Background: Peroxisomal ABC transporters are predicted to function as homodimers in mammals.
Results: ABCD1 interacts with ABCD2. Chimeric proteins mimicking full-length dimers represent novel tools for functional study. Artificial homodimers and heterodimers are functional.
Conclusion: Interchangeability between ABCD1 and ABCD2 is confirmed, but PUFA transport depends on ABCD2.
Significance: For the first time, heterodimers in mammals are proven to be functional.
Keywords: ABC Transporter, Fatty Acid, Peroxisome, Protein Chimera, Protein-Protein Interaction
Abstract
ABCD1 and ABCD2 are two closely related ATP-binding cassette half-transporters predicted to homodimerize and form peroxisomal importers for fatty acyl-CoAs. Available evidence has shown that ABCD1 and ABCD2 display a distinct but overlapping substrate specificity, although much remains to be learned in this respect as well as in their capability to form functional heterodimers. Using a cell model expressing an ABCD2-EGFP fusion protein, we first demonstrated by proximity ligation assay and co-immunoprecipitation assay that ABCD1 interacts with ABCD2. Next, we tested in the pxa1/pxa2Δ yeast mutant the functionality of ABCD1/ABCD2 dimers by expressing chimeric proteins mimicking homo- or heterodimers. For further structure-function analysis of ABCD1/ABCD2 dimers, we expressed chimeric dimers fused to enhanced GFP in human skin fibroblasts of X-linked adrenoleukodystrophy patients. These cells are devoid of ABCD1 and accumulate very long-chain fatty acids (C26:0 and C26:1). We checked that the chimeric proteins were correctly expressed and targeted to the peroxisomes. Very long-chain fatty acid levels were partially restored in transfected X-linked adrenoleukodystrophy fibroblasts regardless of the chimeric construct used, thus demonstrating functionality of both homo- and heterodimers. Interestingly, the level of C24:6 n-3, the immediate precursor of docosahexaenoic acid, was decreased in cells expressing chimeric proteins containing at least one ABCD2 moiety. Our data demonstrate for the first time that both homo- and heterodimers of ABCD1 and ABCD2 are functionally active. Interestingly, the role of ABCD2 (in homo- and heterodimeric forms) in the metabolism of polyunsaturated fatty acids is clearly evidenced, and the chimeric dimers provide a novel tool to study substrate specificity of peroxisomal ATP-binding cassette transporters.
Introduction
X-linked adrenoleukodystrophy (X-ALD,2 OMIM 300100), the most frequent peroxisomal disorder, is associated with neurodegenerative symptoms and characterized by the accumulation of very long-chain fatty acids (VLCFA, mainly C26:0 and C26:1) (1). X-ALD is caused by mutations in the ABCD1 gene, which encodes a peroxisomal member of the ATP-binding cassette (ABC) transporter subfamily D called ALDP (adrenoleukodystrophy protein) or ABCD1 (2). There are two other peroxisomal ABC transporters called ALDRP or ABCD2 (3) and PMP70 (peroxisomal membrane protein) or ABCD3 (4).
These proteins are half-transporters with one transmembrane domain (TMD) followed by one nucleotide-binding domain. They must at least dimerize to form a functional unit because ATP binding requires the cooperation of two nucleotide-binding domains (5). Sucrose density gradient and co-immunoprecipitation experiments have shown that ABCD1 and ABCD3 occur predominantly as homodimers (6). Functional complementation assays in yeast pxa1/pxa2Δ double mutants also suggested the functionality of ABCD1, ABCD2, and ABCD3 as homodimers (7–9). However, yeast two-hybrid, co-immunoprecipitation, FRET, and proximity ligation assays have provided evidence for the existence of heterodimers formed between ABCD1 and one of the two other peroxisomal ABC transporters (10–14). Therefore, it cannot be excluded that heterodimers are functional especially in tissues where at least two ABCD transporters are highly co-expressed. Although expression of ABCD3 is quite ubiquitous, ABCD1 and ABCD2 harbor a tissue-specific but overlapping expression pattern (3, 15) characterized by a mirror expression pattern when specific cell types are analyzed (16). Moreover, because ABCD2 expression is highly inducible (17), both ABCD1 and ABCD2 proteins can be found in the same cells and tissues upon stimulation, thus rendering ABCD1/ABCD2 heterodimerization possible.
The three peroxisomal ABC transporters allow the uptake of the different lipid compounds metabolized into the peroxisomes. Concerning their substrate specificity, a partial functional redundancy has been demonstrated, especially between ABCD1 and ABCD2 (18–20). However, based on lipid analyses from X-ALD patients, from mouse models in which the genes Abcd1, Abcd2, and/or Abcd3 have been deleted, and from various cell models, some substrate preferences have been described for each of the transporters. The uptake of saturated and monounsaturated fatty acyl-CoA would be preferentially done by ABCD1 (7). ABCD2 would be involved in the transport of the same VLCFA but also in the transport of C20:0, C22:0, C22:1, and polyunsaturated fatty acids such as DHA (C22:6 n-3) and its immediate precursor C24:6 n-3 (8–10, 21–23). Finally, ABCD3 would preferentially transport branched-chain fatty acids, bile acid precursors, and dicarboxylic acids (9, 24, 25). The relationships between the substrate specificity of the peroxisomal ABC transporters and their state of dimerization (homo- or heterodimerization) have remained unexplored.
In this study, our objective was to clarify the protein-protein interactions between ABCD1 and ABCD2 and then further study the functional relevance of alternative dimerization. Taking advantage of the H4IIEC3 Tet-On cell line expressing ABCD2-EGFP fusion protein (26), we performed proximity ligation assays (PLA, Duolink) and co-immunoprecipitation experiments. To explore the functionality of the dimers, we generated plasmid constructs allowing the expression of chimeric proteins mimicking homodimeric or heterodimeric states between ABCD1 and ABCD2. The functionality of the chimeric proteins was measured by their capability to restore β-oxidation in pxa1/pxa2Δ yeast strains and to restore VLCFA levels in X-ALD skin fibroblasts.
EXPERIMENTAL PROCEDURES
Cell Culture
Rat H4IIEC3 cells stably expressing ABCD2-EGFP (clone 28.38) (10, 26) were cultured in DMEM/Ham's F-12 (1:1) supplemented with 5% FCS at 37 °C in a humidified atmosphere of 5% CO2 in the presence of 200 μg/ml G418 (InvivoGen) and 200 μg/ml hygromycin B (InvivoGen). Culture of these cells under doxycycline treatment permits a dose-dependent induction of the expression of ABCD2-EGFP. Noteworthy, at the highest dose of antibiotic used (2 μg/ml), the ABCD2-EGFP expression cannot be detected by Coomassie Blue staining on the SDS-polyacrylamide gel indicating that there is no overexpression of this protein.
Murine microglial BV-2 cells (Banca Biologica e Cell Factory-IST, Genova, Italy) were grown in RPMI 1640 medium (Lonza) supplemented with 10% heat-inactivated FCS (Eurobio), 1% antibiotics (penicillin/streptomycin) (Pan Biotech), and 2 mm l-glutamine (Eurobio) at 37 °C in a humidified atmosphere of 5% CO2.
Human skin X-ALD fibroblasts (Coriell Institute GM17819) and human skin WT fibroblasts (Coriell Institute GM03348) were grown in DMEM supplemented with 10% FCS at 37 °C in a humidified atmosphere of 5% CO2 in the absence of antibiotics.
Plasmid Constructs
For X-ALD fibroblast transfection experiments, plasmids were obtained by cloning rat Abcd1 and/or Abcd2 cDNAs in pEGFP-N3 vector (Clontech). First, the cDNA were amplified by PCR using the following forward (F) and reverse (R) primers containing restriction sites at their 5′ end for further directional cloning: ABCD1-N, F (SalI) 5′-GTCGACATGCCGGTACTCTCCACT-3′ and R (NotI) 5′-GCGGCCGCAGTGGGGATGCCTGGGAC-3′; ABCD1-C, F (NotI) 5′-GCGGCCGCCATGCCGGTACTCTCCACTC-3′ and R (BamHI) 5′-GGATCCAGTGGGGATGCCTGGGACCAG-3′; ABCD2-N, F (SalI) 5′-GGGGTCGACTGGAAAAATGATACACATGCT-3′ and R (NotI) 5′-TGCGGCCGCGGATGTGTCCTCTGCAGTTT-3′; and ABCD2-C, F (NotI) 5′-TGCGGCCGCAATGATACACATGCTAAATG-3′ and R (BamHI) 5′-GGGGATCCGGATGTGTCCTCTGCAGTTT-3′. The primers were designed to generate cDNA without the Stop codon to permit fusion protein expression. The amplification products were cloned into pGEM-T Easy vector (Promega) and analyzed by bidirectional DNA sequencing to check for the absence of PCR-introduced errors.
Then the cDNA coding for the N- and C-terminal moieties were obtained after enzymatic cleavage of the pGEM-T Easy constructs with SalI/NotI and NotI/BamHI, respectively. After purification, the N- and C-terminal moieties were cloned into pEGFP-N3 between SalI and BamHI sites after a 3-partner ligation. All the junctions were checked by sequencing, and the plasmids were verified by restriction analysis and electrophoresis. The NotI junction between the N- and C-terminal moieties generates a hinge coding for three alanine residues.
In parallel, the plasmid encoding single ABCD1-EGFP was obtained after digestion of pABCD1-ABCD1-EGFP by BstXI, which cuts inside the ABCD1 sequence, removal of the BstXI fragment, and subsequent ligation. The plasmid encoding single ABCD2-EGFP was obtained by directional cloning of the SacII/BamHI fragment from the ABCD2-C-pGEM-T Easy plasmid into pEGFP-N3.
For yeast experiments, rat Abcd1 and/or rat Abcd2 cDNAs were subcloned into the pEL30 (Pca31) expression vector that contains an oleate-inducible catalase promoter (27). A step of codon optimization was performed for the N-terminal sequence of ABCD1 according to the preferred codon usage of Saccharomyces cerevisiae. The rat Abcd1 cDNA was amplified by PCR using the following primers: F (KpnI/SmaI) 5′-GGTACCCGGGATGCCAGTTTGTCTACTCCCTCCAGACCCTCGAGAGTGACCACGCTT-3′ and R (NotI) 5′-GCGGCCGCAGTGGGGATGCCTGGGAC-3′. The amplicon was cloned into pGEM-T Easy vector. Then, the N- and C-terminal moieties were obtained after enzymatic cleavage of the pGEM-T Easy constructs with KpnI/NotI (for optimized Abcd1-N) or SalI/NotI (for ABCD2-N) and NotI/BamHI (for ABCD1-C and ABCD2-C). After purification, the N- and C-terminal moieties were cloned into pEL30 between KpnI or SalI sites (in 5′) and BamHI sites (in 3′).
Transfection
X-ALD fibroblasts (500,000 cells) were transfected with the different ABCD-EGFP plasmids (2 μg) using the Amaxa electroporation kit “NHDF” according to the manufacturer's instructions to yield a maximum efficiency of transfection (estimated at 80–95% from fluorescence microscopic observations). After transfection, cells were cultivated at 30 or 37 °C in RPMI 1640 medium supplemented with 10% FCS and 1 mm sodium pyruvate for 24 h and then in DMEM supplemented with 10% FCS for additional 44 h. Ten transfections were performed for each plasmid construct to get sufficient material for further analysis. Nontransfected fibroblasts and WT fibroblasts were cultivated at 37 °C in DMEM supplemented with 10% FCS during the same time. To check transfection efficiency, transfected fibroblasts (50,000 cells) were seeded on glass slide, cultivated during 68 h, washed in PBS, fixed in 4% paraformaldehyde, pH 7.5, for 10 min at room temperature, and rinsed in PBS before mounting with FluorSaveTM mounting medium (Merck).
Immunofluorescence
Freshly transfected X-ALD fibroblasts (50,000 cells) were seeded on glass slide and cultivated during 68 h. Then they were washed in PBS, fixed in 4% paraformaldehyde, pH 7.5, for 10 min at room temperature, rinsed in PBS, incubated two times in PBS, 0.1 m glycine for 5 min, permeabilized with FACS permeabilizing solution (BD Biosciences) for 10 min at room temperature, and rinsed in PBS, 0.05% saponin. Immunostaining was performed by incubating cells in the presence of a primary antibody specific for catalase (Abcam, Ab16771 dilution, 1:100) in PBS, 0.05% saponin for 20 min. After washes in PBS, 0.05% saponin, cells were incubated with the 1:1,000 diluted Alexa594 goat anti-mouse secondary antibody (Invitrogen) for 20 min and finally washed in PBS before mounting with FluorSaveTM mounting medium (Merck).
In Situ PLA (Duolink)
H4IIEC3 cells stably expressing ABCD2-EGFP (clone 28.38) upon doxycycline treatment were seeded at 200,000 cells per well in 16-well Lab-Tek chambers slides (Nunc) pre-coated with 0.1 mg/ml poly-l-lysine. After an attachment period of 24 h, cells were treated or not with doxycycline (2 μg/ml) and incubated during 48 h at 37 °C. Fixation, permeabilization, PLA, and slide preparation were performed as described previously (10) using the Duolink kit (Olink Biosciences) following manufacturer's instructions. The following antibodies were used: 1:500 diluted mouse anti-GFP antibody (Roche Applied Science); 1:1,000 diluted rabbit polyclonal anti-ABCD1 antibody (28); 1:100 diluted rabbit polyclonal anti-PMP22 (10). Negative control experiments (with either one or both primary antibodies omitted) were performed in parallel and were checked for the absence of PLA signal. Finally, the samples were mounted with Duolink mounting medium.
Microscopy
Slides of transfected X-ALD fibroblasts were analyzed in fluorescence microscopy using Axio-scope A1 (Zeiss). Immunofluorescence and PLA slides were analyzed in confocal microscopy using TCS SP2 AOBS confocal laser microscope (Leica Microsystemes SA) and a 40× oil-immersion objective. Image processing and analysis were done using the Leica confocal software.
Co-immunoprecipitation Assays
H4IIEC3 cells stably expressing ABCD2-EGFP (clone 28.38) after overnight induction with 2 μg/ml doxycycline or not and BV-2 cells were homogenized in solubilization buffer (100 mm Tris-HCl, pH 8, 100 mm NaCl, 10 mm EDTA, 1% Triton X-100) containing 1% PMSF and protease inhibitor mixtures (Roche Applied Science). Nuclei were removed by centrifugation for 10 min at 4 °C and at 1,000 × g. The cell lysate was first incubated twice with agarose beads for 1 h at 4 °C with gentle rotation. After this pre-clearing step, cell lysate was incubated with rabbit polyclonal anti-ABCD1 (serum 029) or rabbit polyclonal anti-ABCD2 (generous gift from Prof. G. Graf, University of Kentucky (23)) antibodies cross-linked to agarose beads via dimethyl pimelimidate (protocol adapted from Bons et al. (29)) or with mouse monoclonal anti-GFP antibody covalently immobilized on agarose beads (MBL) for 1 h at 4 °C with gentle rotation. The anti-ABCD1 antibody (serum 029) produced in the laboratory with ThermoFisher facilities is directed against a synthetic peptide containing the C-terminal 37 amino acids of mouse ABCD1 (residues 700–736) synthesized with an added cysteine residue to the N terminus for conjugation to keyhole limpet hemocyanin using the coupling agent m-maleimidobenzoyl-N-hydroxysuccinimide ester (Covalab, Villeurbanne, France). The beads were washed three times with solubilization buffer (100 mm Tris-HCl, pH 8, 100 mm NaCl, 10 mm EDTA, and 1% Triton X-100), and bound proteins were released with Laemmli buffer.
Western Blotting
Immunoprecipitated proteins or proteins from total cell lysate (clone 28.38, nontransfected or transfected X-ALD fibroblasts, prepared in solubilization buffer as described above) were separated on a 7.5% SDS-PAGE and transferred onto a PVDF membrane. The membrane was first incubated in 5% skimmed milk dissolved in PBS, 0.1% Tween 20 (PBS/T) for 1 h at room temperature. The membrane was probed with 1:250 diluted mouse anti-GFP antibody (Roche Applied Science) or with rabbit polyclonal anti-ABCD1 (1:5,000) antibody (serum 029) or with rabbit monoclonal anti-tubulin (1:1,000 Cell Signaling) and then with the appropriate secondary antibody coupled to horseradish peroxidase (1:5,000 Santa Cruz Biotechnology) in PBS, 0.1% Tween 20, 1% skimmed milk, before being revealed by ECL (Santa Cruz Biotechnology). Images processing and analysis were done using Chemi Doc XRS + Imaging System (Bio-Rad) and the Image Lab software.
GC-MS Analysis
X-ALD fibroblasts transfected with ABCD-GFP plasmids, nontransfected X-ALD fibroblasts, and WT fibroblasts were collected by trypsinization 68 h after transfection. Fatty acid content were analyzed as pentafluorobenzyl esters by gas chromatography/mass spectrometry in negative chemical ionization mode as described previously (30).
Yeast Strains and Culture Conditions
The wild-type strain (WT) used was S. cerevisiae BJ1991 (Matα, leu2, trp1, ura3-251, prb1-1122, pep4-3, gal2). The deletion stains used were fox1Δ (or pox1Δ, peroxisomal acyl-CoA oxidase) and double mutant pxa1Δ and pxa2Δ (peroxisomal ABC transporters 1 and 2). The mutants were constructed from BJ1991 as described previously (7–9). Yeast transformants containing the expression plasmids with the different chimeric constructs of ABCD1 and ABCD2 were selected and grown in minimal medium containing 6.7 g/liter yeast nitrogen base without amino acids (YNB-WO), supplemented with 3 g/liter glucose and amino acids (20 mg/l) if required. For the induction of peroxisome proliferation, cells were shifted to YPO medium containing 5 g/liter potassium phosphate buffer, pH 6.0, 3 g/liter yeast extract, 5 g/liter peptone supplemented with 1.2 g/liter oleate, and 2 g/liter Tween 80. Prior to shifting to these media, the cells were grown in minimal 3 g/liter glucose medium for at least 24 h.
Yeast and Fatty Acid β-Oxidation Assays
β-Oxidation assays in intact yeast cells were performed as described previously by Van Roermund et al. (7–9). Cells were grown overnight in media containing oleate to induce fatty acid β-oxidation. The β-oxidation capacity was measured in 50 mm MES, pH 6.0, supplemented with 10 μm 1-14C-labeled fatty acids and is expressed as the sum of [14C]CO2 trapped in 2 m NaOH and water-soluble products. The 1-14C-labeled fatty acids were purchased from American Radiolabeled Chemicals.
RESULTS
ABCD1 Interacts with ABCD2
To obtain experimental evidence showing that ABCD1 interacts with ABCD2, we used two complementary approaches. First, we analyzed in situ the proximity of ABCD1 and ABCD2-EGFP by PLA (Duolink). The technique is based on the use of primary antibodies obtained in different species. Secondary antibodies targeting either rabbit or mouse antibodies, as in our case, are coupled to oligonucleotides, which serve as proximity probes. If the distance between the proteins is less than 40 nm, probes can hybridize with subsequently added connector oligonucleotides and guide the formation of a circular DNA template, which is amplified and detected by fluorescence hybridization. The PLA experiments resulted in a positive labeling only when expression of ABCD2-EGFP was induced and with the antibodies directed against ABCD1 and GFP (Fig. 1). This result demonstrates the proximity between ABCD1 and ABCD2-EGFP. It is unlikely that this labeling is due to the overabundance of proteins in a membrane context because no PLA labeling was obtained between ABCD2-EGFP and PMP22, with this protein being one of the most represented proteins of the peroxisomal membrane (31).
FIGURE 1.
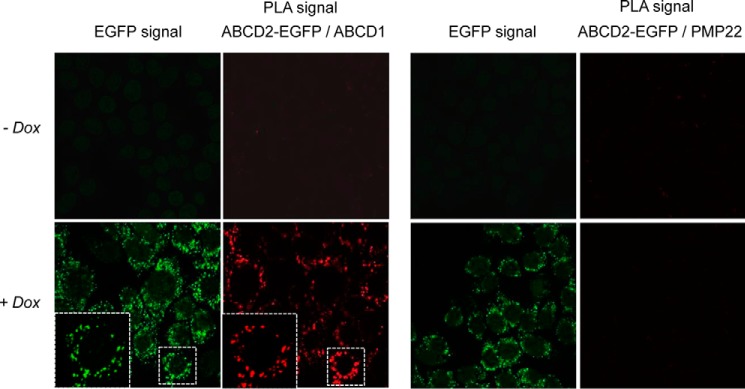
In situ analysis of the proximity of ABCD2-EGFP and ABCD1. Duolink proximity ligation assay between ABCD2-EGFP and ABCD1 or PMP22 in clone 28.38 (H4IIEC3 cells stably expressing ABCD2-EGFP upon doxycycline treatment) treated or not with doxycycline (Dox) (2 μg/ml) for 48 h was observed by confocal microscopy. The PLA signal demonstrates the proximity between ABCD2-EGFP and ABCD1. Negative control experiment shows the absence of PLA signal between ABCD2-EGFP and PMP22, an abundant peroxisomal membrane protein.
To confirm that proximity between ABCD1 and ABCD2-EGFP is due to a physical interaction, cross co-immunoprecipitation experiments were carried out from cell lysates obtained from the clone 28.38 cultivated in the presence of doxycycline. As shown in Fig. 2A, ABCD1 was co-immunoprecipitated with ABCD2-EGFP by anti-GFP antibody only in the doxycycline-treated cell clone 28.38. In the cross experiment, ABCD2-EGFP was co-immunoprecipitated with ABCD1 by anti-ABCD1 antibody only in the doxycycline-treated cell clone 28.38 demonstrating interaction. Although the anti-GFP (or anti-ABCD1) antibody immunoprecipitated the totality of ABCD2-EGFP (or ABCD1) proteins from the cell lysate, only a small fraction of ABCD1 (or ABCD2-EGFP) was co-immunoprecipitated. Hence, the interaction of ABCD1 and ABCD2-EGFP is a minority within the pool of ABCD1 and ABCD2-EGFP proteins in the cell, suggesting that, in addition to ABCD1/ABCD2-EGFP interaction, homo-interactions and/or hetero-interactions with ABCD3 can occur. Because one could argue that the 28.38 clone represents an artificial system and that the EGFP tag may play a role in this interaction, we explored the protein interactions of ABCD1 and ABCD2 in BV-2 mouse microglial cells, which express both ABCD1 and ABCD2 at a high level. As shown in Fig. 2B, cross co-immunoprecipitation of ABCD1 and ABCD2 was carried out and confirmed the interaction in native cells.
FIGURE 2.
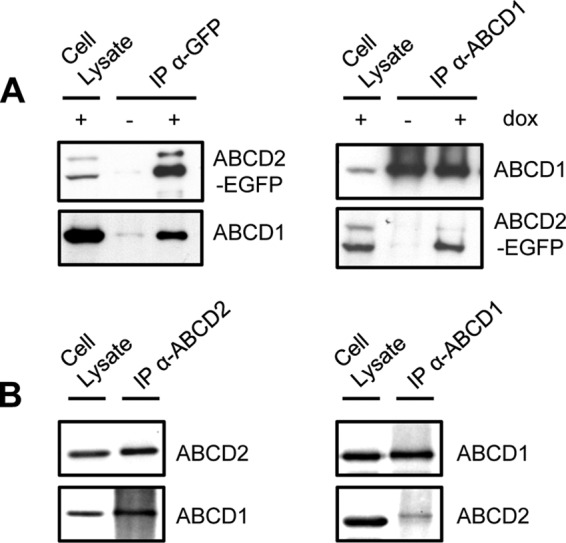
Co-immunoprecipitation of ABCD2 with ABCD1 demonstrating their interaction. Cross co-immunoprecipitation assays were performed in clone 28.38 (rat H4IIEC3 cells stably expressing ABCD2-EGFP upon doxycycline treatment) (A) or in mouse BV-2 cells (B). A, immunoprecipitation (IP) was performed using anti-GFP (left panel) or anti-ABCD1 (right panel) antibodies from cell lysates (800 and 1,600 μg, respectively) from clone 28.38 treated or not with doxycycline (dox) (2 μg/ml for 48 h). 100 μg of cell lysate (+dox) and eluted proteins were separated on 7.5% SDS-polyacrylamide gels and blotted, and PVDF membranes were probed with anti-GFP or anti-ABCD1 antibodies. In the doxycycline-treated cell clone 28.38, the anti-GFP antibody immunoprecipitated the totality of ABCD2-EGFP and co-immunoprecipitated a small fraction of ABCD1. The anti-ABCD1 antibody immunoprecipitated the totality of ABCD1 and co-immunoprecipitated a small fraction of ABCD2-EGFP. B, immunoprecipitation was performed using anti-ABCD2 (left panel) or anti-ABCD1 (right panel) antibodies from 200 μg of cell lysates from mouse BV-2 cells. 40 μg of cell lysate and eluted proteins were separated on 7.5% SDS-polyacrylamide gels and blotted, and PVDF membranes were probed with anti-ABCD2 or anti-ABCD1 antibodies. The anti-ABCD2 antibody immunoprecipitated ABCD2 and co-immunoprecipitated ABCD1. The anti-ABCD1 antibody immunoprecipitated ABCD1 and co-immunoprecipitated a small fraction of ABCD2.
Engineering of Plasmid Constructs to Express Chimeric Dimers
Alternative dimerization of ABC half-transporters has already been shown to be linked to modifications of the substrate specificity in the case of Drosophila proteins, White, Brown, and Scarlet (32). Similarly, alternative dimerization of ABCD proteins could be associated with a change in the lipid substrates that are translocated into the peroxisome. To explore this possibility, we generated chimeric constructs based on a strategy used to investigate the oligomeric state of ABCG2, another ABC half-transporter (33). The N- and C-terminal moieties of ABCD1 or ABCD2 were covalently linked using an Ala-Ala-Ala tripeptide linker to allow the expression of chimeric proteins mimicking homo- or heterodimers (ABCD1-ABCD1, ABCD2-ABCD2, ABCD1-ABCD2, and ABCD2-ABCD1). We generated plasmid constructs in the pEGFP vector (Clontech) leading to express chimeric dimers fused to EGFP in human cells (Fig. 3). Untagged constructs were also obtained in the pEL30 expression vector to allow expression of chimeric dimers in yeast.
FIGURE 3.
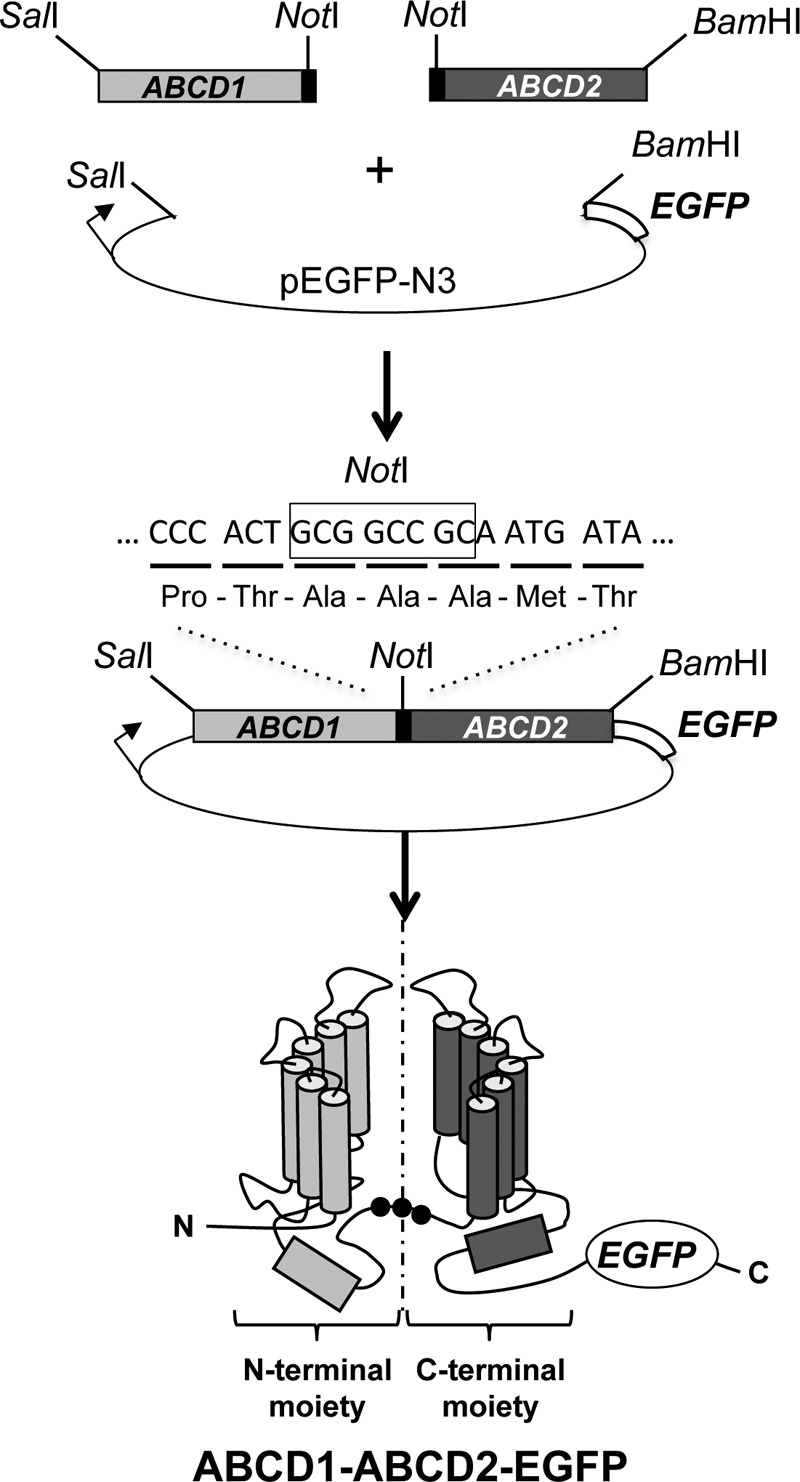
Cloning strategy used to generate the plasmid encoding for the ABCD1-ABCD2-EGFP chimeric fusion protein. The rat cDNAs (devoid of stop codon) of ABCD1 flanked by SalI and NotI and of ABCD2 flanked by NotI and BamHI were introduced after a 3-partner ligation into pEGFP-N3 (Clontech) at the SalI and BamHI restriction sites. The NotI linker results in the introduction of three Ala residues between the last amino acids of ABCD1 (Pro and Thr) and the first amino acids of ABCD2 (Met and Thr). A similar strategy was used to generate the other chimeric constructs (ABCD1-ABCD1, ABCD2-ABCD2, and ABCD2-ABCD1).
Chimeric Proteins Are Functional in Yeast pxa1/pxa2Δ Mutant
In plants, the full-length peroxisomal ABC transporter CTS can be considered to be a fused heterodimer consisting of two homologous but distinct halves (34). Heterologous expression of the full-length CTS protein was shown to complement the pxa1/pxa2Δ yeast mutant for β-oxidation of fatty acids (35). To prove the functionality of the rat full-length ABCD transporters, we expressed the chimeric dimers in pxa1/pxa2Δ yeast cells. All the chimeric constructs of ABCD1 and ABCD2 partially restored C22:0 β-oxidation in pxa1/pxa2Δ cells, which indicates that the full-length constructs are active and localized into the peroxisomal membrane (Fig. 4). Altogether, the chimeric constructs can be used as a valuable tool to study the substrate specificity of peroxisomal ABC transporters.
FIGURE 4.
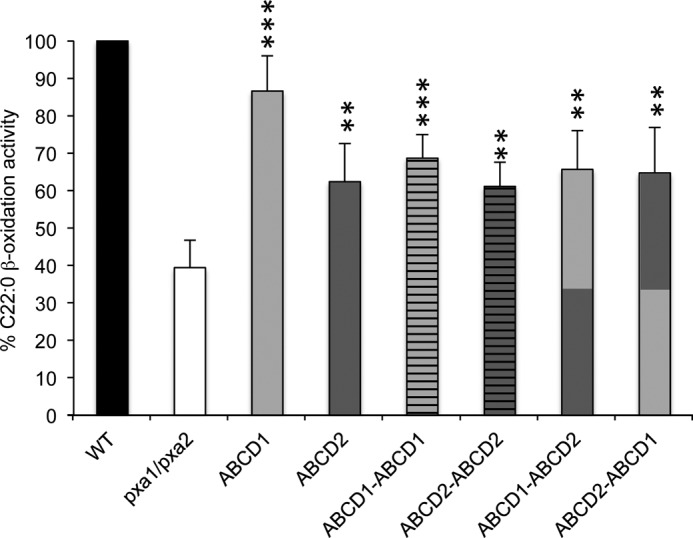
Rescue of the fatty acid oxidation in pxa1/pxa2Δ strain by ABCD1 and ABCD2 chimeric dimers. Cells grown on oleate medium were incubated with behenic acid (C22:0), and β-oxidation rates were measured. Strains shown are as follows: pxa1/pxa2Δ + empty vector, pxa1/pxa2Δ + ABCD1, pxa1/pxa2Δ + ABCD2, pxa1/pxa2Δ + ABCD1-ABCD1, pxa1/pxa2Δ + ABCD2-ABCD2, pxa1/pxa2Δ + ABCD1-ABCD2, and pxa1/pxa2Δ + ABCD2-ABCD1. The activity in wild-type cells (WT) was taken as reference (100%). Data are presented as mean ± S.D. (n = 5). Statistically significant variations (Student's t test) are indicated by asterisks (**, p < 0.01; ***, p < 0.001). See “Experimental Procedures” for strains used and experimental details.
Chimeric Proteins Are Expressed and Correctly Targeted to Peroxisomes in X-ALD Fibroblasts
Before testing the effect of the expression of chimeric constructs on the fatty acid levels in X-ALD fibroblasts, we first investigated whether the transient transfection of these plasmids in human cells permits the expression of chimeric proteins. Western blotting experiment revealed a very low level of expression of the chimeric proteins as compared with the expression level of monomeric proteins (Fig. 5A). Because low temperature was shown to rescue the expression of ABCD1 proteins with missense mutation in X-ALD fibroblasts, probably by correcting stability and folding difficulties (36), we repeated the expression analysis in fibroblasts cultivated at 30 °C. In these conditions, the expression level of chimeric proteins was considerably increased (Fig. 5B). However, expression was much less compared with the ABCD1 and ABCD2 monomers. We then examined the subcellular localization of the chimeric proteins by confocal microscopy. In agreement with the Western blotting observations, the GFP fluorescence signal was considerably higher in cells expressing monomeric proteins than in cells expressing chimeric dimers. Whatever the plasmid transfected, we observed a green punctate staining co-localized with the red signal of the catalase labeling indicating that all the chimeric proteins are correctly targeted to peroxisomes (Fig. 6).
FIGURE 5.
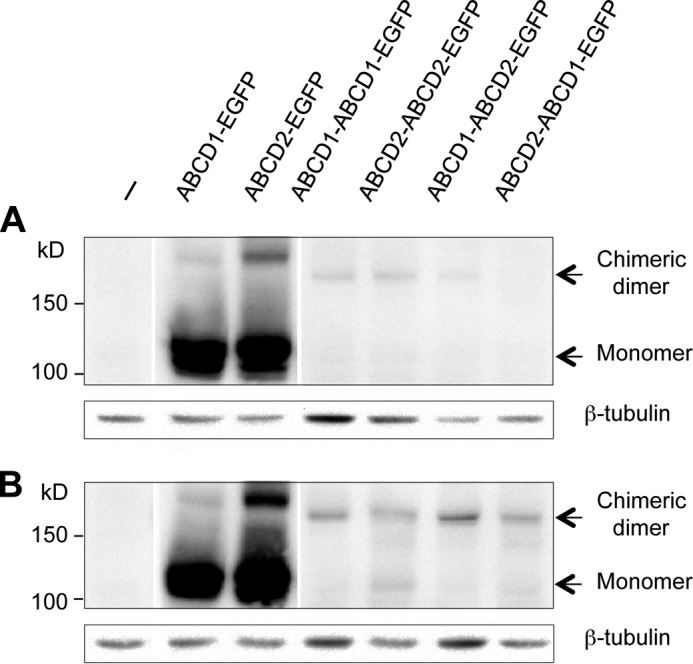
Western blotting analysis of the expression of chimeric proteins in X-ALD fibroblasts cultivated at 37 or 30 °C. Nontransfected (−) or transfected X-ALD fibroblasts with pEGFP plasmids containing single or double cDNA sequences of ABCD1 and/or ABCD2 were cultivated at 37 °C (A) or 30 °C (B) during 68 h. Total cell proteins were solubilized and separated by SDS-PAGE followed by Western blot analysis with anti-GFP antibody. β-Tubulin was used as a loading control.
FIGURE 6.
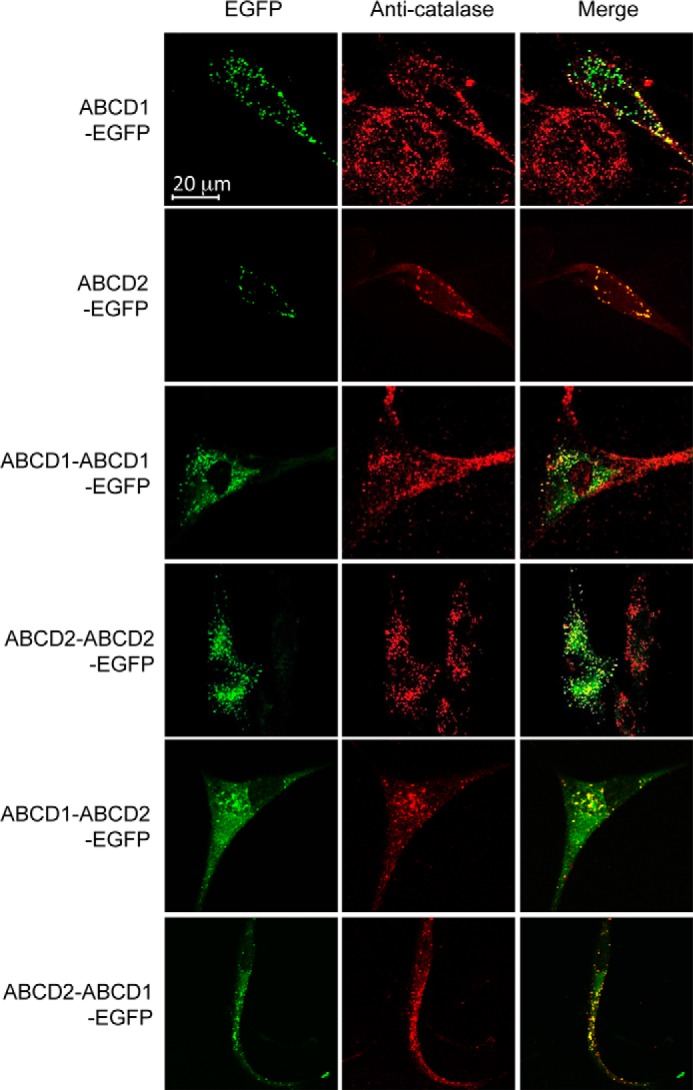
Peroxisomal localization of ABCD-EGFP fusion proteins. X-ALD fibroblasts transfected with pEGFP plasmids containing single or double cDNA sequences of ABCD1 and/or ABCD2 were cultivated at 30 °C during 68 h. Cells were fixed, permeabilized, and subjected to immunostaining with anti-catalase antibody. EGFP fluorescence signal from fusion proteins and catalase signal were visualized by confocal microscopy. Yellow spots in the merge image show co-localization of EGFP-fusion proteins and catalase.
Chimeric ABCD Dimers Are Functional as Shown by Correction of the VLCFA Levels in Transfected X-ALD Fibroblasts
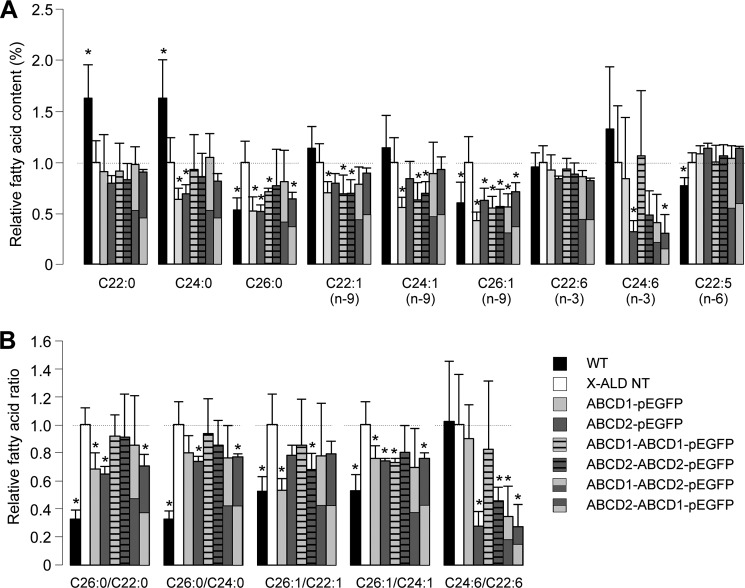
GC-MS analysis of the fatty acid content was performed in X-ALD fibroblasts transfected or not with the different plasmid constructs and cultivated at 30 °C. The analysis was performed in parallel in WT fibroblasts. For each fatty acid analyzed (saturated fatty acids: C20:0, C22:0, C24:0, and C26:0; n-9 monounsaturated fatty acids: C20:1, C22:1, C24:1, and C26:1; n-3 and n-6 PUFA: C18:3 n-3, C20:5 n-3, C22:6 n-3, C24:6 n-3, C18:3 n-6, C20:4 n-6, and C22:5 n-6), the mass was calculated and expressed as a percentage of the total fatty acid content. The effect of the expression of monomeric and chimeric peroxisomal ABC transporters on the VLCFA content is presented in Fig. 7A. Fatty acid ratios (C26:0/C22:0, C26:0/C24:0, C26:1/C22:1, C26:1/C24:1,and C24:6/C22:6 n-3) were calculated and are presented in Fig. 7B. As expected, C26:0 and C26:1 levels were significantly higher in X-ALD fibroblasts than in WT fibroblasts, and this difference was also observed for the ratios. Surprisingly, C22:0 and C24:0 levels were higher in control WT cells, but the other fatty acids were not significantly different between the two genotypes. Expression of either ABCD1-EGFP or ABCD2-EGFP significantly corrected the C26:0 and C26:1 levels in X-ALD fibroblasts. Although there was no effect on the C22:0 level, we observed a decreased percentage of C24:0 and C22:1 in cells expressing ABCD1 or ABCD2-EGFP. A decrease of C24:1 was also observed, but it was only significant in cells expressing ABCD1-EGFP. Concerning ω3 PUFA, we did not observe any significant changes in the content of C22:6 n-3 but found a considerable reduction of the content in C24:6 n-3 in cells expressing ABCD2-EGFP. The results of ratios (Fig. 7B) were coherent with results expressed in percentage of fatty acids (Fig. 7A). Expression of chimeric dimers led to a decreased level of C26:0 and C26:1 even if it was less pronounced than that observed with monomers. Statistical differences were observed in all the conditions except for a few points, and they probably reflect the fact that the expression level of chimeric proteins is much smaller than that of monomers. The results prove the functionality of the chimeric proteins and their ability to complement ABCD1 deficiency. If we look at the results in detail, there was no significant change in the content in C22:0, C24:0, and C22:6 n-3. The levels of C22:1 and C24:1 were significantly decreased upon expression of ABCD1-ABCD1-EGFP and ABCD2-ABCD2-EGFP, whereas the decrease was not significant with the chimeric proteins mimicking heterodimers. Interestingly, as seen in cells expressing ABCD2-EGFP, the level of C24:6 n-3 was significantly decreased by expression of chimeric proteins containing at least one ABCD2 moiety (Fig. 7A). The results were in agreement with the observed changes of the C24:6/C22:6 n-3 ratio (Fig. 7B).
FIGURE 7.
Very long-chain fatty acid content (A) and ratios (B) in ABCD-EGFP-transfected X-ALD fibroblasts. WT, nontransfected (NT) X-ALD fibroblasts, and X-ALD fibroblasts transfected with pEGFP plasmids containing single or double cDNA sequences of ABCD1 and/or ABCD2 were cultivated at 30 °C during 68 h. Cells were collected, and fatty acid content was analyzed by GC-MS. The fatty acid content (A) was calculated as a percentage of each fatty acid/total fatty acid. Ratios (B) were calculated from the % of fatty acids. The mean results (± S.D.) corresponding to 5–8 independent transfections were expressed relatively to the result obtained in the nontransfected X-ALD fibroblasts arbitrarily taken equal to 1. Statistically significant variations (Student's t test) are indicated by asterisks (*, p < 0.05).
DISCUSSION
Using a cell model allowing the expression of ABCD2 fused to EGFP, PLA experiments have now permitted the in situ detection of the proximity between the ABCD1 and ABCD2-EGFP proteins. Co-immunoprecipitation assays confirmed the physical interaction between ABCD1 and ABCD2 both in the doxycycline-treated H4IIEC3-modified cells (clone 28.38) and in native cells (BV-2 microglial cells). These results are in line with previous results suggesting the existence of heterodimers (12, 14). However, they do not formally prove that the detected interactions result from heterodimerization. For instance, proximity and physical interactions could be detected in high molecular weight complexes, possibly tetrameric complexes composed of the association of two different homodimers, ABCD1/ABCD1 and ABCD2/ABCD2. In line with this possibility, the full-length CTS ortholog in plants was found in high molecular weight complexes (37), and other ABC half-transporters such as ABCG2 are thought to belong to tetrameric complexes (38). If tetramers of ABCD1 and ABCD2 exist, we have to remind ourselves that the functionality and the stability of ABC transporters strictly depend on the formation of dimers. MRP1, a full-length ABC transporter, was found to exist in the plasma membrane as a dimer (39). Interestingly, the structure analysis of this dimer revealed a single “side-by-side” organization of the two monomers. If we can speculate on the possibility that different homodimers or heterodimers of ABCD transporters yield heterotetrameric structures, we have to keep in mind that such structures would conserve intact the original dimeric functional units.
If heterodimers are effectively formed, the functionality of such complexes remained to be studied. The generated chimeric constructs mimicking homo- or heterodimers, whatever their arrangement (ABCD1-ABCD1, ABCD1-ABCD2, ABCD2-ABCD1, or ABCD2-ABCD2), were correctly targeted to the peroxisome in yeast and in human skin fibroblasts. Peroxisomal ABC transporters are inserted into the peroxisomal membrane thanks to the membrane peroxisomal targeting signal, located in the cytosol before the first transmembrane segment (40–42). Thanks to this motif, peroxisomal ABC transporters interact with PEX19, which plays a role as chaperone and permit their integration into the peroxisomal membrane (43, 44). Our results showed that artificially forced dimerization does not interfere with the peroxisomal targeting, and therefore, interaction with PEX19 is not compromised.
Despite this low abundance in transfected cells, expression of chimeric dimers resulted in a partial restoration of VLCFA levels (C26:0 and C26:1). The functionality of chimeric dimers was also validated in yeast pxa1/pxa2Δ mutants with the ability to restore growth on oleate and β-oxidation of C22:0. Besides the fact that the β-oxidation process is only peroxisomal in yeast, the yeast pxa1/pxa2Δ model presents the advantage to be completely devoid of peroxisomal ABC transporters. It is not the case in transfected X-ALD fibroblasts that express ABCD3. This could be one reason explaining why we did not observe significant changes of C22:0 level in transfected X-ALD fibroblasts. Concerning the C26:0 and C26:1 levels in transfected X-ALD fibroblasts, we did not notice major differences between the different chimeric dimers. Expression of the forced homo- or heterodimers led to the decrease of C26:0 and C26:1 levels suggesting that chimeric full transporters permit the entry of the corresponding acyl-CoA for further β-oxidation.
This is in agreement with the functional redundancy existing between ABCD1 and ABCD2, but this also suggests functional interchangeability of ABCD1 and ABCD2 proteins within a full transporter. It is well known that two nucleotide-binding domains must bind together to allow ATP binding and to constitute a functional energizer domain (45). Our data showed that both the forced homo- and heterodimeric interactions of the rat proteins were able to energize transport of acyl-CoA. Of note, although the co-expressed N- and C-terminal pseudo-halves of CTS were fully functional in yeast mutant, their expression as separate polypeptides demonstrated stability and peroxisomal targeting but failed to complement mutant (46). Concerning the substrate-binding site that is supposed to belong to the TMDs (47), the results suggest that C26:0-CoA and C26:1-CoA can bind whatever the arrangement. Structural studies in other ABC transporters such as P-glycoprotein have shown that TMDs are tightly associated to constitute the membrane-anchoring domain as well as the substrate-binding sites and the translocation pathway (48). It has been shown that α-helices of one TMD are in close proximity with α-helices of the other. Further experiments are obviously needed to elucidate the structure of peroxisomal ABC transporters and to clarify where and how acyl-CoAs bind to the transporters. However, our results suggest that interactions between TMDs of ABCD1 and ABCD2 yield a functional binding site for acyl-CoA as in the case of homodimerization.
Interestingly, if there was no difference for C26:0 and C26:1 (rather equivalent levels of complementation are obtained with the different chimeric dimers), we observed different C24:6 levels depending on the transfected constructs. The level of C24:6 was decreased only in the presence of ABCD2 and ABCD2-ABCD2, ABCD1-ABCD2, or ABCD2-ABCD1 chimeric proteins but not in the presence of ABCD1 or ABCD1-ABCD1 chimeric dimers. This result reinforces the supposed important role of ABCD2 in PUFA metabolism (8, 10, 49) and is in agreement with the fact that ABCD2 expression is regulated by PUFA (50). It also surprisingly shows that one TMD from ABCD2 is sufficient to ensure C24:6-CoA transport. Noteworthy, the content of DHA, which is produced after one turn of peroxisomal β-oxidation of C24:6 (51), was not modified. From the increased β-oxidation of C24:6, we could have expected an increase of DHA. However, we previously demonstrated that ABCD2 expression increased β-oxidation of DHA (10). It is therefore consistent to find that expression of ABCD2 or of chimeric proteins containing at least one ABCD2 moiety result in equilibrium. As said previously, in normal conditions, heterodimerization of ABCD1 and ABCD2 may occur in a few cell types due to mirror expression of both proteins (16). However, upon hormonal, nutritional, or pharmacological stimulation, the induction of ABCD2 expression in tissues and cells containing ABCD1 may allow the formation of functional heterodimers that could contribute to favor a shift of peroxisomal functions toward PUFA metabolism. Further studies are needed to explore these aspects in details.
In conclusion, we have provided clear evidence in favor of the physical and functional interactions between ABCD1 and ABCD2. We generated functional chimeric constructs that constitute a valuable tool to study the substrate specificity of these transporters both in human and yeast cells. Accurate determination of the substrate specificity of peroxisomal ABC transporters would be facilitated in the yeast model with β-oxidation assays. The complementation experiments with these chimeric proteins demonstrated for the first time that both forced homo- and heterodimers of peroxisomal ABC transporters are functional. Besides a new proof of functional redundancy toward VLCFA and interchangeability between ABCD1 and ABCD2, we conclude that ABCD2 plays a major role in PUFA metabolism.
Acknowledgments
We thank G. Emgoue Emani for technical assistance. We are grateful to D. Methy-Gonnod who participated in the initial steps of the project and to G. Bessede (Lipidomic Analytical Platform, CHU, 21079 Dijon) for technical assistance in lipid analysis.
This work was supported by grants from the regional council of Burgundy.
- X-ALD
- X-linked adrenoleukodystrophy
- ABC
- ATP-binding cassette
- DHA
- docosahexaenoic acid
- EGFP
- enhanced green fluorescent protein
- PLA
- proximity ligation assay
- PUFA
- polyunsaturated fatty acids
- TMD
- transmembrane domain
- VLCFA
- very long-chain fatty acid
- F
- forward
- R
- reverse.
REFERENCES
- 1. Trompier D., Savary S. (2013) X-linked Adrenoleukodystrophy, Vol. 2, pp. 1–134, Morgan & Claypool Life Sciences, Princeton, NJ [Google Scholar]
- 2. Mosser J., Douar A. M., Sarde C. O., Kioschis P., Feil R., Moser H., Poustka A. M., Mandel J. L., Aubourg P. (1993) Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361, 726–730 [DOI] [PubMed] [Google Scholar]
- 3. Lombard-Platet G., Savary S., Sarde C. O., Mandel J. L., Chimini G. (1996) A close relative of the adrenoleukodystrophy (ALD) gene codes for a peroxisomal protein with a specific expression pattern. Proc. Natl. Acad. Sci. U.S.A. 93, 1265–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamijo K., Taketani S., Yokota S., Osumi T., Hashimoto T. (1990) The 70-kDa peroxisomal membrane protein is a member of the Mdr (P-glycoprotein)-related ATP-binding protein superfamily. J. Biol. Chem. 265, 4534–4540 [PubMed] [Google Scholar]
- 5. Dawson R. J., Locher K. P. (2006) Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 [DOI] [PubMed] [Google Scholar]
- 6. Guimarães C. P., Domingues P., Aubourg P., Fouquet F., Pujol A., Jimenez-Sanchez G., Sá-Miranda C., Azevedo J. E. (2004) Mouse liver PMP70 and ALDP: homomeric interactions prevail in vivo. Biochim. Biophys. Acta 1689, 235–243 [DOI] [PubMed] [Google Scholar]
- 7. van Roermund C. W., Visser W. F., Ijlst L., van Cruchten A., Boek M., Kulik W., Waterham H. R., Wanders R. J. (2008) The human peroxisomal ABC half-transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 22, 4201–4208 [DOI] [PubMed] [Google Scholar]
- 8. van Roermund C. W., Visser W. F., Ijlst L., Waterham H. R., Wanders R. J. (2011) Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochim. Biophys. Acta 1811, 148–152 [DOI] [PubMed] [Google Scholar]
- 9. van Roermund C. W., Ijlst L., Wagemans T., Wanders R. J., Waterham H. R. (2014) A role for the human peroxisomal half-transporter ABCD3 in the oxidation of dicarboxylic acids. Biochim. Biophys. Acta 1841, 563–568 [DOI] [PubMed] [Google Scholar]
- 10. Genin E. C., Geillon F., Gondcaille C., Athias A., Gambert P., Trompier D., Savary S. (2011) Substrate specificity overlap and interaction between adrenoleukodystrophy protein (ALDP/ABCD1) and adrenoleukodystrophy-related protein (ALDRP/ABCD2). J. Biol. Chem. 286, 8075–8084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith K. D., Kemp S., Braiterman L. T., Lu J. F., Wei H. M., Geraghty M., Stetten G., Bergin J. S., Pevsner J., Watkins P. A. (1999) X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem. Res. 24, 521–535 [DOI] [PubMed] [Google Scholar]
- 12. Liu L. X., Janvier K., Berteaux-Lecellier V., Cartier N., Benarous R., Aubourg P. (1999) Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J. Biol. Chem. 274, 32738–32743 [DOI] [PubMed] [Google Scholar]
- 13. Tanaka A. R., Tanabe K., Morita M., Kurisu M., Kasiwayama Y., Matsuo M., Kioka N., Amachi T., Imanaka T., Ueda K. (2002) ATP binding/hydrolysis by and phosphorylation of peroxisomal ATP-binding cassette proteins PMP70 (ABCD3) and adrenoleukodystrophy protein (ABCD1). J. Biol. Chem. 277, 40142–40147 [DOI] [PubMed] [Google Scholar]
- 14. Hillebrand M., Verrier S. E., Ohlenbusch A., Schäfer A., Söling H. D., Wouters F. S., Gärtner J. (2007) Live cell FRET microscopy: homo- and heterodimerization of two human peroxisomal ABC transporters, the adrenoleukodystrophy protein (ALDP, ABCD1) and PMP70 (ABCD3). J. Biol. Chem. 282, 26997–27005 [DOI] [PubMed] [Google Scholar]
- 15. Berger J., Albet S., Bentejac M., Netik A., Holzinger A., Roscher A. A., Bugaut M., Forss-Petter S. (1999) The four murine peroxisomal ABC-transporter genes differ in constitutive, inducible and developmental expression. Eur. J. Biochem. 265, 719–727 [DOI] [PubMed] [Google Scholar]
- 16. Troffer-Charlier N., Doerflinger N., Metzger E., Fouquet F., Mandel J. L., Aubourg P. (1998) Mirror expression of adrenoleukodystrophy and adrenoleukodystrophy related genes in mouse tissues and human cell lines. Eur. J. Cell Biol. 75, 254–264 [DOI] [PubMed] [Google Scholar]
- 17. Trompier D., Gondcaille C., Lizard G., Savary S. (2014) Regulation of the adrenoleukodystrophy-related gene (ABCD2): Focus on oxysterols and LXR antagonists. Biochem. Biophys. Res. Commun. 446, 651–655 [DOI] [PubMed] [Google Scholar]
- 18. Netik A., Forss-Petter S., Holzinger A., Molzer B., Unterrainer G., Berger J. (1999) Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum. Mol. Genet. 8, 907–913 [DOI] [PubMed] [Google Scholar]
- 19. Flavigny E., Sanhaj A., Aubourg P., Cartier N. (1999) Retroviral-mediated adrenoleukodystrophy-related gene transfer corrects very long chain fatty acid metabolism in adrenoleukodystrophy fibroblasts: implications for therapy. FEBS Lett. 448, 261–264 [DOI] [PubMed] [Google Scholar]
- 20. Pujol A., Ferrer I., Camps C., Metzger E., Hindelang C., Callizot N., Ruiz M., Pàmpols T., Giròs M., Mandel J. L. (2004) Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum. Mol. Genet. 13, 2997–3006 [DOI] [PubMed] [Google Scholar]
- 21. Weber F. D., Wiesinger C., Forss-Petter S., Regelsberger G., Einwich A., Weber W. H., Köhler W., Stockinger H., Berger J. (2014) X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism is severely impaired in monocytes but not in lymphocytes. Hum. Mol. Genet. 23, 2542–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J., Liang S., Liu X., Brown J. A., Newman K. E., Sunkara M., Morris A. J., Bhatnagar S., Li X., Pujol A., Graf G. A. (2012) The absence of ABCD2 sensitizes mice to disruptions in lipid metabolism by dietary erucic acid. J. Lipid Res. 53, 1071–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J., Sabeva N. S., Bhatnagar S., Li X. A., Pujol A., Graf G. A. (2010) ABCD2 is abundant in adipose tissue and opposes the accumulation of dietary erucic acid (C22:1) in fat. J. Lipid Res. 51, 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jimenez-Sanchez G., Hebron K. J., Thomas G., Valle D. (1999) Targeted disruption of the 70kDa peroxisomal membrane protein (PMP70) in mouse is associated with an increase in the related P70R protein, deficiency of hepatic glycogen and a dicarboxylic aciduria. Pediatr. Res. 45, 139A [Google Scholar]
- 25. Jimenez-Sanchez G., Silva-Zolezzi I., Hebron K. J., Mihalik S., Watkins P., Moser A., Thomas G., Wood P. A., Valle D. (2000) Defective phytanic and pristanic acids metabolism in PMP70 deficient mice results in defective nonshivering thermogenesis and dicarboxylic aciduria. J. Inherited Metab. Dis. 23, 256 [Google Scholar]
- 26. Gueugnon F., Volodina N., Taouil J. E., Lopez T. E., Gondcaille C., Grand A. S., Mooijer P. A., Kemp S., Wanders R. J., Savary S. (2006) A novel cell model to study the function of the adrenoleukodystrophy-related protein. Biochem. Biophys. Res. Commun. 341, 150–157 [DOI] [PubMed] [Google Scholar]
- 27. van Roermund C. W., Hettema E. H., van den Berg M., Tabak H. F., Wanders R. J. (1999) Molecular characterization of carnitine-dependent transport of acetyl-CoA from peroxisomes to mitochondria in Saccharomyces cerevisiae and identification of a plasma membrane carnitine transporter, Agp2p. EMBO J. 18, 5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fouquet F., Zhou J. M., Ralston E., Murray K., Troalen F., Magal E., Robain O., Dubois-Dalcq M., Aubourg P. (1997) Expression of the adrenoleukodystrophy protein in the human and mouse central nervous system. Neurobiol. Dis. 3, 271–285 [DOI] [PubMed] [Google Scholar]
- 29. Bons J. A., Michielsen E. C., de Boer D., Bouwman F. G., Jaeken J., van Dieijen-Visser M. P., Rubio-Gozalbo M. E., Wodzig W. K. (2008) A specific immunoprecipitation method for isolating isoforms of insulin-like growth factor binding protein-3 from serum. Clin. Chim. Acta 387, 59–65 [DOI] [PubMed] [Google Scholar]
- 30. Baarine M., Andréoletti P., Athias A., Nury T., Zarrouk A., Ragot K., Vejux A., Riedinger J. M., Kattan Z., Bessede G., Trompier D., Savary S., Cherkaoui-Malki M., Lizard G. (2012) Evidence of oxidative stress in very long chain fatty acid–treated oligodendrocytes and potentialization of ROS production using RNA interference-directed knockdown of ABCD1 and ACOX1 peroxisomal proteins. Neuroscience 213, 1–18 [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto T., Kuwabara T., Usuda N., Nagata T. (1986) Purification of membrane polypeptides of rat liver peroxisomes. J. Biochem. 100, 301–310 [DOI] [PubMed] [Google Scholar]
- 32. Ewart G. D., Cannell D., Cox G. B., Howells A. J. (1994) Mutational analysis of the traffic ATPase (ABC) transporters involved in uptake of eye pigment precursors in Drosophila melanogaster. Implications for structure-function relationships. J. Biol. Chem. 269, 10370–10377 [PubMed] [Google Scholar]
- 33. Bhatia A., Schäfer H. J., Hrycyna C. A. (2005) Oligomerization of the human ABC transporter ABCG2: evaluation of the native protein and chimeric dimers. Biochemistry 44, 10893–10904 [DOI] [PubMed] [Google Scholar]
- 34. Footitt S., Slocombe S. P., Larner V., Kurup S., Wu Y., Larson T., Graham I., Baker A., Holdsworth M. (2002) Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 21, 2912–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nyathi Y., De Marcos Lousa C., van Roermund C. W., Wanders R. J., Johnson B., Baldwin S. A., Theodoulou F. L., Baker A. (2010) The Arabidopsis peroxisomal ABC transporter, comatose, complements the Saccharomyces cerevisiae pxa1 pxa2Delta mutant for metabolism of long-chain fatty acids and exhibits fatty acyl-CoA-stimulated ATPase activity. J. Biol. Chem. 285, 29892–29902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang X., De Marcos Lousa C., Schutte-Lensink N., Ofman R., Wanders R. J., Baldwin S. A., Baker A., Kemp S., Theodoulou F. L. (2011) Conservation of targeting but divergence in function and quality control of peroxisomal ABC transporters: an analysis using cross-kingdom expression. Biochem. J. 436, 547–557 [DOI] [PubMed] [Google Scholar]
- 37. De Marcos Lousa C., van Roermund C. W., Postis V. L., Dietrich D., Kerr I. D., Wanders R. J., Baldwin S. A., Baker A., Theodoulou F. L. (2013) Intrinsic acyl-CoA thioesterase activity of a peroxisomal ATP binding cassette transporter is required for transport and metabolism of fatty acids. Proc. Natl. Acad. Sci. U.S.A. 110, 1279–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu J., Liu Y., Yang Y., Bates S., Zhang J. T. (2004) Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J. Biol. Chem. 279, 19781–19789 [DOI] [PubMed] [Google Scholar]
- 39. Rosenberg M. F., Mao Q., Holzenburg A., Ford R. C., Deeley R. G., Cole S. P. (2001) The structure of the multidrug resistance protein 1 (MRP1/ABCC1). crystallization and single-particle analysis. J. Biol. Chem. 276, 16076–16082 [DOI] [PubMed] [Google Scholar]
- 40. Landgraf P., Mayerhofer P. U., Polanetz R., Roscher A. A., Holzinger A. (2003) Targeting of the human adrenoleukodystrophy protein to the peroxisomal membrane by an internal region containing a highly conserved motif. Eur. J. Cell Biol. 82, 401–410 [DOI] [PubMed] [Google Scholar]
- 41. Rottensteiner H., Kramer A., Lorenzen S., Stein K., Landgraf C., Volkmer-Engert R., Erdmann R. (2004) Peroxisomal membrane proteins contain common Pex19p-binding sites that are an integral part of their targeting signals. Mol. Biol. Cell 15, 3406–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kashiwayama Y., Asahina K., Shibata H., Morita M., Muntau A. C., Roscher A. A., Wanders R. J., Shimozawa N., Sakaguchi M., Kato H., Imanaka T. (2005) Role of Pex19p in the targeting of PMP70 to peroxisome. Biochim. Biophys. Acta 1746, 116–128 [DOI] [PubMed] [Google Scholar]
- 43. Pinto M. P., Grou C. P., Alencastre I. S., Oliveira M. E., Sá-Miranda C., Fransen M., Azevedo J. E. (2006) The import competence of a peroxisomal membrane protein is determined by Pex19p before the docking step. J. Biol. Chem. 281, 34492–34502 [DOI] [PubMed] [Google Scholar]
- 44. Shibata H., Kashiwayama Y., Imanaka T., Kato H. (2004) Domain architecture and activity of human Pex19p, a chaperone-like protein for intracellular trafficking of peroxisomal membrane proteins. J. Biol. Chem. 279, 38486–38494 [DOI] [PubMed] [Google Scholar]
- 45. Zoghbi M. E., Altenberg G. A. (2014) ATP binding to two sites is necessary for dimerization of nucleotide-binding domains of ABC proteins. Biochem. Biophys. Res. Commun. 443, 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nyathi Y., Zhang X., Baldwin J. M., Bernhardt K., Johnson B., Baldwin S. A., Theodoulou F. L., Baker A. (2012) Pseudo half-molecules of the ABC transporter, COMATOSE, bind Pex19 and target to peroxisomes independently but are both required for activity. FEBS Lett. 586, 2280–2286 [DOI] [PubMed] [Google Scholar]
- 47. Guimarães C. P., Sá-Miranda C., Azevedo J. E. (2005) Probing substrate-induced conformational alterations in adrenoleukodystrophy protein by proteolysis. J. Hum. Genet. 50, 99–105 [DOI] [PubMed] [Google Scholar]
- 48. Ward A. B., Szewczyk P., Grimard V., Lee C. W., Martinez L., Doshi R., Caya A., Villaluz M., Pardon E., Cregger C., Swartz D. J., Falson P. G., Urbatsch I. L., Govaerts C., Steyaert J., Chang G. (2013) Structures of P-glycoprotein reveal its conformational flexibility and an epitope on the nucleotide-binding domain. Proc. Natl. Acad. Sci. U.S.A. 110, 13386–13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fourcade S., Ruiz M., Camps C., Schlüter A., Houten S. M., Mooyer P. A., Pàmpols T., Dacremont G., Wanders R. J., Giròs M., Pujol A. (2009) A key role for the peroxisomal ABCD2 transporter in fatty acid homeostasis. Am. J. Physiol. Endocrinol. Metab. 296, E211–E221 [DOI] [PubMed] [Google Scholar]
- 50. Leclercq S., Skrzypski J., Courvoisier A., Gondcaille C., Bonnetain F., André A., Chardigny J. M., Bellenger S., Bellenger J., Narce M., Savary S. (2008) Effect of dietary polyunsaturated fatty acids on the expression of peroxisomal ABC transporters. Biochimie 90, 1602–1607 [DOI] [PubMed] [Google Scholar]
- 51. Ferdinandusse S., Denis S., Mooijer P. A., Zhang Z., Reddy J. K., Spector A. A., Wanders R. J. (2001) Identification of the peroxisomal beta-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J. Lipid Res. 42, 1987–1995 [PubMed] [Google Scholar]