Background: Understanding of molecular mechanism of TAK1 activation is incomplete.
Results: PKACα and PRKX phosphorylate TAK1 at serine 412 to regulate TAK1 activation in the IL-1 receptor (IL-1R) and Toll-like receptor (TLR) signaling pathways.
Conclusion: TAK1 activation requires phosphorylation by PKACα and PRKX.
Significance: This study advanced our understanding of the molecular mechanism of TAK1 activation.
Keywords: Inflammation, Innate Immunity, Mitogen-activated Protein Kinase (MAPK), NF-kappaB, Protein Kinase A (PKA), Serine/Threonine Protein Kinase, Toll-like Receptor (TLR), Tumor Necrosis Factor (TNF), IL-1R, TAK1
Abstract
TGF-β-activated kinase 1 (TAK1) is a key kinase in mediating Toll-like receptors (TLRs) and interleukin-1 receptor (IL-1R) signaling. Although TAK1 activation involves the phosphorylation of Thr-184 and Thr-187 residues at the activation loop, the molecular mechanism underlying the complete activation of TAK1 remains elusive. In this work, we show that the Thr-187 phosphorylation of TAK1 is regulated by its C-terminal coiled-coil domain-mediated dimerization in an autophosphorylation manner. Importantly, we find that TAK1 activation in mediating downstream signaling requires an additional phosphorylation at Ser-412, which is critical for TAK1 response to proinflammatory stimuli, such as TNF-α, LPS, and IL-1β. In vitro kinase and shRNA-based knockdown assays reveal that TAK1 Ser-412 phosphorylation is regulated by cAMP-dependent protein kinase catalytic subunit α (PKACα) and X-linked protein kinase (PRKX), which is essential for proper signaling and proinflammatory cytokine induction by TLR/IL-1R activation. Morpholino-based in vivo knockdown and rescue studies show that the corresponding site Ser-391 in zebrafish TAK1 plays a conserved role in NF-κB activation. Collectively, our data unravel a previously unknown mechanism involving TAK1 phosphorylation mediated by PKACα and PRKX that contributes to innate immune signaling.
Introduction
TGF-β-activated kinase 1 (TAK1),5 also known as MAP3K7 and MEKK7, is a very important signaling protein involved in the regulation of innate and adaptive immune responses, neural fold morphogenesis, vascular development, and tumorigenesis (1–3). As a serine/threonine kinase, TAK1 can be activated by TGF-β, bone morphogenetic proteins, a variety of inflammatory cytokines (such as TNF-α and IL-1β), ligands of CD40, TLRs, and T- and B-cell receptors. One important and also probably the most extensively studied function of TAK1 is its regulation of proinflammatory and innate immune signaling pathways, such as the TNF receptor, IL-1R, and TLR pathways (4). Upon activation, TAK1 phosphorylates IκB kinase (IKK) complex, p38, JNK, and ERK, which leads to the activation of nuclear factor (NF)-κB and MAPK signaling pathways. Consequently, proinflammatory cytokines and chemokines, such as TNF-α, IL-1β, IL-8, and RANTES, are induced (5). The importance of TAK1 in mediating TLRs, IL-1R signaling pathways, and innate immune responses has been demonstrated by genetic studies using TAK1 knock-out (KO) mice (1, 6).
TAK1 forms a stable complex with TAB1 and TAB2 (or TAB3, the TAB2 homolog) (7, 8). TAB1 regulates TAK1 activation by osmotic stress (9), whereas TAB2 and TAB3 regulate TAK1 activation by proinflammatory stimuli (1). Numerous studies have suggested that site-specific ubiquitination and phosphorylation on TAK1 regulate TAK1 kinase activation (3). In particular, the phosphorylation of residues Thr-184 and Thr-187 located at the TAK1 kinase domain is required for TAK1 kinase activity (10, 11). However, a few researchers have suggested that the phosphorylation of TAK1 Thr-187 is insufficient for its activation (12–15). Thus, additional post-translational modifications are needed. Moreover, numerous phosphorylation sites of TAK1 outside the activation loop have also been recorded in the PhosphoSitePlus Web site and in literature (16–18). Whether these phosphorylation events regulate TAK1 activation and downstream signaling remains to be determined.
Protein kinase A (PKA), which consists of five catalytic members (PKACα, PKACβ, PKACγ, X-linked protein kinase (PRKX), and Y-linked protein kinase (PRKY)) and two major regulatory subunits (RI and RII), belongs to the AGC protein Ser/Thr kinase family. This family has a strong preference for the phosphorylation of Ser and Thr residues located in a consensus sequence containing the basic amino acid Lys or Arg (19). They are activated by an elevated cellular concentration of the second messenger cAMP (20), although their activation independent of cAMP has also been reported (21, 22). Many studies have shown that the cAMP-PKA module is involved in the regulation of NF-κB signaling through the phosphorylation of p65 or p105 in a positive or negative manner, depending on cell type (16, 23–26). Determination of whether PKA also targets other components in NF-κB signaling pathways requires more study.
Zebrafish (Danio rerio) is increasingly becoming a unique in vivo model system to study infectious, innate immune and inflammatory responses that can complement studies in mouse and human cell lines (27, 28). Components of the main innate immune signaling pathways, such as the major pattern recognition receptors, key adaptor proteins (such as MyD88 and TNF receptor-associated factors in the TLR and IL-1R pathways), and the downstream transcription factors NF-κB and AP1 are all found to be highly conserved among humans, mice, and zebrafish. This finding strongly implies that similar signal transduction mechanisms exist among the three species (28, 29). Particularly, the mammalian essential kinases TAK1 and IKK complexes in the TLR and IL-1R signaling pathways display the most apparent orthologies in zebrafish (29), although functional data are not yet derived. Thus, zebrafish is an attractive model for the in vivo investigation of TAK1 activities.
We have recently demonstrated that protein phosphatase 1 (PP1), together with its adaptor GADD34, modulates IL-1R/TLR signaling through the dephosphorylation of TAK1 at Ser-412 (30). In this report, we aimed to further study the regulation of TAK1 activation and Ser-412 phosphorylation. We found that TAK1 Thr-187 phosphorylation occurred through dimerization through its C-terminal coiled-coil domain. Ser-412 phosphorylation is required for TAK1 full activation, downstream signaling, and the induction of proinflammatory cytokines in TLR and IL-1R signaling. Ser-412 is phosphorylated by protein kinases PKACα and PRKX. Using a zebrafish (D. rerio) embryo system, we also provided genetic evidence to demonstrate that the phosphorylation of zebrafish TAK1 (DrTAK1) at Ser-391, which is the conserved site corresponding to mouse TAK1 (mTAK1) Ser-412, was also required for LPS- and IL-1β-induced NF-κB activation. Our data collectively unraveled the pivotal role of TAK1 Ser-412 phosphorylation and its regulation by PKACα and PRKX in innate immune and proinflammatory signaling pathways.
EXPERIMENTAL PROCEDURES
Antibodies, Reagents, and Plasmids
Antibodies against phospho-TAK1 (Thr-187), -TAK1 (Ser-412), -IκBα, -JNK, -ERK1/2, and -p38 were all purchased from Cell Signaling Technology and diluted (1:1000) according to the manufacturer's protocols. Antibodies against total TAK1 (1:1000), PKACα (1:50,000), and PRKX (1:1000) were from Epitomics. Anti-FLAG (1:5000), -Myc (1:5000), -HA (1:4000), and -GST (1:5000) antibodies were from Abmart (Shanghai, China). Anti-FLAG (M2)-agarose beads were from Sigma. Recombinant human IL-1β was purchased from PeproTech. LPS (0111:B4 for RAW264.7 stimulation and 055:B5 for microinjection in zebrafish embryos) and poly(I:C) were from Sigma. CpG oligodeoxynucleotide (ODN) 1826 was synthesized and purified as described previously (31). PKA inhibitor H-89 was purchased from Beyotime (China). Expression plasmids encoding human PRKX, PKACα, TAB1, and mouse TAK1 were amplified by PCR and ligated into pcDNA3.1-FLAG/Myc/HA to generate expression constructs. cDNA encoding full-length mouse TAK1 was inserted into a modified pGEX-4T-1-GST-His10 vector containing an N-terminal GST tag and a C-terminal His10 tag. Mutants of TAK1 were generated by using the QuikChange site-directed mutagenesis protocol (Stratagene).
The zebrafish TAK1 was amplified using PrimeSTAR DNA polymerase (Takara) from cDNA prepared from 1-day-old zebrafish embryos, using the following primers: DrTAK1-F, 5′-ATTAGCGGCCGCAATGCTGGAAACACCGCCTATGTATC-3′; DrTAK1-R, 5′-ATTTAGCGATCGCTGAGGTGCCCTGTCTCTTCTGC-3′. Mutants of zebrafish TAK1 (K45W, T169A, and S391A) were generated by using the QuikChange site-directed mutagenesis protocol (Stratagene).
Cell Culture and Transfection
RAW264.7, HEK293T, MEF, and HeLa cells were grown in Dulbecoo's modified Eagle's medium (DMEM) supplemented with 10% FBS at 37 °C in a humidified incubator with 5% CO2. HEK293T cells were transfected with Lipofectamine 2000 (Invitrogen) or polyethyleneimine (Mr 40,000; Polysciences), and RAW264.7 cells were transfected with JetPEI-Macrophage (Polyplus) according to the manufacturers' protocols. Cre-mediated-TAK1 KO MEFs were a gift from Dr. H Xiao (Institute Pasteur of Shanghai, Chinese Academy of Sciences), and transfected with the MEF Nucleofector Kit (Lonza). For inhibition of PKA by H-89, HeLa cells were pretreated with H-89 (10 μm) or DMSO for 1 h and then stimulated with IL-1β for the indicated time.
In Vitro Kinase Assay
FLAG-tagged kinases (PKACα, PRKX, or TAK1 (WT, K63W, T187A, S412A, or aa 1–303 or 1–436 truncation mutants)) were transiently overexpressed in 293T cells and purified using anti-FLAG (M2)-agarose beads according to the manufacturer's protocol. GST-TAK1 or His-MKK6(K82A) was expressed in Escherichia coli BL21(DE3) and purified using glutathione beads (GE Healthcare) or nickel beads (Pierce), respectively, according to the manufacturers' protocols.
The PKA in vitro kinase assay was carried out in 10 μl of reaction mixture containing 50 mm Tris-HCl, pH 7.5, 2 mm ATP, 5 mm MgCl2, 0.2 μm OA, GST-TAK1 as the substrates, and FLAG-tagged kinase (PKACα, PRKX, or TAK1). The mixture was incubated at 30 °C for 60 min and analyzed by immunoblotting with antibodies against phospho-TAK1 (Ser-412) and GST.
The TAK1 activity assay was carried out in 20 μl of reaction mixture containing 50 mm Tris-HCl, pH 7.5, 2 mm ATP, 5 mm MgCl2, MKK6 (K82A) as the substrate, and FLAG-tagged kinase TAK1 (WT, K63W, T187A, S412A, or aa 1–303 or 1–436 truncation mutants). The mixture was incubated at 25 °C for 20 min and analyzed by immunoblotting with antibodies against phospho-MKK6, phospho-TAK1 (Ser-412), phospho-TAK1 (Thr-187), and FLAG. For immunoprecipitation kinase assays, FLAG-tagged WT TAK1 or its mutant S412A was immunoprecipitated by M2-agarose beads from RAW264.7 cells stably expressing TAK1 WT or S412A mutant, and then used under the same conditions as for the TAK1 activity assays.
LPS- or IL-1β-induced NF-κB Activation in Zebrafish
Wild-type AB zebrafish (D. rerio), 1 year old of both sexes, weighing ∼0.5–1 g, with a body length of 1–2 cm, were purchased from National Zebrafish Resources of China, kept in the recirculating water at 28 °C, and fed with commercial pellets at a daily ration of 0.7% of their body weight. All fish were held for at least 2 weeks before experiments for acclimatization and evaluation of overall fish health. Only the healthy fish, as determined by general appearance and level of activity, were used for the studies.
Capped mRNAs for microinjection encoding DrTAK1 and its mutants were synthesized in vitro using the Message Machine Kit (Ambion) according to the supplier's manual. NF-κB activation was examined in zebrafish embryos as reported previously (32). In brief, LPS (2 ng/embryo, E. coli 055:B5) (or 200 pg/embryo IL-1β mRNA in the case of IL-1β, empty vector mRNA, or DrTAK1 WT mRNA, or mutant mRNA (100 pg/embryo)), together with the NF-κB firefly luciferase reporter gene (40 pg/embryo), were diluted in the microinjection buffer (0.5% phenol red, 240 mm KCl, and 40 mm HEPES, pH 7.4) and injected into one-cell-stage embryos (2 nl/embryo) using a microinjector (ASI MPPI-3). The pRL-TK Renilla luciferase reporter plasmid was used as the internal control. After injection, the embryos were rinsed once with E3 medium (5 mm NaCl, 0.17 mm KCl, 0.33 mm CaCl2, and 0.33 mm MgSO4) in a 28.5 °C incubator. The firefly and Renilla luciferase activities were assayed 24 h postmicroinjection with 5–10 replicates (each containing extracts from 50–100 embryos) according to the manufacturer's instructions. NF-κB activation was normalized to pRL-TK activity and expressed as -fold stimulation relative to the control.
The sequences for DrTAK1 and its control morpholinos (MOs) were described previously (2), and the MOs were synthesized by Gene Tools, LLC (Philomath, OR). For MO knockdown and mRNA rescue experiments, DrTAK1 MOs or control MOs (6 ng/embryo), with or without the indicated kinds of mRNA (200 pg/embryo), were included in the injection mixture. To detect interruption of splicing by the DrTAK1 MOs, cDNA was made from 24-h morpholino-injected fish and amplified by PCR using the following primers. The forward primer used for the ORF cloning was still used here, and a reverse primer (5′-TCCATCATCTAGACCAGGAAC-3′) corresponding to a sequence inside intron 6 was designed to detect inclusion of intron 6.
shRNA-mediated Stable Knockdown of PKACα, PRKX, and PKACβ
pLKO.1-based lentiviral shRNA vectors (TRCN0000012459 and TRCN0000012461 for mouse PKACα, TRCN0000022785 and TRCN0000022786 for mouse PRKX, TRCN0000022814 and TRCN0000022816 for mouse PKACβ) were from Sigma. Production of viral particles was conducted according to standard protocols. To generate stable knockdown cells, RAW264.7 were infected with lentiviral particles in the presence of Polybrene (Sigma) and selected in the presence of puromycin (5 μg/ml) for 2 weeks. Pools of puromycin-resistant cells were used in the experiments.
siRNA
PRKX, PKACα, and TAK1 siRNA oligos were synthesized by Genepharma (Shanghai, China). siRNA duplexes were transfected into HeLa cells by using RNAimax (Invitrogen) according to the manufacturer's protocol. The following sequences of siRNA oligonucleotides were used (only the sense strands are shown): human PKACα, siRNA 1 (5′-CAGCCCACUUGGAUCAGUUUGdTdT-3′) and siRNA 2 (5′-GCUCCCUUCAUACCAAAGUdTdT-3′); human PRKX, siRNA 1 (5′-GGAGCAACACGUACACAAUdTdT-3′) and siRNA 2 (5′-CAACCCGUUUGGCAUUUAUdTdT-3′).
ELISA Detection of IL-6 and TNF-α
Induction of IL-6 and TNF-α level in the culture supernatants was measured with ELISA kits (eBioscience) according to the manufacturer's protocols.
Quantitative RT-PCR
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's instructions. RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Takara). Quantitative PCR was performed using SYBR Green (Takara) and the ABI 7500 real-time PCR system (Applied Biosystems). The following primers were used: human β-actin-F, 5′- catgtacgttgctatccaggc-3′; human β-actin-R, 5′-ctccttaatgtcacgcacgat-3′; human IL-8-F, 5′-ataaagacatactccaaacctttccac-3′; human IL-8-R, 5′-aagctttacaataatttctgtgttggc-3′; human TNF-α-F, 5′-cccagggacctctctctaatca-3′; human TNF-α-R, 5′-gcttgagggtttgctacaacatg-3′; human MCP-1-F, 5′-actctcgcctccagcatgaa-3′; human MCP-1-R, 5′-ttgattgcatctggctgagc-3′; human RANTES-F, 5′-tttgcctacattgcccgc-3′; human RANTES-R, 5′-tttcgggtgacaaagacgact-3′.
Luciferase Reporter Assays
The luciferase activities in total cell lysates were measured with the Dual-Luciferase reporter assay system (Promega) according to the manufacturer's manual. A Renilla luciferase reporter plasmid (pRL-TK) was used as an internal control.
Statistical Analysis
Unpaired, two-tailed Student's t test was used to determine the statistical differences between the data sets. p ≤ 0.05 was considered statistically significant. In the mouse endotoxin shock study, p values for differences in survival between groups were calculated by a log-rank test and analyzed for statistical significance with GraphPad Prism version 4.0 software.
RESULTS
The Phosphorylation of TAK1 Ser-412 Is Essential for TLR Signaling and Cytokine Production
Previous studies have suggested that TAK1 activation requires Thr-184 and Thr-187 phosphorylation in its kinase activation domain. However, some studies have shown that the phosphorylation of these sites is insufficient for TAK1 activation (14, 15). Consistent with this notion, our recent investigation with PP1 implied that the phosphorylation of TAK1 Ser-412 is important for TAK1 activation and function (30).
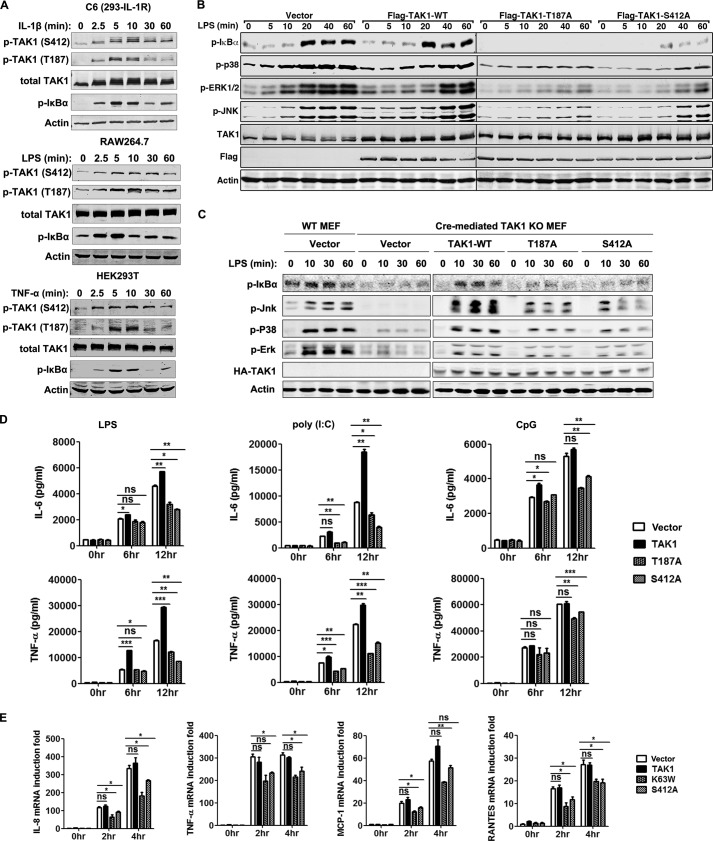
To determine whether TAK1 Ser-412 was phosphorylated and its relationship to TAK1 activation in response to TLR and IL-1R activation, we stimulated C6 cells (HEK293 derivatives stably expressing IL-1R) with IL-1β, RAW264.7 cells with LPS, or HEK293T cells with TNF-α and determined the activation status of TAK1 and its downstream signaling component IκBα using their respective phospho-specific antibodies in the three signaling pathways. In addition to TAK1 Thr-187 and IκBα phosphorylation, which are indications of TAK1 and IKK activation, all of the treatment induced the phosphorylation of Ser-412 in a time-dependent manner, as detected with a TAK1 phospho-Ser-412-specific antibody (Fig. 1A). This finding suggested that TAK1 Ser-412 phosphorylation may be a general event during TAK1 and IKK activation in response to proinflammatory stimulation in various cell types.
FIGURE 1.
TAK1 Ser-412 phosphorylation is essential for TLR signaling and induction of proinflammatory cytokines. A, IL-1R-stably expressing HEK293T cells (C6), RAW264.7 cells, and HEK293T cells were treated by IL-1β (10 ng/ml), LPS (100 ng/ml), or TNF-α (10 ng/ml), respectively, for the indicated time. Cell lysates were then used for immunoblotting with the indicated antibodies. B, RAW264.7 cells were transiently transfected with 4 μg of empty vector, FLAG-tagged TAK1 wild type (TAK1-WT), TAK1 Thr-187 to alanine mutant (T187A), or TAK1 Ser-412 to alanine mutant (S412A) vector. After 48 h, cells were treated with LPS (100 ng/ml) for the indicated time. Proteins in the NF-κB and MAPK pathways were detected by immunoblotting. C, Cre-mediated TAK1 knock-out MEF cells were transfected with empty vector or TAK1-WT, T187A, or S412A vector by using the MEF Nucleofector Kit. After 24 h, cells were treated with LPS (1 μg/ml) for the indicated time. Cell lysates were then used for immunoblotting with the indicated antibodies. D, Cells were transfected as in B but treated with LPS (100 ng/ml), poly(I:C) (20 μg/ml), or CpG ODN (0.3 μm) for longer duration. Secretion of IL-6 and TNF-α in the supernatant was analyzed by ELISA. Data are plotted as means ± S.E. (error bars); *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus controls. ns, no significance. All experiments were performed at least three times. E, C6 cells were transiently transfected with 1 μg of empty vector or vectors expressing FLAG-tagged TAK1, K63W, or S412A. After 48 h, cells were treated with IL-1β (10 ng/ml) for the indicated time. Total RNA were then extracted and used to determine induction of IL-8, TNF-α, MCP-1, and RANTES by using quantitative real-time PCR (Q-RT-PCR). Data are plotted as means ± S.E.; *, p < 0.05; **, p < 0.01 versus controls. All experiments were performed at least three times. p-, phosphorylated.
We next investigated the significance of the phosphorylation of TAK1 Ser-412 in the NF-κB and MAPK signaling pathways. We transiently overexpressed TAK1 wild type (WT), the Ser-412 to the alanine (S412A) mutant, or the Thr-187 to alanine (T187A) mutant in RAW264.7 cells to test their effect in the LPS signaling pathway. The TAK1 T187A mutant is a known TAK1 kinase-dead mutant that can function as a dominant-negative mutant. LPS treatment of RAW264.7 cells transfected with empty vector or TAK1 WT resulted in TAK1 activation, which in turn led to the phosphorylation of the downstream signaling proteins IκBα, p38, JNK, and ERK1/2 (Fig. 1B). The phosphorylation of IκBα, p38, JNK, and ERK1/2 was significantly reduced by the transient overexpression of the TAK1 S412A mutant, which was comparable with reduction by the overexpression of the TAK1 T187A mutant (Fig. 1B). Similarly, transient overexpression of TAK1 WT in MEF cells derived from TAK1 KO mice rescued phosphorylation of IκBα, JNK, p38, and ERK1/2 following LPS treatment (Fig. 1C), which was comparable with that of WT MEF cells. However, transient overexpression of T187A or S412A mutants could not rescue LPS-induced phosphorylation of these proteins (Fig. 1C). The overexpression of TAK1 T187A and S412A but not of WT in RAW264.7 cells also reduced the production of IL-6 and TNF-α in response to LPS, poly(I:C), or CpG treatment (Fig. 1D). Similarly, the treatment of C6 cells with IL-1β induced the mRNA production of TNF-α, IL-8, MCP-1, and RANTES based on quantitative RT-PCR analysis, which was significantly suppressed by the overexpression of TAK1 T187A and S412A mutants but not of WT (Fig. 1E). These results indicated that S412A is a kinase-dead mutant and that Ser-412 phosphorylation is important for TAK1 kinase activation, downstream signaling, and proinflammatory cytokine induction.
The Phosphorylations of TAK1 Thr-187 and Ser-412 Are Independent of Each Other
Both Thr-187 and Ser-412 were phosphorylated in a parallel fashion in response to proinflammatory stimulation (Fig. 1A). Thus, we next investigated the relationship between the phosphorylation of these two sites to see whether they were coupled or independent. We transfected TAK1 WT and the mutants K63W, S412A, S412D, T187A, or T187D, in the absence or presence of TAB1, into 293T cells and checked for the phosphorylation status at Thr-187 and Ser-412. As shown in Fig. 2A, only TAK1 WT and the mutants S412A and S412D showed comparable levels of Thr-187 phosphorylation. Interestingly, except for TAK1 S412A mutant, all other proteins showed similar levels of Ser-412 phosphorylation. This result suggested that TAK1 Thr-187 and Ser-412 phosphorylation events were independent from each other, although they showed parallel phosphorylation patterns in response to proinflammatory stimulation. This result also indicated that TAK1 Ser-412 phosphorylation should be mediated by other kinase(s).
FIGURE 2.
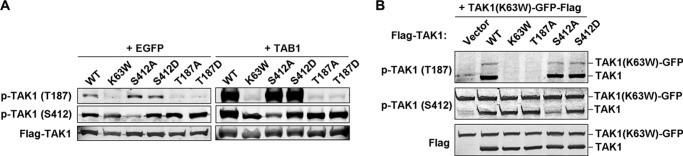
Ser-412 and Thr-187 phosphorylation events are independent from each other. A, 500 ng of TAK1 WT and mutant K63W, S412A, S412D, T187A, or T187D vector was transfected into 293T cells together with 500 ng of vector encoding EGFP or TAB1. After 48 h, TAK1 phosphorylation states at Thr-187 and Ser-412 were detected with the indicated phosphorylation-specific antibodies. B, 500 ng of FLAG-tagged empty vector, TAK1 WT, or its mutant K63W, T187A, S412A, or S412D vector was co-transfected with TAK1-K63W-GFP-FLAG (as the substrates) into 293T cells. After 36 h, cells were collected and lysed for immunoblotting with the indicated antibodies. p-, phosphorylated.
To further verify this point, we co-transfected into 293T cells TAK1 WT or the mutants together with TAK1(K63W)-GFP, which was used as a substrate and fused to GFP to increase its size difference from the other TAK1 proteins during immunoblotting. As shown in Fig. 2B, the co-transfection of TAK1(K63W)-GFP with TAK1 WT, S412A mutant, or S412D mutant but not with the two kinase-dead mutants K63W and T187A resulted in its phosphorylation at Thr-187. However, all proteins except S412A showed similar levels of phosphorylation at Ser-412.
These data strongly suggested that the phosphorylations of Thr-187 and Ser-412 were two independent events (i.e. Thr-187 underwent autophosphorylation, and Ser-412 was phosphorylated by another kinase).
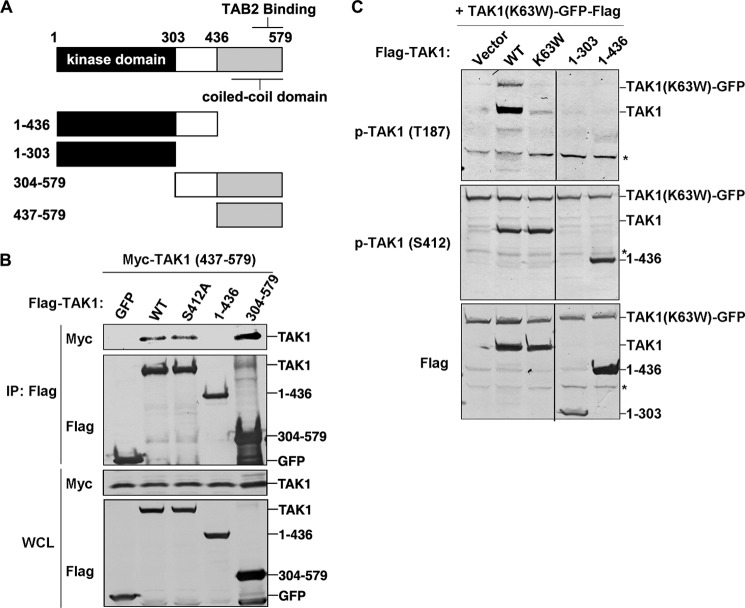
TAK1 Thr-187 Phosphorylation Requires Putative C-terminal Coiled-coil Domain-mediated Dimerization
Detailed secondary structure analysis using several bioinformatics tools (such as COILS) revealed a high-probability coiled-coil domain in the category of dimerization (Fig. 3A) at the C terminus of TAK1. This finding suggested that TAK1 could self-dimerize through this coiled-coil domain. To test this possibility, we made deletion mutants (Fig. 3A), co-transfected them into 293T cells, and then performed co-immunoprecipitation. In the FLAG-based immunoprecipitation experiments (Fig. 3B), the FLAG-tagged full-length TAK1 WT, S412A, or the deletion mutant TAK1(304–579) co-immunoprecipitated with Myc-tagged deletion mutant TAK1(437–579), suggesting that TAK1 can form a dimer through the coiled-coil domain. A previous study has shown that this region was also responsible for the interaction with TAB2 (33).
FIGURE 3.
The C-terminal coiled-coil domain of TAK1 is essential for TAK1 intermolecular interaction and autophosphorylation. A, domain organization of mouse TAK1 and the truncation mutants. The kinase and putative coiled-coil domains are indicated. B, 500 ng of FLAG-tagged GFP or TAK1 WT, S412A, or aa 1–436 or 304–579 truncation mutant vector was transfected with 500 ng of Myc-tagged TAK1 aa 437–579 truncation mutant vector into 293T cells for 36 h and then subjected to immunoprecipitation by using M2 FLAG antibody-agarose beads. Immunoprecipitated proteins and whole cell lysates (WCL) were analyzed with anti-FLAG or anti-Myc antibody. C, 500 ng of FLAG-tagged TAK1 WT or its mutants K63W, T187A, S412A, or S412D or the truncation mutant (aa 1–303 or 1–436) vector was co-transfected with 500 ng of TAK1-K63W-GFP-FLAG vector into 293T cells. After 36 h, cell lysates were used for immunoblotting with the indicated antibodies. *, nonspecific bands. IP, immunoprecipitation; p-, phosphorylated.
To study the function of this coiled-coil domain-mediated dimerization of TAK1, we determined whether it was required for TAK1 autophosphorylation. We transfected TAK1(K63W)-GFP with TAK1 WT, TAK1(1–303), or TAK1(1–436) into 293T cells and examined the phosphorylation status at Thr-187 of TAK1(K63W)-GFP. As shown in Fig. 3C, only the co-transfection of TAK1 WT and not that of TAK1(1–303) or TAK1(1–436) led to the Thr-187 phosphorylation of TAK1(K63W)-GFP. These data suggested that TAK1 autophosphorylation depends on dimerization through its coiled-coil domain.
Ser-412 Phosphorylation Regulates TAK1 Kinase Activity
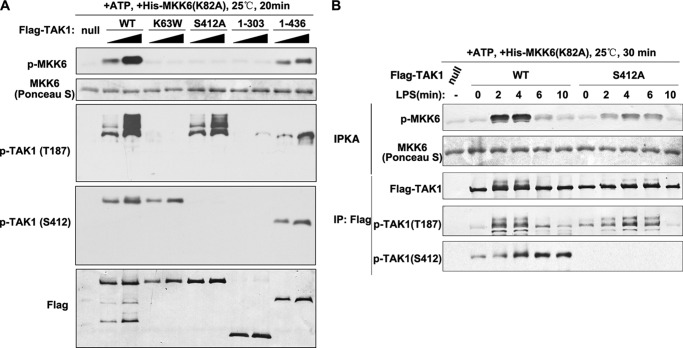
Our data indicated that TAK1 Ser-412 phosphorylation was important for its kinase activity. Accordingly, we designed two experiments to further investigate this hypothesis. First, we performed in vitro kinase assays using TAK1 purified from transiently transfected 293T cells as the catalytic kinase and the recombinant kinase-dead MKK6 (K82A mutant) isolated from E. coli as the substrate (34). The incubation of TAK1 WT kinase with MKK6 readily led to the site-specific phosphorylation of MKK6 in a dose-dependent manner (Fig. 4A). As expected, the catalytically inactive TAK1 K63W did not cause MKK6 phosphorylation. Interestingly, TAK1 S412A, as well as TAK1(1–303) and TAK1(1–436) deletion mutants, also did not cause detectable MKK6 phosphorylation, in agreement with our hypothesis that Ser-412 phosphorylation was required for TAK1 kinase activity (Fig. 4A). In our second experiment, we generated cell lines stably expressing FLAG-TAK1 WT or S412A mutant in RAW264.7 cells for immunoprecipitation protein kinase assays (Fig. 4B, IPKA). We stimulated the two cell lines with LPS for different periods of time and immunoprecipitated TAK1 using anti-FLAG antibody-conjugated agarose beads. The kinase activities of the immunoprecipitated TAK1 were then evaluated by its phosphorylation toward MKK6 in the immunoprecipitation kinase assay. As shown in Fig. 4B, TAK1 WT showed time-dependent and robust activities, whereas S412A mutant showed only residual activities on MKK6 phosphorylation. Taken together, these data suggested that Ser-412 phosphorylation was required for TAK1 kinase activity.
FIGURE 4.
Ser-412 phosphorylation and the C-terminal coiled-coil domain are essential for TAK1 kinase activities. A, FLAG-tagged TAK1 WT, K63W, S412A, and aa 1–303 and 1–436 truncation mutants were expressed and purified from 293T cells and used in the in vitro kinase assay by using kinase-dead MKK6 purified from E. coli as the substrate. After the reaction, proteins were detected with the indicated antibodies. B, RAW264.7 cells stably expressing FLAG-TAK1 WT or S412A mutant were stimulated for the indicated time. FLAG-TAK1 was then immunoprecipitated by using M2 FLAG antibody-agarose beads and used in the kinase assay as in A. IPKA, immunoprecipitation protein kinase assay. IP, immunoprecipitation; p-, phosphorylated.
PKACα and PRKX Regulate TAK1 Kinase Activity by Phosphorylating Ser-412
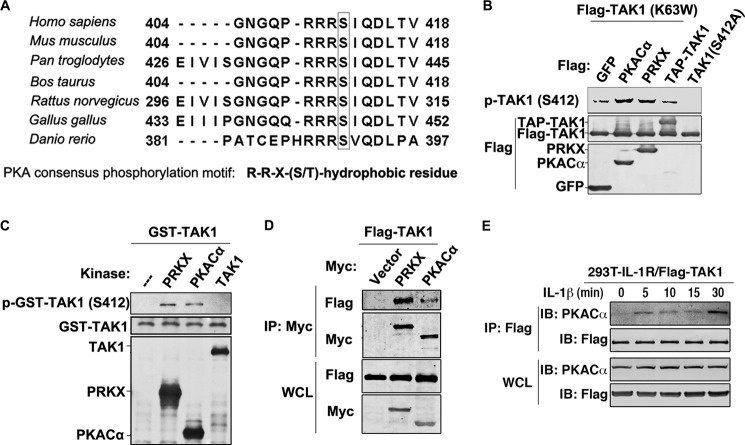
The importance of Ser-412 phosphorylation for TAK1 activation, which should be mediated by another kinase, led us to search for the kinase(s) responsible for its phosphorylation. To identify the putative kinase(s) that phosphorylated Ser-412, we conducted Scansite prediction, and results suggested the PKA and Aurora kinase families as the most likely candidates. AMP-activated protein kinase and Akt families were also putative candidates with lowered stringency. In fact, this residue and the surrounding ones were highly conserved in humans, mice, rats, and even zebrafish, and they matched very well with the consensus sequence of PKA substrates. Thus, PKA may regulate TAK1 kinase activation (Fig. 5A) (20). We tested all catalytic members of the four kinase families by overexpression experiments. The overexpression of TAK1 K63W, which served as the substrate, with PKACα or PRKX, but not with the other kinases, including TAK1 WT (in this case, a TAP-tagged TAK1 was used because it differed from K63W in size), led to strong phosphorylation of Ser-412 (Fig. 5B) (data not shown). To further verify that PKACα and PRKX can phosphorylate TAK1 at the Ser-412 site, we performed in vitro kinase assays using PKACα and PRKX as the kinases transiently overexpressed in and purified from 293T cells and GST-TAK1 expressed in and purified from E. coli as the substrate. As shown in Fig. 5C, incubation of PKACα or PRKX, but not TAK1 (which was also transiently overexpressed and purified from 293T cells), with GST-TAK1 led to strong phosphorylation of TAK1 Ser-412 detected by the phospho-Ser-412-specific antibody. This in vitro kinase assay also suggested that PKACα and PRKX could directly phosphorylate TAK1 (Fig. 5C). In support of this, PKACα and PRKX co-immunoprecipitated with TAK1 when they were co-overexpressed in 293T cells (Fig. 5D). Furthermore, the interaction between TAK1 and PKACα is stimulation-dependent. As shown in Fig. 5E, a co-immunoprecipitation assay using C6 cells stably expressing FLAG-TAK1 shows there was no interaction between TAK1 and PKACα without stimulation. However, upon IL-1β treatment, TAK1 immunoprecipitated PKACα, and this interaction lasted for the period of time tested.
FIGURE 5.
PKACα and PRKX mediate TAK1 phosphorylation at Ser-412. A, peptide sequences of TAK1 from the indicated species were aligned by using ClustalW to show the consensus motif (RRX(S/T) motif) that can be recognized by PKA kinases. B, 500 ng of FLAG-tagged GFP, PKACα, PRKX, or TAP-TAK1 vector was co-transfected with 500 ng of FLAG-TAK1-K63W vector into 293T cells. After 36 h, cell lysates were used for immunoblotting with the indicated antibodies. FLAG-TAK1 S412A was also transfected alone as a negative control for phospho-TAK1 Ser-412 antibody. C, FLAG-tagged PRKX, PKACα, and TAK1 were expressed and purified from 293T cells and used in the in vitro kinase assay using GST-TAK1 purified from E. coli as substrate. After the reaction, proteins were detected with the indicated antibodies. D, 1 μg of FLAG-tagged TAK1 vector alone or with 1 μg of Myc-tagged PRKX or PKACα vector was transfected into 293T cells for 36 h and then subjected to immunoprecipitation by using anti-Myc antibody. Immunoprecipitated proteins and whole cell lysates (WCL) were analyzed with anti-FLAG or anti-Myc antibody. E, C6 cells stably expressing FLAG-TAK1 were treated by IL-1β (10 ng/ml) for the indicated time and then subjected to immunoprecipitation by using anti-FLAG antibody. Immunoprecipitated proteins and whole cell lysates were analyzed with anti-FLAG or anti-PKACα antibody. IP, immunoprecipitation; p-, phosphorylated.
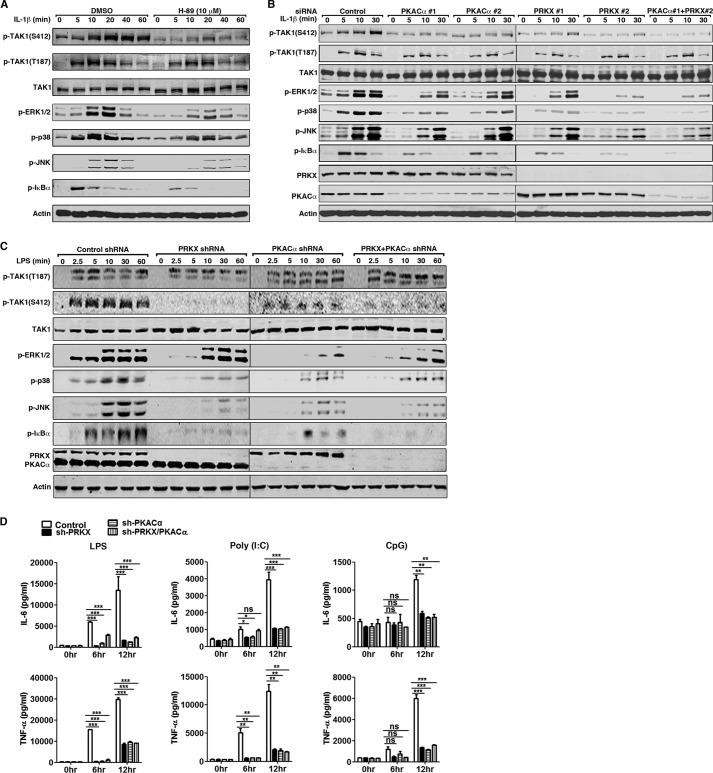
We next determined the requirement of PKACα and PRKX for TAK1 Ser-412 phosphorylation. We treated HeLa cells with H-89, a widely used PKA kinase inhibitor and tested if this would inhibit IL-1β-induced TAK1 activation and signaling. As shown in Fig. 6A, H-89 treatment strongly inhibited phosphorylation of TAK1 Ser-412 and its downstream targets ERK1/2, p38, JNK, and IκBα compared with control (DMSO) treatment. siRNA-based transient knockdown of PKACα, PRKX, or both PKACα and PRKX in HeLa cells reduced IL-1β-induced phosphorylation of TAK1 Ser-412, ERK1/2, p38, JNK, and IκBα (Fig. 6B). We also generated shRNA-based stable knockdown cell lines in RAW264.7 cells. As shown in Fig. 6C, in PKACα, PRKX, or PKACα/PRKX double knockdown cells, the phosphorylation of TAK1 Ser-412, ERK, JNK, p38, and IκBα was dramatically reduced in response to LPS treatment compared with shRNA control cells. Consequently, the production of IL-6 and TNF-α was significantly reduced in PKACα and PRKX stable knockdown cells upon stimulation by LPS, poly(I:C), or CpG ODN (Fig. 6D). Consistent with our initial screening result, the stable knockdown of another PKA catalytic member PKACβ in RAW264.7 cells did not affect the phosphorylation of TAK1 Ser-412 and downstream signaling proteins (data not shown). As expected, TAK1 Thr-187 phosphorylation was not affected by H-89 treatment or by RNAi-based knockdown of PKACα and PRKX (Fig. 6, A–C). These data suggested that the PKA kinase family members PKACα and PRKX phosphorylated TAK1 at Ser-412 to regulate its kinase activity and directly participate in TLR and IL-1R signaling.
FIGURE 6.
PKACα and PRKX regulate TAK1 kinase activity by phosphorylating TAK1 Ser-412. A, HeLa cells were treated with DMSO or H-89 (10 μm) for 1 h and then stimulated with IL-1β (10 ng/ml) for the indicated time. Cell lysates were subjected to immunoblotting with the indicated antibodies. B, HeLa cells were transfected with scrambled siRNA, two distinct siRNAs against human PRKX or PKACα (40 nm final siRNA concentration) for 48 h, and stimulated with IL-1β (10 ng/ml) for the indicated time. Cell lysates were subjected to immunoblotting with the indicated antibodies. C, stable RAW264.7 cells harboring control shRNA or shRNA against PRKX, PKACα, or both PRKX and PKACα were stimulated with LPS (100 ng/ml) for the indicated time. Cell lysates were collected and used for immunoblotting with the indicated antibodies. D, as in C, RAW264.7 cells were treated with LPS (100 ng/ml), poly(I:C) (20 μg/ml), or CpG ODN (0.3 μm) for 6 or 12 h. Secretion of IL-6 and TNF-α in the supernatant was analyzed by ELISA. Data are plotted as means ± S.E. (error bars). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus controls. ns, no significance. All experiments were performed at least three times. p-, phosphorylated.
DrTAK1 Ser-391 Phosphorylation Plays a Conserved Role in Innate Immune Signaling in Zebrafish
To obtain genetic evidence that TAK1 Ser-412 phosphorylation was important for its kinase activity and signaling, we studied its homolog DrTAK1 in zebrafish embryos. Mouse TAK1 and DrTAK1 showed 71% identity and 79% similarity at the amino acid level (2). The amino acid residue corresponding to mouse TAK1 Ser-412 was Ser-391 in DrTAK1 (Fig. 5A).
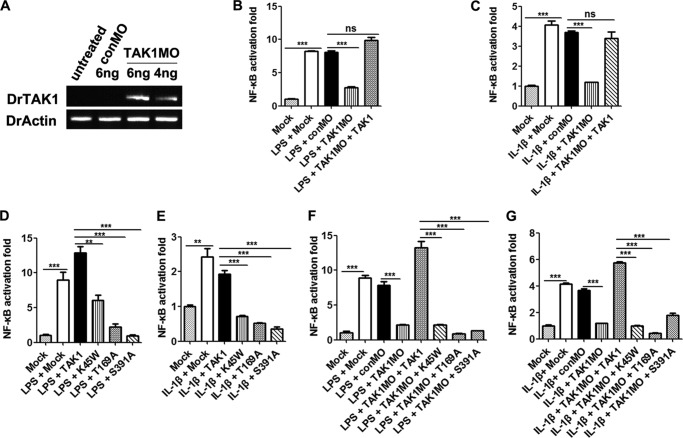
We first studied if DrTAK1 was required for NF-κB activation, which had not yet been reported. The injection of LPS or IL-1β mRNA led to robust NF-κB activation (Fig. 7, B and C). Activation was abolished when MO against DrTAK1 was co-injected (Fig. 7, A–C), which was rescued well by the simultaneous injection of mRNA for DrTAK1 (Fig. 7, B and C). The DrTAK1 MO used in this study did not have any detectable adverse effect on embryo development (data not shown). These results demonstrated that DrTAK1 was important for LPS- and IL-1β-mediated NF-κB activation in zebrafish.
FIGURE 7.
DrTAK1 Ser-391 phosphorylation is essential in innate immune signaling in zebrafish. A, RT-PCR analysis to detect inclusion of intron 6 in DrTAK1 transcripts upon morpholino injection. Zebrafish actin (DrActin) was used as control. B and C, one-cell stage embryos were injected with NF-κB luciferase reporter vectors, DrTAK1 MO, or control MO, together with LPS (B) or capped IL-1β mRNA (C). The firefly and Renilla luciferase activities were assayed 24 h postmicroinjection with 4–5 replicates (each containing 50–100 embryos). D and E, activation of NF-κB signal pathway after injection of one-cell stage embryos with NF-κB luciferase reporter vectors, LPS (D), or IL-1β mRNA (E) plus empty vector, TAK1, the K45W mutant, the T169A mutant, or the S391A mutant mRNAs. The firefly and Renilla luciferase activities were assayed 24 h postmicroinjection with 4–5 replicates (each containing 50–100 embryos). F and G, activation of the NF-κB signal pathway after injection with NF-κB luciferase reporter vectors, LPS (F), or IL-1β mRNA (G) plus empty vector mRNA, control MO, or DrTAK1 MO alone or with the above mentioned TAK1 mRNAs. The firefly and Renilla luciferase activities were assayed 24 h postmicroinjection with 4–5 replicates (each containing 50–100 embryos). Data from B–G are plotted as means ± S.E. (error bars). **, p < 0.01; ***, p < 0.001 versus controls. ns, no significance.
We then determined whether Ser-391 was important for DrTAK1 activation. The overexpression of DrTAK1 WT by mRNA injection did not affect LPS- and IL-1β-induced NF-κB activation. However, activation was dramatically reduced by the overexpression of DrTAK1 S391A mutant, similar to the kinase-dead mutants DrTAK1 K45W and T169A (Figs. 7, D and E). Furthermore, the MO-mediated knockdown of DrTAK1 rendered embryos unresponsive to LPS or IL-1β stimulation, which was rescued very well by DrTAK1 WT but not by K45W, T169A, or S391A mutants (Fig. 7, F and G). These data demonstrated that DrTAK1 Ser-391 phosphorylation was important for DrTAK1 activation and TLR and IL-1β signaling in zebrafish embryos in vivo.
DISCUSSION
TAK1 Ser-412 Phosphorylation for TAK1 Activation
As the first kinase for which its kinase activity is known to be required, TAK1 is highly important in mediating IKK/NF-κB and MAPK activation in proinflammatory and innate immune signaling pathways. Numerous studies have shown that TAK1 activation required the autophosphorylation of key residues (especially Thr-184 and Thr-187) in its kinase domain mediated by TAB2 (or TAB3) binding to free Lys-63-linked polyubiquitin chains (34). In this study, we further showed that TAK1 can dimerize through its C-terminal coiled-coil domain, which was required for the TAK1 autophosphorylation of its kinase domain. We also investigated the importance of Ser-412 phosphorylation on TAK1 kinase activity in the IL-1R and TLR signaling pathways. We showed that TAK1 Ser-412 was phosphorylated in these pathways and that its phosphorylation was important for TAK1 kinase activation in TLR and IL-1R signaling. This regulation was conserved in evolution, as evidenced by our studies using zebrafish embryos. Furthermore, we presented data confirming that the PKA kinase family members PKACα and PRKX were responsible for TAK1 Ser-412 phosphorylation. Our current studies have advanced our understanding of the regulation of TAK1 activation in proinflammatory and innate immune signaling pathways.
TAK1 Ser-412 phosphorylation has been previously reported (16–18), and the database PhosphositePlus has recorded this phenomenon 94 times. However, the functional significance of TAK1 Ser-412 phosphorylation has been unclear until our studies. In this work, we unraveled its importance for TAK1 activation and its role in the TLR and IL-1R signal transduction pathways for the first time. Determination of whether TAK1 Ser-412 phosphorylation occurs and is important for its function in other pathways involving TAK1 requires more investigation. Although we showed that TAK1 Ser-412 phosphorylation was important for its kinase activity, we did not determine how the phenomenon occurred. Ser-412 was near the C terminus of TAK1, which was far from the kinase domain. Structural information should be collected before we can gain a better understanding about the matter. Notably, this kind of mechanism is not unique to TAK1. Kinases in the Akt, NDR, and PKC families also require the phosphorylation of Ser/Thr residues outside of their respective kinase domains (20).
TAK1 Phosphorylation by PKA Kinases
Our data showed that mTAK1 Ser-412 phosphorylation was mediated by the PKA kinase family members PKACα and PRKX. Either PKACα or PRKX can phosphorylate and show comparable activity toward TAK1 Ser-412 in vitro, suggesting that they may work independently from each other, and either one could phosphorylate Ser-412 in vivo. Interestingly, either a single knockdown of PKACα or PRKX or a double knockdown of both PKACα and PRKX resulted in aberrant Ser-412 phosphorylation to a hard-to-detect level in vivo, implying that neither PKACα nor PRKX was sufficient for Ser-412 phosphorylation. The reason for this observation is not known at present. One possibility is that the cellular concentration of PKACα or PRKX alone is insufficient to phosphorylate Ser-412 to a detectable level under our immunoblotting conditions; thus, the difference between single and double knockdown conditions was not revealed. Notably, immunoblotting showed that PRKX knockdown did not change the PKACα expression level and vice versa. The other possibility is that PKACα and PRKX may need to work together, as a heterodimer for example, to phosphorylate Ser-412. A similar phenomenon has been reported for histone H3 Lys-9 methylation by the methyltransferases G9a and GLP, in which either one can methylate histone H3 Lys-9 in vitro with purified proteins, but the KO of either one leads to the defect of histone H3 Lys-9 methylation in mice. Detailed biochemical studies have revealed that in vivo, G9a and GLP have to form a heterodimer as a functional H3K9 methyltransferase (35). Whether PKACα and PRKX phosphorylate TAK1 Ser-412 in a similar manner warrants further study.
In the present work, we showed TAK1 Ser-412 phosphorylation by PKACα and PRKX in IL-1R and several TLR pathways without co-stimulation by known PKA activation agents. This finding suggested that PKACα and PRKX could be activated by ligands of IL-1R and TLRs. Determination of how they are activated in response to IL-1R and TLR activation requires further investigation. In general, the activation of PKA kinase members is in response to an elevated cellular cAMP concentration. Whether cAMP is involved in the activation of PKACα and PRKX or if they are activated in a cAMP-independent mechanism in the IL-1R/TLR pathways is yet to be determined. Kobayashi et al. (16) previously reported that in RAW264.7 cells, cAMP and PKA, through the phosphorylation of TAK1 Ser-412, mediate the effect of prostaglandin E2 on the enhancement of osteoclastic differentiation stimulated by RANK ligand (RANKL) and on IL-6 production stimulated by LPS. However, RANKL or LPS treatment alone did not result in Ser-412 phosphorylation.
In summary, the present study together with our recent report (30) showing a negative regulation of TAK1 by the phosphatase PP1-GADD34 complex provided strong evidence that TAK1 activation required the phosphorylation of TAK1 Ser-412 in the IL-1R and TLR signaling pathways. PKA kinases, by directly phosphorylating TAK1, were found to be important regulators of proinflammatory and innate immune responses.
Acknowledgments
We thank Dr. H Xiao for the TAK1−/− MEF cells and Dr. Brian Skaug for critical reading of the manuscript.
This work was supported by National Basic Research Program of China (973) Grants 2013CB945004 and 2011CB910800, National Natural Science Foundation of China Grant 31071242, and Natural Science Foundation of Zhejiang Province Grant R2110588.
- TAK1
- TGF-β-activated kinase 1
- aa
- amino acid(s)
- IKK
- IκB kinase
- IL-1R
- interleukin-1 receptor
- MEKK/MKK
- MAP/ERK kinase kinase
- MO
- morpholino
- NF-κB
- nuclear factor κ-light chain enhancer of activated B cells
- ODN
- oligodeoxynucleotide
- PP1
- protein phosphatase 1
- TLR
- Toll-like receptor
- RANTES
- regulated on activation normal T cell expressed and secreted
- PRKX and PRKY
- X- and Y-linked protein kinase, respectively
- PKAC
- protein kinase A catalytic subunit
- MEF
- mouse embryo fibroblast.
REFERENCES
- 1. Shim J. H., Xiao C., Paschal A. E., Bailey S. T., Rao P., Hayden M. S., Lee K. Y., Bussey C., Steckel M., Tanaka N., Yamada G., Akira S., Matsumoto K., Ghosh S. (2005) TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jadrich J. L., O'Connor M. B., Coucouvanis E. (2006) The TGF β activated kinase TAK1 regulates vascular development in vivo. Development 133, 1529–1541 [DOI] [PubMed] [Google Scholar]
- 3. Ajibade A. A., Wang H. Y., Wang R. F. (2013) Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 34, 307–316 [DOI] [PubMed] [Google Scholar]
- 4. Chen Z. J. (2005) Ubiquitin signalling in the NF-κB pathway. Nat. Cell Biol. 7, 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghosh S., Hayden M. S. (2012) Celebrating 25 years of NF-κB research. Immunol. Rev. 246, 5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 [DOI] [PubMed] [Google Scholar]
- 7. Ishitani T., Takaesu G., Ninomiya-Tsuji J., Shibuya H., Gaynor R. B., Matsumoto K. (2003) Role of the TAB2-related protein TAB3 in IL-1 and TNF signaling. EMBO J. 22, 6277–6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kanayama A., Seth R. B., Sun L., Ea C. K., Hong M., Shaito A., Chiu Y. H., Deng L., Chen Z. J. (2004) TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 [DOI] [PubMed] [Google Scholar]
- 9. Inagaki M., Omori E., Kim J. Y., Komatsu Y., Scott G., Ray M. K., Yamada G., Matsumoto K., Mishina Y., Ninomiya-Tsuji J. (2008) TAK1-binding protein 1, TAB1, mediates osmotic stress-induced TAK1 activation but is dispensable for TAK1-mediated cytokine signaling. J. Biol. Chem. 283, 33080–33086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singhirunnusorn P., Suzuki S., Kawasaki N., Saiki I., Sakurai H. (2005) Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-β-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J. Biol. Chem. 280, 7359–7368 [DOI] [PubMed] [Google Scholar]
- 11. Scholz R., Sidler C. L., Thali R. F., Winssinger N., Cheung P. C., Neumann D. (2010) Autoactivation of transforming growth factor β-activated kinase 1 is a sequential bimolecular process. J. Biol. Chem. 285, 25753–25766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu Y., Ge N., Xie M., Sun W., Burlingame S., Pass A. K., Nuchtern J. G., Zhang D., Fu S., Schneider M. D., Fan J., Yang J. (2008) Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFκB and AP-1 activation as well as IL-6 gene expression. J. Biol. Chem. 283, 24497–24505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sakurai H., Nishi A., Sato N., Mizukami J., Miyoshi H., Sugita T. (2002) TAK1-TAB1 fusion protein: a novel constitutively active mitogen-activated protein kinase kinase kinase that stimulates AP-1 and NF-κB signaling pathways. Biochem. Biophys. Res. Commun. 297, 1277–1281 [DOI] [PubMed] [Google Scholar]
- 14. Sakurai H., Miyoshi H., Mizukami J., Sugita T. (2000) Phosphorylation-dependent activation of TAK1 mitogen-activated protein kinase kinase kinase by TAB1. FEBS Lett. 474, 141–145 [DOI] [PubMed] [Google Scholar]
- 15. Kishimoto K., Matsumoto K., Ninomiya-Tsuji J. (2000) TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. J. Biol. Chem. 275, 7359–7364 [DOI] [PubMed] [Google Scholar]
- 16. Kobayashi Y., Mizoguchi T., Take I., Kurihara S., Udagawa N., Takahashi N. (2005) Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J. Biol. Chem. 280, 11395–11403 [DOI] [PubMed] [Google Scholar]
- 17. Kim S. I., Kwak J. H., Wang L., Choi M. E. (2008) Protein phosphatase 2A is a negative regulator of transforming growth factor-β1-induced TAK1 activation in mesangial cells. J. Biol. Chem. 283, 10753–10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prickett T. D., Ninomiya-Tsuji J., Broglie P., Muratore-Schroeder T. L., Shabanowitz J., Hunt D. F., Brautigan D. L. (2008) TAB4 stimulates TAK1-TAB1 phosphorylation and binds polyubiquitin to direct signaling to NF-κB. J. Biol. Chem. 283, 19245–19254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor S. S., Keshwani M. M., Steichen J. M., Kornev A. P. (2012) Evolution of the eukaryotic protein kinases as dynamic molecular switches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 2517–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pearce L. R., Komander D., Alessi D. R. (2010) The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 11, 9–22 [DOI] [PubMed] [Google Scholar]
- 21. Dulin N. O., Niu J., Browning D. D., Ye R. D., Voyno-Yasenetskaya T. (2001) Cyclic AMP-independent activation of protein kinase A by vasoactive peptides. J. Biol. Chem. 276, 20827–20830 [DOI] [PubMed] [Google Scholar]
- 22. Zieger M., Tausch S., Henklein P., Nowak G., Kaufmann R. (2001) A novel PAR-1-type thrombin receptor signaling pathway: cyclic AMP-independent activation of PKA in SNB-19 glioblastoma cells. Biochem. Biophys. Res. Commun. 282, 952–957 [DOI] [PubMed] [Google Scholar]
- 23. Gerlo S., Kooijman R., Beck I. M., Kolmus K., Spooren A., Haegeman G. (2011) Cyclic AMP: a selective modulator of NF-κB action. Cell. Mol. Life Sci. 68, 3823–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mosenden R., Taskén K. (2011) Cyclic AMP-mediated immune regulation: overview of mechanisms of action in T cells. Cell. Signal. 23, 1009–1016 [DOI] [PubMed] [Google Scholar]
- 25. Zhong H., Voll R. E., Ghosh S. (1998) Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1, 661–671 [DOI] [PubMed] [Google Scholar]
- 26. Wall E. A., Zavzavadjian J. R., Chang M. S., Randhawa B., Zhu X., Hsueh R. C., Liu J., Driver A., Bao X. R., Sternweis P. C., Simon M. I., Fraser I. D. (2009) Suppression of LPS-induced TNF-α production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci. Signal. 2, ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trede N. S., Langenau D. M., Traver D., Look A. T., Zon L. I. (2004) The use of zebrafish to understand immunity. Immunity 20, 367–379 [DOI] [PubMed] [Google Scholar]
- 28. van der Vaart M., Spaink H. P., Meijer A. H. (2012) Pathogen recognition and activation of the innate immune response in zebrafish. Adv. Hematol. 2012, 159807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stein C., Caccamo M., Laird G., Leptin M. (2007) Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol. 8, R251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gu M., Ouyang C., Lin W., Zhang T., Cao X., Xia Z., Wang X. (2014) Phosphatase holoenzyme PP1/GADD34 negatively regulates TLR response by inhibiting TAK1 serine 412 phosphorylation. J. Immunol. 192, 2846–2856 [DOI] [PubMed] [Google Scholar]
- 31. An H., Zhao W., Hou J., Zhang Y., Xie Y., Zheng Y., Xu H., Qian C., Zhou J., Yu Y., Liu S., Feng G., Cao X. (2006) SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity 25, 919–928 [DOI] [PubMed] [Google Scholar]
- 32. Xiong R., Nie L., Xiang L. X., Shao J. Z. (2012) Characterization of a PIAS4 homologue from zebrafish: insights into its conserved negative regulatory mechanism in the TRIF, MAVS, and IFN signaling pathways during vertebrate evolution. J. Immunol. 188, 2653–2668 [DOI] [PubMed] [Google Scholar]
- 33. Takaesu G., Kishida S., Hiyama A., Yamaguchi K., Shibuya H., Irie K., Ninomiya-Tsuji J., Matsumoto K. (2000) TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol. Cell 5, 649–658 [DOI] [PubMed] [Google Scholar]
- 34. Xia Z. P., Sun L., Chen X., Pineda G., Jiang X., Adhikari A., Zeng W., Chen Z. J. (2009) Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tachibana M., Ueda J., Fukuda M., Takeda N., Ohta T., Iwanari H., Sakihama T., Kodama T., Hamakubo T., Shinkai Y. (2005) Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 19, 815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]