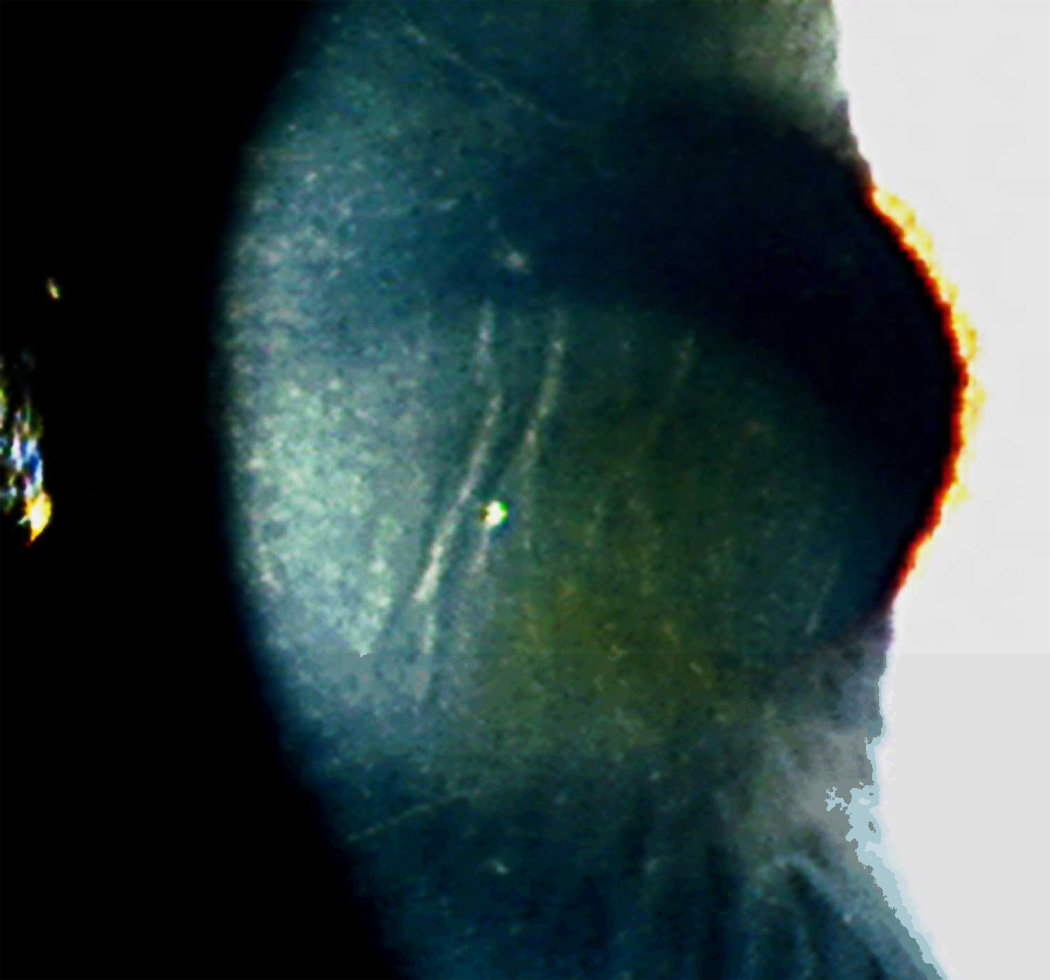
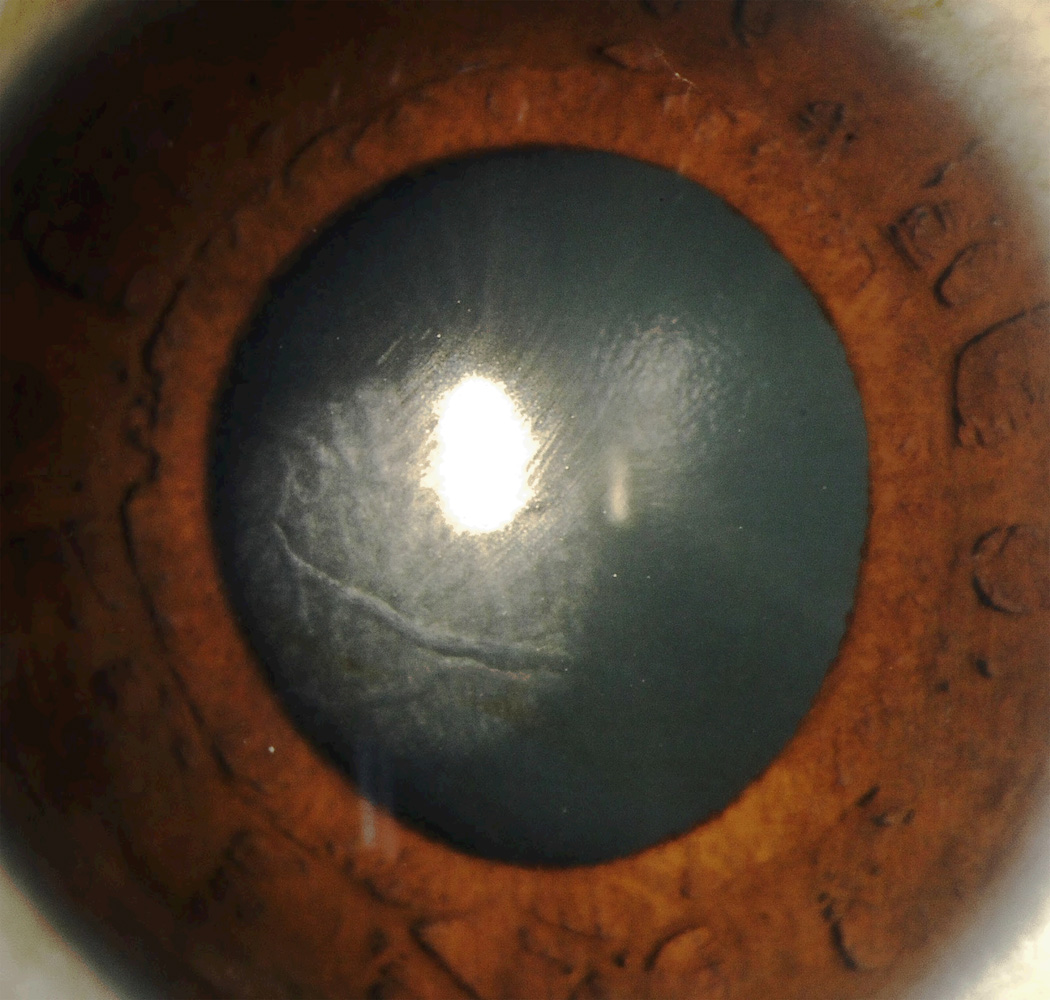
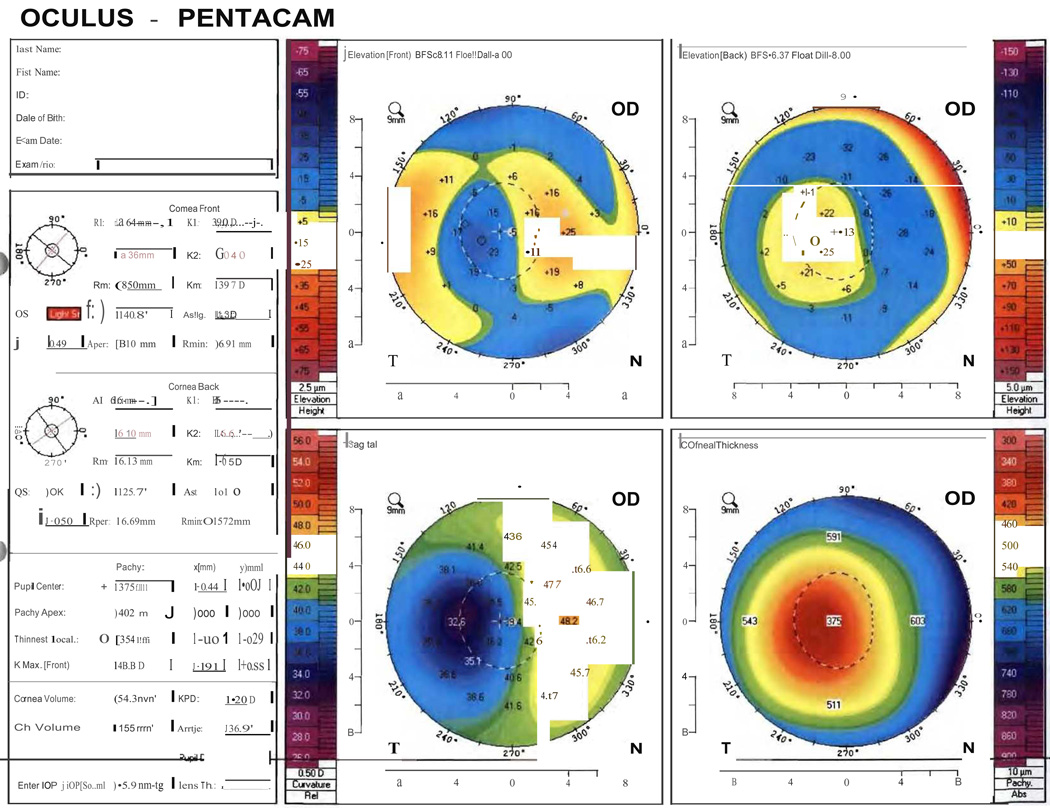
CTK is a rare, acute, non-inflammatory central corneal opacification that can occur within days after uneventful LASIK or PRK (Figure 5).56–62 Etiology is unknown but may be related to enzymatic degradation of keratocytes.57, 60 Confocal microscopy has demonstrated activated keratocytes without inflammatory cells, with initial keratocyte loss from the stromal bed, with gradual repopulation over time.63, 64 CTK has been reported to demonstrate anterior curvature flattening without alteration of posterior curvature in anterior segment tomography65; however, some cases do appear to alter all tomographic findings, likely as measurement artifact [Figure 10]. The onset is acute without worsening over time, as opposed to most other interface entities.
Figure 5.
Central toxic keratopathy (CTK). Note the focal corneal haze with a striate appearance within the center of the haze. (Image courtesy of Joung Y. Kim, M.D.)
Figure 10.
Pentacam image of central toxic keratopathy, demonstrating significant focal flattening noted in sagittal curvature map corresponding to focal corneal haze. Note that the corneal thickness (A) and posterior elevation maps (A&B) are also significantly focally altered, likely due to measurement artifact. These changes resolved over time (not shown).
Management
The first critical step in appropriate management is distinguishing CTK from stage 4 DLK.58 CTK is almost always painless, as opposed to DLK, which in almost all cases has at least a moderate foreign body sensation, and CTK is acute in onset, as opposed to the progression over time to stage 4 DLK. CTK is self-limited and treatment is not warranted.57 While some have advocated aggressive topical steroid use61 or flap lift and irrigation,66 most feel that surgical intervention will not help and may worsen the final outcome.56, 57, 60
Outcomes
Most individuals nearly fully recover after a variable, often protracted clinical course; however, most patients retain some faint corneal haze, and some have a visually significant, persistent hyperopic shift.57, 60, 65
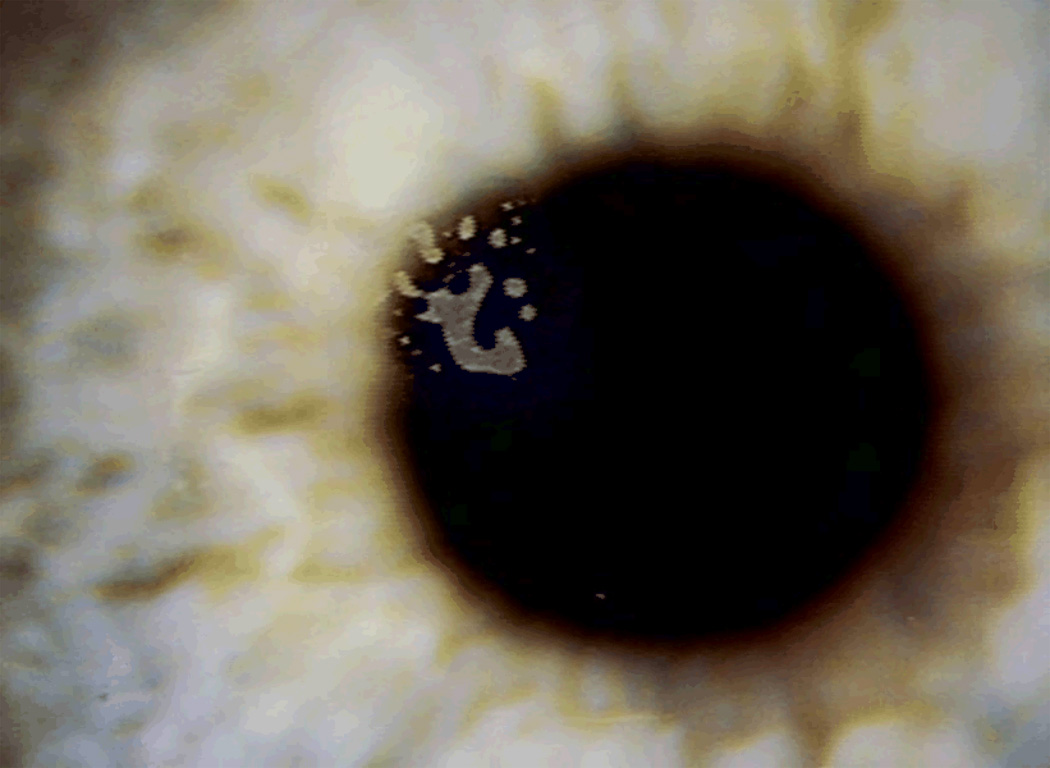
Epithelial Ingrowth
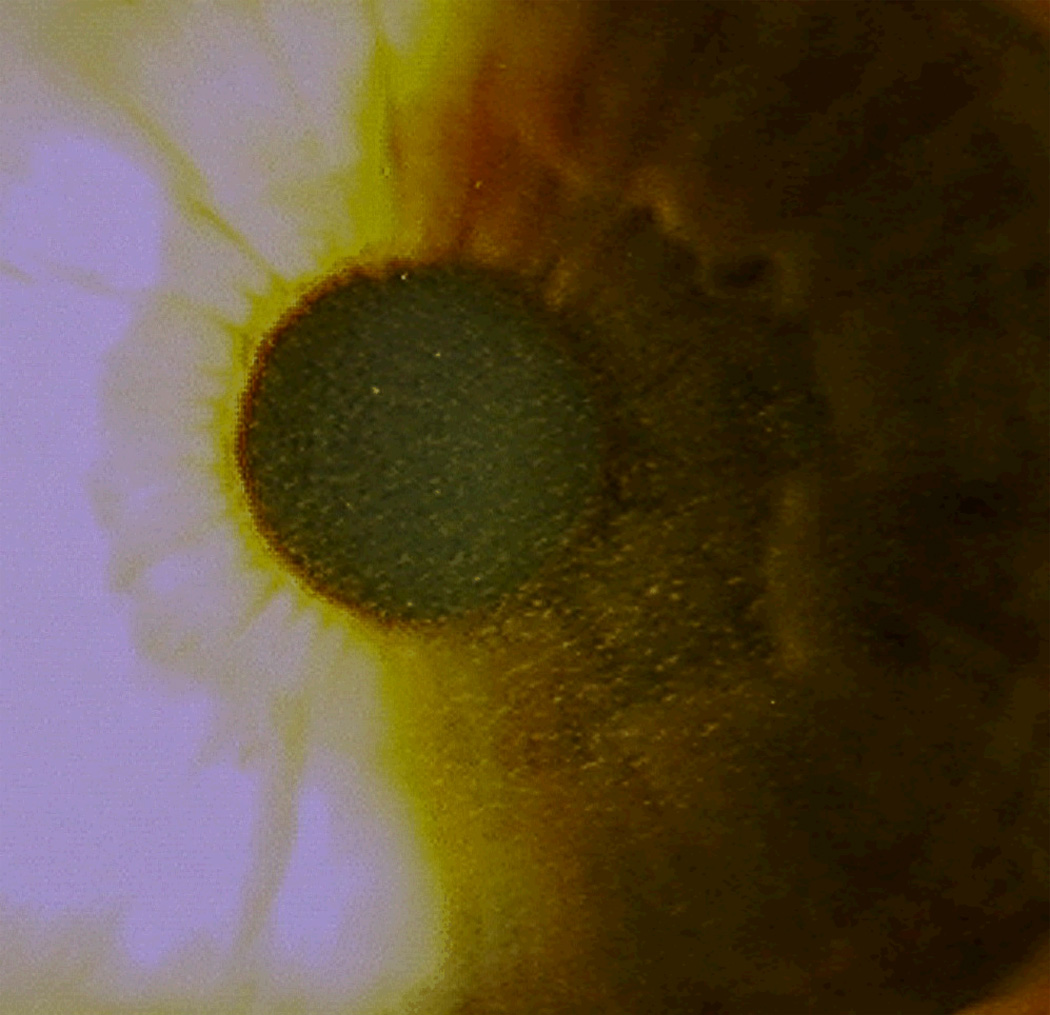
Etiology
Epithelial ingrowth at the far periphery is a normal healing response to LASIK flap creation,1 but clinically-relevant epithelial ingrowth occurs when a fistula develops under the flap allowing epithelial cell growth into the interface.67 Most cases can be observed without requiring intervention.67 With femtosecond laser flap creation, the overall incidence of visually significant epithelial ingrowth has decreased.68 In some eyes, epithelial ingrowth may require treatment for a variety of reasons listed in Table 8 [Figure 11].67, 69 For primary LASIK, increased epithelial ingrowth incidence is associated with mechanical microkeratomes as compared to femtosecond lasers for flap creation,33 hyperopic LASIK treatment,70 LASIK after RK,71 epithelial defects during surgery,72 and older age.73 For LASIK retreatment, increased epithelial ingrowth incidence is associated with the use of contact lenses after retreatment73 and flap-lift retreatment performed 3 or more years after primary LASIK.74
Table 8.
Reasons for Intervention for Epithelial Ingrowth
| Finding | Symptom |
|---|---|
| Extension into visual axis | Decreased acuity |
| Extension to edge of pupil | Increased glare |
| Focal flap elevation (paracentral) | Induced irregular astigmatism |
| Focal flap elevation (flap edge) | Foreign body sensation |
| Induced keratolysis | Focal flap melt |
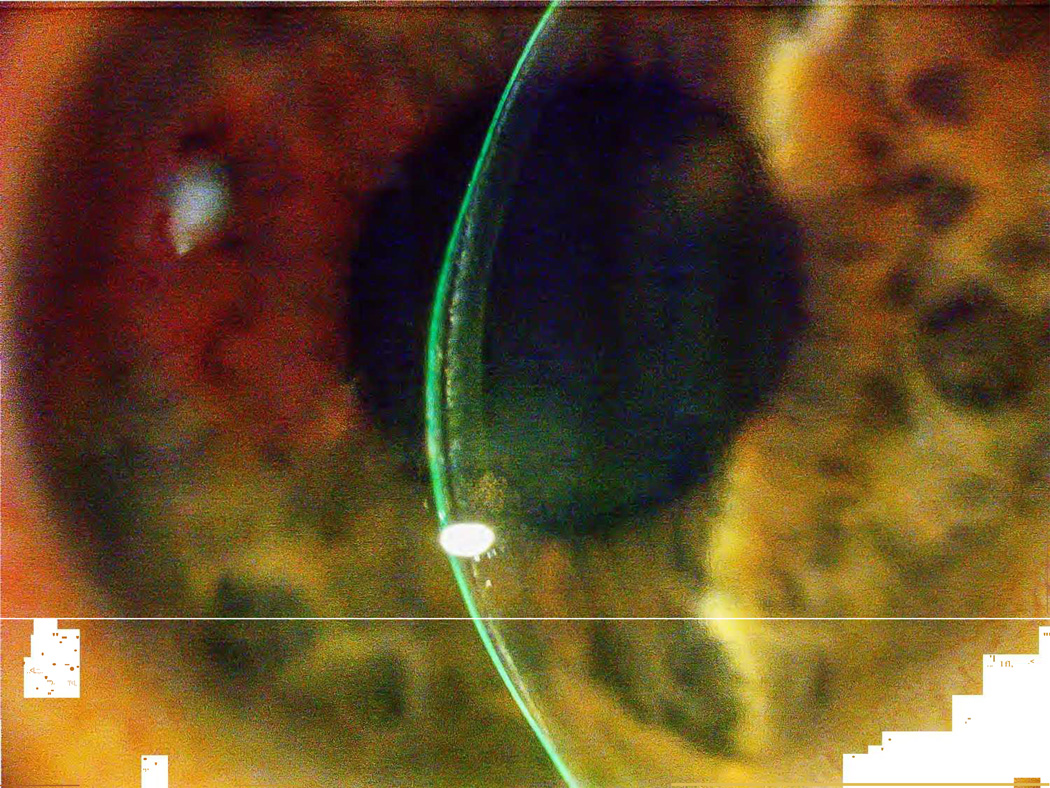
Figure 11.
A) Focal area of epithelial ingrowth noted on slit lamp exam with B) corresponding focal irregular astigmatism noted on topography. (Reprinted from Randleman JB. Chapter 8: Etiology and clinical presentations of irregular astigmatism after keratorefractive surgery. In: Wang M, ed. Irregular Astigmatism: Diagnosis and Treatment. SLACK Inc., Thorofare, NJ 2007: 79–90)
Management
Treatment depends on the clinical situation. Majority of cases of mild, clinically insignificant ingrowth are managed with observation. Initial surgical treatment for epithelial ingrowth is performed with flap lift, removal of epithelial cells from the posterior surface of the flap and the stromal bed with a blade or similar instrument, and replacement of the flap without sutures or tissue glue.67, 75 With recurrent episodes of epithelial ingrowth, additional measures are typically taken, including flap suture,76, 77 fibrin glue,78, 79 or YAG laser treatment.80
Outcomes
As long as flap melt does not occur, patients typically retain UDVA and CDVA following appropriate management with low rates of recurrence.
Distinguishing between Various Interface Complications
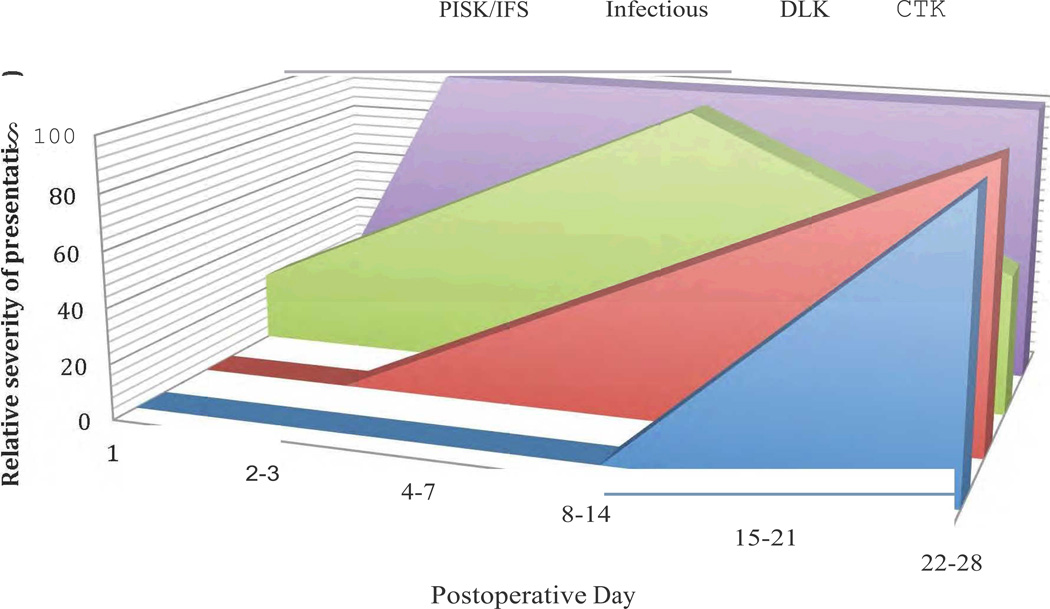
The key to appropriate management of LASIK interface disorders is distinguishing them from one another. Epithelial ingrowth has a highly characteristic appearance and is therefore rarely mistaken for any other condition; however, the other four entities may have overlapping presentations. In addition to presenting symptoms and appearance [Table 1], timing of onset is important in distinguishing between various entities [Figure 12].
Figure 12.
Schematic representation of relative onset and severity of the overlapping interface complications. The Y-axis represents that time point by which that relative percentage of cases usually present and the relative severity of each complication; the decline in percentage on the Y-axis for DLK indicates the likelihood that DLK should begin to evolve and resolve with appropriate management over that time course.
Distinguishing Infectious Keratitis from other possible etiologies
The appearance of a focal infiltrate at any time point, or a new infiltrate of any configuration more than one week after LASIK, should be presumed infectious until proven otherwise. Infectious etiologies are most commonly symptomatic, accompanied by increased foreign body sensation and some degree of visual reduction, with significantly increased conjunctival erythema, which is unique among interface disorders [Table 1]. The infiltrate is focal in nature with surrounding inflammatory cells with haze, as opposed to the more diffuse appearance of DLK. Unlike DLK, the inflammation associated infectious keratitis persists despite topical corticosteroid use. Infectious keratitis is dissimilar from PISK or CTK.
Distinguishing DLK from other possible etiologies
At various times in its clinical course, DLK may mimic benign interface debris (DLK Stage 1–2) [Figures 1 & 2], PISK (DLK Stage 3) [Figure 4], and CTK (DLK Stage 4) [Figure 5], and has been confused with infectious etiologies at various stages. The prominent element of DLK is early onset, evolution in appearance over time, and dramatic response to steroids; these features should facilitate distinguishing DLK from all other interface entities.
Benign interface debris is asymptomatic and does not change over time, with or without steroid use. PISK takes at least 2 weeks to develop, as compared to the early onset of DLK, and does not improve with steroid use.39–41, 50 All patients with the presumptive diagnosis of DLK managed on aggressive steroids should have that diagnosis reconsidered if there is no dramatic change in clinical appearance within 2 weeks. IOP measurement is critical when steroids are used for more than one week after LASIK. CTK is painless, presents almost immediately with a stage 4 DLK-like appearance within the first few days after surgery, and does not evolve over time, as opposed to DLK, which takes longer to evolve to this appearance.
Summary
A variety of complications can present in the LASIK interface, with overlapping clinical features but unique mechanisms, time courses, and management strategies. Differentiating between these interface disorders by taking a careful history of symptoms and onset, combined with a focused clinical exam, allows for accurate diagnosis and treatment on presentation. Close attention to these specific features should lead the clinician rapidly to the appropriate diagnosis and optimal management.
Figure 3.
Diffuse lamellar keratitis (DLK), stage 3. Note the diffuse, granular haze throughout the central cornea.
Table 6.
Pressure Induces Stromal Keratopathy (PISK)
| Study | Year | Term Used |
# Patients |
Age Range |
IOP range (mmHg) |
Method | Interface fluid |
Time to diagnosis |
VF loss |
Visual Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Belin30 | 2002 | Pressure induced stromal keratitis | 4 (7 eyes) | 33–48 | 27–38 | PN | No | 13 days – 4 months | Not reported | UDVA: 14.3%≥20/20 57.1%≥20/25 |
| Hamilton39 | 2002 | Interface fluid | 4 (6 eyes) | 19–58 | 30–57 | TP | Yes – 4 eyes | <4 weeks – 6 months | 4 (marked visual field loss in 1 case) | CDVA: LP (1 eye) 20/400 (2 eyes) 3 eyes ≥ 20/50 |
| Nordlund31 | 2004 | Pressure induced interface keratitis | 6 (10 eyes) | 28–55 | 32–42 | Not reported | No | 1 week – 9 weeks | Not reported | CDVA: 80% ≥ 20/20 90% ≥ 20/30 |
| Galal38 | 2006 | Steroid induced elevation of intraocular pressure | 8 (13 eyes) | 31.4±5.3* (mean) | 24–56* | GAT | Yes - 2 eyes | 2 weeks – 3 months | 2 eyes | UDVA: (logMAR) 0.9± 0.18* |
PN: Pneumatonometer
TN: Tono-pen
GAT: Goldmann Applanation Tonometry
VF = visual field
: Results reported include patients in Group 1: misdiagnosed initially as DLK
Table 7.
Central Toxic Keratopathy (CTK)
Acknowledgments
Financial Support: Supported in part by NIH NEI P30EY06360 and an unrestricted departmental grant from Research to Prevent Blindness, Inc.
Footnotes
The Authors have no financial interests in the products or topics in this manuscript
References
- 1.Dawson DG, Kramer TR, Grossniklaus HE, Waring GO, 3rd, Edelhauser HF. Histologic, ultrastructural, and immunofluorescent evaluation of human laser-assisted in situ keratomileusis corneal wounds. Archives of ophthalmology. 2005;123:741–756. doi: 10.1001/archopht.123.6.741. [DOI] [PubMed] [Google Scholar]
- 2.Llovet F, de Rojas V, Interlandi E, et al. Infectious keratitis in 204 586 LASIK procedures. Ophthalmology. 2010;117:232–238. e1–e4. doi: 10.1016/j.ophtha.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Stulting RD, Randleman JB, Couser JM, Thompson KP. The epidemiology of diffuse lamellar keratitis. Cornea. 2004;23:680–688. doi: 10.1097/01.ico.0000127477.14304.de. [DOI] [PubMed] [Google Scholar]
- 4.Chang MA, Jain S, Azar DT. Infections following laser in situ keratomileusis: an integration of the published literature. Survey of ophthalmology. 2004;49:269–280. doi: 10.1016/j.survophthal.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Donnenfeld ED, Kim T, Holland EJ, et al. ASCRS White Paper: Management of infectious keratitis following laser in situ keratomileusis. Journal of cataract and refractive surgery. 2005;31:2008–2011. doi: 10.1016/j.jcrs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Freitas D, Alvarenga L, Sampaio J, et al. An outbreak of Mycobacterium chelonae infection after LASIK. Ophthalmology. 2003;110:276–285. doi: 10.1016/S0161-6420(02)01643-3. [DOI] [PubMed] [Google Scholar]
- 7.Karp CL, Tuli SS, Yoo SH, et al. Infectious keratitis after LASIK. Ophthalmology. 2003;110:503–510. doi: 10.1016/S0161-6420(02)01760-8. [DOI] [PubMed] [Google Scholar]
- 8.Moshirfar M, Welling JD, Feiz V, Holz H, Clinch TE. Infectious and noninfectious keratitis after laser in situ keratomileusis Occurrence, management, and visual outcomes. Journal of cataract and refractive surgery. 2007;33:474–483. doi: 10.1016/j.jcrs.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Solomon R, Donnenfeld ED, Azar DT, et al. Infectious keratitis after laser in situ keratomileusis: results of an ASCRS survey. Journal of cataract and refractive surgery. 2003;29:2001–2006. doi: 10.1016/s0886-3350(03)00512-1. [DOI] [PubMed] [Google Scholar]
- 10.Solomon R, Donnenfeld ED, Perry HD, et al. Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. American journal of ophthalmology. 2007;143:629–634. doi: 10.1016/j.ajo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Garg P, Chaurasia S, Vaddavalli PK, Muralidhar R, Mittal V, Gopinathan U. Microbial keratitis after LASIK. J Refract Surg. 2010;26:209–216. doi: 10.3928/1081597X-20100224-07. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi T, Bissen-Miyajima H, Hori-Komai Y, et al. Infectious keratitis outbreak after laser in situ keratomileusis at a single laser center in Japan. Journal of cataract and refractive surgery. 2011;37:894–900. doi: 10.1016/j.jcrs.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Mozayan A, Madu A, Channa P. Laser in-situ keratomileusis infection: review and update of current practices. Current opinion in ophthalmology. 2011;22:233–237. doi: 10.1097/ICU.0b013e3283479ed4. [DOI] [PubMed] [Google Scholar]
- 14.Hyon JY, Joo MJ, Hose S, Sinha D, Dick JD, O'Brien TP. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarithromycin in the treatment of experimental Mycobacterium chelonae keratitis. Archives of ophthalmology. 2004;122:1166–1169. doi: 10.1001/archopht.122.8.1166. [DOI] [PubMed] [Google Scholar]
- 15.Sarayba MA, Shamie N, Reiser BJ, et al. Fluoroquinolone therapy in Mycobacterium chelonae keratitis after lamellar keratectomy. Journal of cataract and refractive surgery. 2005;31:1396–1402. doi: 10.1016/j.jcrs.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 16.Woodward M, Randleman JB. Bilateral methicillin-resistant Staphylococcus aureus keratitis after photorefractive keratectomy. Journal of cataract and refractive surgery. 2007;33:316–319. doi: 10.1016/j.jcrs.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 17.Smith RJ, Maloney RK. Diffuse lamellar keratitis. A new syndrome in lamellar refractive surgery. Ophthalmology. 1998;105:1721–1726. doi: 10.1016/S0161-6420(98)99044-3. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JD, Harissi-Dagher M, Pineda R, Yoo S, Azar DT. Diffuse lamellar keratitis: incidence, associations, outcomes, and a new classification system. Journal of cataract and refractive surgery. 2001;27:1560–1566. doi: 10.1016/s0886-3350(01)00958-0. [DOI] [PubMed] [Google Scholar]
- 19.Buhren J, Baumeister M, Cichocki M, Kohnen T. Confocal microscopic characteristics of stage 1 to 4 diffuse lamellar keratitis after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2002;28:1390–1399. doi: 10.1016/s0886-3350(02)01307-x. [DOI] [PubMed] [Google Scholar]
- 20.Shah MN, Misra M, Wihelmus KR, Koch DD. Diffuse lamellar keratitis associated with epithelial defects after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2000;26:1312–1318. doi: 10.1016/s0886-3350(00)00570-8. [DOI] [PubMed] [Google Scholar]
- 21.Gritz DC. LASIK interface keratitis: epidemiology, diagnosis and care. Current opinion in ophthalmology. 2011;22:251–255. doi: 10.1097/ICU.0b013e3283477b52. [DOI] [PubMed] [Google Scholar]
- 22.Holland SP, Mathias RG, Morck DW, Chiu J, Slade SG. Diffuse lamellar keratitis related to endotoxins released from sterilizer reservoir biofilms. Ophthalmology. 2000;107:1227–1233. doi: 10.1016/s0161-6420(00)00246-3. discussion 33-4. [DOI] [PubMed] [Google Scholar]
- 23.Javaloy J, Alio JL, Rodriguez A, Gonzalez A, Perez-Santonja JJ. Epidemiological analysis of an outbreak of diffuse lamellar keratitis. J Refract Surg. 2011;27:796–803. doi: 10.3928/1081597X-20110411-01. [DOI] [PubMed] [Google Scholar]
- 24.Peters NT, Iskander NG, Anderson Penno EE, Woods DE, Moore RA, Gimbel HV. Diffuse lamellar keratitis: isolation of endotoxin and demonstration of the inflammatory potential in a rabbit laser in situ keratomileusis model. Journal of cataract and refractive surgery. 2001;27:917–923. doi: 10.1016/s0886-3350(00)00779-3. [DOI] [PubMed] [Google Scholar]
- 25.Villarrubia A, Palacin E, Gomez del Rio M, Martinez P. Description, etiology, and prevention of an outbreak of diffuse lamellar keratitis after LASIK. J Refract Surg. 2007;23:482–486. doi: 10.3928/1081-597X-20070501-11. [DOI] [PubMed] [Google Scholar]
- 26.Yuhan KR, Nguyen L, Wachler BS. Role of instrument cleaning and maintenance in the development of diffuse lamellar keratitis. Ophthalmology. 2002;109:400–403. doi: 10.1016/s0161-6420(01)00876-4. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 27.Nakano EM, Nakano K, Oliveira MC, Portellinha W, Simonelli R, Alvarenga LS. Cleaning solutions as a cause of diffuse lamellar keratitis. J Refract Surg. 2002;18:S361–S363. doi: 10.3928/1081-597X-20020502-16. [DOI] [PubMed] [Google Scholar]
- 28.Shen YC, Wang CY, Fong SC, Tsai HY, Lee YF. Diffuse lamellar keratitis induced by toxic chemicals after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2006;32:1146–1150. doi: 10.1016/j.jcrs.2005.12.142. [DOI] [PubMed] [Google Scholar]
- 29.Wilson SE, Ambrosio R., Jr Sporadic diffuse lamellar keratitis (DLK) after LASIK. Cornea. 2002;21:560–563. doi: 10.1097/00003226-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Jin GJ, Lyle WA, Merkley KH. Late-onset idiopathic diffuse lamellar keratitis after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2005;31:435–437. doi: 10.1016/j.jcrs.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 31.Kymionis GD, Diakonis VF, Bouzoukis DI, Lampropoulou I, Pallikaris AI. Idiopathic recurrence of diffuse lamellar keratitis after LASIK. J Refract Surg. 2007;23:720–721. doi: 10.3928/1081-597X-20070901-12. [DOI] [PubMed] [Google Scholar]
- 32.Linebarger EJ, Hardten DR, Lindstrom RL. Diffuse lamellar keratitis: diagnosis and management. Journal of cataract and refractive surgery. 2000;26:1072–1077. doi: 10.1016/s0886-3350(00)00468-5. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Feng Y, Stojanovic A, Jankov MR, 2nd, Wang Q. IntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysis. J Refract Surg. 2012;28:15–24. doi: 10.3928/1081597X-20111228-02. [DOI] [PubMed] [Google Scholar]
- 34.Choe CH, Guss C, Musch DC, Niziol LM, Shtein RM. Incidence of diffuse lamellar keratitis after LASIK with 15 KHz, 30 KHz, and 60 KHz femtosecond laser flap creation. Journal of cataract and refractive surgery. 2010;36:1912–1918. doi: 10.1016/j.jcrs.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moshirfar M, Gardiner JP, Schliesser JA, et al. Laser in situ keratomileusis flap complications using mechanical microkeratome versus femtosecond laser: retrospective comparison. Journal of cataract and refractive surgery. 2010;36:1925–1933. doi: 10.1016/j.jcrs.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman RS, Fine IH, Packer M. Incidence and outcomes of lasik with diffuse lamellar keratitis treated with topical and oral corticosteroids. Journal of cataract and refractive surgery. 2003;29:451–456. doi: 10.1016/s0886-3350(02)01835-7. [DOI] [PubMed] [Google Scholar]
- 37.MacRae SM, Rich LF, Macaluso DC. Treatment of interface keratitis with oral corticosteroids. Journal of cataract and refractive surgery. 2002;28:454–461. doi: 10.1016/s0886-3350(01)01325-6. [DOI] [PubMed] [Google Scholar]
- 38.Javaloy J, Alio JL, El Kady B, Munoz G, Barraquer RI, Maldonado MJ. Refractive outcomes and quality of vision related to an outbreak of diffuse lamellar keratitis. J Refract Surg. 2011;27:804–810. doi: 10.3928/1081597X-20110411-02. [DOI] [PubMed] [Google Scholar]
- 39.Belin MW, Hannush SB, Yau CW, Schultze RL. Elevated intraocular pressure-induced interlamellar stromal keratitis. Ophthalmology. 2002;109:1929–1933. doi: 10.1016/s0161-6420(02)01163-6. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton DR, Manche EE, Rich LF, Maloney RK. Steroid-induced glaucoma after laser in situ keratomileusis associated with interface fluid. Ophthalmology. 2002;109:659–665. doi: 10.1016/s0161-6420(01)01023-5. [DOI] [PubMed] [Google Scholar]
- 41.Nordlund ML, Grimm S, Lane S, Holland EJ. Pressure-induced interface keratitis: a late complication following LASIK. Cornea. 2004;23:225–234. doi: 10.1097/00003226-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 42.Dawson DG, Schmack I, Holley GP, Waring GO, 3rd, Grossniklaus HE, Edelhauser HF. Interface fluid syndrome in human eye bank corneas after LASIK: causes and pathogenesis. Ophthalmology. 2007;114:1848–1859. doi: 10.1016/j.ophtha.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Lyle WA, Jin GJ, Jin Y. Interface fluid after laser in situ keratomileusis. J Refract Surg. 2003;19:455–459. doi: 10.3928/1081-597X-20030701-13. [DOI] [PubMed] [Google Scholar]
- 44.Cheng AC, Law RW, Young AL, Lam DS. In vivo confocal microscopic findings in patients with steroid-induced glaucoma after LASIK. Ophthalmology. 2004;111:768–774. doi: 10.1016/j.ophtha.2003.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Kurian M, Shetty R, Shetty BK, Devi SA. In vivo confocal microscopic findings of interlamellar stromal keratopathy induced by elevated intraocular pressure. Journal of cataract and refractive surgery. 2006;32:1563–1566. doi: 10.1016/j.jcrs.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 46.Tourtas T, Cursiefen C. "PISK-itis" or "PISK-opathy"? Cornea. 2011 doi: 10.1097/ICO.0b013e3182215992. [DOI] [PubMed] [Google Scholar]
- 47.Davidson RS, Brandt JD, Mannis MJ. Intraocular pressure-induced interlamellar keratitis after LASIK surgery. Journal of glaucoma. 2003;12:23–26. doi: 10.1097/00061198-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Galal A, Artola A, Belda J, et al. Interface corneal edema secondary to steroid-induced elevation of intraocular pressure simulating diffuse lamellar keratitis. Journal of refractive surgery. 2006;22:441–447. doi: 10.3928/1081-597X-20060501-04. [DOI] [PubMed] [Google Scholar]
- 49.Randleman JB, Lesser GR. Glaucomatous Damage from Pressure-induced Stromal Keratopathy After LASIK. J Refract Surg. 2012;28:378–379. doi: 10.3928/1081597X-20120517-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tourtas T, Kopsachilis N, Meiller R, Kruse FE, Cursiefen C. Pressure-induced interlamellar stromal keratitis after laser in situ keratomileusis. Cornea. 2011;30:920–923. doi: 10.1097/ICO.0b013e3182032002. [DOI] [PubMed] [Google Scholar]
- 51.Chang DH, Stulting RD. Change in intraocular pressure measurements after LASIK the effect of the refractive correction and the lamellar flap. Ophthalmology. 2005;112:1009–1016. doi: 10.1016/j.ophtha.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 52.Wheeldon CE, Hadden OB, Niederer RL, McGhee CN. Presumed late diffuse lamellar keratitis progressing to interface fluid syndrome. Journal of cataract and refractive surgery. 2008;34:322–326. doi: 10.1016/j.jcrs.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 53.Park HJ, Uhm KB, Hong C. Reduction in intraocular pressure after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2001;27:303–309. doi: 10.1016/s0886-3350(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 54.Fogla R, Rao SK, Padmanabhan P. Interface fluid after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2001;27:1526–1528. doi: 10.1016/s0886-3350(00)00881-6. [DOI] [PubMed] [Google Scholar]
- 55.Pepose JS, Feigenbaum SK, Qazi MA, Sanderson JP, Roberts CJ. Changes in corneal biomechanics and intraocular pressure following LASIK using static, dynamic, and noncontact tonometry. American journal of ophthalmology. 2007;143:39–47. doi: 10.1016/j.ajo.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Moshirfar M, Hazin R, Khalifa YM. Central toxic keratopathy. Current opinion in ophthalmology. 2010;21:274–279. doi: 10.1097/ICU.0b013e32833a8cb2. [DOI] [PubMed] [Google Scholar]
- 57.Sonmez B, Maloney RK. Central toxic keratopathy: description of a syndrome in laser refractive surgery. American journal of ophthalmology. 2007;143:420–427. doi: 10.1016/j.ajo.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Hazin R, Daoud YJ, Khalifa YM. What is Central Toxic Keratopathy Syndrome if it is not Diffuse lamellar Keratitis Grade IV? Middle East African journal of ophthalmology. 2010;17:60–62. doi: 10.4103/0974-9233.61218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fraenkel GE, Cohen PR, Sutton GL, Lawless MA, Rogers CM. Central focal interface opacity after laser in situ keratomileusis. J Refract Surg. 1998;14:571–576. doi: 10.3928/1081-597X-19980901-17. [DOI] [PubMed] [Google Scholar]
- 60.Hainline BC, Price MO, Choi DM, Price FW., Jr Central flap necrosis after LASIK with microkeratome and femtosecond laser created flaps. J Refract Surg. 2007;23:233–242. doi: 10.3928/1081-597X-20070301-05. [DOI] [PubMed] [Google Scholar]
- 61.Lyle WA, Jin GJ. Central lamellar keratitis. Journal of cataract and refractive surgery. 2001;27:487–490. doi: 10.1016/s0886-3350(01)00818-5. [DOI] [PubMed] [Google Scholar]
- 62.Parolini B, Marcon G, Panozzo GA. Central necrotic lamellar inflammation after laser in situ keratomileusis. J Refract Surg. 2001;17:110–112. doi: 10.3928/1081-597X-20010301-03. [DOI] [PubMed] [Google Scholar]
- 63.Hau SC, Allan BD. In vivo confocal microscopy findings in central toxic keratopathy. Journal of cataract and refractive surgery. 2012;38:710–712. doi: 10.1016/j.jcrs.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Thornton IL, Foulks GN, Eiferman RA. Confocal Microscopy of Central Toxic Keratopathy. Cornea. 2012 doi: 10.1097/ICO.0b013e3181f7f109. [DOI] [PubMed] [Google Scholar]
- 65.Sikder S, Khalifa YM, Neuffer MC, Moshirfar M. Tomographic corneal profile analysis of central toxic keratopathy after LASIK. Cornea. 2012;31:48–51. doi: 10.1097/ICO.0b013e31821de341. [DOI] [PubMed] [Google Scholar]
- 66.Tu KL, Aslanides IM. Surgical intervention in central toxic keratopathy. European journal of ophthalmology. 2012;0 doi: 10.5301/ejo.5000155. [DOI] [PubMed] [Google Scholar]
- 67.Wang MY, Maloney RK. Epithelial ingrowth after laser in situ keratomileusis. American journal of ophthalmology. 2000;129:746–751. doi: 10.1016/s0002-9394(00)00357-3. [DOI] [PubMed] [Google Scholar]
- 68.Kamburoglu G, Ertan A. Epithelial ingrowth after femtosecond laser-assisted in situ keratomileusis. Cornea. 2008;27:1122–1125. doi: 10.1097/ICO.0b013e3181731439. [DOI] [PubMed] [Google Scholar]
- 69.Asano-Kato N, Toda I, Hori-Komai Y, Takano Y, Tsubota K. Epithelial ingrowth after laser in situ keratomileusis: clinical features and possible mechanisms. American journal of ophthalmology. 2002;134:801–807. doi: 10.1016/s0002-9394(02)01757-9. [DOI] [PubMed] [Google Scholar]
- 70.Mohamed TA, Hoffman RS, Fine IH, Packer M. Post-laser assisted in situ keratomileusis epithelial ingrowth and its relation to pretreatment refractive error. Cornea. 2011;30:550–552. doi: 10.1097/ico.0b013e3182000ac3. [DOI] [PubMed] [Google Scholar]
- 71.Randleman JB, Banning CS, Stulting RD. Persistent epithelial ingrowth. Ophthalmology. 2006;113:1468 e1–1468 e3. doi: 10.1016/j.ophtha.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Jabbur NS, Chicani CF, Kuo IC, O'Brien TP. Risk factors in interface epithelialization after laser in situ keratomileusis. J Refract Surg. 2004;20:343–348. doi: 10.3928/1081-597X-20040701-07. [DOI] [PubMed] [Google Scholar]
- 73.Chan CC, Boxer Wachler BS. Comparison of the effects of LASIK retreatment techniques on epithelial ingrowth rates. Ophthalmology. 2007;114:640–642. doi: 10.1016/j.ophtha.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 74.Caster AI, Friess DW, Schwendeman FJ. Incidence of epithelial ingrowth in primary and retreatment laser in situ keratomileusis. Journal of cataract and refractive surgery. 2010;36:97–101. doi: 10.1016/j.jcrs.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 75.Rapuano CJ. Management of epithelial ingrowth after laser in situ keratomileusis on a tertiary care cornea service. Cornea. 2010;29:307–313. doi: 10.1097/ICO.0b013e3181b7f3c5. [DOI] [PubMed] [Google Scholar]
- 76.Rojas MC, Lumba JD, Manche EE. Treatment of epithelial ingrowth after laser in situ keratomileusis with mechanical debridement and flap suturing. Archives of ophthalmology. 2004;122:997–1001. doi: 10.1001/archopht.122.7.997. [DOI] [PubMed] [Google Scholar]
- 77.Spanggord HM, Epstein RJ, Lane HA, et al. Flap suturing with proparacaine for recurrent epithelial ingrowth following laser in situ keratomileusis surgery. Journal of cataract and refractive surgery. 2005;31:916–921. doi: 10.1016/j.jcrs.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 78.Anderson NJ, Hardten DR. Fibrin glue for the prevention of epithelial ingrowth after laser in situ keratomileusis. Journal of cataract and refractive surgery. 2003;29:1425–1429. doi: 10.1016/s0886-3350(02)01989-2. [DOI] [PubMed] [Google Scholar]
- 79.Yeh DL, Bushley DM, Kim T. Treatment of traumatic LASIK flap dislocation and epithelial ingrowth with fibrin glue. American journal of ophthalmology. 2006;141:960–962. doi: 10.1016/j.ajo.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Ayala MJ, Alio JL, Mulet ME, De La Hoz F. Treatment of laser in situ keratomileusis interface epithelial ingrowth with neodymium:yytrium-aluminum-garnet laser. American journal of ophthalmology. 2008;145:630–634. doi: 10.1016/j.ajo.2007.11.022. [DOI] [PubMed] [Google Scholar]