Background: Sirt1 activity, like osteoblast number and bone mass, declines with age.
Results: Mice with Sirt1 deletion in osteoprogenitor cells have low cortical bone mass due to decreased bone formation resulting from increased β-catenin sequestration by FoxOs.
Conclusion: Sirt1 increases Wnt signaling and bone formation by a mechanism involving FoxOs.
Significance: Sirt1 in osteoprogenitor cells could be a therapeutic target for osteoporosis.
Keywords: Animal Model, Apoptosis, Cell Proliferation, Osteoporosis, Wnt Signaling
Abstract
A decline of the levels and activity of Sirtuin1 (Sirt1), a NAD+ class III histone deacetylase, with age contributes to the development of several diseases including type 2 diabetes, neurodegeneration, inflammation, and cancer. The anti-aging effects of Sirt1 evidently result from the deacetylation of many transcription factors and co-factors including members of the Forkhead box O (FoxO) family and β-catenin. Wnt/β-catenin is indispensable for osteoblast generation. FoxOs, on the other hand, sequester β-catenin and inhibit osteoprogenitor proliferation. Here, we have deleted Sirt1 in osteoprogenitors expressing Osterix1 (Osx1)-Cre and their descendants. Sirt1ΔOsx1 mice had lower cortical thickness in femora and vertebrae because of reduced bone formation at the endocortical surface. In line with this, osteoprogenitor cell cultures from the Sirt1ΔOsx1 mice exhibited lower alkaline phosphatase activity and mineralization, as well as decreased proliferation and increased apoptosis. These changes were associated with decreased Wnt/β-catenin signaling and expression of cyclin D1 and resulted from increased binding of FoxOs to β-catenin. These findings demonstrate that Sirt1-induced deacetylation of FoxOs unleashes Wnt signaling. A decline in Sirt1 activity in osteoblast progenitors with aging may, therefore, contribute to the age-related loss of bone mass. Together with evidence that Sirt1 activators increase bone mass in aged mice, our results also suggest that Sirt1 could be a therapeutic target for osteoporosis.
Introduction
Sirtuin1 (Sirt1)3 is an NAD+-dependent deacetylase that slows aging in lower organisms and opposes the development of aging-associated diseases in mammals (1, 2). Sirt1 affects a variety of biological functions, including DNA repair, energy metabolism, tumor suppression, and mitochondrial homeostasis. These effects have been linked to the deacetylation of important transcription factors and co-factors including members of the Forkhead box O (FoxO) family and β-catenin, a co-activator of canonical Wnt signaling (3).
Osteoblasts, the cells responsible for the formation and mineralization of the bone matrix, are terminally differentiated and short-lived. Therefore, bone growth and maintenance requires their continuous replacement with new ones originating from mesenchymal progenitors (4). This replenishment process is accomplished by the proliferation and differentiation of committed progenitors expressing the osteoblast lineage specific transcription factors Runx2 and Osterix1 (Osx1). The progression of the committed progenitors to mature osteoblasts is governed by Wnt signaling (5). Canonical Wnt signaling is initiated by binding of Wnt ligands to Frizzled and low density lipoprotein receptor-related protein 5 or 6 receptors in the cell membrane. This process prevents the destruction of β-catenin and promotes its accumulation. In the nucleus, β-catenin associates with the T-cell factor (TCF)/lymphoid-enhancing factor (Lef) family of transcription factors and promotes the expression of genes involved in proliferation and differentiation. Wnt signaling can be down-regulated by several extracellular and intracellular inhibitors. The latter group includes FoxOs. FoxOs downregulate Wnt signaling by binding to β-catenin and preventing the association of β-catenin with TCF/Lef (6). Attenuation of β-catenin/TCF transcription by FoxOs in osteoblast progenitors restrains their proliferation and decreases bone formation and mass (7).
Germline deletion or overexpression of Sirt1 decreases or increases bone mass, respectively (8, 9). In line with this evidence, Sirt1 stimulators, such as resveratrol or SRT2140, prevent the loss of bone mass caused by unloading (10, 11), ovariectomy (12), or aging (11, 13). The effects of Sirt1 on bone are mediated, at least in part, via actions in cells of the osteoblast lineage. Mice with targeted deletion of Sirt1 in osteoblast lineage cells, similar to mice with germline deletion, exhibit low bone mass (14, 15). Moreover, deletion of Sirt1 in all cells of mesenchymal lineage, decreases cortical bone mass in aged mice; surprisingly, however, such deletion had no effect in young mice (14). In that earlier study, the low bone mass caused by Sirt1 deletion was attributed to the acetylation, and thereby, retention of β-catenin in the cytoplasm. However, Wnt/β-catenin signaling is indispensable for osteoblastogenesis (16). Hence, Sirt1 inactivation in osteoblast progenitors should affect the skeleton early in life. Herein, we examined the role of Sirt1 in committed osteoblast progenitors using mice with Sirt1 deletion in Osx1-Cre expressing cells. We present evidence that Sirt1 potentiates osteoblast formation and the accrual of bone mass at the endocortical surface by decreasing the binding of FoxO to β-catenin, thereby unleashing Wnt signaling and the proliferation of osteoblast progenitors.
EXPERIMENTAL PROCEDURES
Animal Experimentation
The experimental mice were generated by a two-step breeding strategy. Hemizygous Osx1-Cre transgenic mice in which a Cre-GFP fusion protein is under the control of Osx1 regulatory elements (16) (C57BL/6 genetic background) were crossed with Sirt1 floxed (f/f) mice (C57BL/6 genetic background) (The Jackson Laboratory) to generate mice heterozygous for the Sirt1 floxed allele with and without the Cre allele. These mice were intercrossed to generate the experimental wild-type, Osx1-Cre, Sirt1f/f, and Sirt1ΔOsx1 mice. Genotypes of the offspring were determined by PCR using primers specific for Cre (7) that detect the wild-type and floxed Sirt1 alleles (17). To quantify bone formation, mice were injected with tetracycline (15 mg/kg of body weight) 8 and 4 days before euthanasia. All procedures were approved by the Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences and the Central Arkansas Veterans Healthcare System.
Bone Imaging and Histology
Microcomputed tomography analysis of vertebrae and femora was done after the bones were dissected, cleaned, fixed in 10% Millonig's formalin, and transferred to 100% ethanol, loaded into 10-mm diameter scanning tubes, imaged (μCT40, Scanco Medical), and analyzed as described previously (18). For histology, femora were fixed in 10% Millonig's formalin, transferred to 100% ethanol, and embedded undecalcified in methyl methacrylate. Endocortical and periosteal bone formations were assessed in longitudinal 5-μm-thick femoral sections using the OsteoMeasure analysis system (OsteoMetrics, Inc.) as described before (18). Cancellous bone formation measurements were restricted to the secondary spongiosa. Osteoclasts (and osteoblasts) were visualized on tartrate-resistant acid phosphatase (TRAP)-stained sections. Data are reported using the nomenclature recommended by the American Society for Bone and Mineral Research (19).
Cell Culture
Bone marrow cells pooled from 3–5 mice from each genotype were cultured with α-minimum Eagle's medium supplemented with 10% fetal bovine serum and 1% each penicillin, streptomycin, and glutamine. The nonadherent cells were collected 24 h later and cultured with 30 ng ml−1 M-CSF for 3 days to generate bone marrow-derived macrophages. To obtain osteoblast progenitors, the adherent cells were cultured in the presence of 1 mm ascorbate-2-phosphate. Half of the medium was replaced every 5 days. Proliferation was assayed by BrdU incorporation with a kit from Roche Diagnostics. Apoptosis was determined in cells pretreated with 10 mm N-acetylcysteine (NAC) for 1 h and then with vehicle or 50 μm H2O2 for 6 h. Caspase-3 activity was quantified by determining the degradation of the fluorometric substrate DEVD (Biomol Research Labs), and protein concentration was measured using a Bio-Rad detergent-compatible kit (Bio-Rad). For the alizarin red assay, bone marrow cells were seeded in 12-well tissue culture plates in presence of 1 mm ascorbate-2-phosphate at 5 × 106 cells/well and cultured for 3 weeks, and the mineralized matrix was stained with 40 mm alizarin red solution. Alkaline phosphatase activity was determined in 3-day cell cultures treated with vehicle or 3 μm SRT2104 (a small synthetic molecule that stimulates Sirt1 activity made by Glaxo Smith-Kline) and lysed in 100 mm glycine, 1 mm MgCl2, and 1% Triton X-100 at pH 10 using a buffer containing 2-amino-2-methylpropanol and p-nitrophenyl phosphate (Sigma-Aldrich). Alkaline phosphatase activity was normalized to total protein concentration measured as described above. Intracellular reactive oxygen species were quantified using dichlorodihydrofluorescein dye (20). For all assays, cells were plated in triplicate.
Generation of an Osteoblastic Cell Line with Inducible FoxO Activity
OPF-iFoxO3 cells were derived from OPF-5, a spontaneously transformed cell line derived from the bone marrow of FoxO1,3,4f/f;Osx1-Cre mice generated in our laboratory. OPF-5 cells were transduced with a retrovirus consisting of a tetracycline-controlled transcriptional activator inserted into the vector pLEN (21) followed by another transduction with a retrovirus expressing human FoxO3 with an amino-terminal hemagglutinin (HA) tag, which was produced from a plasmid generated by inserting the HA-FoxO3 coding sequence (Addgene plasmid 1787) into pRev-TRE (BD Biosciences/Clontech). OPF-iFoxO3 cells express the HA-FoxO3 only in the absence of doxycycline.
Transfection Studies
OPF-iFoxO3 or ST2 cells were plated on a 48-well plate and 16 h later transfected with 0.2 μg of reporter plasmid FoxO-luciferase (luc) or TCF-luc and 0.01 μg of Renilla (control reporter), and then co-transfected with 0.2 μg of pcDNA, 0.2 μg of FoxO1 or FoxO3 and with or without 0.2 μg of Sirt1, using Lipofectamine Plus (Invitrogen). Twenty-four hours later, the cells not carrying the Sirt1 plasmid were treated with vehicle or 3 μm SRT2104 for another 24 h. ST2 cells were also transfected with Flag-FoxO1-WT, Flag-FoxO1-6KR, and Flag-FoxO1–6KQ (Addgene plasmids 12148, 17560 and 17562). Luciferase activity was determined using the Dual-Luciferase® reporter assay system (Promega), according to the manufacturer's instructions. Light intensity was measured with a luminometer, and luciferase activity was divided by the control reporter to normalize for transfection efficiency.
Lentiviral Transduction of TCF-luc
Viral particles were generated by transfecting 293T cells with a plasmid expressing TCF-luc (Addgene plasmid 24308) along with lentiviral packaging vectors (Addgene plasmids 12259 and 12260) using Lipofectamine Plus. Supernatants containing viral particles were collected between 48 and 72 h after transfection, filtered through a 0.45-μm filter, and either used immediately or stored at −80 °C. Subconfluent primary bone marrow osteoprogenitor cell cultures were infected overnight with the lentiviral particles expressing TCF-luc in the presence of Polybrene. Twenty-four hours later, cells were treated with vehicle or 3 μm SRT2104 for another 24 h, and luciferase activity was determined as described above.
RNA Analysis
Tissues from mice were frozen immediately upon harvest. Osx1-GFP+ calvaria cells were obtained as described previously (7). Total RNA was extracted from tissues and cultured cells using TRIzol (Invitrogen) and reverse-transcribed using the High-Capacity cDNA archive kit (Applied Biosystems) according to the manufacturer's instructions. Primers and probes for the different genes were manufactured by the TaqMan® gene expression assays service (Applied Biosystems). TaqMan quantitative PCR was performed to determine mRNA levels using the assays Mm00490758_m1 (Sirt1); Mm00443610_m1 (Axin2); Mm00802276_m1 (Ccrn4l); Mm00802276_m1 (Rnd3); Mm00490672_m1 (FoxO1); Mm00490673_m1 (FoxO3); Mm00840140_g1 (FoxO4); Mm00432359_m1 (Ccnd1); Mm0050158_m1 (Runx2); Mm00801666_g1 (Col1a1); Mm00470479_m1 (Sost); and Mm00475528_m1 (ribosomal protein S2). Osx1, Ocn, and Dkk-1 mRNA levels were determined using custom-made TaqMan Assay by Design primer sets 5′-ATCTGACTTTGCTCCCCTTAACC-3′ and 5′-GGGCCCTGGTTGCAAGA-3′; 5′-GCTGCGCTCTGTCTCTCTGA-3′ and 5′-TGCTTGGACATGAAGGCTTTG-3′; and 5′-GGGCTGTGTTGTGCAAGACA-3′ and 5′-GGTGCACACCTGACCTTCTTTAA-3′, respectively. The reporter probe sequences for the above mentioned genes were 5′-TACCCAGCGCCCCAC-3′; 5′-AAGCCCAGCGGCC-3′; and 5′-TTCTGGTCCAAGATCT-3′, respectively. The mRNA levels were calculated by normalizing to the housekeeping gene ribosomal protein S2 using the ΔCt method (22).
Immunoprecipitation and Western Blot
Bone marrow-derived osteoblastic cell culture lysates from Osx1-Cre or Sirt1ΔOsx1 mice were immunoprecipitated with an anti-β-catenin antibody (BD Transduction Laboratories, 610154) or a nonspecific IgG control antibody (Santa Cruz Biotechnology sc-2003). Immunoprecipitates were resolved by SDS-PAGE, and co-immunoprecipitating FoxO1 and FoxO3 were analyzed by Western blotting with anti-FoxO1 and anti-FoxO3 antibody (Cell Signaling 9454 and 9476). Antibodies against FoxO4 (Cell Signaling 9472), Sirt1, β-actin, and lamin B (Santa Cruz Biotechnology, sc-15404, sc-4778, and sc-373918) were used to detect their corresponding protein levels. Acetylated FoxO1 was detected using an antibody recognizing Lys-259, Lys-262, and Lys-271 Ac-FoxO1 (Santa Cruz Biotechnology, sc-49437), and phosphorylated p66shc was detected using an antibody recognizing Ser-36(P)-p66shc (Abcam, 566807). Quantification of the intensity of the bands in the autoradiograms was done using a VersaDocTM imaging system (Bio-Rad). Nuclear and cytosolic extracts were obtained using the nuclear extract kit (Active Motif) and the manufacturer's instructions.
Statistical Analysis
Group mean values were compared, as appropriate, by Student's two-tailed t test or two-way ANOVA with Tukey's test, after determining that the data were normally distributed and exhibited equivalent variances. All t tests were two-sided. A p value ≤0.05 was considered significant for all statistical comparisons.
RESULTS
Sirt1 Deletion in Osteoprogenitors Decreases Cortical Bone Mass
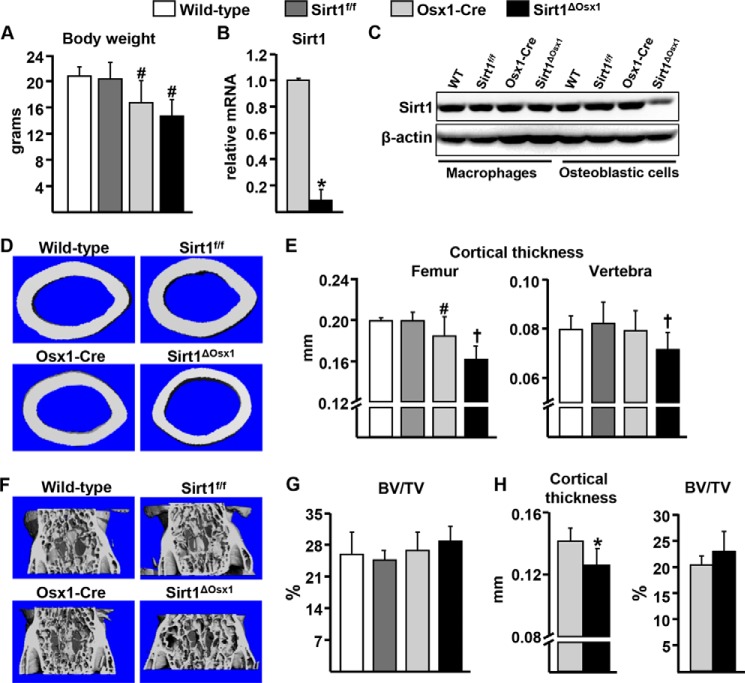
Sirt1 was deleted in committed osteoblast progenitors expressing Osx1-Cre. The Osx1-Cre transgene is expressed in osteoblast progenitors present in the bone-forming regions of the perichondrium and primary spongiosa as well as in hypertrophic chondrocytes (16). Mice lacking Sirt1 in the Osx1-Cre-expressing cells, hereafter referred to as Sirt1ΔOsx1, were born at the expected Mendelian frequencies. All mice carrying the Osx1-Cre transgene had lower body weight when compared with the wild-type and Sirt1f/f littermate controls (Fig. 1A) in agreement with previous reports that the Osx1-Cre transgene decreases body size (18, 23). Sirt1 deletion, however, did not affect body weight as Sirt1ΔOsx1 mice were indistinguishable from the Osx1-Cre littermates. Sirt1 mRNA was reduced by ∼90% in Osx1-GFP+ cells isolated by flow cytometry from neonatal calvaria cell cultures from Sirt1ΔOsx1 mice when compared with cells from Osx1-Cre mice (Fig. 1B). In addition, Sirt1 protein levels were greatly reduced in bone marrow-derived osteoblastic cells from Sirt1ΔOsx1 mice when compared with cells from wild-type, Sirt1f/f, and Osx1-Cre littermates (Fig. 1C). In contrast, Sirt1 levels in bone marrow-derived macrophages were similar between the four genotypes, demonstrating the specificity of Sirt1 deletion. Female mice expressing Osx1-Cre had lower cortical thickness at the femur when compared with wild-type or Sirt1f/f littermate controls at 12 weeks of age (Fig. 1, D and E). Despite the effects of the Osx1-Cre transgene alone, cortical thickness was further decreased by Sirt1 deletion in Sirt1ΔOsx1 mice (approx. 12%) when compared with Osx1-Cre controls. Cortical thickness at the spine was also decreased in Sirt1ΔOsx1 mice. In contrast, cancellous bone mass (bone volume per tissue volume, BV/TV) was unaffected (Fig. 1, F and G). The reduction of cortical thickness in the femur was observed as early as 8 weeks of age (Fig. 1H). However, similar to our findings with 12-week-old mice, no effects were seen in cancellous bone mass at 8 weeks. Bone mass in male mice was unaffected by Sirt1 deletion (data not shown).
FIGURE 1.
Sirt1 deletion in osteoprogenitors decreases cortical bone mass. A, body weight of 12-week-old females (n = 6–8/group). B, GFP+ osteoprogenitor cell cultures derived from neonatal calvaria. C, Sirt1 protein levels in bone marrow-derived macrophages cultured in the presence of M-CSF, and osteoblastic cells cultured with ascorbate, from mice of the indicated genotypes. D and E, representative microcomputed tomography images of femoral midshaft (D) and femoral and vertebral cortical thickness (E) of mice described in A. F and G, representative microcomputed tomography images (F) and cancellous BV/TV (G) in vertebrae of mice described in A. H, cortical thickness of femur and BV/TV of vertebrae from 8-week-old females (n = 5–7/group). #, p < 0.05 versus wild-type and Sirt1f/f mice; †, p < 0.05 versus wild-type, Sirt1f/f and Osx1-Cre mice by two-way ANOVA. *, p < 0.05 by Student's t test. Bars represent mean and S.D. (error bars).
Sirt1 Deletion Decreases Bone Formation
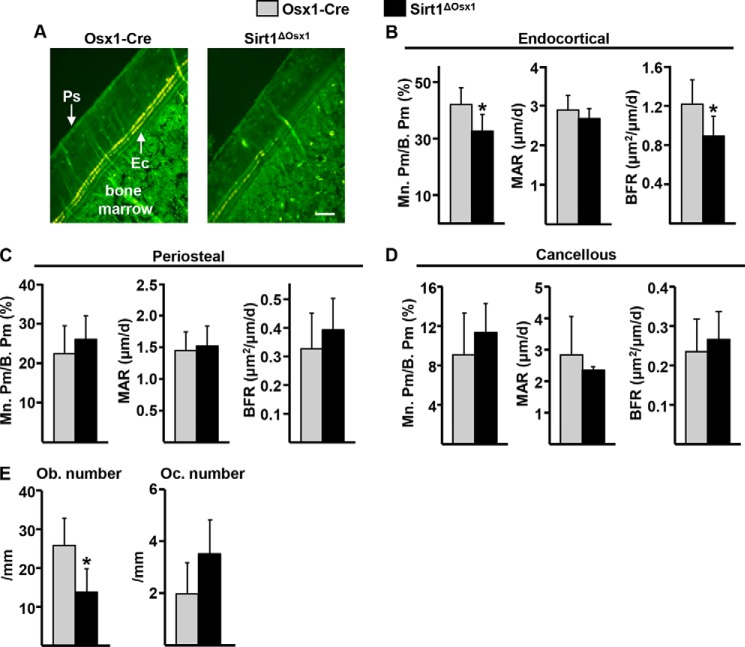
Dynamic histomorphometric analysis revealed that bone formation was lower in femoral cortical bone of 8-week old Sirt1ΔOsx1 female mice as measured by the mineralizing (tetracycline-labeled) perimeter (Mn.Pm/B.Pm) (Fig. 2, A and B). Mineral apposition rate (MAR), the distance between the tetracycline labels, was unaffected. Bone formation rate (BFR = MAR × Mn.Pm/B.Pm) was, therefore, significantly reduced by 27% at the endocortical surface when compared with the control littermates. Bone formation was not affected by Sirt1 deletion at the periosteal surface (Fig. 2C) or in cancellous bone (Fig. 2D). In line with the reduced bone formation, the number of osteoblasts at the endocortical surface was decreased (Fig. 2E). The number of osteoclasts was unaffected.
FIGURE 2.
Sirt1ΔOsx1 mice have decreased endocortical bone formation. A, representative photomicrographs of cortical bone labeled with tetracycline (yellow). Ps, periosteal surface; Ec, endocortical surface. Scale bar, 50 μm. B–D, mineralizing surface (M. Pm/B. Pm), MAR, and BFR as determined by tetracycline labels at the endocortical (B), periosteal (C), and cancellous (D) bone surfaces in longitudinal undecalcified femur sections from 8-week-old females (n = 5–7/group). E, osteoblast (Ob) and osteoclast (Oc) number in endocortical bone surface. *, p < 0.05 by Student's t test. Bars represent mean and S.D. (error bars).
Sirt1 Promotes Osteoblast Progenitor Proliferation, Differentiation, and Survival
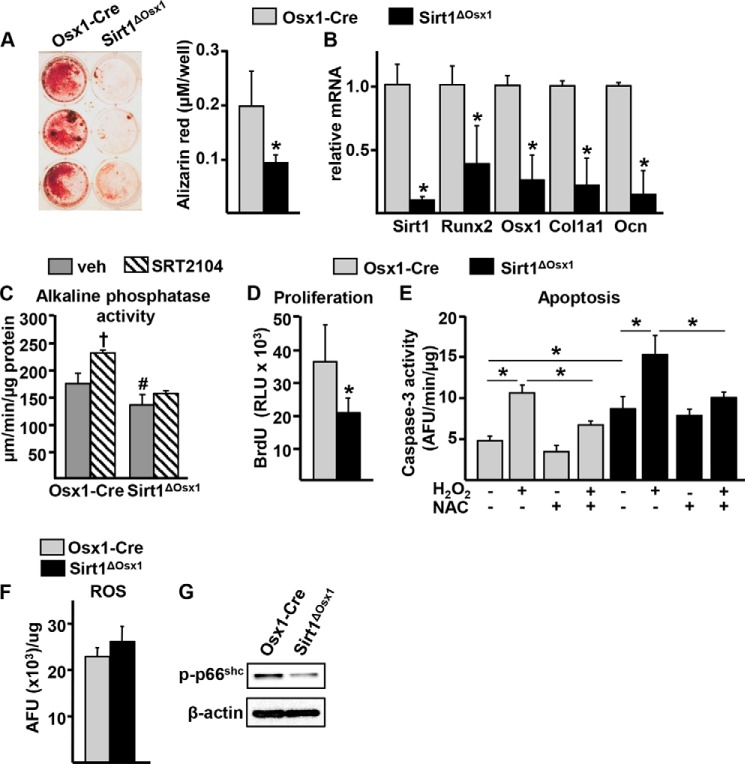
To determine the cellular mechanisms by which Sirt1 promotes bone formation, we first examined the differentiation of cultured bone marrow-derived osteoprogenitor cells isolated from femurs. Consistent with the reduced osteoblast numbers and BFR, osteoprogenitor cells from the Sirt1ΔOsx1 mice formed markedly fewer mineralized nodules (Fig. 3A). Moreover, the expression of osteoblast specific genes, such as Runx2, Osx1, Col1a1, and Ocn, was also lower in the Sirt1ΔOsx1 cultures (Fig. 3B). To further examine the actions of Sirt1 on osteoblastogenesis, we used SRT2104, a synthetic small molecule activator of Sirt1 (24). SRT2104 stimulated alkaline phosphatase activity in cultured bone marrow-derived osteoblast progenitor cells obtained from Osx1-Cre mice (Fig. 3C). The pro-osteogenic actions of SRT2104 were prevented in cells from Sirt1ΔOsx1 mice, confirming that Sirt1 mediates the effects of the compound in our model. In line with the above findings, Sirt1 deletion alone attenuated alkaline phosphatase activity.
FIGURE 3.
Sirt1 promotes osteoblastogenesis. A, alizarin red staining of bone marrow-derived osteoprogenitor cells cultured with 1% ascorbate for 21 days. B, gene expression in cells described in A. C, osteoprogenitor cells cultured for 3 days with 1% ascorbate in the presence of vehicle (veh) or SRT2104. D, BrdU incorporation in osteoprogenitor cell cultures. RLU, relative luminescence units. E, caspase-3 activity in osteoprogenitor cells cultured in the presence or absence of NAC and H2O2, as indicated. AFU, arbitrary fluorescence units. F and G, reactive oxygen species (ROS) (F) and phosphorylated p66shc (G) in osteoprogenitor cells. *, p < 0.05 by Student's t test. †, p < 0.05 effect of treatment within each genotype; #, p < 0.05 versus the equivalent treatment in Osx1-Cre by two-way ANOVA. Bars represent mean and S.D. (error bars).
We next examined whether Sirt1 deletion altered osteoprogenitor proliferation and apoptosis. The rate of cell proliferation, as measured by BrdU incorporation, was decreased by Sirt1 deletion (Fig. 3D). In addition, Sirt1 deletion increased apoptosis of the osteoprogenitors by 2-fold, as assayed by caspase-3 activity (Fig. 3E). Because Sirt1 can prevent apoptosis via antioxidant mechanisms (25), we used the antioxidant NAC to examine whether oxidative stress was responsible for the increased apoptosis seen in osteoblast progenitors from Sirt1ΔOsx1 mice. As expected, NAC greatly attenuated the pro-apoptotic actions of H2O2, used as a control, in cells from Osx1-Cre or Sirt1ΔOsx1 mice. However, NAC had no effect on the increased apoptosis of the cells from Sirt1ΔOsx1 mice. In addition, the levels of reactive oxygen species were unaffected (Fig. 3F) and the phosphorylation of the adaptor protein p66shc, a reliable marker of oxidative stress (26), was decreased (Fig. 3G) in cells from Sirt1ΔOsx1 mice. These findings strongly suggest that oxidative stress is not responsible for the increased apoptosis in osteoprogenitors lacking Sirt1.
Sirt1 Potentiates TCF-Mediated Transcription by Attenuating the Association between β-Catenin and FoxO
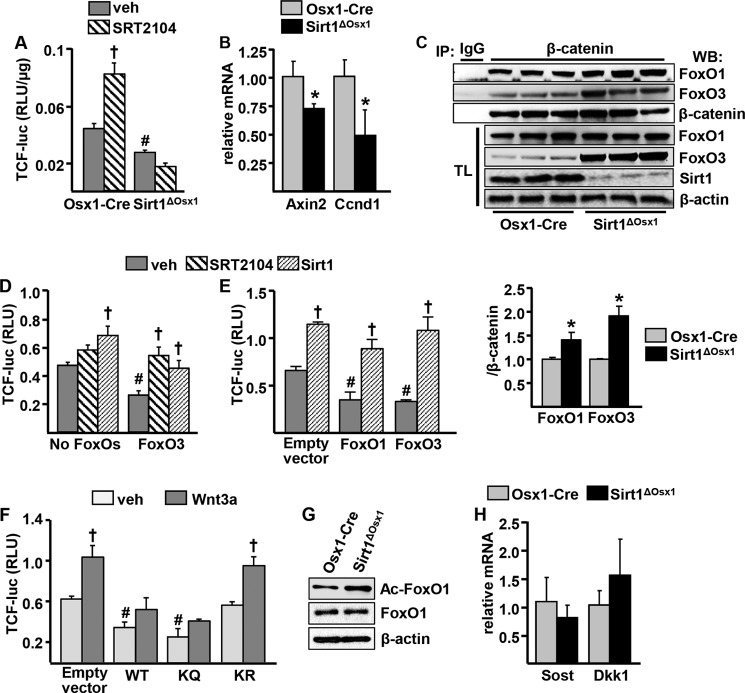
We next investigated whether Sirt1 promotes osteoblastogenesis by potentiating Wnt signaling, as previously suggested by others (14). To this end, we first examined the activity of a TCF-luc construct transduced into bone marrow-derived osteoprogenitor cells cultured from Sirt1ΔOsx1 mice or Osx1-Cre control littermates. Cells lacking Sirt1 had decreased TCF-luc activity (Fig. 4A), and activation of Sirt1 with SRT2104 potentiated TCF-mediated transcription in the control cells but had no effect in Sirt1ΔOsx1 cells. In agreement with the lower proliferation in cells lacking Sirt1, the mRNA expression of the Wnt target gene Ccnd1, as well as Axin2, was decreased in calvaria-derived Osx1-Cre GFP+ cells from Sirt1ΔOsx1 mice (Fig. 4B).
FIGURE 4.
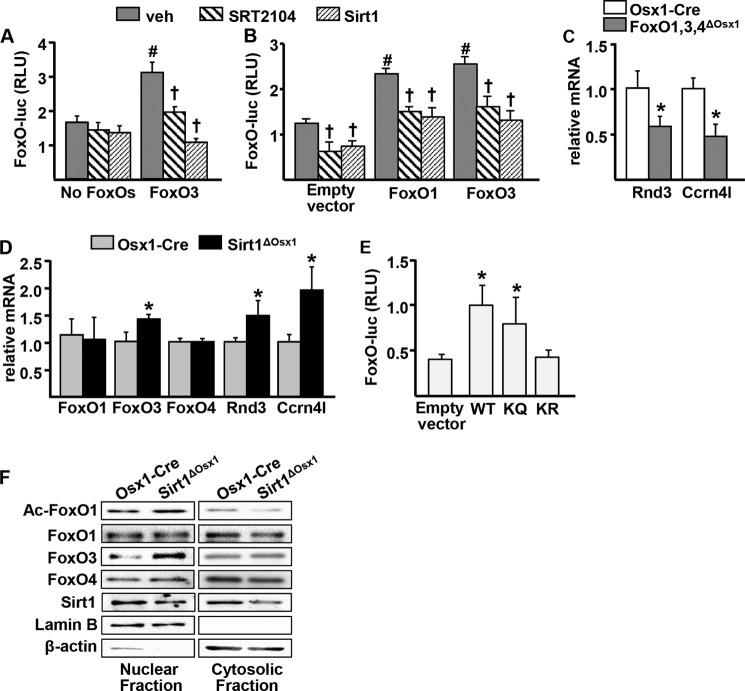
Sirt1 potentiates TCF-mediated transcription by attenuating FoxO/β-catenin association. A, bone marrow-derived osteoprogenitor cells were transduced with a TCF-luc reporter construct and cultured in the presence of vehicle (veh) or SRT2104 for 24 h. RLU, relative luminescence units. B, GFP+ cell cultures derived from neonatal calvaria. C, top panel, osteoprogenitor cell culture lysates immunoprecipitated (IP) with an anti-β-catenin or -IgG antibody and probed with anti-FoxO1, anti-FoxO3, and anti-β-catenin antibodies. TL, total cell lysates; WB, Western blot. Bottom panel, relative amount of FoxO1 and FoxO3 in β-catenin immunoprecipitates. D, FoxO3 expression was induced in the OPF-iFoxO3 cell line by withdrawal of doxycycline, and cells were transfected with a TCF-luc reporter construct and with a Sirt1 expression plasmid, as indicated. Twenty-four hours later, vehicle or SRT2104 was added to the cultures for another 24 h. E, ST2 cells transfected with TCF-luc and with empty vector, FoxO1, or FoxO3 expression plasmids and co-transfected with Sirt1, as indicated. F, ST2 cells transfected with TCF-luc and with empty vector, wild-type (WT) FoxO1, acetylation mimic (KQ), or acetylation mutant (KR) and cultured in the presence of vehicle or 25 ng/ml Wnt3a for 24 h. G, acetylated FoxO1 (Ac-FoxO1) in bone marrow-derived osteoprogenitor cell cultures by Western blot. H, mRNA from vertebral bone homogenates of 8-week-old females. †, p < 0.05 versus respective vehicle; #, p < 0.05 versus vehicle in control group by two-way ANOVA. *, p < 0.05 by Student's t test. Bars represent mean and S.D. (error bars).
In contrast to Sirt1, FoxOs inhibit Wnt signaling in osteoprogenitors by binding to β-catenin and preventing β-catenin/TCF-mediated transcription (7, 27). Because Sirt1 can deacetylate FoxOs and/or β-catenin, it is conceivable that this post-translational modification alters the association of FoxOs with β-catenin. To test this possibility, we immunoprecipitated β-catenin in Osx1-Cre or Sirt1ΔOsx1 bone marrow-derived osteoprogenitor cells and assessed the amount of FoxO1 or FoxO3 bound to β-catenin. The abundance of β-catenin did not differ between the two genotypes, but Sirt1 deletion increased the amount of FoxO1 or FoxO3 associated with β-catenin (Fig. 4C). The protein levels of FoxO3 were also increased in the total cell lysates from Sirt1ΔOsx1 mice. This finding is in agreement with evidence that deacetylation of FoxO3 by Sirt1 promotes its ubiquitination and proteasomal degradation (28). The levels of FoxO1 and FoxO4 were not affected by Sirt1 deletion. To examine the functional significance of FoxOs on the stimulation of TCF-mediated transcription by Sirt1, we used an osteoblastic cell line derived from the bone marrow of mice lacking FoxO1, 3 and 4, in which human FoxO3 is stably expressed in a doxycycline-regulated manner (OPF-iFoxO3). Consistent with previous evidence (27, 29), FoxO3 expression decreased TCF-luc activity (Fig. 4D). SRT2104 increased TCF-luc activity in the presence of FoxO3, by 2-fold, but had no effect in the absence of FoxOs. Sirt1 overexpression increased TCF-luc activity in the absence or presence of FoxOs. However, in the absence of FoxOs, the effect of Sirt1 was greatly attenuated. These findings suggest that Sirt1 can stimulate TCF activity via FoxO-dependent and -independent mechanisms. We also overexpressed Sirt1 along with FoxO1 or FoxO3 in ST2 cells. Overexpression of Sirt1 increased, whereas overexpression of FoxO1 or FoxO3 attenuated TCF-luc activity (Fig. 4E). In addition, Sirt1 prevented the inhibitory actions of FoxO1 or FoxO3 on TCF activity.
We next examined the role of acetylation in the actions of FoxO on Wnt signaling, using FoxO1 mutants in which six lysine residues corresponding to proposed FoxO acetylation sites (Lys-242, Lys-245,Lys-K259, Lys-262, Lys-271, and Lys-291) (25, 30) were mutated to arginine or to glutamine to prevent or mimic acetylation, respectively (31). Transfection of the acetylation mimic FoxO1 mutant (KQ) into ST2 cells, similar to wild-type FoxO1, inhibited both basal and Wnt3a-induced TCF-luc activity (Fig. 4F). In contrast, the acetylation mutant of FoxO1 (KR) had no effect on TCF-mediated transcription. In agreement with evidence that FoxOs are deacetylated by Sirt1, FoxO1 acetylation was greatly increased in cells lacking Sirt1 (Fig. 4G).
Cohen-Kfir et al. (8) have previously suggested that Sirt1-mediated repression of Sost expression, one of the secreted inhibitors of the Wnt pathway, contributes to the stimulatory actions of Sirt1 on bone formation. To examine whether this was the case in our model, we quantified the mRNA levels of Sost in vertebral bone lysates from Sirt1ΔOsx1 mice. Sost mRNA was unaffected by Sirt1 deletion (Fig. 4H). Likewise, the mRNA levels of Dkk1, another secreted inhibitor of Wnt signaling, were unchanged in the bone of Sirt1ΔOsx1 mice. Collectively, these data suggest that Sirt1 potentiates β-catenin/TCF-mediated transcription by decreasing FoxO3 levels and by deacetylating FoxOs, thereby suppressing FoxO/β-catenin association.
Sirt1 Inhibits FoxO-mediated Transcription in Osteoblast Progenitors
Finally, we examined the role of Sirt1 on FoxO-mediated transcription in our cell models. In the OPF-iFoxO3 cell line, expression of FoxO3 by removal of doxycycline increased FoxO-mediated transcription by 2-fold, as determined by the activity of a FoxO-luc reporter construct (Fig. 5A). Interestingly, either SRT2104 or overexpression of Sirt1 attenuated FoxO3-induced luciferase activity. Similar results were obtained in ST2 cells transfected with an empty vector control, FoxO1, or FoxO3 expression plasmids (Fig. 5B). We also examined the effects of Sirt1 on the expression of endogenous FoxO target genes in osteoprogenitor cells. Cultured Osx1-Cre GFP+ osteoprogenitor cells derived from mice lacking FoxO1, FoxO3, and FoxO4, described previously (7), exhibited decreased expression of Ccrn4l and Rnd3 (Fig. 5C). In agreement with the inhibitory actions of Sirt1 on FoxO-luc activity, Osx1-Cre GFP+ cells from Sirt1ΔOsx1 mice exhibited increased expression of nocturnin and rnd3 (Fig. 5D). The mRNA level of FoxO3 was also increased by Sirt1 deletion, in line with evidence that FoxO3 can self-amplify (32). The mRNA levels of FoxO1 and FoxO4 were not affected by Sirt1.
FIGURE 5.
Sirt1 inhibits FoxO activity. A, FoxO3 expression was induced in the OPF-iFoxO3 cell line by withdrawal of doxycycline, and cells were transfected with a FoxO-luc reporter construct and a Sirt1 expression plasmid, as indicated. Twenty-four hours later, vehicle (veh) or SRT2104 was added to the cultures for another 24 h. RLU, relative luminescence units. B, ST2 cells transfected with an empty vector control (pcDNA), FoxO1, or FoxO3 expression plasmids and co-transfected with a Sirt1 plasmid followed by treatment as in A. C and D, GFP+ cell cultures derived from neonatal calvaria of FoxO1,3,4 (C) and Sirt1 (D) conditional deletion mouse models. E, ST2 cells transfected with FoxO-luc and with empty vector, wild-type (WT) FoxO1, acetylation mimic (KQ), or acetylation mutant (KR). †, p < 0.05 versus respective vehicle; #, p < 0.05 versus vehicle in control group by two-way ANOVA. *, p < 0.05 by Student's t test. Bars represent mean and S.D. (error bars).
To further clarify the role of acetylation on FoxO transcriptional activity, we used the FoxO1 acetylation mimic and mutant plasmids. Transfection of the acetylation mimic KQ plasmid stimulated FoxO-luc activity similar to WT FoxO1, whereas the acetylation mutant KR had no transcriptional activity (Fig. 5E). Furthermore, the levels of acetylated FoxO1 were increased in the nucleus and decreased in the cytoplasm of cells lacking Sirt1 (Fig. 5F). FoxO3 was increased in the nucleus, whereas no changes were detected for FoxO1 and FoxO4. Together, these data suggest that Sirt1 inhibits FoxO-mediated transcription in osteoblast progenitors by decreasing FoxO3 levels and by deacetylating FoxOs.
DISCUSSION
Despite considerable progress in elucidating the role of Sirt1 in skeletal homeostasis, the target cells of Sirt1 as well as its mechanisms of action remain unclear. In the present report we show that during growth Sirt1 deletion in osteoblast progenitors attenuates the accrual of cortical bone at the endocortical surface as a result of decreased bone formation. The effects of Sirt1 deletion were readily seen in females but not in males. Similar female-specific effects of Sirt1 in the skeleton have been reported by others (8). A potential explanation for these findings could be cross-talk between Sirt1 and estrogen receptor α (ERα), documented in other cell types (33). Nonetheless, in earlier work of ours, deletion of ERα in osteoprogenitor cells did not affect endocortical bone formation (18), suggesting that the effects of Sirt1 in osteoprogenitors are independent of ERα.
We have previously shown that FoxOs decrease β-catenin binding to TCF in Osx1 expressing cells; and thereby, attenuate Ccnd1 expression and proliferation and decrease bone mass (7). In line with these earlier results, we found here that Sirt1 stimulates Ccnd1 and proliferation in osteoprogenitors. Deacetylation of FoxOs by Sirt1 promotes or inhibits FoxO-mediated transcription depending on the cellular context and the target genes (34). For example, Sirt1-mediated FoxO1 deacetylation promotes the expression of the antioxidant gene manganese superoxide dismutase (Mn-SOD) and protects the heart from ischemia/reperfusion injury (35). In contrast, Sirt1-mediated FoxO deacetylation prevents FoxO-induced expression of atrogin 1 and MuRF1 and attenuates muscle atrophy (36, 37). The present findings suggest that in osteoprogenitors Sirt1 decreases the binding of FoxOs to β-catenin. β-catenin itself can also be deacetylated by Sirt1; it is, therefore, possible that deacetylation of β-catenin contributes to its decreased binding to FoxOs. In support of this idea, Simic et al. (14) have reported that in mesenchymal progenitors deacetylation of β-catenin promotes its translocation to the nucleus. Sirt1 may also promote bone formation by repressing the expression of the Wnt signaling-antagonist Sost (8), a secreted protein produced by osteocytes (38). However, the expression of Sost was unaffected in the mice of the present work, suggesting that Sost could not be responsible for the bone phenotype. In other cell types, Sirt1 can stimulate Wnt signaling via regulating Dishevelled proteins (39) and by promoting epigenetic silencing of Wnt antagonists (40). It remains unknown whether these alternative mechanisms of Wnt signaling potentiation by Sirt1 play a role in osteoprogenitors.
Sirt1 deletion in all cells of the mesenchymal lineage including mesenchymal stem cells (MSC), osteoblast progenitors, osteoblasts, and osteocytes, using a Prx1-Cre transgene, accelerates loss of cortical bone mass with aging (14). The results of this report show that Sirt1 expressed in committed osteoblast progenitors is required for optimal accrual of cortical bone during growth. This effect is evidently independent of actions on MSC because the Osx1-Cre transgene does not affect these early progenitors (41, 42). The skeletal effect of Sirt1 deletion in MSC using Prx1-Cre is similar to the effect reported here using Osx1-Cre. This evidence along with the finding that cortical bone is unaffected in mice lacking Sirt1 in mature osteoblasts or osteoclasts (15) suggests that Sirt1 activity in osteoblast progenitors, as opposed to more mature cells, is responsible for the effects of Sirt1 on cortical bone. Importantly, we have previously shown that inducible germline deletion of Sirt1 in adult mice promotes the loss of cortical bone mass (11). Together with the results of the present report, this earlier finding supports the contention that the actions of Sirt1 in osteoblast progenitors are required not just for the acquisition of bone during growth, but for the maintenance of the skeleton throughout life as well. Deletion of Sirt1 in osteoprogenitors had no effect on cancellous bone mass. A similar finding was seen in mice in which Sirt1 was deleted using Prx1-Cre (14). In contrast, deletion of Sirt1 in mature osteoblasts (Col1-Cre expressing cells) decreases cancellous bone mass (15). Deletion of Sirt1 from Prx1-Cre or Osx1-Cre inexorably deletes Sirt1 in downstream descendants, such as the Col1 expressing cells. We currently have no explanation for why in our studies cancellous bone was unaffected whereas it did decrease in mice lacking Sirt1 in Col1-Cre expressing cells. Different genetic backgrounds and age of the animals may account for this discrepancy. Be that as it may, Sirt1 deletion in osteoclasts only affects cancellous bone mass (15). Together with the evidence presented herein, this result strongly suggests that Sirt1 protects the cortical and cancellous bone compartments via actions on different cell types and different mechanisms. Notably, different cell types and mechanisms are also responsible for the protective effects of estrogens on cancellous versus cortical bone (43).
Sirt1 is implicated in the prevention of several age-related pathologies, such as type 2 diabetes, neurodegeneration, inflammation, and cancer (1, 2, 44). In addition, we and others have recently shown that Sirt1 activators or overexpression of Sirt1 attenuate the loss of bone mass with aging (9, 11, 13), a condition characterized by decreased osteoblast production. The present findings support the notion that the beneficial effects of Sirt1 on bone are mediated, at least in part, by direct actions in osteoprogenitors that promote their proliferation and survival. Sirt1 activity declines with age as evidenced by a decrease in the mRNA levels of Sirt1 in bone (15), as well as a decrease in the levels of NAD+, required for Sirt1 deacetylase activity (45). This evidence is consistent with our conclusion that a decline in Sirt1 activity in osteoblast progenitors plays a pathogenetic role in the age-related decrease in osteoblast numbers and bone loss. Hence, pharmacologic activation of Sirt1 represents a mechanistically rational approach to the prevention and treatment of involutional osteoporosis.
Acknowledgments
We thank A. Warren, S. Berryhill, and J. Crawford for technical assistance, and Leah Elrod for help with the preparation of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AR56679 (to M. A.), P01 AG13918 (to S. C. M.), F32 AR061956-02 (to S. M. B.); Biomedical Laboratory Research and Development Service of the Veteran's Administration Office of Research and Development Grant I01 BX001405 (to S. C. M.); and University of Arkansas for Medical Sciences Tobacco Funds and Translational Research Institute Grant 1UL1RR029884.
- Sirt1
- Sirtuin1
- FoxO
- Forkhead box O
- Osx1
- Osterix1
- TCF
- T-cell factor
- Lef
- lymphoid-enhancing factor
- f/f
- floxed
- Sirt1ΔOsx1
- Sirtuin1 conditional deletion
- NAC
- N-acetylcysteine
- luc
- luciferase
- BV/TV
- bone volume/tissue volume
- MAR
- mineral apposition rate
- BFR
- bone formation rate
- ERα
- estrogen receptor α
- MSC
- mesenchymal stem cells
- ANOVA
- analysis of variance.
REFERENCES
- 1. Haigis M. C., Sinclair D. A. (2010) Mammalian sirtuins: biological insights and disease relevance. Annu. Rev Pathol. 5, 253–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baur J. A., Ungvari Z., Minor R. K., Le Couteur D. G., de Cabo R. (2012) Are sirtuins viable targets for improving healthspan and lifespan? Nat. Rev. Drug Discov. 11, 443–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michan S., Sinclair D. (2007) Sirtuins in mammals: insights into their biological function. Biochem. J 404, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manolagas S. C. (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137 [DOI] [PubMed] [Google Scholar]
- 5. Long F. (2012) Building strong bones: molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 13, 27–38 [DOI] [PubMed] [Google Scholar]
- 6. Almeida M. (2011) Unraveling the role of FoxOs in bone: insights from mouse models. Bone 49, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iyer S., Ambrogini E., Bartell S. M., Han L., Roberson P. K., de Cabo R., Jilka R. L., Weinstein R. S., O'Brien C. A., Manolagas S. C., Almeida M. (2013) FoxOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest. 123, 3409–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen-Kfir E., Artsi H., Levin A., Abramowitz E., Bajayo A., Gurt I., Zhong L., D'Urso A., Toiber D., Mostoslavsky R., Dresner-Pollak R. (2011) Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology 152, 4514–4524 [DOI] [PubMed] [Google Scholar]
- 9. Herranz D., Muñoz-Martin M., Cañamero M., Mulero F., Martinez-Pastor B., Fernandez-Capetillo O., Serrano M. (2010) Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Momken I., Stevens L., Bergouignan A., Desplanches D., Rudwill F., Chery I., Zahariev A., Zahn S., Stein T. P., Sebedio J. L., Pujos-Guillot E., Falempin M., Simon C., Coxam V., Andrianjafiniony T., Gauquelin-Koch G., Picquet F., Blanc S. (2011) Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 25, 3646–3660 [DOI] [PubMed] [Google Scholar]
- 11. Mercken E. M., Mitchell S. J., Martin-Montalvo A., Minor R. K., Almeida M., Gomes A. P., Scheibye-Knudsen M., Palacios H. H., Licata J. J., Zhang Y., Becker K. G., Khraiwesh H., González-Reyes J. A., Villalba J. M., Baur J. A., Elliott P., Westphal C., Vlasuk G. P., Ellis J. L., Sinclair D. A., Bernier M., de Cabo R. (2014) SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 10.1111/acel.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su J. L., Yang C. Y., Zhao M., Kuo M. L., Yen M. L. (2007) Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J. Biol. Chem. 282, 19385–19398 [DOI] [PubMed] [Google Scholar]
- 13. Pearson K. J., Baur J. A., Lewis K. N., Peshkin L., Price N. L., Labinskyy N., Swindell W. R., Kamara D., Minor R. K., Perez E., Jamieson H. A., Zhang Y., Dunn S. R., Sharma K., Pleshko N., Woollett L. A., Csiszar A., Ikeno Y., Le Couteur D., Elliott P. J., Becker K. G., Navas P., Ingram D. K., Wolf N. S., Ungvari Z., Sinclair D. A., de Cabo R. (2008) Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simic P., Zainabadi K., Bell E., Sykes D. B., Saez B., Lotinun S., Baron R., Scadden D., Schipani E., Guarente L. (2013) SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol Med 5, 430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edwards J. R., Perrien D. S., Fleming N., Nyman J. S., Ono K., Connelly L., Moore M. M., Lwin S. T., Yull F. E., Mundy G. R., Elefteriou F. (2013) Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling. J Bone Miner Res 28, 960–969 [DOI] [PubMed] [Google Scholar]
- 16. Rodda S. J., McMahon A. P. (2006) Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, 3231–3244 [DOI] [PubMed] [Google Scholar]
- 17. Li H., Rajendran G. K., Liu N., Ware C., Rubin B. P., Gu Y. (2007) SirT1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res. 9, R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almeida M., Iyer S., Martin-Millan M., Bartell S. M., Han L., Ambrogini E., Onal M., Xiong J., Weinstein R. S., Jilka R. L., O'Brien C. A., Manolagas S. C. (2013) Estrogen receptor-α signaling in osteoblast progenitors stimulates cortical bone accrual. J. Clin. Invest. 123, 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang X., Frenkel K., Klein C. B., Costa M. (1993) Nickel induces increased oxidants in intact cultured mammalian cells as detected by dichlorofluorescein fluorescence. Toxicol. Appl. Pharmacol. 120, 29–36 [DOI] [PubMed] [Google Scholar]
- 21. O'Brien C. A., Gubrij I., Lin S.-C., Saylors R. L., Manolagas S. C. (1999) STAT3 activation in stromal/osteoblastic cells is required for induction of the receptor activator of NF-kB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-Dihydroxyvitamin D3 or parathyroid hormone. J. Biol. Chem. 274, 19301–19308 [DOI] [PubMed] [Google Scholar]
- 22. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 23. Davey R. A., Clarke M. V., Sastra S., Skinner J. P., Chiang C., Anderson P. H., Zajac J. D. (2012) Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic. Res 21, 885–893 [DOI] [PubMed] [Google Scholar]
- 24. Milne J. C., Lambert P. D., Schenk S., Carney D. P., Smith J. J., Gagne D. J., Jin L., Boss O., Perni R. B., Vu C. B., Bemis J. E., Xie R., Disch J. S., Ng P. Y., Nunes J. J., Lynch A. V., Yang H., Galonek H., Israelian K., Choy W., Iffland A., Lavu S., Medvedik O., Sinclair D. A., Olefsky J. M., Jirousek M. R., Elliott P. J., Westphal C. H. (2007) Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 26. Trinei M., Berniakovich I., Beltrami E., Migliaccio E., Fassina A., Pelicci P., Giorgio M. (2009) P66Shc signals to age. Aging (Albany, NY) 1, 503–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Almeida M., Han L., Martin-Millan M., O'Brien C. A., Manolagas S. C. (2007) Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor- to Forkhead box O-mediated transcription. J. Biol. Chem. 282, 27298–27305 [DOI] [PubMed] [Google Scholar]
- 28. Wang F., Chan C. H., Chen K., Guan X., Lin H. K., Tong Q. (2012) Deacetylation of FOXO3 by SIRT1 or SIRT2 leads to Skp2-mediated FOXO3 ubiquitination and degradation. Oncogene 31, 1546–1557 [DOI] [PubMed] [Google Scholar]
- 29. Hoogeboom D., Essers M. A., Polderman P. E., Voets E., Smits L. M., Burgering B. M. (2008) Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J. Biol. Chem. 283, 9224–9230 [DOI] [PubMed] [Google Scholar]
- 30. Daitoku H., Hatta M., Matsuzaki H., Aratani S., Ohshima T., Miyagishi M., Nakajima T., Fukamizu A. (2004) Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl. Acad. Sci. U.S.A. 101, 10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kitamura Y. I., Kitamura T., Kruse J. P., Raum J. C., Stein R., Gu W., Accili D. (2005) FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab 2, 153–163 [DOI] [PubMed] [Google Scholar]
- 32. Essaghir A., Dif N., Marbehant C. Y., Coffer P. J., Demoulin J. B. (2009) The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J. Biol. Chem. 284, 10334–10342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore R. L., Dai Y., Faller D. V. (2012) Sirtuin 1 (SIRT1) and steroid hormone receptor activity in cancer. J. Endocrinol. 213, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Horst A., Burgering B. M. (2007) Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 8, 440–450 [DOI] [PubMed] [Google Scholar]
- 35. Hsu C. P., Zhai P., Yamamoto T., Maejima Y., Matsushima S., Hariharan N., Shao D., Takagi H., Oka S., Sadoshima J. (2010) Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122, 2170–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S. H., Goldberg A. L. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee D., Goldberg A. L. (2013) SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 288, 30515–30526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baron R., Kneissel M. (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19, 179–192 [DOI] [PubMed] [Google Scholar]
- 39. Holloway K. R., Calhoun T. N., Saxena M., Metoyer C. F., Kandler E. F., Rivera C. A., Pruitt K. (2010) SIRT1 regulates Dishevelled proteins and promotes transient and constitutive Wnt signaling. Proc. Natl. Acad. Sci. U.S.A. 107, 9216–9221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pruitt K., Zinn R. L., Ohm J. E., McGarvey K. M., Kang S. H., Watkins D. N., Herman J. G., Baylin S. B. (2006) Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet 2, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenbaum A., Hsu Y. M., Day R. B., Schuettpelz L. G., Christopher M. J., Borgerding J. N., Nagasawa T., Link D. C. (2013) CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park D., Spencer J. A., Koh B. I., Kobayashi T., Fujisaki J., Clemens T. L., Lin C. P., Kronenberg H. M., Scadden D. T. (2012) Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10, 259–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Manolagas S. C., O'Brien C. A., Almeida M. (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9, 699–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Longo V. D., Kennedy B. K. (2006) Sirtuins in aging and age-related disease. Cell 126, 257–268 [DOI] [PubMed] [Google Scholar]
- 45. Gomes A. P., Price N. L., Ling A. J., Moslehi J. J., Montgomery M. K., Rajman L., White J. P., Teodoro J. S., Wrann C. D., Hubbard B. P., Mercken E. M., Palmeira C. M., de Cabo R., Rolo A. P., Turner N., Bell E. L., Sinclair D. A. (2013) Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]