Abstract
Objective
Catechol-O-methyltransferase (COMT), a key enzyme in catecholamine metabolism, is implicated in cardiovascular, sympathetic, and endocrine pathways. This study aimed to confirm preliminary association of COMT genetic variation with incident cardiovascular disease (CVD). It further aimed to evaluate whether aspirin, a commonly used CVD prevention agent, modified the potential association of COMT with incident CVD.
Approach and Results
We examined COMT polymorphism rs4680 (MAF=0.47), encoding a non-synonymous methionine (met)-to-valine (val) substitution, in the Women's Genome Health Study (WGHS), a large population-based cohort of women with randomized allocation to aspirin or vitamin E compared with placebo and 10 years follow-up. Rs4680 effects were confirmed with COMT polymorphism rs4818 and also examined in CARDIoGRAM/C4D, consortia for genome-wide association studies of coronary artery disease.
Among WGHS women allocated to placebo (135 events/N=5811), the rs4680 val allele was protective against incident CVD relative to the met, (HR[95%CI]=0.66[0.51-0.84], p=0.0007); an association also observed in CARDIoGRAM and C4D (combined p=2.4×10-5). In the WGHS, the rs4680 association was abolished by randomized allocation to aspirin, such that val/val women experienced higher CVD rates with aspirin allocation compared to placebo (HR[95%CI]=1.85[1.05-3.25], p=0.033) while met/met women experienced lower rates (HR[95%CI]=0.60[0.39-0.93], p=0.023). Allocation to vitamin E also conferred higher but non-significant CVD rates on val/val (HR[95%CI]=1.50 [0.83-2.70], p=0.180) compared with significantly lower rates on met/met (HR[95%CI]=0.53[0.34-0.84], p=0.006) women. Rs4818 results were similar.
Conclusions
Common COMT polymorphisms were associated with incident CVD, and this association was modified by randomized allocation to aspirin or vitamin E. Replication of these findings is required.
Keywords: aspirin, cardiovascular disease prevention, catecholamine, genetic association and women
Introduction
The catecholamines, epinephrine, norepinephrine, dopamine and catechol estrogen, play a critical role in cardiovascular, sympathetic, and endocrine pathways. Variation in the levels of these signaling molecules is implicated in a broad spectrum of disorders, including cardiovascular conditions, i.e., acute coronary syndrome1, stress cardiomyopathy2, hyperhomocysteinemia3, and preeclampsia4. The enzyme catechol-O-methyltransferase (COMT) modulates the function of catecholamines. We wanted to confirm preliminary evidence that genetic variation in COMT might affect susceptibly to cardiovascular disease (CVD)1, 5-7. Aspirin is commonly prescribed for CVD prevention. It is not known whether any potential association of genetic variation in COMT with incident CVD might be modified by aspirin treatment.
COMT degrades catecholamines by catalyzing the transfer of a methyl group donated by S-adenosyl methionine onto catechol moieties, resulting in their deactivation. The COMT genetic variant rs4680 (val158met) is an extensively studied single nucleotide polymorphism (SNP) that encodes a valine (G)- to-methionine (A) substitution at amino acid 158 in the membrane and 108 in the secreted form of the enzyme8. This functional polymorphism results in the met variant having a 3-4 fold lower enzymatic activity than the val variant, and is, therefore, inversely correlated with endogenous levels of dopamine9 and other COMT substrates, both at rest and with exercise10 or cardiac surgery-induced stress11. In addition, several small population-based studies have found genetic variation in COMT to be associated with coronary heart disease1 and hypertension in men5-7. A second COMT SNP, rs4818, is a C- to G-transversion in the same exon as rs4680. Rs4818, in partial linkage disequilibrium with rs4680, has been associated with differential stability of COMT mRNA secondary structure12 as well as a series of clinical outcomes some of which are shared with rs468013.
Aspirin is the gold standard for antiplatelet therapy and is widely prescribed because it is considered a safe treatment for CVD prevention. Despite demonstrated benefit of aspirin in primary and secondary CVD prevention14, 15, particularly among men16, the Women's Health Study (WHS), a large placebo-controlled trial (N=39,876) of aspirin in primary prevention among initially healthy, middle-aged women, found only a 9% non-significant reduction of major CVD events compared to placebo over 10 years of follow-up17. Given that aspirin like catecholamines18 interacts with multiple pathways to affect CVD, e.g., platelet activation, we hypothesized that genetic variation in COMT might also affect response to aspirin treatment for prevention of major CVD. In the Women's Genome Health Study (WGHS)17, 19, 20, a subset of the WHS for genome-wide genetic analysis, we therefore, performed a candidate association study of COMT SNPs rs4680 and rs4818 for association with incident CVD and potential interaction with randomized allocation to placebo or aspirin. The 2×2 factorial design of the WHS also allowed exploration of the association of COMT and incident CVD in women randomly allocated to vitamin E.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
The primary population for the study is the Women's Genome Health Study (WGHS), a large prospective cohort for genetics of CVD derived from the Women's Health Study (WHS), a randomized trial of aspirin and vitamin E compared with placebo in a balanced 2×2 factorial design. Random allocation to aspirin or vitamin E in the WHS allowed exploration of the association of genetic variation in COMT with incident CVD in the four treatment arms: placebo plus placebo (N= 5,811); aspirin plus placebo (N=5,810); vitamin E plus placebo (N=5,856); and aspirin plus vitamin E (N=5,796), (See methods).
Demographics and baseline characteristics in this study did not differ by treatment arm as expected by randomization (Supplementary Table I). COMT SNPs rs4680 (val158met) and rs4818 were found to be in Hardy Weinberg equilibrium, and minor allele frequencies of rs4680 (G or val) and rs4818 (G) were 0.47 and 0.39 respectively. In the WGHS, the linkage disequilibrium between these SNPs was moderate (r2=0.70, D′=1.00).
Among WGHS participants allocated exclusively to placebo (N=5,811), there was a 34% lower age-adjusted incidence rate of the primary outcome, major CVD, associated with each additional val allele in the COMT rs4680 (val158met) polymorphism, (HR[95%CI]=0.66[0.51-0.84], p=0.0007) (Table 1 and Figure 1). The val allele was also associated with a decreased rate of age-adjusted secondary outcomes of total CVD (HR[95%CI]=0.77[0.63-0.93], p=0.0075) and myocardial infarction (HR[95%CI]=0.60[0.41-0.90], p= 0.0130), but not stroke (HR[95%CI]=0.76 [0.53-1.09], p=0.14) or coronary heart disease (CHD) (HR[95%CI]=0.81[0.63-1.04], p= 0.103) (Table 1 and Figure 1). The incident cardiovascular event rates associated with the rs4818 minor allele among women allocated to the placebo arm were similar to the rs4680 val allele (Table 1 and Supplementary Figure I). Results were essentially equivalent when Cox models were adjusted for standard risk factors (age, systolic blood pressure, diastolic pressure, LDL-cholesterol, HDL-cholesterol, triglycerides, family history of myocardial infarction, family history of diabetes, smoking history, and use of hormone replacement therapy) (Table 1).
Table 1.
Hazard Ratios (HR) for major cardiovascular disease (CVD), total CVD, coronary heart disease, stroke and myocardial infarction by COMT SNPs rs4680 (val158met) and rs4818 among women exclusively allocated to placebo.
| Endpoint* | Events | rs4680† HR[95% CI], p | rs4818† HR[95% CI], p |
|---|---|---|---|
| Age-adjusted models | N=5,811 | N=5,795 | |
| Major CVD | 135 | 0.66 [0.51-0.84], p=0.0007 | 0.67 [0.51-0·86], p=0.0022 |
| Total CVD | 204 | 0.77 [0.63-0.93], p=0.0075 | 0.70 [0.57-0.87], p=0.0010 |
| Coronary Heart Disease | 126 | 0.81 [0.63-1.04], p=0.1030 | 0.69 [0.53-0.90], p=0.0068 |
| Stroke | 59 | 0.76 [0.53-1.09], p=0.1400 | 0·80 [0.55-1.17], p=0.2580 |
| Myocardial Infarction | 53 | 0.60 [0.41-0.90], p=0.0130 | 0·59 [0.39-0.91], p=0.0162 |
| Fully-adjusted models‡ | N=5,136 | N=5,120 | |
| Major CVD | 116 | 0.65 [0.50-0.85], p=0.0016 | 0.69 [0.52-0.91], p=0.0095 |
| Total CVD | 174 | 0.73 [0.59-0.91], p=0.0042 | 0.69 [0.55-0.87], p=0.0016 |
| Coronary Heart Disease | 108 | 0.73 [0.56-0.96], p=0.0248 | 0.65 [0.38-0.87], p=0.0038 |
| Stroke | 50 | 0.76 [0.51-1.12], p=0.1669 | 0.79 [0.52-1.20], p=0.2764 |
| Myocardial Infarction | 47 | 0.53 [0.35-0.82], p=0.0047 | 0.58 [0.37-0.91], p=0.0168 |
Major CVD, the primary WHS outcome is a composite of myocardial infarction, stroke or death from cardiovascular causes. Total CVD, is a composite of revascularization procedures (percutaneous transluminal coronary angioplasty and coronary bypass graft) in addition to events in the primary outcome. Coronary heart disease (CHD) is a composite of nonfatal MI or fatal CHD plus revascularization procedures.
rs4680 coded allele = G(val), reference allele = A(met); rs4818 coded allele = G, reference allele = C.
Fully-adjusted Cox models were adjusted for standard cardiovascular risk factors: age, systolic blood pressure, diastolic pressure, LDL-cholesterol, HDL-cholesterol, triglycerides, family history of myocardial infarction, family history of diabetes, smoking and use of hormone replacement therapy. Observations with incomplete data were not included in the analysis.
Figure 1.
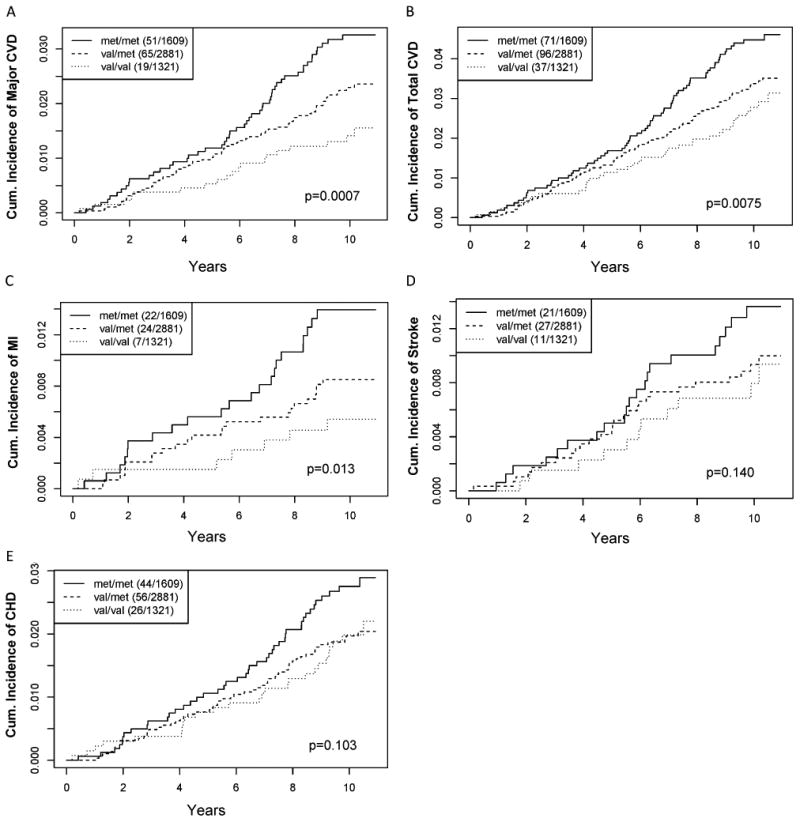
Kaplan–Meier estimates of the cumulative incidence of WGHS women in the placebo arm (N=5,811) according to COMT rs4680 genotype with a first ever (A) major CVD (B) total CVD (C) myocardial infarction (D) ischemic stroke or (E) coronary heart disease event. Legends indicate genotype strata and number of cases/total number in each stratum.
In CARDIoGRAM (Coronary ARtery DIsease Genome wide Replication and Meta-analysis)21, a large-scale meta-analysis of 13 studies consisting of 22,233 cases of coronary artery disease (CAD), a composite including myocardial infarction, revascularization, angina, and/or angiographic stenosis cases and 64,762 controls, the rs4680 val allele was associated with a decreased rate of CAD, (OR[95%CI]=0.960[0.935-0.988], p=0.0047), (Table 2). Among the additional and independent 34 CAD sample collections of C4D22, comprising 41,513 cases and 65,919 controls of European or South Asian ancestry, the association was also significant, with the val allele again conferring protection (OR[95% CI]=0.964[0.943-0.986], p=0.0017). Overlapping confidence intervals of the effect estimates in the gender stratified cohort suggested that there was no significant difference between males and females (Supplementary Table II). In a meta-analysis of CARDIoGRAM plus C4D, the significance of the association was p=5.2×10-5. Despite the consistency of the risk allele across all three studies, the evident heterogeneity in the COMT SNP effect, i.e., HR=0.66 in WGHS (Table 1), OR= 0.96 CARDiOGRAM, OR=0.96 C4D (Table 2), precluded the possibility of meta-analysis.
Table 2.
Meta-analysis of COMT associated CVD Protection in CARDIoGRAM, C4D and CARDIoGRAM+C4D.
| SNP§ | Stage 1* CARDIoGRAM | Stage 2† C4D | Stage 3‡ CARDIoGRAM+C4D | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| N | OR [SE], p | N | OR [SE], p | Fisher p | p‖ | |
|
|
|
|
||||
| rs4680 | 13 | 0.96 [0.014], 0.0047 | 34 | 0.96 [0.012], 0.0017 | 5.2×10-5 | 2.4×10-5 |
| rs4818 | 9 | 0.96 [0.016], 0.0081 | NA | NA | NA | NA |
Stage 1: CARDIoGRAM data set consisted of 22,233 coronary artery disease (CAD) cases and 64,762 controls. CAD is a composite of myocardial infarction, revascularization, angina, and/or angiographic stenosis.
Stage 2: Analysis of 34 additional CAD sample collections of European or South Asian descent (41,513 cases and 65,919 controls).
Stage 3: Meta-analysis of CARDIoGRAM+C4D database.
rs4680 coded allele = G(val), reference allele = A(met); rs4818 coded allele = G, reference allele = C; rs4818 was not available in Stage 2.
Inverse variance p-value.
In the whole WGHS cohort, univariate analyses of rs4680 and 14 cardiovascular biomarkers measured at baseline23 revealed significant associations after correction for multiple hypothesis testing (p ≤ 0.004 [=0.05/14 risk factors]). At baseline, (log) triglycerides (beta[SE]=-0.018[0.005], p=0.0004) and systolic blood pressure (beta[SE]=‐0.367[0.127] mMHg, p=0.004) were significantly associated with the val allele, consistent with less CV risk for both risk factors (Supplementary Table III). Similarly the minor allele of rs4818 was significant for (log) triglycerides (beta[SE]=-0.018[0.005], p=0.0004) and nominally significant for systolic blood pressure (beta[SE]=-0.335[0.130] mMHg, p=0.01), as well as apolipoprotein B (beta[SE] =-0.631[0.282] μmol/L, p=0.025) and sICAM1 (beta[SE]=-1.707[0.837] μmol/L p=0.041). Despite these associations, rs4680 and rs4818 associations with incident major CVD were essentially unaffected in Cox models further adjusted by these risk factors (Table 1, fully adjusted models and data not shown). COMT rs4680 associations with triglycerides (p= 0.028) and systolic blood pressure (p= 0.0059) were confirmed in published results from the Global Lipids Genetics Consortium24 and the International Consortium for Blood Pressure Genome-Wide Association Studies25, respectively.
The 2×2 design of the WGHS allowed us to evaluate how random allocation to aspirin or vitamin E might influence the association of COMT with incident CVD. Among WGHS participants randomly allocated exclusively to aspirin, the protective rs4680 val allele association was not observed (HR[95%CI=1.13[0.88-1.45], p=0.34) (Table 3 and Figure 2A). Comparison of these aspirin-allocated participants to those randomly allocated exclusively to placebo revealed a significant interaction between rs4680 and aspirin (pint=0.0022). Similarly, random allocation exclusively to vitamin E also abolished the association of rs4680 with incident CVD (HR[95%CI]=1.08[0.84-1.38], p=0.52), revealing a significant interaction of rs4680 with vitamin E allocation compared with placebo-only allocation (pint=0.004) (Table 3 and Figure 2B). Results for rs4818 were similar (Table 3 and Supplementary Figure II).
Table 3.
Age-adjusted Cox models relating COMT rs4680 val allele (met allele as reference) and rs4818 G allele associations (C allele as reference) to incident major CVD and total CVD, stratified by randomized treatment assignment.
| SNP* | Outcome† | Treatment Arm | HR[95% CI], p‡ | Gene-drug int. p§ |
|---|---|---|---|---|
| rs4680 | Major CVD | Placebo‖ | 0.66 [0.51-0.84], p=0.0007 | -- |
| Aspirin only | 1.13 [0.88-1.45], p=0.34 | 0.002 | ||
| Vitamin E only | 1.08 [0.84-1.38], p= 0.52 | 0.004 | ||
|
| ||||
| Total CVD | Placebo | 0.77 [0.63-0.93], p=0.0075 | -- | |
| Aspirin only | 1.10 [0.90-1.40], p=0.23 | 0.011 | ||
| Vitamin E only | 1.09 [0.90-1.32], p=0.38 | 0.011 | ||
|
| ||||
| rs4818 | Major CVD | Placebo | 0.67 [0.51-0·86], p=0.0022 | -- |
| Aspirin only | 1.22 [0.95-1.56], p=0.11 | 0.0009 | ||
| Vitamin E only | 1.03 [0.80-1.32], p=0.80 | 0.016 | ||
|
| ||||
| Total CVD | Placebo | 0.70 [0.57-0.87], p=0.001 | -- | |
| Aspirin only | 1.14 [0.93-1.40], p=0.20 | 0.001 | ||
| Vitamin E only | 1.06 [0.87-1.29], p=0.57 | 0.005 | ||
rs4680 coded allele = G(val), reference allele = A(met); rs4818 coded allele = G, reference allele = C.
Major CVD, the primary WHS outcome is a composite of myocardial infarction, stroke or death from cardiovascular causes. Total CVD, is a composite of revascularization procedures (percutaneous transluminal coronary angioplasty, coronary bypass graft), in addition to events in the primary outcome.
Hazard ratios refer to SNP associations with incident CVD in designated treatment arms.
Gene-drug interaction p-value refers to the significance of the difference between the SNP association among placebo allocated WGHS participants and participants allocated to the designated treatment arm.
Placebo results are also reported in Table 1.
Figure 2.
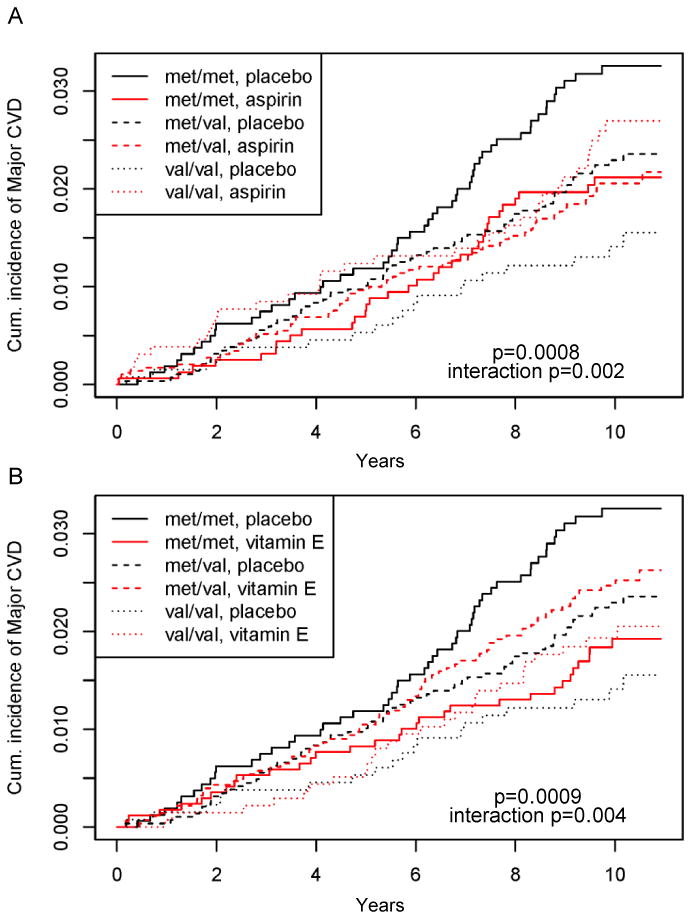
Kaplan–Meier estimates of the cumulative incidence of WGHS women according to COMT rs4680 genotype with a first ever major CVD event in the (A) aspirin vs. placebo arms, and (B) vitamin E vs. placebo arms. P-values are for the whole model and interaction p-values are for the drug by genotype interaction terms.
The consequences of the aspirin and vitamin E allocation on risk differed by rs4680 genotype. For COMT rs4680 met allele homozygotes, exclusive allocation to aspirin or vitamin E compared with placebo resulted in age-adjusted lower rates of incident CVD of 40% (95%CI=‐7% to -61%, p=0.023) and 47% (95%CI=-16% to -68%, p=0.006), respectively (Table 4). In contrast, val allele homozygotes had higher CVD rates of 85% (95%CI=5% to 325%, p=0.033) and 50% (95% CI=-17% to 170%, p=0.18), respectively, although only the aspirin allocation increase was significant. Among heterozygotes, allocation to either aspirin or vitamin E did not affect the incidence rate. For met allele homozygotes allocated to both aspirin and vitamin E, the difference in rates compared to those allocated to placebo was not significant, implying a further interaction for allocation to both agents compared with either alone. This further interaction was significant (p=0.006, Table 4). Fully-adjusted models revealed similar effects except for diminished significance of the effect of aspirin on met allele homozygotes (p=0.1, data not shown). Results for rs4818 were similar (Table 4 and Supplementary Figure II).
Table 4.
Age-adjusted Cox models of major CVD* stratified by COMT SNP genotype within the three drug treatment arms compared to the placebo allocated arm (reference).
| Genotype Stratum | Treatment Arm (HR[95%CI], p-value)† | Drug int. p‡ | |||
|---|---|---|---|---|---|
|
| |||||
| Placebo | Aspirin only | Vitamin E only | Aspirin + Vitamin E | ||
| rs4680 | |||||
| met/met | ref. | 0.60 [0.39-0.93], 0.02 | 0.53 [0.34-0.84], 0.006 | 0.80 [0.53-1.21], 0.30 | 0.006 |
| val/met | ref. | 0.89 [0.63-1.27], 0.52 | 1.06 [0.76-1.49], 0.73 | 0.91 [0.64-1.29], 0.58 | 0.85 |
| val/val | ref. | 1.85 [1.05-3.25], 0.03 | 1.50 [0.83-2.70], 0.18 | 1.54 [0.87-2.74], 0.14 | 0.15 |
|
| |||||
| rs4818 | |||||
| C/C | ref. | 0.69 [0.47-1.00], 0.05 | 0.67 [0.46-0.98], 0.04 | 0.77 [0.53-1.12], 0.17 | 0.08 |
| G/C | ref. | 0.81 [0.56-1.18], 0.28 | 1.04 [0.73-1.47], 0.85 | 1.02 [0.72-1.46], 0.89 | 0.44 |
| G/G | ref. | 2.98 [1.45-6.09], 0.003 | 1.81 [0.84-3.93], 0.13 | 1.67 [0.77-3.63], 0.19 | 0.02 |
Major CVD, the primary WHS outcome is a composite of myocardial infarction, stroke or death from cardiovascular causes.
Hazard ratio[95%CI] and p-value for each genotype by drug strata relative to placebo in age-adjusted models.
Drug Interaction p-value for interaction of aspirin and vitamin E across all three drug allocations within each SNP genotype stratum relative to placebo.
Discussion
This is the first study to show a significant association of the COMT rs4680 (val158met) polymorphism with incident CVD in a population-based sample of women. The COMT val allele conferred a lower rate of events in the prospective setting of the WGHS compared with the met allele. The association was significantly replicated in two additional studies, CARDIoGRAM21 and C4D26, and was also demonstrated for the first time in any study with the COMT rs4818, a SNP in partial LD with rs4680. Furthermore, we found that randomized allocation to aspirin eliminated the COMT val allele protective association with CVD, resulting in an 85% increase in the rate of incident CVD for rs4680 val allele homozygotes allocated to aspirin compared to placebo. Conversely, a 40% decrease in the rate of incident CVD was observed for the met allele homozygotes allocated to aspirin compared to placebo. Randomized allocation to vitamin E also modified the COMT CVD association as a non-significant increase in the rate of incident CVD in val allele homozygotes compared to placebo, and a significant 47% rate reduction for the met allele homozygotes. Similar COMT effect modification by both aspirin and vitamin E was observed for another COMT SNP, rs4818.
The treatment effects described here resulting in a reduction in the rate of incident CVD for the 28% of the WGHS population that was homozygous for the met allele are modest. Yet, over the approximately 10 years of follow-up in the WGHS, they translated into number-needed-to-treat estimates of 91 for aspirin or 74 for vitamin E compared to 581 or 674, respectively, for met allele homozygotes allocated exclusively to placebo. Conversely, for the 23% of the WGHS population that was homozygous for the val allele, where the drug effects were less significant than for the met homozygotes, the number-needed-to-harm estimates were 91 for aspirin and 189 for vitamin E, again compared to the population allocated to placebo. Our findings of differential CVD risk, thus, may be interpreted in the context of personalized medicine in which a sub-population defined by COMT genotype would be identified for potential benefit or harm by either of these treatments.
Several plausible catecholamine-mediated cardiovascular functions could account for the cardiovascular protection we observed in association with the high activity COMT val allele. COMT is present in platelets27 and in endothelial and vascular smooth muscle cells28, where the attenuated COMT activity of met allele homozygotes could increase catecholamine flux and oxidant stress, thus lowering the threshold for platelet activation and endothelial dysfunction. At the same time, our findings that baseline biomarkers of cardiovascular risk, including triglycerides, systolic blood pressure, sICAM1, and apolipoprotein B, were associated with COMT SNPs suggest a potential pleiotropy of COMT effects in the pathophysiology underlying its association with incident CVD. In addition to these associated mechanisms, COMT activity may be modified by plasma homocysteine concentrations, thereby potentiating the adverse effects of hyperhomocysteinemia by decreasing catecholamine O-methylation and inactivation: elevated levels of homocysteine lead to an increase in S-adenosylhomocysteine, which is a noncompetitive inhibitor of COMT3. Evidence for the effects of this theoretical interaction between COMT activity and homocysteine levels was demonstrated in the Kuopio Ischaemic Heart Disease Risk Factor Study1, as well as studies of venous thrombosis risk29 and preeclampsia30. Interestingly a recent trial that examined the effect of aspirin on hyperhomocysteinemia also reported effect modification of aspirin inhibition of platelet aggregation by homocysteine level31.
How aspirin modifies CVD protection associated with COMT is not known, but candidate mechanisms include effects on platelet function or homocysteine levels, and may support the hypothesis that differential response to aspirin therapy in a variety of settings is a heritable trait32, 33. Such genetic effects on aspirin response have precedent in our previous finding that carriers of an apolipoprotein(a) gene variant had a doubling of incident CVD rate and appeared to benefit more from aspirin therapy than non-carriers34.
In principle, the lack of an overall effect of vitamin E on CVD risk in the original WHS report19 does not preclude the possibility that subgroups defined on the basis of genetic strata experience benefit or harm, such as implied by the novel associations reported here. The underlying mechanisms of the COMT-drug interactions are yet to be elucidated. Recent data showing platelet COMT-mediated methylation-dependent inactivation of the common dietary antioxidant, quercetin35, may be indicative of a potential mechanism by which vitamin E effects may also depend on COMT genotype and activity. The COMT interaction with vitamin E is hypothesis generating, and may potentially offer some insight into the conflicting observations between animal and in-vitro studies36 that support a role for vitamin E in minimizing cardiovascular risk, and overall null findings in CVD trials19. Thus, mechanistic studies that could account for this differential aspirin and vitamin E treatment effect by genotype are warranted, and may include analyses of platelet and vascular cell prostanoid and eicosanoid metabolism, oxidant stress, nitric oxide signaling, and platelet and endothelial function assessment over a wider range of doses. Moreover, the results suggest that any future studies exploring aspirin or vitamin E treatment in disease prevention or therapy should be mindful of COMT genotype and other genetic variation in the catecholamine metabolic pathway. More broadly, the results illustrate how gene-drug interactions may influence the interpretation of a major clinical trial.
The strengths of our study are the prospective and homogeneous nature of the WGHS cohort, the validation of cardiovascular endpoints by physician review of medical records, the randomized allocation of WGHS participants to aspirin or vitamin E, and the replication of the COMT rs4680 association with cardiovascular disease rate in CARDIoGRAM and C4D. In addition, our inclusion of another directly genotyped SNP, rs4818, provided a mutually confirmatory safeguard against artifacts due to genotyping errors. Despite the epidemiologic strengths of the WGHS in support of the association with rs4680, the effect in CARDIoGRAM was weaker than expected given its much larger number of cases; understanding this discrepancy may be revealing for the mechanism of COMT action. In this regard, it may be relevant to note differences between the WGHS and CARDIoGRAM/C4D in CVD endpoint definitions and population composition. There were a variety of CVD endpoints in the studies contributing the CARDIoGRAM/C4D meta-analysis that focused on cases of CAD, a composite including myocardial infarction, revascularization, angina, and/or angiographic stenosis, but variously recruited with or without additional criteria related to age and family history37. The studies in C4D similarly included cases recruited for a variety of CAD definitions, including angina, and also recruited from both South Asian and European populations22, a strategy that may have introduced subtle heterogeneity, e.g., from environmental interactions, that could have degraded power of already weak associations. In contrast, the primary endpoint in the WGHS was major CVD, a composite of non-fatal myocardial infarction or stroke, or cardiovascular death, and the population had homogeneous European ancestry. A secondary endpoint in the WGHS, CHD, defined as myocardial infarction or coronary revascularization but not angina, is somewhat closer to the CAD used by CARDIoGRAM/C4D and had a less strong association with rs4680 in the WGHS than the primary major CVD outcome. The differences likely do not reflect a sex-specific effect since, although CARDIoGRAM included both men and women (in contrast to the all-female composition of the WGHS), there was no evidence for rs4680 differential effects according to sex. Moreover, the association between rs4680 and CVD is consistent with results from the Kuopio Ischemic Heart Disease Study, where there was a significant association of met homozygotes compared to val carriers among 69 acute coronary events in a population of 792 men1. Another possible explanation might be related to the interactions we observed with aspirin and vitamin E. Exposures to these common drugs and others that may abrogate the COMT rs4680 association with CAD were not recorded in CARDIoGRAM/C4D but may have nevertheless contributed to the weaker association observed in this study.
Genetic analysis offers one route to a deeper understanding of the underlying pathophysiology of CVD. Our findings of a robust association between COMT variation and incident CVD add to a range of other clinical outcomes influenced by the catecholamine pathway. Finally the modulation of CVD risk conferred by COMT through random allocation to aspirin and vitamin E may have implications for personalized medicine and development of strategies in attenuating CVD risk.
Supplementary Material
Significance.
Preliminary evidence suggests genetic variation in the gene encoding catechol-O-methyltransferase (COMT) is associated with cardiovascular disease (CVD). It is not known whether potential association of genetic variation in COMT with incident CVD is further modified by aspirin. In the Women's Genome Health Study (N=23,294), a large population-based prospective cohort of women with randomized allocation to aspirin or vitamin E compared to placebo, we reinforce preliminary evidence for COMT association with incident CVD over 10 years of follow-up. We further demonstrate modification of this association by randomization to aspirin and vitamin E, such that individuals with some COMT genotypes had significantly higher rates of incident CVD by allocation to drug. Given that aspirin is widely prescribed and that the COMT target genetic variant described here is common (MAF 47%), this study underscores the importance of adapting a pharmacogenetic approach to understanding and treating underlying CVD pathophysiology. These results also illustrate how gene-drug interactions can influence interpretation of major clinical trials.
Acknowledgments
We thank Valerie Stone, Christina Wee, Karin Jansen, Dale Abel, and James Meigs for helpful discussions.
Sources of Funding: The WGHS is supported by HL 043851 and HL080467 from the National Heart, Lung, and Blood Institute and CA 047988 from the National Cancer Institute, and the Donald W. Reynolds Foundation, with collaborative scientific support and funding for genotyping provided by Amgen. KTH and TJK are supported by NCCAM-NIH grants T32AT000051, R01AT004662, K24AT004095, R21AT002860 and 3R01AT004662-02S1. TJK is also partially supported from Blue Guitar Foundation. RBD and MAM are supported by Harvard Catalyst through NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health. No funding sources contributed to the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Nonstandard Abbreviations and Acronyms
- COMT
catechol-O-methyltransferase
- CVD
cardiovascular disease
- met
methionine
- val
valine
- A
adenine
- G
guanine
- WGHS
Women's Genome Health Study
- WHS
Women's Health Study
- CARDIoGRAM
Coronary ARtery DIsease Genome wide Replication and Meta-analysis
- C4D
The Coronary Artery Disease Genetics Consortium
- CAD
coronary artery disease
- HR
hazard ratio
- OR
odds ratio
- SE
standard error
- pint
p-value of the interaction term
- sICAM
soluble intracellular adhesion molecule
Footnotes
Disclosures: Drs. Chasman, Nelson, Davis, Buring, Kirsch, Mittleman, Loscalzo, Samani and Ridker report no disclosures. Dr. Hall and Kaptchuk are scientific advisors to Biometheus, LLC.
References
- 1.Voutilainen S, Tuomainen TP, Korhonen M, Mursu J, Virtanen JK, Happonen P, Alfthan G, Erlund I, North KE, Mosher MJ, Kauhanen J, Tiihonen J, Kaplan GA, Salonen JT. Functional comt val158met polymorphism, risk of acute coronary events and serum homocysteine: The kuopio ischaemic heart disease risk factor study. PLoS One. 2007;2:e181. doi: 10.1371/journal.pone.0000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittstein IS. Stress cardiomyopathy: A syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. 2012;32:847–857. doi: 10.1007/s10571-012-9804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu BT. On the mechanism of homocysteine pathophysiology and pathogenesis: A unifying hypothesis. Histol Histopathol. 2002;17:1283–1291. doi: 10.14670/HH-17.1283. [DOI] [PubMed] [Google Scholar]
- 4.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, Strauss JF, Kalluri R. Deficiency in catechol-o-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–1121. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 5.Miyaki K, Htun NC, Song Y, Ikeda S, Muramatsu M, Shimbo T. The combined impact of 12 common variants on hypertension in japanese men, considering gwas results. J Hum Hypertens. 2012;26:430–436. doi: 10.1038/jhh.2011.50. [DOI] [PubMed] [Google Scholar]
- 6.Htun NC, Miyaki K, Song Y, Ikeda S, Shimbo T, Muramatsu M. Association of the catechol-o-methyl transferase gene val158met polymorphism with blood pressure and prevalence of hypertension: Interaction with dietary energy intake. Am J Hypertens. 2011;24:1022–1026. doi: 10.1038/ajh.2011.93. [DOI] [PubMed] [Google Scholar]
- 7.Annerbrink K, Westberg L, Nilsson S, Rosmond R, Holm G, Eriksson E. Catechol o-methyltransferase val158-met polymorphism is associated with abdominal obesity and blood pressure in men. Metabolism. 2008;57:708–711. doi: 10.1016/j.metabol.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-o-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-o-methyltransferase: Effects on mrna, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghimire LV, Kohli U, Li C, Sofowora GG, Muszkat M, Friedman EA, Solus JF, Wood AJ, Stein CM, Kurnik D. Catecholamine pathway gene variation is associated with norepinephrine and epinephrine concentrations at rest and after exercise. Pharmacogenetics and genomics. 2012;22:254–260. doi: 10.1097/FPC.0b013e328350a274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase-Fielitz A, Haase M, Bellomo R, Lambert G, Matalanis G, Story D, Doolan L, Buxton B, Gutteridge G, Luft FC, Schunck WH, Dragun D. Decreased catecholamine degradation associates with shock and kidney injury after cardiac surgery. J Am Soc Nephrol. 2009;20:1393–1403. doi: 10.1681/ASN.2008080915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-o-methyltransferase haplotypes modulate protein expression by altering mrna secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 13.Bialecka M, Kurzawski M, Klodowska-Duda G, Opala G, Tan EK, Drozdzik M. The association of functional catechol-o-methyltransferase haplotypes with risk of parkinson's disease, levodopa treatment response, and complications. Pharmacogenet Genomics. 2008;18:815–821. doi: 10.1097/FPC.0b013e328306c2f2. [DOI] [PubMed] [Google Scholar]
- 14.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuster V, Sweeny JM. Aspirin: A historical and contemporary therapeutic overview. Circulation. 2011;123:768–778. doi: 10.1161/CIRCULATIONAHA.110.963843. [DOI] [PubMed] [Google Scholar]
- 16.Final report on the aspirin component of the ongoing physicians' health study. Steering committee of the physicians' health study research group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 18.von Kanel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000;65:357–369. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin e in the primary prevention of cardiovascular disease and cancer: The women's health study: A randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE. Rationale, design, and methodology of the women's genome health study: A genome-wide association study of more than 25,000 initially healthy american women. Clinical chemistry. 2008;54:249–255. doi: 10.1373/clinchem.2007.099366. [DOI] [PubMed] [Google Scholar]
- 21.Schunkert H, Konig IR, Kathiresan S, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A genome-wide association study in europeans and south asians identifies five new loci for coronary artery disease. Nat Genet. 2011;43:339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 23.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the women's health study. J Womens Health Gend Based Med. 2000;9:19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 24.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deloukas P, Kanoni S, Willenborg C, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stramentinoli G, Gualano M, Algeri S, de Gaetano G, Rossi EC. Catechol-o-methyl transferase in human and rat platelets. Thromb Haemost. 1978;39:238–239. [PubMed] [Google Scholar]
- 28.Spatz M, Kaneda N, Sumi C, Nagatsu I, Creveling CR, Nagatsu T. The presence of catechol-o-methyltransferase activity in separately cultured cerebromicrovascular endothelial and smooth muscle cells. Brain Res. 1986;381:363–367. doi: 10.1016/0006-8993(86)90090-9. [DOI] [PubMed] [Google Scholar]
- 29.Gellekink H, Muntjewerff JW, Vermeulen SH, Hermus AR, Blom HJ, den Heijer M. Catechol-o-methyltransferase genotype is associated with plasma total homocysteine levels and may increase venous thrombosis risk. Thromb Haemost. 2007;98:1226–1231. [PubMed] [Google Scholar]
- 30.Hill LD, York TP, Kusanovic JP, Gomez R, Eaves LJ, Romero R, Strauss JF., 3rd Epistasis between COMT and MTHFR in maternal-fetal dyads increases risk for preeclampsia. PLoS One. 2011;6:e16681. doi: 10.1371/journal.pone.0016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karolczak K, Kamysz W, Karafova A, Drzewoski J, Watala C. Homocysteine is a novel risk factor for suboptimal response of blood platelets to acetylsalicylic acid in coronary artery disease: A randomized multicenter study. Pharmacological Research. 2013;74:7–22. doi: 10.1016/j.phrs.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Herrera-Galeano JE, Becker DM, Wilson AF, Yanek LR, Bray P, Vaidya D, Faraday N, Becker LC. A novel variant in the platelet endothelial aggregation receptor-1 gene is associated with increased platelet aggregability. Arterioscler Thromb Vasc Biol. 2008;28:1484–1490. doi: 10.1161/ATVBAHA.108.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Jeong HH, Cho BY, Kim M, Lee HY, Lee J, Wee K, Park HS. Association of four-locus gene interaction with aspirin-intolerant asthma in korean asthmatics. J Clin Immunol. 2008;28:336–342. doi: 10.1007/s10875-008-9190-7. [DOI] [PubMed] [Google Scholar]
- 34.Chasman DI, Shiffman D, Zee RY, Louie JZ, Luke MM, Rowland CM, Catanese JJ, Buring JE, Devlin JJ, Ridker PM. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis. 2009;203:371–376. doi: 10.1016/j.atherosclerosis.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright B, Gibson T, Spencer J, Lovegrove JA, Gibbins JM. Platelet-mediated metabolism of the common dietary flavonoid, quercetin. PLoS One. 2010;5:e9673. doi: 10.1371/journal.pone.0009673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higashi O, Ishigaki W. Effects of vitamin e on the platelet aggregation induced by combined adenosine diphosphate and hydrogen peroxide. Tohoku J Exp Med. 1977;121:41–46. doi: 10.1620/tjem.121.41. [DOI] [PubMed] [Google Scholar]
- 37.Preuss M, Konig IR, Thompson JR, et al. Design of the coronary artery disease genome-wide replication and meta-analysis study: A genome-wide association meta-analysis involving more than 22 000 cases and 60 000 controls. Circ Cardiovasc Genet. 2010;3:475–483. doi: 10.1161/CIRCGENETICS.109.899443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


