Abstract
Objective:
We performed a prospective study to evaluate the value of contrast-enhanced (CE) ultrasound in quantitative evaluation of renal cortex perfusion in patients with chronic kidney dysfunction (CKD Stage I–II).
Methods:
The present study was approved by the institutional ethics committee. The study focused on 41 consecutive patients (males, 32; females, 9; mean age, 55.0 ± 5.0 years) with clinical suspicion of CKD (Stages I–II). For both kidneys, CE ultrasound was performed after intravenous bolus injection of 1.0 ml SonoVue® (Bracco Imaging S.p.A., Milan, Italy). Time–intensity curves (TICs) and quantitative indexes were created with Qlab software (Philips, Bothell, WA). 45 healthy volunteers were included as control group. All statistical analyses were performed with SPSS® v. 15.0 software package (SPSS, Chicago, IL). A difference was considered statistically significant with p < 0.05.
Results:
Patients with CKD (Stages I–II) had no obvious change in the shape of TICs. Among all quantitative indexes, the changes of area under the curve (AUC), derived peak intensity (DPI) and slope rate of elevation curve (A) were statistically significant (p < 0.05). DPI <12 dB, A >2 and AUC >1300 dB s had high utility in the evaluation of CKD, with 81%, 73% and 78% specificities and 76%, 73% and 77% sensitivities.
Conclusion:
CE ultrasound might be valuable in the early evaluation of CKD. AUC, A and DPI might be valuable quantitative indexes.
Advances in knowledge:
Quantitative CE ultrasound analysis can be used for the standardized and early evaluation of renal dysfunction.
Throughout the world, chronic kidney dysfunction (CKD) is a growing health concern because of its increasing prevalence and incidence rate.1 Since CKD primarily involves perfusion changes in the renal cortex, assessment of tissue perfusion is an important component for the evaluation of CKD.2 Early and detailed visualizations of perfusion changes of the renal cortex yield information about organ viability and function, which would be crucial to make diagnosis and to initiate early drug therapy.3
Different non-invasive imaging modalities, such as multidetector CT,4 positron emission tomography,5 MRI6 and single-photon emission CT with 99mTc-diethylenetriamine pentaacetic acid7 are used in the quantifications of tissue perfusions. However, high costs, reduced availability, long examination periods, patients' exposure to radiation or nuclear tracers limited clinical applications of these techniques.4–7 Greyscale renal ultrasound combined with colour Doppler flow imaging (CDFI) had become the main non-invasive imaging methods for evaluating the renal anatomy and blood flow.8 However, CDFI parameters such as the resistance index (RI) and peak systolic velocity (PSV) provided only indirect macrovasculature parameters, which could not directly assess renal cortex perfusion and were of limited diagnostic use in the CKD.9 To date, there was no reliable, accurate and convenient method to determine renal blood perfusion in vivo, thereby leading to difficulty in early and accurate diagnosis of CKD.
In recent years, low mechanical index (MI) real-time contrast-enhanced (CE) ultrasound has been proposed as an alternative imaging technique in this area.10 Because microbubbles are blood-pool agents, when injected intravenously, they remain entirely intravascular, mix uniformly with blood in the circulation and possess the same intravascular rheology as red blood cells.11 The advantages of CE ultrasound include the absence of ionizing radiation or nephrotoxicity, and the widespread availability. When CE ultrasound is performed immediately after a non-conclusive ultrasound study, only a short time was needed to arrive at a final diagnosis.12 CE ultrasound has been recently used as a new imaging technique for quantifying tissue perfusion changes in the liver,13 heart14 and kidney.15 The large blood supply of the kidney was a good base for contrast studies, as >90% of kidney blood flow supplied the renal cortex by the renal arterioles and capillaries.16 Since CE ultrasound microbubbles remain strictly inside the vessels, they can be viewed as blood-pool markers enabling functional imaging of the kidney.17 The increase in echo signal intensity after microbubble injection may be quantified by dedicated software packages to produce time–intensity curves (TICs). Enhancement-based representations had been used to assess unilateral kidney dysfunction such as in renal artery stenosis by a simple analysis of the tracer concentration curve.18 These features made low MI CE ultrasound a promising technique in evaluation of renal cortex perfusion.
The purpose of this initial study was to evaluate the feasibility of CE ultrasound to assess renal cortex tissue perfusion in the early stages of CKD (Stages I–II) by means of TICs. The diagnostic efficacy gained by quantitative CE ultrasound was compared with that of renal arterial PSV and RI measured by CDFI.
METHODS AND MATERIALS
Patients
This study was performed with the approval of the ethics committee of Zhongshan Hospital, Fudan University, Shanghai, China; all patients gave their full informed consent to participate in our study. They were examined using standardized procedures of CE ultrasound.
41 consecutive patients (males, 32; females, 9; mean age, 55.0 ± 5.0 years), from June 2008 to December 2011 with normal renal appearances on ultrasound scanning, were studied prospectively using CDFI and CE ultrasound. CKD (Stages I–II) was suspected clinically in these patients at the nephropathy department of our hospital. The inclusion criteria for suspected early renal dysfunction were (1) the patient had evidence of kidney damage for 3 or more months [e.g. proteinuria, hypertension, structural changes on biopsy, elevated blood serum urea nitrogen levels (BUN >7.0 mmol l−1) or serum creatinine levels (SCr >130 μmol l−1)]. (2) In clinical evaluations, glomerular filtration rates (GFRs) were estimated by the modification of diet in renal disease (MDRD) equation, which was derived from the patient's SCr value, age and sex. These patients had kidney damage with normal or mild decrease in GFR (>60 ml min−1 per 1.73 m−2). According to the Kidney Disease Outcome Quality Initiatives clinical practice guidelines for chronic kidney disease, these patients were classified as CKD (Stages I–II).19 The exclusion criteria were contrast agent allergy, severe heart or pulmonary disease and pregnancy. Patients who could not hold their breath during CE ultrasound procedure were also excluded (Table 1).
Table 1.
Baseline characteristics
| Variables | Control group (n = 45) | Chronic kidney dysfunction patients (n = 41) |
|---|---|---|
| Mean age (years) | 56.5 ± 3 | 55.0 ± 5 |
| Male, n (%) | 35 (77.8) | 60 (74.1) |
| Female, n (%) | 10 (22.2) | 21 (25.9) |
| Estimated glomerular filtration rate (MDRD equation) (ml min−1 per 1.73 m2) | 105.0 ± 13.5 | 85.0 ± 15.6a |
| BUN (mmol l−1) | 4.8 ± 2.3 | 5.6 ± 1.2 |
| Serum creatinine (μmol l−1) | 80.0 ± 39.1 | 80.3 ± 27.6 |
| Systolic pressure (mmHg) | 101.7 ± 10.3 | 115.2 ± 15.0a |
| Diastolic pressure (mmHg) | 64.9 ± 8.9 | 78.7 ± 10.4 |
BUN, blood serum urea nitrogen level; MDRD, modification of diet in renal disease.
p < 0.05.
45 sex- and age-matched healthy volunteers (males, 35; females, 10) were enrolled as the control group; mean age, 56.5 ± 3.0 years. These healthy adults visited our hospital for routine physical examinations. They had no history of nephropathy with normal renal appearances on ultrasound scanning, normal BUN (<7.0 mmol l−1) and Scr levels (<130 μmol l−1). The recruitment of all healthy subjects was in accordance with the institutional review board-approved guidelines. All subjects voluntarily received CE ultrasound examinations and signed the consent forms prior to enrolment.
Protocol
All ultrasound examinations were performed with Philips iU22 unit (Philips, Bothell, WA), using a C5-2 3.5-MHz transducer. All the scanning procedures were performed by one experienced radiologist with 10 years' experience in diagnostic ultrasound of the kidney. All patients were examined by ultrasound according to a standard three-step protocol in the supine position. First, we performed greyscale ultrasound examinations of both the kidneys, both longitudinally and cross sectionally. The echo, the size (length and width) and the thickness of the renal cortex were noted. We determined a maximum longitudinal scanning plane for CE ultrasound that included the entire kidney. Second, CDFI was used to assess the renal blood flow, and RI and PSV were measured. Third, after injection of the microbubble ultrasound contrast agent, we performed CE ultrasound. First for the right kidney, and after a time interval for about 20 min, the same CE ultrasound procedure was performed for the left kidney. All images were stored digitally on a hard disk and then transferred to a personal computer (PC). Renal perfusion images obtained with CE ultrasound were analysed offline, using a commercially available software tool (Qlab release v. 4.1; Philips). The perfusion analysis and quantification procedures were performed by another experienced radiologist with 5 years' experience of CE ultrasound imaging analysis.
Before the ultrasound examinations, patients rested in a sitting position for 10 min, and their blood pressure was taken in the right upper extremity. In cases of patients scheduled for kidney biopsy, CE ultrasound was performed before the biopsy. BUN and SCr levels were measured at the time of CE ultrasound examination.
CONTRAST-ENHANCED ULTRASOUND EXAMINATION
Image acquisition
CE ultrasound was performed using contrast harmonic imaging at a low MI of <0.1 and a minimum tissue signal. The focal zone was set at the level of the deep cortex. The acoustic power was set at the default setting, and the gain was 80%. We performed CE ultrasound using SonoVue® (Bracco Imaging S.p.A., Milan, Italy), a second generation contrast agent composed of sulfur-hexafluoride microbubbles stabilized by a phospholipid shell. A white, milky suspension of sulfur-hexafluoride-filled microbubbles was obtained by the addition of 5 ml of physiological saline solution (0.9% of sodium chloride) to a lyophilized powder (25 mg) containing phospholipids, according to the protocol proposed by the pharmaceutical company. The suspension was then left to settle for at least 2 min. The bubble concentration was in the range of 1–5 × 108 microbubbles per millilitre, with 90% of bubbles <8 μm in diameter. SonoVue was administered as a 1-ml bolus together with 5 ml of saline solution over 4–5 s. At the same time, digital cine clips (180 s in duration) were stored in a hard disk as digital image communications in medicine images. Imaging of renal perfusion was obtained in a maximum longitudinal scanning plane including the entire kidney. Throughout the examination, we manually held the transducer in the same scanning plane, and patients were instructed to breathe quietly.
Quantification procedure
Digital cine loops were transferred to a PC system for offline quantification using proprietary analysis software (Qlab; Philips). This software took into account the exact compression algorithm and supplies absolute value data in a linear, uncompressed scale. A region of interest (ROI) was drawn over the mid superficial peripheral renal cortex. The investigator ensured selection of a similar sized ROI (5 × 5 mm2) and placed the ROI in a similar depth on the kidney cortex for each subject while avoiding inclusion of the interlobar and arcuate arteries. For quantitative analysis of renal tissue perfusion, signal intensity in the ROI was measured, and TICs were automatically generated. We used the “breath compensation” function in Qlab to identify and correct placements of the ROIs. For each kidney, analyses were repeated three times, and we took the average of three ROI observations; in order to minimize the transitional distance caused by respiration and for accuracy of the analyses.
After SonoVue was administered as a bolus, shapes of the TICs observed in the renal cortex reflected a highly skewed gaussian curve. The γ-variate function: I(t) = A × t × exp(−αt) + C was the suitable curve fit approximation.20 In such a formula, I(t) represented the pixel intensity as a function of time, the slope rate of ascending curve (A) was the scaling factor related to the wash-in of TICs and the slope rate of descending curve (α) was a rate constant reflected to the width of TICs. The area the under curve (AUC) was derived from this equation as A/α2, and the derived peak intensity (DPI) was calculated as (A/α × exp1). Time to peak (TTP) occurred at 1/α. The asymptotic value of C represented the baseline intensity. Quantitative perfusion parameters were measured using TICs to estimate renal blood flow.
CONVENTIONAL COLOUR DOPPLER FLOW IMAGING PARAMETERS
The same C5-2 3.5-MHz convex array probe with a pulse repetition frequency of 2.5 kHz was used to assess the RI and PSV by CDFI. Intrarenal Doppler signals were obtained from three representative interlobular arteries in the upper, middle and lower poles of the renal cortex. The median values of PSV and RI were assessed by using three different measurements performed by a single investigator.
ANALYSES AND STATISTICS
Contrast-enhanced ultrasound data analyses
Data are expressed as mean ± standard deviation. Before performing any statistical analyses between groups, data were checked for normal distribution using the Kolmogorov–Smirnov Z-test.
The two-samples Student's t-test was applied for the comparison between the CKD group and the control group, also between two kidneys. All statistical analyses were performed with SPSS® v. 15.0 software package (SPSS Inc., Chicago, IL). A difference was considered statistically significant with p < 0.05.
Receiver operating characteristic analysis
For further analyses of the predictive power of ultrasound quantitative indexes, we performed a receiver operating characteristic (ROC) analysis as a predictor of CKD (n = 41) compared with the normal group (n = 45). To assess diagnostic performance, χ2 tests were performed to compare the sensitivities, specificities and overall accuracies of the CE ultrasound perfusion indexes. Estimated GFR (MDRD) was considered as the gold standard. Sensitivity was calculated as true positive/true positive + false negative, and specificity was calculated as true negative/true negative + false positive, while overall accuracy was calculated as true positive + true negative/total number. A difference was considered statistically significant with p < 0.05.
RESULTS
No effects of sonographic contrast material were noted, and there was no haematuria or local pain.
Real-time observation of renal cortical perfusion
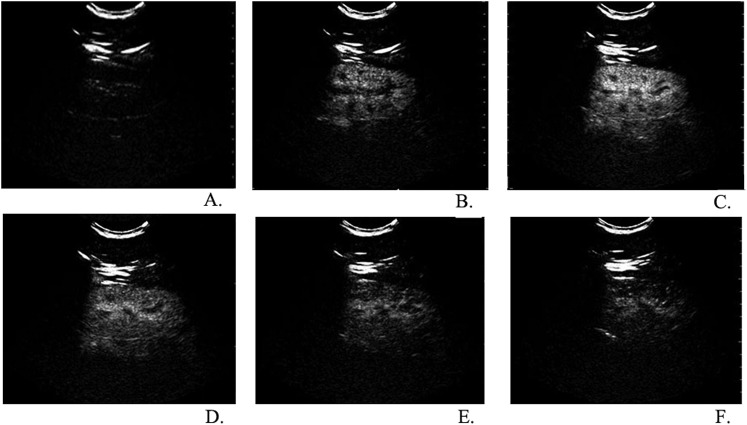
After SonoVue administration, in vivo greyscale ultrasound showed real-time flow and perfusion in the kidney. We obtained very clear and detailed renal perfusion images of the two kidneys in all patients. From the longitudinal plane, we observed quick and intense enhancement of the cortex owing to the high renal blood flow. Initial visualization of the contrast agent occurred after a time of 15–25 s, from segmental renal arteries to small interlobular arteries, and immediately followed by an intense and uniform enhancement of the renal cortex. After the enhancement of the cortex, the pyramids were gradually filled in with contrast agents and became isoechoic with the cortex in about 20–30 s. In about 180 s, the renal enhancing effect decreased as the contrast concentration decreased (Figure 1).
Figure 1.
Real-time observation of renal cortical perfusion in different stages after a bolus injection of SonoVue® (Bracco Imaging S.p.A., Milan, Italy); from the longitudinal plane, we observed a quick and intense enhancement of the cortex (a). Initial visualization of the contrast agent occurred after 15–25 s, from segmental renal arteries to small interlobular arteries and immediately followed by an intense and uniform enhancement of the renal cortex (b). After the enhancement of the cortex, the pyramids were gradually filled in with contrast agents and became isoechoic with the cortex. In about 30–45 s, enhancement of the renal cortex reached peak intensity (c). In 50–180 s, the renal cortex enhancement gradually decreased as the contrast concentration decreased (d–f). No significant delay was observed in the perfusion of the renal cortex between patients with early chronic kidney dysfunction and the control group.
No significant delay was observed in the perfusion of renal cortex between the early CKD patients (Stages I–II) and the control group.
Quantitative renal perfusion data
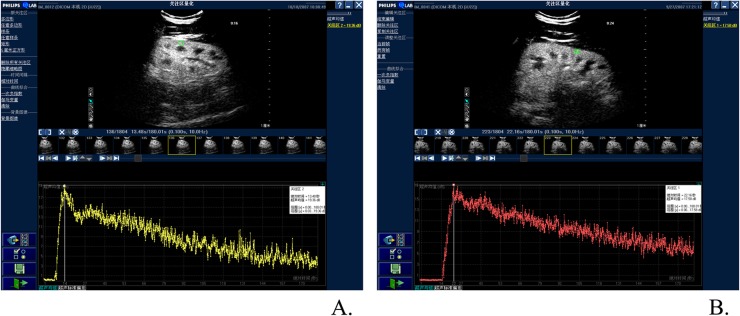
CE ultrasound renal perfusion images were then converted into TICs. On bolus study, TICs followed a typical dilution shape, which had the same wash-in and wash-out shape as renography. Compared with normal groups, the curves of patients with early CKD had no obvious change in the shape of TICs. In both the groups, TIC of renal cortex perfusion was an asymmetrical curve with an obvious steep ascending slope, a peak and a flat descending slope (Figure 2).
Figure 2.
Comparison of time–intensity curves (TICs) between patients with early chronic kidney dysfunction (CKD) and the control group; compared with the normal groups, the curves of patients with early CKD had no obvious change in the shape of TICs. In both the groups, TICs of renal cortex perfusion were asymmetrical curves with obvious steep ascending slope, peak and flat descending slope. (a) TIC of patients with CKD (Stages I–II); (b) TIC of the control group.
Compared with normal volunteers, quantitative indexes of patients with CKD (Stages I–II) were characterized by a delayed (AUC and TTP increased) and decreased enhancement (DPI decreased) of a comparable magnitude in the renal cortex. The slope rates of both ascending and descending curves were low (A gradually increased and α decreased). It took more time to reach the peak intensity (TTP increased).
Among all the quantitative indexes, in both the kidneys, AUC and A were significantly increased (p < 0.01) and DPI was significantly decreased (p < 0.05). However, changes of TTP and α were not significant in the renal cortex (Table 2).
Table 2.
Comparison of quantitative renal perfusion indexes between chronic kidney dysfunction (CKD) (Stages I–II) patients with healthy volunteers
| Quantitive renal perfusion indexes | Area under the curve (dB s) | Time to peak (s) | Derived peak intensity (dB) | A (dB s−1) | α (1 s−1) |
|---|---|---|---|---|---|
| Left kidney | |||||
| CKD patients (n = 41) | 1308.76 ± 503.65 | 22.04 ± 6.34 | 14.61 ± 1.84 | 2.46 ± 0.67 | 0.01 ± 0.02 |
| Control group (n = 45) | 1178.99 ± 468.28 | 22.21 ± 6.04 | 16.01 ± 0.45 | 2.24 ± 0.69 | 0.01 ± 0.01 |
| p-value | 0.030 | 0.584 | 0.035 | 0.047 | 0.765 |
| Right kidney | |||||
| CKD patients (n = 41) | 1261.22 ± 430.44 | 21.15 ± 4.71 | 14.69 ± 0.63 | 2.63 ± 0.89 | 0.02 ± 0.00 |
| Control group (n = 45) | 1156.22 ± 352.59 | 21.68 ± 5.78 | 15.64 ± 0.45 | 2.17 ± 0.96 | 0.01 ± 0.00 |
| p-value | 0.044 | 0.792 | 0.033 | 0.046 | 0.434 |
A, slope rate of ascending curve; α, slope rate of descending curve.
Conventional colour Doppler flow imaging parameters
Compared with the control group, patients with CKD (Stages I–II), by unenhanced ultrasound scanning, demonstrated no change in morphologic appearance with good differentiation of the medulla and cortex.
For the CDFI measurements, statistical analyses revealed a non-significant difference in RI and PSV between the normal control group (n = 45) and patients with CKD (Stages I–II) (n = 41) (Table 3).
Table 3.
Comparison of color Doppler flow imaging parameters between chronic kidney dysfunction (CKD) (Stages I–II) patients with healthy volunteers
| Colour Doppler flow imaging parameters | Peak systolic velocity(cm s−1) | Resistance index |
|---|---|---|
| Control group (n = 45) | 54.30 ± 21.00 | 0.60 ± 1.00 |
| CKD patients (n = 41) | 67.00 ± 15.54 | 0.63 ± 0.20 |
| p-value | 0.677 | 0.796 |
Receiver operating characteristic analysis
ROC curve analysis indicated that the diagnostic accuracies of DPI, A and AUC were better than 0.7. Among them, DPI had the biggest area under the ROC; therefore, we assumed that this quantitative index had the best diagnostic accuracy.
Assuming 12 dB as the cut-off value (>12 dB, no disease; <12 dB, disease), DPI measurements in the 41 patients yielded 33 true positives, 35 true negatives, 10 false positives and 8 false negatives [sensitivity, 81%; specificity, 76%; positive predictive value (PPV), 80%; and negative predictive value (NPV), 77%].
Assuming 2 as the cut-off value (<2, no disease; >2, disease), A measurements in the 41 patients yielded 28 true positives, 35 true negatives, 10 false positives and 13 false negatives (sensitivity, 72%; specificity, 73%; PPV, 68%; and NPV, 78%).
Assuming 1300 dB s as the cut-off value (<1300 dB s, no disease; >1300 dB s, disease), AUC measurements in the 41 patients yielded 31 true positives, 36 true negatives, 9 false positives and 10 false negatives (sensitivity, 77%; specificity, 78%; PPV, 75%; and NPV, 80%) (Table 4).
Table 4.
Comparison of sensitivities, specificities and overall accuracy of contrast-enhanced ultrasound perfusion indexes by χ2 tests
| Cut-off value | Accuracy (%) | Sensitivity (%) | Specificities (%) | Positive predictive value (%) | Negative predictive value (%) | p-value |
|---|---|---|---|---|---|---|
| Derived peak intensity <12 dB | 79.07 (68/86) | 76.74 (33/43) | 81.40 (35/43) | 80.49 (33/41) | 77.78 (35/45) | 0.012 |
| A >2 | 73.26 (63/86) | 73.68 (28/38) | 72.92 (35/48) | 68.29 (28/41) | 77.78 (35/45) | 0.499 |
| Area under the curve >1300 dB s | 77.91 (67/86) | 77.50 (31/40) | 78.26 (36/46) | 75.61 (31/41) | 80.00 (36/45) | 0.037 |
A, slope rate of ascending curve.
DISCUSSION
The kidney is a highly vascularized organ, with very rapid contrast enhancement and rapid wash-out. Our results showed that CE ultrasound provided very clear and detailed views of the renal perfusions, with full evaluations of renal vascularization including deep pole areas. CE ultrasound allowed real-time observation of tissue enhancement associated with real-time identification of blood flow. It provided excellent spatial resolution and higher temporal resolution than all other imaging modalities. The enhancement and perfusion images of renal cortex could be rapidly and continuously discriminated both in patients with CKD (Stages I–II) and healthy volunteers.
Chronic renal ischaemia is one of the key factors in the development of CKD.3 Pathologically, in the early stage of CKD, with the microvascular changes in renal cortex, the haemodynamic impedance of microcirculation in the renal cortex also increased. Owing to the renal cortical hypoperfusion, renal autoregulation mechanism was activated to keep the balance of perfusion. As a result, renal perfusion was reduced and fewer contrast microbubbles entered the renal cortex.1,3 In our study, among all five quantitative indexes of patients with early CKD (Stages I–II), statistical analyses revealed that AUC and A were significantly increased and DPI was significantly decreased compared with that of normal volunteers. These results indicated that less contrast microbubbles entered the renal cortex microvascular bed in unit time. The results of our study suggested that intravenous injection of microbubble-based ultrasound contrast agents allowed estimation of relative blood flow and fractional microvasculature blood volume in ROIs. This technique may be a valuable tool to quantify the impaired microcirculation perfusion in patients with early CKD.
ROC curve analysis indicated that the diagnostic accuracy of DPI, A and AUC were better than 0.7, and among them, DPI had the biggest area under the ROC. Thus, we assumed that this quantitative index had the best diagnostic accuracy. Assuming 12 dB as the cut-off value of the DPI, 2 as the cut-off value of A and 1300 dB s as the cut-off value of the AUC, we got relatively satisfactory diagnostic predictive power of those CE ultrasound quantitative indexes. Changes in quantitative indexes provided better insight into the perfusion of the renal cortex. They might reflect vascular perfusion damage in the early stage of CKD. Some CE ultrasound quantitative indexes of TICs in the renal cortex could be used for the standardized and early diagnosis of renal dysfunction.
CDFI was commonly used to assess renal perfusion non-invasively, but this method was limited by its low sensitivity in detecting low velocity flow of small vessels (<2 mm in diameter)11,21,22 and could not evaluate cortical perfusion. RI was influenced by the patients' vessels and elasticity.23 CDFI was also examiner dependent, limited by the deep location of the kidney.21–23 In our study, no significant difference could be found in RI and PSV. Furthermore, χ2 tests indicated that the diagnostic performances of CE ultrasound quantitative indexes were much better than that of RI and PSV as mentioned in literature.8,24 Our results also provided the evidence that quantitative CE ultrasound evaluation of CKD in the early period might be superior to previous ultrasound assessments, including ultrasound scan, RI and PSV determination.
Some basic limitations of CE ultrasound methods may exist for quantitative perfusion. First, patients must co-operate, since the transducer must be held stably without moving. Second, experienced examiners are needed to define comparable ROIs. Other limitations included the different causes of patients with CKD; whether taking drugs may alter haemodynamics of renal cortex and affect the final quantitative results. Although confirmation by a larger series is required, our preliminary findings are encouraging for the performance of CE ultrasound quantitative analysis as a screening test for patients with early CKD. CE ultrasound renal perfusion had potential utility in patient management evaluation.
FUNDING
This study was supported by the Youth Scientific Project of Shanghai Municipal Health Bureau, China. (No. 20114Y175).
REFERENCES
- 1.Levin A, Stevens LA. Executing change in the management of chronic kidney disease: perspectives on guidelines and practice. Med Clin North Am 2005; 89: 701–9. doi: 10.1016/j.mcna.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Bauer C, Melamed ML, Hostetter TH. Staging of chronic kidney disease: time for a course correction. J Am Soc Nephrol 2008; 19: 844–6. doi: 10.1681/ASN.2008010110 [DOI] [PubMed] [Google Scholar]
- 3.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int 2009; 75: 1009–14. doi: 10.1038/ki.2009.49 [DOI] [PubMed] [Google Scholar]
- 4.Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 2007; 243: 405–12. [DOI] [PubMed] [Google Scholar]
- 5.Green MA, Mathias CJ, Willis LR, Handa RK, Lacy JL, Miller MA, et al. Assessment of Cu-ETS as a PET radiopharmaceutical for evaluation of regional renal perfusion. Nucl Med Biol 2007; 34: 247–55. doi: 10.1016/j.nucmedbio.2007.01.002 [DOI] [PubMed] [Google Scholar]
- 6.Prowle JR, Molan MP, Hornsey E, Bellomo R. Ciné phase-contrast magnetic resonance imaging for the measurement of renal blood flow. Contrib Nephrol 2010; 165: 329–36. [DOI] [PubMed] [Google Scholar]
- 7.Ma YC, Zuo L, Zhang CL, Wang M, Wang RF, Wang HY. Comparison of 99mTc-DTPA renal dynamic imaging with modified MDRD equation for glomerular filtration rate estimation in Chinese patients in different stages of chronic kidney disease. Nephrol Dial Transplant 2007; 22: 417–23. doi: 10.1093/ndt/gfl603 [DOI] [PubMed] [Google Scholar]
- 8.Le Dorze M, Bouglé A, Deruddre S, Duranteau J. Renal Doppler ultrasound: a new tool to assess renal perfusion in critical illness. Shock 2012; 37: 360–5. doi: 10.1097/SHK.0b013e3182467156 [DOI] [PubMed] [Google Scholar]
- 9.Kavakli HS, Koktener A, Yilmaz A. Diagnostic value of renal resistive index for the assessment of renal colic. Singapore Med J 2011; 52: 271–3. [PubMed] [Google Scholar]
- 10.Quaia E. Classification and safety of microbubble-based contrast agents. In: Quaia E, ed. Contrast media in ultrasonography: basic principles and clinical applications. New York, NY: Springer; 2005. pp. 3–14. [Google Scholar]
- 11.Cosgrove D, Lassau N. Imaging of perfusion using ultrasound. Eur J Nucl Med Mol Imaging 2010; 37(Suppl. 1): S65–85. doi: 10.1007/s00259-010-1537-7 [DOI] [PubMed] [Google Scholar]
- 12.Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol 2009; 193: 55–60. doi: 10.2214/AJR.09.2553 [DOI] [PubMed] [Google Scholar]
- 13.Ridolfi F, Abbattista T, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound evaluation of hepatic microvascular changes in liver diseases. World J Gastroenterol 2012; 18: 5225–30. doi: 10.3748/wjg.v18.i37.5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulte CS, Slikkerveer J, Meijer RI, Gort D, Kamp O, Loer SA, et al. Contrast-enhanced ultrasound for myocardial perfusion imaging. Anesth Analg 2012; 114: 938–45. doi: 10.1213/ANE.0b013e318248e261 [DOI] [PubMed] [Google Scholar]
- 15.Schneider AG, Hofmann L, Wuerzner G, Glatz N, Maillard M, Meuwly JY, et al. Renal perfusion evaluation with contrast-enhanced ultrasonography. Nephrol Dial Transplant 2012; 27: 674–81. doi: 10.1093/ndt/gfr345 [DOI] [PubMed] [Google Scholar]
- 16.Hoeffel C, Mulé S, Huwart L, Frouin F, Jais JP, Helenon O, et al. Renal blood flow quantification in pigs using contrast-enhanced ultrasound: an ex vivo study. Ultraschall Med 2010; 31: 363–9. doi: 10.1055/s-0029-1245238 [DOI] [PubMed] [Google Scholar]
- 17.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol 2007; 17: 1995–2008. doi: 10.1007/s00330-007-0623-0 [DOI] [PubMed] [Google Scholar]
- 18.Ciccone MM, Cortese F, Fiorella A, Scicchitano P, Cito F, Quistelli G, et al. The clinical role of contrast-enhanced ultrasound in the evaluation of renal artery stenosis and diagnostic superiority as compared to traditional echo-color-Doppler flow imaging. Int Angiol 2011; 30: 135–9. [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 4. Definition and classification of stages of chronic kidney disease. Am J Kidney Dis 2002; 39(2 Suppl. 1): S1–266. [PubMed] [Google Scholar]
- 20.Whittingham T. Contrast-specific imaging techniques: technical perspective. In: Quaia E, ed. Contrast media in ultrasonography: basic principles and clinical application. New York, NY: Springer; 2005. pp. 43–70. [Google Scholar]
- 21.Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, Schwarz A, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med 2003; 349: 115–24. doi: 10.1056/NEJMoa022602 [DOI] [PubMed] [Google Scholar]
- 22.Quaia E. Assessment of tissue perfusion by contrast-enhanced ultrasound. Eur Radiol 2011; 21: 604–15. [DOI] [PubMed] [Google Scholar]
- 23.Heine GH, Gerhart MK, Ulrich C, Köhler H, Girndt M. Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int 2005; 68: 875–85. doi: 10.1111/j.1523-1755.2005.00470.x [DOI] [PubMed] [Google Scholar]
- 24.Schwenger V, Korosoglou G, Hinkel UP, Morath C, Hansen A, Sommerer C, et al. Real-time contrast-enhanced sonography of renal transplant recipients predicts chronic allograft nephropathy. Am J Transplant 2006; 6: 609–15. doi: 10.1111/j.1600-6143.2005.01224.x [DOI] [PubMed] [Google Scholar]