Abstract
Purpose
Anti-angiogenic agents combined with histone deacetylase inhibitors act synergistically in vitro and in vivo. We conducted a phase I study of the combination of the anti-VEGF monoclonal antibody bevacizumab and histone deacetylase inhibitor valproic acid in patients with advanced cancers.
Methods
Bevacizumab was administered at escalating doses of 2.5–11 mg/kg on days 1 and 15, and oral valproic acid at doses of 5.3–10 mg/kg on days 1–28 every 28 days to determine the maximum tolerated dose (MTD). Pharmacodynamic (PD) parameters were assessed in peripheral blood mononuclear cells (histone H3 acetylation) and serum (valproic acid levels).
Results
Fifty-seven patients were enrolled. Dose-limiting toxicities were grade 3 altered mental status (n=2), related to valproic acid. Bevacizumab 11 mg/kg given on days 1 and 15 and valproic acid 5.3 mg/kg daily was the recommended phase II dose. Stable disease (SD) ≥ 6 months was reported in 4/57 (7%) of patients, including 2 patients with colorectal cancer who had progressed previously on bevacizumab. Of the 39 patients evaluated for histone acetylation, 2 of 3 (67%) patients with SD ≥ 6 months showed histone acetylation, while 8 of 36 (22%) without SD ≥ 6 months demonstrated histone acetylation (p=0.16). Patients with any grade of hypertension, compared to others, had a prolonged median survival (11.1 months versus 5.8 months; p=0.012).
Conclusions
The combination of bevacizumab 11 mg/kg and valproic acid 5.3 mg/kg is safe in patients with advanced malignancies, with activity in colorectal, gastroesophageal junction and prostate cancer. Patients with hypertension had improved overall survival.
Keywords: bevacizumab, histone deacetylase, hypertension, VEGF, valproic acid
Introduction
Angiogenesis is a fundamental process in the growth and metastatic progression of solid tumors [1]. The vascular endothelial growth factor (VEGF) is a heparin-binding peptide that acts as a potent angiogenic factor, with specific mitogenic activity in epithelial cells [2]. Bevacizumab, a humanized monoclonal antibody against VEGF, has demonstrated activity in breast [3], colorectal [4], lung [5], brain [6], and kidney [7] cancers.
Histone deacetylases are enzymes that regulate chromatin structure and function by catalyzing removal of the acetyl modification of lysine residues in histones [8]. Inhibiting histone deacetylase activity results in histone acetylation, which is associated with up-regulated gene expression [9]. Valproic acid, a short-chain fatty acid commonly used to treat epilepsy and other neurologic disorders, is also a histone deacetylase inhibitor. Valproic acid induces differentiation, growth inhibition, and apoptosis by promoting gene transcription in different cellular systems [10]. Histone deacetylase inhibitors have also been shown to down-regulate angiogenesis-related gene expression in endothelial and tumor cells [11,12]. Preclinical in vitro and in vivo studies demonstrate synergistic antiangiogenic activity with a combination of a histone deacetylase inhibitor and VEGF pathway inhibition [13].
This phase I study (NCT00530907) was designed to evaluate the combination of bevacizumab and valproic acid, including an exploratory analysis of biomarkers (histone acetylation and hypertension).
Patients and Methods
Study group
Patients entered in the study were required to have a pathologically confirmed cancer that was metastatic or unresectable and refractory to standard therapy or for whom there was no standard therapy for their cancer that resulted in a 3-month survival advantage. Other eligibility criteria were: adequate performance status (Eastern Cooperative Oncology Group ≤ 2) [14], adequate cardiac function (New York Heart Association classes III and IV were excluded) [15] and adequate bone marrow, liver, and kidney function (absolute neutrophil count >1,000/UL, platelets >50,000/UL, total bilirubin <2.0 mg/dL, and creatinine <2.0 mg/dL). The study was approved and conducted at the University of Texas MD Anderson Cancer Center and was therefore performed in accordance with the ethical standards laid down by the 1964 Declaration of Helsinki and its later amendments. All patients were enrolled after giving written informed consent in accordance with our Institutional Review Board requirements
Interventions
A toxicity adaptive dosing (TAD) algorithm, with 6 patients enrolled per dose level, was used to determine the maximum tolerated dose (MTD)/recommended phase II dose (RP2D) [16]. Bevacizumab, at the starting dose of 2.5 mg/kg (50% of FDA approved dose at the time of study initiation), was administered by IV on an outpatient basis on day 1 and day 15 of each cycle with dose escalation according to the TAD algorithm and no intra-patient dose escalation was allowed. Valproic acid was administered orally at a starting dose of 10 mg/kg/d once daily with dose titration, with the goal of achieving therapeutic plasma trough levels between 75 and 100 µg/mL. This concentration range was predicted to increase histone acetylation in preclinical models [17].
According to the TAD algorithm, 6 patients were treated at the starting dose and evaluated for toxicity. If there was no drug-related toxicity, the next cohort of 6 patients was treated with a 100% dose escalation. If grade 1 toxicity that was at least possibly drug related was seen, the next cohort was treated with a 50% dose escalation. If grade 2 toxicity was observed or if 1 of the 6 patients treated at a determined dose experienced a dose-limiting toxicity or toxicities, the next cohort of 6 patients was treated with a 25% dose escalation. If 2 or more of 6 patients at a determined dose experienced dose-limiting toxicities, the MTD was deemed to have been exceeded. The prior lower dose level was then established as the MTD (incidence of dose-limiting toxicities < 33%).
After establishing the MTD, additional patients with tumor types demonstrating stable disease (SD) ≥ 6 months and/or partial or complete response (PR, CR) were treated in the expansion cohort [18]. Pharmacodynamic (PD) parameters were assessed in PBMC (histone H3 acetylation). Courses of therapy were repeated no earlier than every 28 days. Patients continued on treatment until disease progression or unacceptable toxicities occurred. Patients received approximately two courses of treatment (~56 days) before their initial re-evaluation for response.
Monitoring and treatment assessment
Regular physical examinations, complete blood counts with differential serum chemistry (liver function tests, electrolytes, urea and creatinine) and valproic acid levels were done at least twice per course of therapy. Valproic acid levels were monitored using a commercially available immunoassay. Toxicity for dose escalation was assessed at the end of the first cycle (28 days) and adverse events were recorded and coded based on the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0, which was most recent version at the time of study initiation. Response to therapy was evaluated according to the Response Evaluation Criteria in Solid Tumors (version 1.0) [18]. Measurements were performed at baseline and after 2 cycles of treatment.
Analysis of histone acetylation
To confirm the induction of histone acetylation by valproic acid, we collected PBMC from patients on days 1 and 15 of each cycle and histone H3 acetylation was analyzed in cell lysates by Western blotting using an antibody directed against acetylated histone H3 (Upstate Biotechnology, Waltham, MA). Whole-cell lysates were prepared in RIPA lysis buffer (Cell Signaling Technology, Danvers, MA). Forty micrograms of protein were separated in 15% SDS–polyacrylamide gels (Bio-Rad, Hercules, CA). Proteins were transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Immunoreactive bands were visualized using a Western blotting luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA). β-Actin was used as an internal control.
Statistical Analysis
Progression-free survival (PFS) was defined as the time interval from the start of therapy to the first observation of disease progression or death, whichever occurred first. Patients alive and without disease progression were censored at the last follow-up date. Overall survival (OS) was defined as the time interval from the start of therapy to the date of death, and patients alive at the time of analysis were censored at the last follow-up date. OS and PFS were estimated using the method of Kaplan and Meier and were compared among the subgroups of patients using a log-rank test [19,20]. All tests were two-sided and p values less than 0.05 were considered statistically significant. All statistical analyses were carried out using SPSS 17 computer software (SPSS, Chicago, IL).
Results
Patient characteristics
Fifty-seven patients were enrolled. Median age was 63 years (range, 41 to 83 years). Patient characteristics are shown in Table 1. The most common malignancies were colorectal cancer (29 patients), prostate cancer (6 patients), breast cancer (5 patients) and melanoma (3 patients). A total of 181 cycles were administered with a median of 3 cycles per patient.
Table 1.
Patient Characteristics
| No. of patients (%) |
|
|---|---|
| Total | 57 (100) |
| Gender | |
| Male | 32 (56) |
| Female | 25 (44) |
| Age (years) | |
| Median | 63 |
| Range | 41–83 |
| Type of cancer Diagnosis | |
| Colorectal | 29 (51) |
| Prostate | 6 (10) |
| Breast | 5 (9) |
| Melanoma | 3 (5) |
| Thyroid | 3 (5) |
| Ovarian | 2 (4) |
| Carcinoid | 2 (4) |
| Head and Neck | 2 (4) |
| Hepatocellular | 1 (2) |
| Leiomyosarcoma | 1 (2) |
| Non-small cell lung | 1 (2) |
| Renal cell | 1 (2) |
| Gastroesophageal | 1 (2) |
| ECOG PS | |
| 0 | 20 (35) |
| 1 | 37 (65) |
| Prior bevacizumab | 29 (51) |
ECOG PS: Eastern Cooperative Oncology Group Performance Status
Toxicity
The dose escalation started at 10 mg/kg of valproic acid and bevacizumab (2.5 mg/kg, 5 mg/kg, 7.5 mg/kg and 11 mg/kg). At dose level 4 (bevacizumab 11 mg/kg and valproic acid 10 mg/kg), the first dose-limiting toxicity of altered mental status (grade 3) related to valproic acid was observed. The patient exhibited slow speech and was unable to identify his location or the day of the week. His pattern of limb movement was hesitant and slow and he was unable to walk in a straight line. As a result, there was an intra-cohort dose reduction of valproic acid to 7.5 mg/kg. Another patient at dose level 4 subsequently experienced a dose-limiting toxicity of grade 3 altered mental status while receiving 7.5 mg/kg of valproic acid. This patient had progressive lethargy with visual hallucinations and inappropriate responses. As a result, no additional patients were enrolled at dose level 4 and another dose level was opened with valproic acid being reduced to 5.3 mg/kg and a bevacizumab dose of 11 mg/kg. While this dose de-escalation schema was unforeseen in the setting of observed neurotoxicity, it was an IRB-approved alteration to ensure patient safety. No further dose-limiting toxicities were observed (within the dose-limiting toxicity window of cycle 1) even after an expansion up to 32 patients and this was considered the RP2D. Although dose level 3 fit the criteria for the MTD, it was not established as the RP2D because the dose-limiting toxicities were related to valproic acid and dose level 3 was a reduction in bevacizumab only; therefore, the protocol was amended (with IRB approval) to reduce valproic acid to 5.3 mg/kg. Bevacizumab was maintained at a dose of 11 mg/kg for the expansion cohort dose as it had not caused any toxicities.
Grade 3 or 4 toxicities other than dose-limiting toxicities included 2 patients with grade 3 proteinuria and 2 patients with grade 3 hypertension, both related to bevacizumab (Table 2). Both of these grade 3 toxicities occurred after the initial dose-limiting toxicity window. There were 8 dose interruptions in 5 patients (1 patient had 4 interruptions). Six of these interruptions were toxicity related and 2 were due to impending surgeries. One patient at the RP2D had a reduction in dose from 11 mg/kg to 7.5 mg/kg of bevacizumab and from 5.3 mg/kg to 2.5 mg/kg of valproic acid due to toxicities.
Table 2.
Number of reported toxicities in each cohort (at least possibly drug-related)
| Dose Level | Bevacizumab | Valproic Acid | Patients (N=57) |
Toxicities occurring during Cycle 1 (DLTs) |
Toxicities occurring after Cycle 1 | ||
|---|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3/4 | Grade 1/2 | Grade 3/4 | ||||
| 1 | 2.5 mg/kg IV D1, 15 q 28 |
10 mg/kg PO D1–28 q 28 |
8a | Hypertension (n=4) Bleeding (n=1) Fatigue (n=2) |
|||
| 2 | 5 mg/kg IV D1, 15 q 28 |
10 mg/kg PO D1–28 q 28 |
6 | Hypertension (n=2) | |||
| 3 | 7.5 mg/kg IV D1, 15 q 28 |
10 mg/kg PO D1–28 q 28 |
6 | Hypertension (n=2) Fatigue (n=2) |
|||
| 4 | 11 mg/kg IV D1, 15 q 28 |
10→7.5 mg/kg PO D1–28 q 28b |
5 | Altered Mental Status (n=2) | Hypertension (n=1) Fatigue (n=2) |
||
| RP2Dc | 11 mg/kg IV D1, 15 q 28 |
5.3 mg/kg PO D1–28 q 28 |
32 | Hypertension (n=24) Headache (n=1) Bleeding (n=2) Deep vein thrombus (n=1) Proteinuria (n=3) |
Hypertension (n=2) Proteinuria (n=2) |
||
Additional patients added to cohort after voluntary disenrollment of 2 patients for non-treatment related reasons
Valproic acid reduced within cohort due to dose-limiting toxicity (DLT)
RP2D: recommended phase 2 dose
Overall Response
No CRs or PRs were observed. Four patients (7%, 95% CI 0.03–0.17) had SD ≥ 6 months. Two patients with colorectal cancer (1 patient at dose level 1 and 1 patient at the RP2D) remained on study for 14.7 months and 10.1 months, respectively. One patient with gastroesophageal junction cancer (at the RP2D) was on study for 7.4 months and 1 patient with prostate cancer (at dose level 2) for 7.6 months. There was no association between rate of SD ≥ 6 months and any grade of hypertension. Two of 30 patients (7%) with hypertension had SD ≥ 6 months compared 2 of 27 patients (7%) without hypertension. There was also no association between SD ≥ 6 months and prior treatment with bevacizumab. Two of 5 patients (40%) with SD ≥ 6 months had prior treatment with bevacizumab compared to 27 of 52 patients (51%) who did not have SD ≥ 6 months (p=0.73). There was no association between grade 3 or 4 toxicity and therapeutic efficacy (p=0.036).
Valproic acid levels
Twenty-one patients received an initial 10 mg/kg/d dose of valproic acid (dose levels 1–4), with the goal of achieving trough levels in the expected therapeutic range of 75 – 100 µg/mL. Fifteen patients had initial trough levels below therapeutic range, resulting in dose escalation for 11 of these patients. Two patients experienced toxicities that prevented dose escalations and continued at the initial dose. One patient experienced a DLT of altered mental status which resulted in a dose decrease to 7.5 mg/kg/d, as previously mentioned. One patient dis-enrolled from the study before trough levels were assessed. Trough levels were not monitored for 36 patients who received 7.5 and 5.3 mg/kg/d valproic acid.
Acetylation of Histones
To assess the effect of valproic acid on histone acetylation, we performed Western blots of acetylated histone H3 on days 1 and 15 of each cycle (whenever patients agreed to provide samples). Acetylation of histone H3 (at least doubling over baseline by densitometry) was observed in 10 of the 39 evaluable patients (26%). Patients with SD ≥ 6 months compared to those who did not achieve SD ≥ 6 months demonstrated a trend to a higher rate of histone acetylation (2/3, 67% vs. 8/39, 22%; p=0.16). Higher valproic acid serum levels (>30 µg/mL) in some patients (measured shortly after day 15 and 28 doses) did not correspond to those patients being more likely to have SD ≥ 6 months (p=0.43) or a higher level of histone deacetylase inhibition (p=0.74). The rate of histone acetylation was not associated with the dose of valproic acid. Thirty-nine patients were tested for histone acetylation. Two of 11 (18%) patients who received 10 mg/kg of valproic acid showed histone acetylation versus 8 of 28 (28%) patients who received 5.3 mg/kg (p=0.504). There was no association between grade 3 or 4 toxicity and treatment efficacy (p=0.036)
Progression-free and overall survival
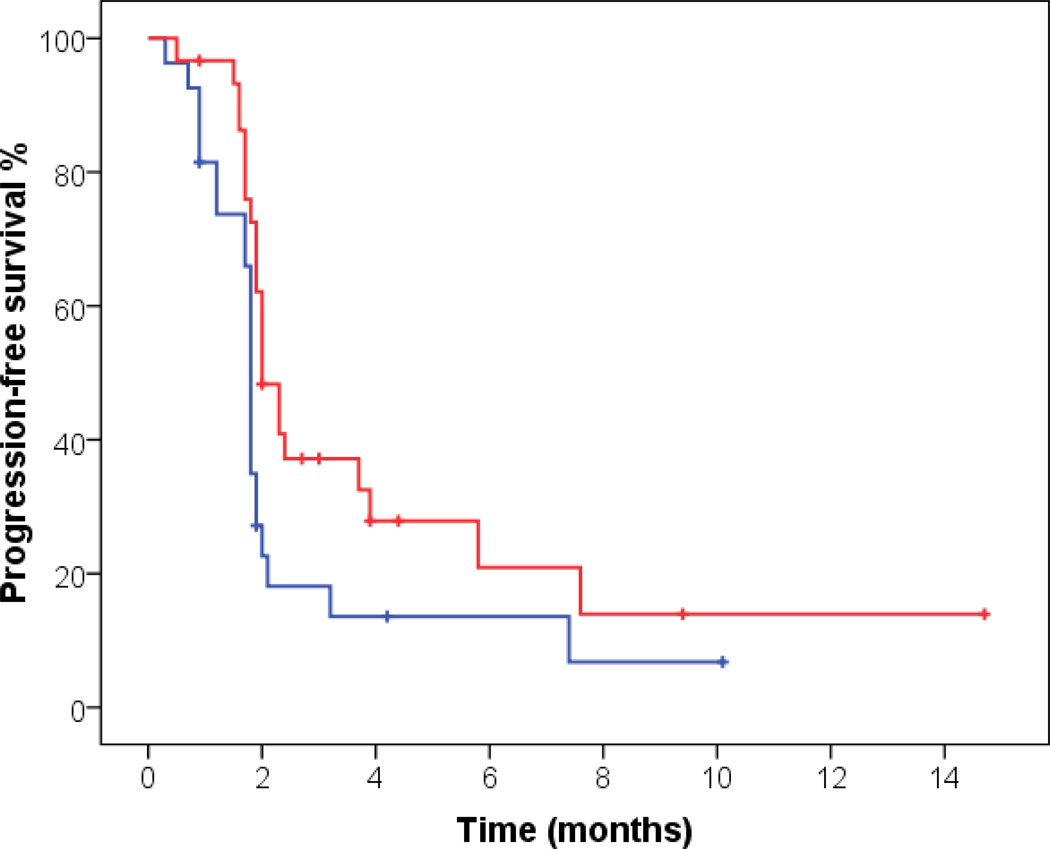
Median PFS was 1.9 months (95% CI 1.8–2.0). Patients with hypertension of any grade at any time during the course of treatment compared to others demonstrated statistically (but not clinically) significant prolonged median PFS (2.0 months, 95% CI 1.7–2.3 vs. 1.8 months, 95% CI 1.7–1.9, p=0.036, Figure 1). Median OS was 7.6 months, (95% CI 5.9–9.3). Patients with hypertension of any grade compared to others demonstrated statistically significant prolonged median OS (11.1 months, 95% CI 6.9–15.2 vs. 5.8 months, 95% CI 4.0–7.6, p=0.012, Figure 2). None of the other factors analyzed (histone deacetylase inhibition, valproic acid doses or dose levels) were associated with improved PFS and OS outcomes.
Fig 1. Kaplan Meier curve for PFS and hypertension of any grade.
Patients with hypertension of any grade (red) compared to others (blue) demonstrated prolonged median PFS (2.0 months, 95% CI 1.7–2.3 vs. 1.8 months, 95% CI 1.7–1.9, p=0.036).
Fig 2. Kaplan Meier curve for OS and hypertension of any grade.
Patients with hypertension of any grade (red) compared to others (blue) demonstrated prolonged median OS (11.1 months, 95% CI 6.9–15.2 vs. 5.8 months, 95% CI 4.0–7.6, p=0.012).
Discussion
The combination of bevacizumab (11 mg/kg IV once every 14 days) and valproic acid (5.3 mg/kg PO daily) is safe and well tolerated. The median PFS and OS were 1.9 and 7.3 months, respectively, for enrolled patients. SD ≥ 6 months was observed in 4 of the 57 patients (7%) treated, including 2 of 29 (7%) with colorectal cancer, 1 of 6 (17%) with prostate cancer and 1 of 1 (100%) with gastroesophageal junction cancer. Reported therapeutic efficacy in our study is in accord with previous reports from phase I trials with targeted therapies, which demonstrated response rates from 4% to 6% in an unselected patient population [21]. Of interest, both patients with colorectal cancer with SD ≥ 6 months had previously received and progressed on bevacizumab. In our study, prior therapy with bevacizumab was not associated with a worse response to therapy as reflected by SD ≥ 6 months, 40% vs. 51% (p=0.73).
Bevacizumab inhibits VEGF signaling in endothelial cells, which can lead to a rapid rise in blood pressure [22]. A meta-analysis of randomized controlled trials indicated that the relative risk of developing hypertension was 3.0 (95% CI 2.2–2.2, p<0.001) for low doses and 7.5 (95% CI 4.2–13.4, p<0.001) for high doses of bevacizumab [23]. Retrospective analyses of data from metastatic breast cancer [24] and advanced, non-squamous, non-small cell lung carcinoma [25] trials with bevacizumab associated the incidence of hypertension with increased OS, whereas studies in colorectal cancer [26] and renal cell carcinoma [27] found an association between hypertension and increased PFS. In agreement with these findings, we also demonstrated that patients who experienced hypertension as a side effect of therapy lived longer than patients without hypertension (11.1 months vs. 5.8 months; p=0.012) and had prolonged PFS (2.0 months vs. 1.8 months; p=0.036).
In previous studies, high intermittent doses of valproic acid were found to modulate histone deacetylase activity in peripheral blood mononuclear cells,[28,29] however these doses were also associated with substantial neurotoxicity (20–26% of patients), mainly somnolence, confusion and fatigue [28,29]. Therefore, we designed a trial with lower continuous doses of valproic acid and found that histone deacetylase was inhibited in 26% of evaluated patients, even after additional dose reductions. In addition, we observed a trend towards association between histone acetylation and SD ≥ 6 months with 2 out of 3 patients (67%) achieving SD ≥ 6 months versus 8 out of 36 patients (22%) not achieving SD ≥ 6 months (p=0.16). Interestingly, there was no relationship between the dose of valproic acid and histone acetylation. The lack of association can be also explained by the qualitative nature of our analysis. Patients with histone acetylation levels that doubled from baseline after initiation of valproic acid treatment were considered to have target inhibition while those whose histone acetylation levels may have been elevated, but not above the specified threshold, were considered as not having target inhibition. A review of the literature indicates that quantitative analyses (degree of histone acetylation versus dose) have shown relationships between the amount of histone acetylation and dose levels [30–33], whereas qualitative analyses (histone acetylation above or below specified threshold versus dose) have not [28,34,29,35].
There are several limitations to this study. First, valproic acid is not the most potent HDAC inhibitor; however, at the time of study design there was evidence of its efficacy as an HDAC inhibitor in cancer [28] there was no other agent with HDAC activity approved by the FDA. Second, an expansion cohort for gastroesophageal cancer was planned but not filled due to the lack of candidates, which did not allow us to further investigate efficacy in this tumor type. Third, we performed histone acetylation analysis in PBMC as a surrogate for the target modulation in the cancer; however, performing these analyses in sequential tumor biopsies might provide a more accurate assessment.”
In conclusion, this study demonstrated that the combination of bevacizumab and valproic acid is safe with SD ≥ 6 months achieved in patients with colorectal, prostate and gastroesophageal cancers, including those with prior bevacizumab treatment. Hypertension of any grade was associated with prolonged PFS and OS. The presence of histone acetylation was independent of valproic acid dose and serum levels and demonstrated a trend toward a higher rate of SD ≥ 6 months. Our results suggest that the combination of bevacizumab and valproic acid warrants further investigation with a focus on biomarkers predicting clinical activity.
Acknowledgements
Supported by Grant Number RR024148 from the National Center for Research Resources, a component of the NIH Roadmap for Medical Research. (http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp).
FJ has served as a consultant/advisor for Trovagene and has funding from Novartis, Roche, Biocartis, Trovagene and Transgenomi.
Footnotes
Disclosures
All authors have not conflicts to disclose.
References
- 1.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 3.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792–799. doi: 10.1200/JCO.2005.05.098. doi:23/4/792 [pii] 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H. Integrating the anti-VEGF-A humanized monoclonal antibody bevacizumab with chemotherapy in advanced colorectal cancer. Clin Colorectal Cancer. 2004;4(Suppl 2):S62–S68. doi: 10.3816/ccc.2004.s.010. [DOI] [PubMed] [Google Scholar]
- 5.Sandler A. Bevacizumab in non small cell lung cancer. Clin Cancer Res. 2007;13(15 Pt 2):s4613–s4616. doi: 10.1158/1078-0432.CCR-07-0647. doi:13/15/4613s [pii] 10.1158/1078-0432.CCR-07-0647. [DOI] [PubMed] [Google Scholar]
- 6.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:JCO.2008.19.8721 [pii] 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi: 10.1016/S0140-6736(07)61904-7. doi:S0140-6736(07)61904-7 [pii] 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 8.Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–168. doi: 10.1016/S0065-230X(04)91004-4. doi:10.1016/S0065-230X(04)91004-4 S0065230X04910044 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 10.Johannessen CU, Johannessen SI. Valproate: past, present, and future. CNS Drug Rev. 2003;9(2):199–216. doi: 10.1111/j.1527-3458.2003.tb00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, Blacher S, Verdin E, Foidart JM, Nusgens BV, Castronovo V. Histone deacetylases inhibitors as antiangiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21(3):427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7(4):437–443. doi: 10.1038/86507. doi:10.1038/86507 86507 [pii] [DOI] [PubMed] [Google Scholar]
- 13.Qian DZ, Wang X, Kachhap SK, Kato Y, Wei Y, Zhang L, Atadja P, Pili R. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64(18):6626–6634. doi: 10.1158/0008-5472.CAN-04-0540. doi:10.1158/0008-5472.CAN-04-0540 64/18/6626 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 15.Criteria, Committee, of, the New, York, Heart, Association. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th edn. Boston: Little, Brown & Co; 1994. [Google Scholar]
- 16.Braiteh F, Soriano AO, Garcia-Manero G, Hong D, Johnson MM, Silva Lde P, Yang H, Alexander S, Wolff J, Kurzrock R. Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res. 2008;14(19):6296–6301. doi: 10.1158/1078-0432.CCR-08-1247. doi:10.1158/1078-0432.CCR-08-1247 14/19/6296 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276(39):36734–36741. doi: 10.1074/jbc.M101287200. doi:10.1074/jbc.M101287200 M101287200 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- 20.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50(3):163–170. [PubMed] [Google Scholar]
- 21.Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, Shoemaker D, Emanuel EJ, Grady C. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352(9):895–904. doi: 10.1056/NEJMsa042220. doi:352/9/895 [pii] 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 22.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102(9):596–604. doi: 10.1093/jnci/djq091. doi:djq091 [pii] 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49(2):186–193. doi: 10.1053/j.ajkd.2006.11.039. doi:S0272-6386(06)01833-6 [pii] 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26(28):4672–4678. doi: 10.1200/JCO.2008.16.1612. doi:26/28/4672 [pii] 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28(6):949–954. doi: 10.1200/JCO.2009.25.4482. doi:JCO.2009.25.4482 [pii] 10.1200/JCO.2009.25.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20(2):227–230. doi: 10.1093/annonc/mdn637. doi:mdn637 [pii] 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 27.Bono P, Elfving H, Utriainen T, Osterlund P, Saarto T, Alanko T, Joensuu H. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol. 2009;20(2):393–394. doi: 10.1093/annonc/mdn729. doi:mdn729 [pii] 10.1093/annonc/mdn729. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Manero G, Kantarjian HM, Sanchez-Gonzalez B, Yang H, Rosner G, Verstovsek S, Rytting M, Wierda WG, Ravandi F, Koller C, Xiao L, Faderl S, Estrov Z, Cortes J, O'Brien S, Estey E, Bueso-Ramos C, Fiorentino J, Jabbour E, Issa JP. Phase 1/2 study of the combination of 5-aza-2'-deoxycytidine with valproic acid in patients with leukemia. Blood. 2006;108(10):3271–3279. doi: 10.1182/blood-2006-03-009142. doi:blood-2006-03-009142 [pii] 10.1182/blood-2006-03-009142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano AO, Yang H, Faderl S, Estrov Z, Giles F, Ravandi F, Cortes J, Wierda WG, Ouzounian S, Quezada A, Pierce S, Estey EH, Issa JP, Kantarjian HM, Garcia-Manero G. Safety and clinical activity of the combination of 5-azacytidine, valproic acid, and all-trans retinoic acid in acute myeloid leukemia and myelodysplastic syndrome. Blood. 2007;110(7):2302–2308. doi: 10.1182/blood-2007-03-078576. doi:blood-2007-03-078576 [pii] 10.1182/blood-2007-03-078576. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Assouline S, Cortes J, Estrov Z, Kantarjian H, Yang H, Newsome WM, Miller WH, Jr, Rousseau C, Kalita A, Bonfils C, Dubay M, Patterson TA, Li Z, Besterman JM, Reid G, Laille E, Martell RE, Minden M. Phase 1 study of the oral isotype specific histone deacetylase inhibitor MGCD0103 in leukemia. Blood. 2008;112(4):981–989. doi: 10.1182/blood-2007-10-115873. doi:blood-2007-10-115873 [pii] 10.1182/blood-2007-10-115873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, Loboda A, Fantin VR, Randolph SS, Hardwick JS, Reilly JF, Chen C, Ricker JL, Secrist JP, Richon VM, Frankel SR, Kantarjian HM. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111(3):1060–1066. doi: 10.1182/blood-2007-06-098061. doi:blood-2007-06-098061 [pii] 10.1182/blood-2007-06-098061. [DOI] [PubMed] [Google Scholar]
- 32.Kelly WK, O'Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. doi:JCO.2005.14.167 [pii] 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan QC, Headlee D, Acharya M, Sparreboom A, Trepel JB, Ye J, Figg WD, Hwang K, Chung EJ, Murgo A, Melillo G, Elsayed Y, Monga M, Kalnitskiy M, Zwiebel J, Sausville EA. Phase I and pharmacokinetic study of MS-275, a histone deacetylase inhibitor, in patients with advanced and refractory solid tumors or lymphoma. J Clin Oncol. 2005;23(17):3912–3922. doi: 10.1200/JCO.2005.02.188. doi:JCO.2005.02.188 [pii] 10.1200/JCO.2005.02.188. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, Hoshino K, Quintas-Cardama A, Richon VM, Garcia-Manero G. Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood. 2006;108(4):1174–1182. doi: 10.1182/blood-2005-09-008086. doi:blood-2005-09-008086 [pii] 10.1182/blood-2005-09-008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadia TM, Yang H, Ferrajoli A, Maddipotti S, Schroeder C, Madden TL, Holleran JL, Egorin MJ, Ravandi F, Thomas DA, Newsome W, Sanchez-Gonzalez B, Zwiebel JA, Espinoza-Delgado I, Kantarjian HM, Garcia-Manero G. A phase I study of vorinostat in combination with idarubicin in relapsed or refractory leukaemia. Br J Haematol. 2010;150(1):72–82. doi: 10.1111/j.1365-2141.2010.08211.x. doi:BJH8211 [pii] 10.1111/j.1365-2141.2010.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]