Abstract
Objective
To describe Surveillance, Epidemiology and End Results (SEER) hepatocellular carcinoma (HCC) incidence trends and United States liver cancer mortality trends by geography, age, race/ethnicity and gender.
Methods
HCC incidence data from SEER 18 registries and liver cancer mortality data from the National Center for Health Statistics were analyzed. Rates and joinpoint trends were calculated by demographic subgroup. State-level liver cancer mortality rates and trends were mapped.
Results
HCC incidence rates in SEER registries did not significantly increase during 2007–2010, however U.S. liver cancer mortality rates did increase. HCC incidence and liver cancer mortality rates increased among black, Hispanic and white men aged 50+ years and decreased among 35–49 year old men in all racial/ethnic groups including Asians/Pacific Islanders. Significantly increasing incidence and mortality rates among women were restricted to blacks, Hispanics and whites aged 50+ years. Asian/Pacific Islander liver cancer mortality rates decreased during 2000–2010 with decreasing rates among women aged 50–64 years and men 35–49 years and stable rates in other groups. During 2006–2010 among person 50–64 years of age, blacks and Hispanics had higher incidence and mortality rates than Asians/Pacific Islanders. Liver cancer mortality rates were highest in Louisiana, Mississippi, Texas and Washington, DC.
Conclusion
Decreasing HCC incidence and liver cancer mortality rates among Asian/Pacific Islanders, men aged 35–49 years, and the non-significant increase in overall HCC incidence rates suggest that the peak of the epidemic may be near or have passed. Findings of geographic variation in mortality rates can inform control efforts.
Keywords: hepatocellular carcinoma, liver cancer
Introduction
Primary liver cancer is the third largest contributor to cancer mortality in the world (1) and the seventh largest contributor in the United States (U.S.) (2). The burden of liver cancer in the U.S. is inequitably distributed by gender, age, and race/ethnicity. Incidence rates of hepatocellular carcinoma (HCC), the predominant form of liver cancer, and mortality rates of liver cancer, rise with age and are roughly three times higher among men than women (3). During 2003–2005 in the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) cancer registries, incidence rates of HCC were more than three times higher among Asians/Pacific Islanders than whites, with intermediate rates among Hispanics, blacks and American Indians/Alaska Natives (4). U.S. liver cancer mortality rates mirror HCC incidence rates, reflecting the poor survival of this cancer. In many countries, including the U.S., HCC incidence rates and liver cancer mortality rates have been increasing for decades. Between 1992 and 2005, HCC incidence rates in SEER registries increased from 3.1 to 5.1 per 100,000 persons, and United States liver cancer mortality rates rose from 3.3 to 4.0 per 100,000 persons (4).
Models based on the prevalence of an important cause of liver cancer in the U.S., chronic infection with hepatitis C virus (HCV), have predicted that HCC incidence will continue to climb for the next several decades (5). However, these models have not considered the changing prevalence of HCV and other risk factors (3). Whether predicted models of HCC trends are accurate remains uncertain. To characterize trends in the U.S. in the early 21st century, HCC incidence and liver cancer mortality rates were examined by demographic characteristics.
Methods
Incidence
Cancer incidence data during 2000–2010 were obtained from all 18 SEER registries, which cover 28% of the U.S. population (6). Liver cancer incidence was defined by International Classification of Diseases for Oncology, Third Edition (7) using topography codes C22.0 and C22.1. HCC cases were restricted to morphology codes 8170–8175. Of 87,988 malignant liver and intrahepatic cancer diagnoses reported during 2000–2010 in SEER 18 registries, 63,735 (72%) were classified as HCCs.
Mortality
United States data on cause of mortality during the years 2000–2010 were reported by the Centers for Disease Control and Prevention, National Center for Health Statistics (8). Deaths due to liver cancer were identified by International Classification for Diseases version 10 codes for the underlying cause of death (9), using codes C22.0–C22.9 (malignant neoplasm of liver and intrahepatic bile ducts), excluding C22.1 (intrahepatic bile duct cancer). To improve completeness of classification, mortality rates among Hispanics and non-Hispanics were restricted to areas that met data quality measures for reporting of Hispanic ethnicity, thereby excluding the populations of New Hampshire, North Dakota, South Carolina, and Washington, D.C which account for approximately 2% of the U.S. population (10). The current analysis was based on 138,326 reported liver cancer deaths, after excluding 27,203 intrahepatic bile duct cancer deaths. Sensitivity analyses of mortality trends that restricted cases and populations to SEER registry areas were performed.
Populations
Data on HCC incidence and liver cancer mortality were linked to Census Bureau population denominator data for 2000 through 2010, with data by geographic location, gender, age, and race/ethnicity (non-Hispanic white, black, Asian/Pacific Islander, and Hispanic) (11). American Indians/Alaska Natives were not included in the current study as small counts yielded unstable rate estimates.
Statistical analysis
Average annual HCC incidence and liver cancer mortality rates per 100,000 persons were estimated for the most recent five-year period of diagnoses, 2006–2010 (SEER*Stat v 7.0.9, Information Management Services; Silver Spring, MD). Rates were age-adjusted by the direct method to the 2000 US standard population (19 age groups) (12). Rates and trends were examined by age group (overall, 35–49, 50–64 and 65+ years of age), gender, non-Hispanic race and Hispanic ethnicity. Joinpoint regression (13) allowing two segments was used to fit age-adjusted trends for 2000–2010 (Joinpoint v 3.5, Information Management Services; Silver Spring, MD). Annual percent change (APC) was considered statistically significant when the regression line slope differed from zero (P<0.05).
State-specific liver cancer mortality rates per 100,000 persons during 2006–2010 were age-adjusted by the direct method to the 2000 US standard population (19 age groups) (12). Maps of overall liver cancer mortality rates used the Jenks natural break classification method (14) to yield six categories: states with 2.3 to 2.9 deaths per 100,000 (5 states), 3.0 to 3.5 deaths per 100,000 (11 states), 3.6 to 4.0 deaths per 100,000 (13 states), 4.1 to 4.5 deaths per 100,000 (12 states), 4.6 to 5.5 deaths per 100,000 (6 states), and 5.6 to 6.8 deaths per 100,000 (3 states and Washington, D.C.).
Maps of liver cancer mortality rate trends for age groups 35–49, 50–64, and 65+ years of age, based on a single joinpoint segment model for the period 2000–2010 categorized states into five groups, based on criteria used in the National Cancer Institute’s Cancer Trends Progress Report (15). Group 1: Significant decrease (Rate decreasing, statistically significant annual percentage change (APC)); Group 2: Non-significant decrease (Rate decrease more than −0.5% per year, APC not statistically significant); Group 3: Stable (Absolute value of rate change less than or equal to 0.5% per year, APC not statistically significant); Group 4: Non-significant increase (Rate increase over 0.5% per year, APC not statistically significant); and Group 5: Significant increase (Rate increasing with a statistically significant APC). National mortality trends for two underlying causes of liver cancer 1) liver disease and cirrhosis and 2) diabetes mellitus were compared with those for liver cancer. Using the five-category trend variable correlations with state-level mortality trends were examined with the CORR procedure (SAS v 9.3, Cary, NC). State-level maps of liver cancer mortality rates and trends were drawn using ArcMap 10.0 (ESRI, Redlands, CA).
Results
HCC incidence
As shown in Table 1, HCC incidence increased with age in all racial/ethnic groups in the interval 2006–2010, with the exception of blacks, for whom rates were higher among persons 50–64 than 65+ years of age. (All reported incidence rates are per 100,000 people). Overall, Asians and Pacific Islanders had the highest incidence rates, followed by Hispanics, blacks and lastly, whites. Among persons aged 35–49 years, Asians and Pacific Islanders had the highest HCC incidence rate (4.7), followed by Hispanics (3.2), blacks (2.5), and whites (1.4). Among persons aged 50–64 years, blacks had the highest incidence rate (26.9), followed by Hispanics (24.3), Asians and Pacific Islanders (23.5) and whites (12.2). The very highest age-specific rates were experienced by Asians and Pacific Islanders aged 65+ years (54.7 per 100,000).
Table 1.
Age-Adjusted HCC Incidence and Liver Cancer Mortality Rates per 100,000 persons, 2006–2010
| Non-Hispanic
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | All Races | White | Black | API | Hispanic | ||||||
|
| |||||||||||
| Outcome | Years | Rate | (95% CI) | Rate | (95% CI) | Rate | (95% CI) | Rate | (95% CI) | Rate | (95% CI) |
| HCC | Overall | 5.9 | (5.8, 5.9) | 4.2 | (4.2, 4.3) | 7.5 | (7.3, 7.8) | 11.7 | (11.3, 12.0) | 9.5 | (9.3, 9.8) |
| Incidence | 35–49 | 2.2 | (2.1, 2.3) | 1.4 | (1.3, 1.5) | 2.5 | (2.2, 2.8) | 4.7 | (4.3, 5.2) | 3.2 | (2.9, 3.4) |
| SEER 18 | 50–64 | 16.5 | (16.2, 16.8) | 12.2 | (11.9, 12.6) | 26.9 | (25.8, 28.1) | 23.5 | (22.4, 24.7) | 24.3 | (23.3, 25.3) |
| 65+ | 22.3 | (21.9, 22.7) | 16.0 | (15.5, 16.4) | 22.4 | (20.9, 23.9) | 54.7 | (52.4, 57.0) | 40.5 | (38.7, 42.4) | |
| Liver Cancer | Overall | 4.3 | (4.3, 4.3) | 3.6 | (3.5, 3.6) | 6.4 | (6.3, 6.6) | 8.2 | (7.9, 8.4) | 7.0 | (6.9, 7.2) |
| Mortality | 35–49 | 1.2 | (1.2, 1.2) | 0.9 | (0.8, 0.9) | 2.0 | (1.9, 2.2) | 2.8 | (2.6, 3.1) | 1.4 | (1.3, 1.5) |
| United States | 50–64 | 9.7 | (9.5, 9.8) | 7.7 | (7.6, 7.8) | 18.6 | (18.2, 19.1) | 13.0 | (12.4, 13.6) | 13.5 | (13.0, 13.9) |
| 65+ | 20.1 | (19.9, 20.3) | 17.2 | (17.0, 17.5) | 24.5 | (23.7, 25.3) | 43.2 | (41.6, 44.8) | 36.7 | (35.6, 37.8) | |
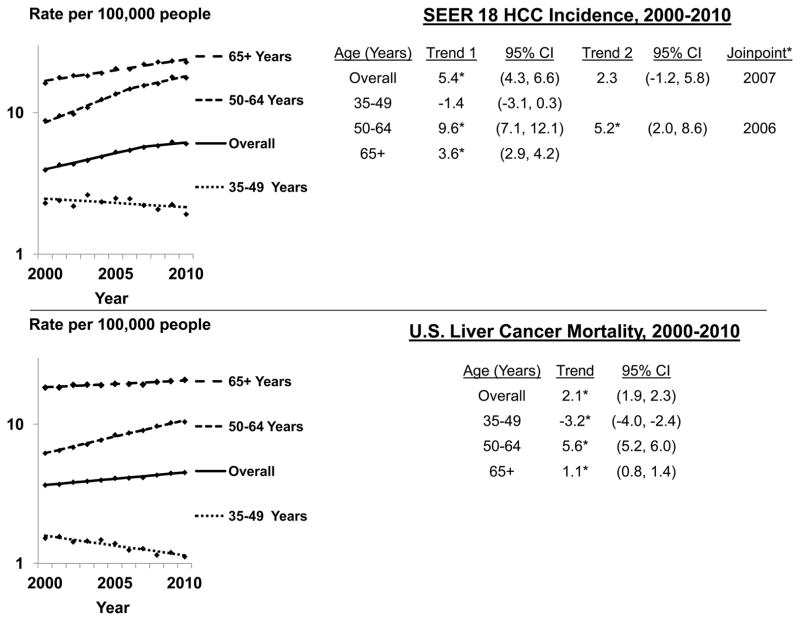
Between 2000 and 2010, incidence significantly increased, by 5.4% per year during 2000–2007, then non-significantly by 2.3% per year during 2007–2010 (Figure 1a). Among persons aged 35–49 years, incidence rates non-significantly decreased by 1.4% per year during 2000–2010. Among persons aged 50–64 years, rates significantly increased by 9.6% per year from 2000 to 2006, then by 5.2% per year from 2006 to 2010. Among persons 65 years+, rates increased 3.6% per year during 2000–2010.
Figure 1.
Age-adjusted SEER 18 HCC incidence and U.S. liver cancer mortality rates by age group and year; 2000–2010
CI=confidence interval
Trend = annual percent change, or APC. Joinpoint regression defines when a trend changes. Up to one joinpoint allowed in the eleven year period.
* Asterisk indicates slope of trend differs from zero (P<0.05)
Overall rates significantly increased among whites during 2000–2008 (5.9% per year), then non-significantly increased by 1.3% per year during 2008–2010 (Table 2). Overall rates increased during 2000–2010 among blacks and Hispanics (5.6% and 3.3% per year respectively). Among Asians/Pacific Islanders overall rates non-significantly increased during 2000–2002 (8.2% per year) followed by a period of borderline statistically significant decrease during 2002–2010 (−1.2% per year).
Table 2.
Age-Adjusted HCC Incidence and Liver Cancer Mortality Joinpoint Trends, 2000–2010 by Age, Race and Ethnicity
| Trend 1 | Trend 2 | ||||||
|---|---|---|---|---|---|---|---|
| Race and Ethnicity | Age | Years | APC | 95% CI | Years | APC | 95% CI |
| A. Incidence Trends (SEER) | |||||||
| non-Hispanic White | Overall | 2000–2008 | 5.9* | 4.9, 6.9 | 2008–2010 | 1.3 | −5.4, 8.6 |
| 35–49 | 2000–2010 | −1.7* | −3.3, 0.0 | ||||
| 50–64 | 2000–2006 | 11.7* | 8.7, 14.8 | 2006–2010 | 6.5* | 2.8, 10.4 | |
| 65+ | 2000–2010 | 3.5* | 2.6, 4.3 | ||||
| non-Hispanic Black | Overall | 2000–2010 | 5.6* | 4.5, 6.7 | |||
| 35–49 | 2000–2010 | −4.4* | −6.9, −1.9 | ||||
| 50–64 | 2000–2010 | 9.0* | 6.7, 11.3 | ||||
| 65+ | 2000–2010 | 4.3* | 2.3, 6.3 | ||||
| non-Hispanic | Overall | 2000–2002 | 8.2 | −6.0, 24.5 | 2002–2010 | −1.2 | −2.5, 0.1 |
| Asians/Pacific Islanders | 35–49 | 2000–2005 | 3.6 | −3.2, −10.9 | 2005–2010 | −8.3* | −14.4, −1.7 |
| 50–64 | 2000–2010 | −0.6 | −2.0, −0.9 | ||||
| 65+ | 2000–2002 | 15.1* | 0.6, 31.6 | 2002–2010 | −1.1 | −2.3, 0.1 | |
| Hispanic | Overall | 2000–2010 | 3.3* | 2.0, 4.6 | |||
| 35–49 | 2000–2010 | −1.7 | −4.1, 0.8 | ||||
| 50–64 | 2000–2005 | 9.9* | 4.0, 16.1 | 2005–2010 | 3.7 | −0.1, 7.7 | |
| 65+ | 2000–2010 | 2.2* | 0.6, 3.9 | ||||
| B. Mortality Trends (U.S.) | |||||||
| non-Hispanic White | Overall | 2000–2010 | 2.1* | 1.9, 2.3 | |||
| 35–49 | 2000–2010 | −2.8* | −4.0, −1.5 | ||||
| 50–64 | 2000–2010 | 6.3* | 5.9, 6.7 | ||||
| 65+ | 2000–2010 | 0.8* | 0.5, 1.1 | ||||
| non-Hispanic Black | Overall | 2000–2010 | 2.4* | 1.9, 2.9 | |||
| 35–49 | 2000–2010 | −5.3* | −6.7, −3.9 | ||||
| 50–64 | 2000–2010 | 6.2* | 5.4, 7.1 | ||||
| 65+ | 2000–2010 | 1.0* | 0.3, 1.7 | ||||
| non-Hispanic | Overall | 2000–2010 | −1.6* | −2.5, −0.6 | |||
| Asians and | 35–49 | 2000–2010 | −4.3* | −6.8, −1.8 | |||
| Pacific Islanders | 50–64 | 2000–2010 | −2.4* | −3.5, −1.2 | |||
| 65+ | 2000–2010 | −0.8 | −2.2, 0.7 | ||||
| Hispanic | Overall | 2000–2010 | 1.3* | 0.7, 1.9 | |||
| 35–49 | 2000–2010 | −4.8* | −6.2, −3.4 | ||||
| 50–64 | 2000–2003 | 10.4* | 0.6, 21.2 | 2003–2010 | 1.9* | 0.1, 3.7 | |
| 65+ | 2000–2010 | 1.0* | 0.3, 1.7 | ||||
Asterisk indicates slope differs from zero, (P<0.05).
APC=Annual Percent Change
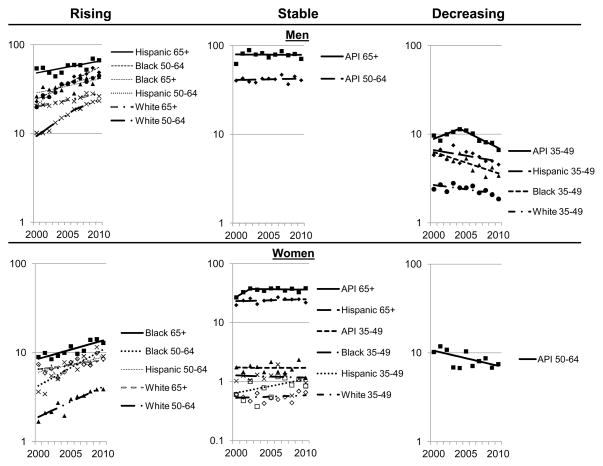
HCC incidence trends by race, age and gender are shown in Figure 2. Age- and race-specific rates were higher among men than women. Significantly increasing trends occurred only among Hispanic, white, and black men and women ages 50–64 and 65+ years, with the exception of a stable trend among Hispanic women aged 65+ years. Rates were also stable among Asian/Pacific Islander women aged 65+ years and women aged 35–49 years in all racial/ethnic groups. Among men rates were stable for Asian/Pacific Islanders aged 50–64 and 65+ years. Significantly decreasing HCC incidence rates were experienced among Asian/Pacific Islander, white and black men aged 35–49 years, with a borderline statistically significant decrease among Hispanic men in this age group. Among women, rates significantly declined only for Asian/Pacific Islanders aged 50–64 years. APCs and confidence intervals for HCC incidence trends are presented in Table 3A.
Figure 2.
Age-adjusted HCC incidence rates per 100,000 by age group, gender, non-Hispanic race and Hispanic ethnicity, SEER 18 registries -- 2000–2010
Table 3.
Age-Adjusted HCC Incidence and Liver Cancer Mortality Joinpoint Trends from 2000 through 2010 by Age, Sex, Racial and Ethnic Origin
| Trend | Direction | Sex | Origin* | Age, Years | Period | Trend 1 | 95% CI | Period | Trend 2 | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| A. Incidence | ||||||||||
| (SEER 18) | 1. Rising | |||||||||
| Men | ||||||||||
| Hispanic | 65+ | 2000–2010 | 3.1* | 0.8, 5.4 | ||||||
| Black | 50–64 | 2000–2010 | 8.7* | 6.6, 10.8 | ||||||
| Black | 65+ | 2000–2010 | 3.7* | 1.4, 6.0 | ||||||
| Hispanic | 50–64 | 2000–2004 | 13.7* | 3.7, 24.6 | 2004–2010 | 3.9* | 0.7, 7.3 | |||
| White | 65+ | 2000–2010 | 3.4* | |||||||
| White | 50–64 | 2000–2006 | 12.0* | 8.5, 15.5 | 2006–2010 | 6.6* | 2.3, 11.0 | |||
| Women | ||||||||||
| Black | 65+ | 2000–2010 | 4.9* | 2.5, 7.2 | ||||||
| Black | 50–64 | 2000–2010 | 9.8* | 5.3, 14.5 | ||||||
| Hispanic | 50–64 | 2000–2010 | 4.0* | 0.9, 7.1 | ||||||
| White | 65+ | 2000–2010 | 2.4* | 1.5, 3.4 | ||||||
| White | 50–64 | 2000–2010 | 8.2* | 5.7, 7.1 | ||||||
| 2. No Significant | ||||||||||
| Change | Men | |||||||||
| API | 65+ | 2000–2010 | −0.1 | −2.1, 2.1 | ||||||
| API | 50–64 | 2000–2010 | 0.4 | −1.2, 2.1 | ||||||
| Women | ||||||||||
| API | 65+ | 2000–2002 | 16.7 | −10.9, 52.9 | 2002–2010 | −0.3 | −2.6, 2.0 | |||
| Hispanic | 65+ | 2000–2010 | 0.7 | −1.3, 2.8 | ||||||
| API | 35–49 | 2000–2010 | 0.0 | −4.8, 5.0 | ||||||
| Black | 35–49 | 2000–2010 | −0.8 | −7.2, 6.0 | ||||||
| Hispanic | 35–49 | 2000–2010 | 5.3 | −1.7, 12.9 | ||||||
| White | 35–49 | 2000–2010 | 1.0 | −2.4, 4.5 | ||||||
| 3. Decreasing | ||||||||||
| Men | ||||||||||
| API | 35–49 | 2000–2004 | 6.8 | −0.6, 14.7 | 2004–2010 | −8.1* | −11.5, −4.5 | |||
| Hispanic | 35–49 | 2000–2010 | −2.9 | −5.8, 0.1 | ||||||
| Black | 35–49 | 2000–2010 | −5.4* | −7.9, −2.8 | ||||||
| White | 35–49 | 2000–2010 | −2.2* | −4.3, −0.2 | ||||||
| Women | ||||||||||
| API | 50–64 | 2000–2010 | −3.8* | −7.3, −0.3 | ||||||
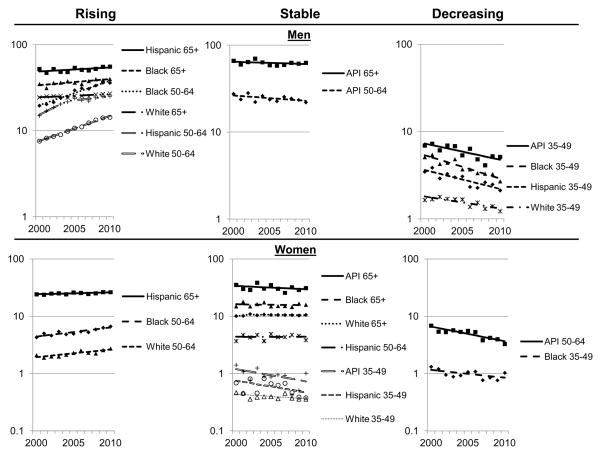
| B. Mortality | ||||||||||
| (U.S.) | 1. Rising | |||||||||
| Men | ||||||||||
| Hispanic | 65+ | 2000–2010 | 1.1* | 0.1, 2.1 | ||||||
| Black | 65+ | 2000–2010 | 1.7* | 0.7, 2.6 | ||||||
| Black | 50–64 | 2000–2010 | 6.7* | 5.6, 7.8 | ||||||
| White | 65+ | 2000–2010 | 0.9* | 0.4, 1.4 | ||||||
| Hispanic | 50–64 | 2000–2004 | 9.6* | 4.3, 15.2 | ||||||
| White | 50–64 | 2000–2010 | 6.9* | 6.4, 7.5 | 2004–2010 | 2.1* | 0.1, 4.0 | |||
| Women | ||||||||||
| Hispanic | 65+ | 2000–2010 | 0.8* | 0.3, 1.4 | ||||||
| Black | 50–64 | 2000–2010 | 3.8* | 2.4, 5.1 | ||||||
| White | 50–64 | 2000–2010 | 3.2* | 1.8, 4.6 | ||||||
| 2. No Significant | ||||||||||
| Change | Men | |||||||||
| API | 65+ | 2000–2010 | −0.6 | −1.7, 0.6 | ||||||
| API | 50–64 | 2000–2010 | −1.4 | −2.9, 0.1 | ||||||
| Women | ||||||||||
| API | 65+ | 2000–2010 | 1.3 | −3.6, 1.1 | ||||||
| Black | 65+ | 2000–2010 | −0.4 | −1.9, 1.2 | ||||||
| White | 65+ | 2000–2002 | 3.3 | −0.6, 7.4 | ||||||
| Hispanic | 50–64 | 2000–2010 | 0.0 | −2.2, 2.3 | 2002–2010 | 0.3 | −0.7, 0.1 | |||
| API | 35–49 | 2000–2010 | −4.9 | −10.2, 0.8 | ||||||
| Hispanic | 35–49 | 2000–2010 | −4.6 | −9.9, 1.1 | ||||||
| White | 35–49 | 2000–2010 | −1.8 | −3.7, 0.2 | ||||||
| 3. Decreasing | API | 35–49 | 2000–2010 | −4.2* | −6.5, −1.8 | |||||
| Men | ||||||||||
| Black | 35–49 | 2000–2010 | −6.0* | −7.6, −4.3 | ||||||
| Hispanic | 35–49 | 2000–2010 | −4.9* | −6.8, −2.9 | ||||||
| White | 35–49 | 2000–2010 | −3.1* | −4.6, −1.5 | ||||||
| Women | ||||||||||
| API | 50–64 | 2000–2010 | −5.7* | −7.9, −3.5 | ||||||
| Black | 35–49 | 2000–2010 | −3.1* | −6.0, −0.1 | ||||||
All racial groups restricted to non-Hispanics, API=Asians and Pacific Islanders
Liver cancer mortality
Between 2006 and 2010, U.S. liver cancer mortality rates increased with age in all racial/ethnic groups (Table 1). (All reported mortality rates are per 100,000 people). Among persons aged 35–49 years, Asians/Pacific Islanders had the highest mortality rates (2.8), followed by blacks (2.0), Hispanics (1.4), and whites (0.9). Among persons aged 50–64 years, blacks had the highest mortality rate (18.6), followed by Hispanics (13.5), Asians/Pacific Islanders (13.0) and whites (7.7). Mortality rates were highest among Asians/Pacific Islanders aged 65+ years (43.2 per 100,000).
As shown in Figure 1b, overall liver cancer mortality rates significantly increased during 2000–2010 (APC=2.1%), with a less rapid increase among persons aged 65+ years (APC=1.1%) than among persons aged 50–64 years (APC=5.6%). There was a significant decrease, however, among persons aged 35–49 years (APC=−3.2%).
United States liver cancer mortality trends by race/ethnic group during 2000–2010 are shown in Table 2B. Overall rates significantly increased during 2000–2010 among whites, blacks and Hispanics (2.1%, 2.4% and 1.3% per year respectively), however rates among Asians/Pacific Islanders significantly decreased (−1.6% per year). Sensitivity analyses restricted to SEER areas revealed mortality trends comparable to U.S. patterns in both magnitude and statistical significance (data not shown).
United States liver cancer mortality trends by race, age and gender are shown in Figure 3. Age- and race-specific rates were higher among men than women. Significantly increasing trends occurred only among Hispanic, white and black men aged 50–64 and 65+ years, Hispanic women aged 65+ years, and black and white women aged 50–64 years. Mortality rates did not significantly change for women in other population subgroups except for decreasing trends among black women aged 35–49 years and Asian/Pacific Islander women aged 50–64 years. Among men, mortality rates were stable for Asian/Pacific Islanders aged 50–64 and 65+ years and significantly decreased among Asian/Pacific Islander, black, Hispanic and white men aged 35–49 years. APCs and confidence intervals for these trends are presented in Table 3B.
Figure 3.
Age-adjusted liver cancer mortality rates per 100,000 by age group, gender, non-Hispanic race and Hispanic ethnicity, United States -- 2000–2010
State-specific liver cancer mortality rates
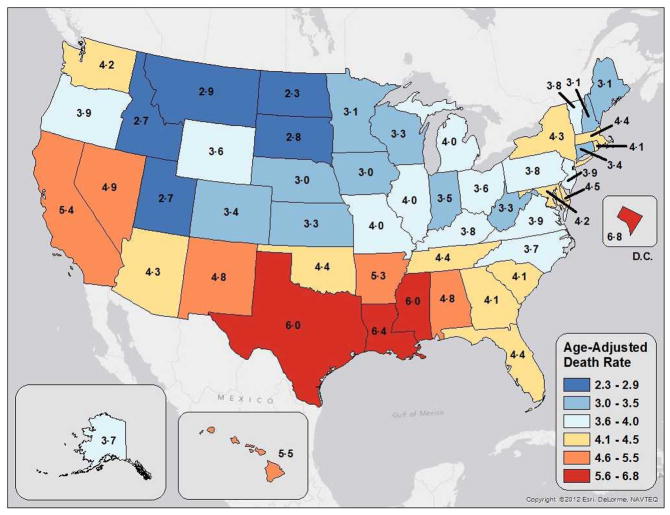
Figure 4 presents state-specific liver cancer mortality rates, which ranged from 2.3 to 6.8. The highest rates (5.5–6.8) were experienced by the populations of Washington, D.C. and three Gulf Coast states (Louisiana, Mississippi, and Texas). The second highest rates were reported in Alabama, Arkansas, California, Hawaii, Nevada, and New Mexico. Coastal, Appalachian, and Midwestern states generally reported higher rates than states in the Northern Plains and Northern Rocky Mountains. Confidence intervals for these rates are presented in Table 4
Figure 4.
Age-adjusted liver cancer mortality rates per 100,000 by state, 2006–2010
Table 4.
Liver Cancer Mortality Rate per 100,000 people, 2006–2010
| State/District | Rate per 100,000 | 95% CI* |
|---|---|---|
| Washington, DC | 6.8 | (5.9, 7.8) |
| Louisiana | 6.4 | (6.1, 6.8) |
| Mississippi | 6.0 | (5.6, 6.4) |
| Texas | 6.0 | (5.9, 6.2) |
| Hawaii | 5.5 | (5, 6.1) |
| California | 5.4 | (5.3, 5.5) |
| Arkansas | 5.3 | (4.9, 5.7) |
| Nevada | 4.9 | (4.5, 5.3) |
| Alabama | 4.8 | (4.6, 5.1) |
| New Mexico | 4.8 | (4.4, 5.2) |
| Delaware | 4.5 | (3.9, 5.1) |
| Florida | 4.4 | (4.3, 4.5) |
| Massachusetts | 4.4 | (4.2, 4.6) |
| Oklahoma | 4.4 | (4.1, 4.7) |
| Tennessee | 4.4 | (4.2, 4.6) |
| Arizona | 4.3 | (4.1, 4.6) |
| New York | 4.3 | (4.2, 4.4) |
| Maryland | 4.2 | (3.9, 4.4) |
| Washington | 4.2 | (4, 4.5) |
| Georgia | 4.1 | (3.9, 4.3) |
| Rhode Island | 4.1 | (3.6, 4.7) |
| South Carolina | 4.1 | (3.9, 4.4) |
| Illinois | 4.0 | (3.8, 4.1) |
| Michigan | 4.0 | (3.9, 4.2) |
| Missouri | 4.0 | (3.8, 4.3) |
| New Jersey | 3.9 | (3.7, 4.1) |
| Oregon | 3.9 | (3.6, 4.2) |
| Virginia | 3.9 | (3.7, 4.1) |
| Kentucky | 3.8 | (3.5, 4) |
| Pennsylvania | 3.8 | (3.7, 3.9) |
| Vermont | 3.8 | (3.2, 4.6) |
| Alaska | 3.7 | (3, 4.5) |
| North Carolina | 3.7 | (3.5, 3.9) |
| Ohio | 3.6 | (3.4, 3.7) |
| Wyoming | 3.6 | (3, 4.4) |
| Indiana | 3.5 | (3.3, 3.7) |
| Colorado | 3.4 | (3.1, 3.6) |
| Connecticut | 3.4 | (3.1, 3.7) |
| Kansas | 3.3 | (3, 3.6) |
| West Virginia | 3.3 | (3, 3.7) |
| Wisconsin | 3.3 | (3.1, 3.5) |
| Maine | 3.1 | (2.8, 3.5) |
| Minnesota | 3.1 | (2.9, 3.4) |
| New Hampshire | 3.1 | (2.7, 3.5) |
| Iowa | 3.0 | (2.7, 3.2) |
| Nebraska | 3.0 | (2.6, 3.3) |
| Montana | 2.9 | (2.5, 3.4) |
| South Dakota | 2.8 | (2.3, 3.3) |
| Idaho | 2.7 | (2.3, 3.1) |
| Utah | 2.7 | (2.4, 3.1) |
| North Dakota | 2.3 | (1.8, 2.8) |
CI=Confidence Interval
State-specific liver cancer mortality trends
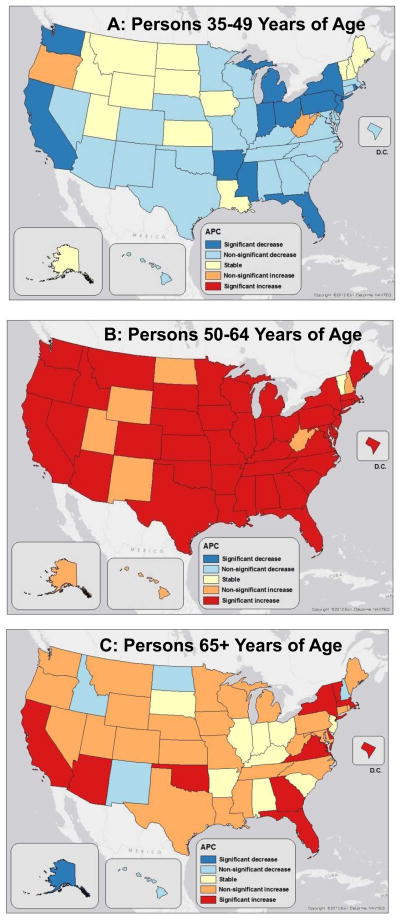
When state-specific liver cancer mortality trends during 2000–2010 were examined by age group, striking age-specific patterns were seen (Figure 5). Among persons aged 35–49 years, rates significantly decreased in 12 states and non-significantly decreased in 23 states and Washington, D.C., with no state experiencing a significant increase (Figure 5A). In contrast, among persons aged 50–64 years, rates significantly increased in 41 states and Washington, D.C. with no state experiencing a significant decrease (Figure 5B). Rates among persons aged 65+ years significantly increased in California, Arizona, Oklahoma, Florida, Georgia, Virginia, the District of Columbia, Delaware, New York, Vermont, and Massachusetts and significantly decreased in Alaska (Figure 5C).
Figure 5.
Annual percent change (APC) in liver cancer mortality rates by state, 2000 to 2010 by age group: (A) 35 to 49, (B) 50 to 64 and (C) 65+ years of age
Table 5 presents national mortality trends for liver cancer and two underlying causes of liver cancer 1) chronic liver disease and cirrhosis and 2) diabetes mellitus. The direction of liver cancer mortality trends were consistent with those for chronic liver disease and cirrhosis in 35–49 year olds, for whom both causes of death significantly decreased and 50–64 year olds, for whom both causes of death are significantly increased. There was also a statistically significant correlation between the liver cancer and chronic liver disease, cirrhosis trends among persons 50–64 year of age, P=0.03.
Table 5.
Annual Percent Change in United States Death Rates, 2000–2010 by Age Group -- Liver Cancer, Chronic Liver Disease and Cirrhosis, and Diabetes Mellitus, with Correlations between State-Level Liver Cancer and Other Cause Mortality Trends
| Measure | Cause of Death | Age Group (Years)
|
||
|---|---|---|---|---|
| 35–49 | 50–64 | 65+ | ||
| APC, (95% CI) | ||||
| Mortality Rate 2000–2010 (United States) | Liver Cancer | −3.3* (−4.1, −2.4) | 5.6* (5.2, 6.1) | 1.1* (0.8, 0.4) |
| Chronic Liver Disease/Cirrhosis | −1.7* (−2.5, −0.9) | 1.5* (1.1, 2.0) | −1.4* (−1.8, −1.0) | |
| Diabetes Mellitus | −0.2 (−0.8, 0.5) | −1.9* (−2.5, −1.3) | −2.4* (−3.2, −1.7) | |
| P-value, Pearson Correlation | ||||
| State-Mortality Trends: Liver Cancer, Other Specified Cause | Chronic Liver Disease/Cirrhosis | 0.16 | 0.03 | 0.36 |
| Diabetes Mellitus | 0.73 | 0.24 | 0.34 | |
APC=Annual Percent Change, CI=Confidence Interval
Discussion
After decades of statistically significant increasing HCC incidence rates, during 2007–2010 the trend was no longer statistically significant. The change was partly explained by decreasing incidence rates among Asians/Pacific Islanders, the racial group most affected by HCC, and among men aged 35–49 years. Incidence rates only increased among black, Hispanic and white men and women aged 50+ years. Geographic variation in liver cancer mortality suggests a need for focused liver cancer control efforts in southern and coastal states. Across states, increases in liver cancer mortality rates were most often seen among persons aged 50–64 years (baby-boomers) and decreasing rates occurred primarily among persons in the next generation, aged 35–49 years. These findings support the hypothesis that HCC incidence and liver cancer mortality trends vary across race, ethnicity, age, gender and geographic groups.
HCC incidence trends are affected by the changing prevalence of risk factors. In the U.S. a leading risk factor is hepatitis C virus (HCV) infection (16), particularly among person born between 1945 and 1965, commonly referred to as “baby-boomers” (17). Other HCC risk factors include chronic hepatitis B virus (HBV) infection (18), obesity (19), diabetes (20), non-alcoholic fatty liver disease (NAFLD) (21), and excessive alcohol use (22), all of which occur at variable frequencies across socio-demographic sectors of the population (23–27). Each of these factors predisposes to cirrhosis, the precursor of most HCC. In the past, persons with cirrhosis tended to die from the disease. As treatment has improved, however, the risk of death from cirrhosis has declined and, as a consequence, the risk HCC developing among persons with cirrhosis may be increasing (28).
Consistent with prior studies of HCC incidence in the United States, the continuing increase in U.S. liver cancer mortality rates was driven by increasing trends among blacks, whites, and Hispanics (4, 29, 30). In this report, liver cancer mortality rates among adults aged 50–64 years were significantly higher for blacks than other racial/ethnic groups. While not statistically significant, for the first time, Hispanics aged 50–64 years had higher HCC incidence and liver cancer mortality rates than Asians/Pacific Islanders. Better estimates of the fraction of HCC cases in affected subgroups attributable to specific etiologies would facilitate screening of people at-risk.
Compared to other racial groups, liver cancer incidence historically has been elevated among Asians/Pacific Islanders. This is attributed to the high rate of chronic HBV infection among older adults who were born outside the United States (16). A previous report observed that liver cancer mortality rates among Asians/Pacific Islanders declined 0.9% per year from 1992–2005 (4). The present report found a more rapid rate of decline (−1.6%) during 2000–2010. Cultural awareness of liver cancer risk may incentivize HBV and liver cancer screening and related therapies in this racial group that will further decrease liver cancer mortality rates.
Declining liver cancer mortality rates among young adults, ages 35–49 years may signal a future decline in overall U.S. liver cancer mortality rates. This was the case in Japan, where liver cancer mortality peaked a generation earlier than in the United States among the Japanese birth cohort born during 1925–1939 (35, 36). A decrease in Japanese liver cancer mortality began around 1985, and was first evident in the sentinel group of young adults, about 40 years of age (36). The reversal of liver cancer mortality trends in Japan has been attributed to declining exposure to HCV-related risk factors that included injected drug use and contaminated blood product transfusion in the post-World War II era (36).
Recent HCV screening guidelines for persons born during 1945–1965 (17) combined with advances in treatment of HCV infection (31, 32) and guidelines for HCC screening and therapy (33) may accelerate progress in reducing U.S. liver cancer mortality rates. While 3.2 million people in the U.S. currently have chronic HCV infection, the rate of new infection has greatly decreased (17, 34). Until recently, the standard of care for HCV-infected persons consisted of lengthy treatment with interferon and ribavirin. Historically, treatment has been a challenge due to limited treatment success and discontinuation due to adverse effects. Progress has been made, however, and future regimens are likely to incorporate multiple direct-acting antiviral drugs. In 2011, the FDA approved two direct-acting antiviral agents for persons infected with HCV genotype 1: telaprevir and boceprevir (31). In addition, there are other promising drugs in development that target HCV encoded proteins. At present, prescribed drug regimens cure approximately 80% of HCV patients. While cost of treatment remains an impediment, improving treatments could have a considerable downward effect on future HCC incidence rates, particularly among the baby-boomer cohort.
In the present study, United States liver cancer mortality rates continued a statistically significant rise while the increase in HCC incidence rates in SEER registries was no longer statistically significant. Several factors could explain the discrepancy. Mortality statistics cover 98% of the U.S., while SEER registries cover 28% and areas with the highest mortality rates including Texas, Mississippi and the District of Columbia are not in the SEER catchment. Underlying trends may differ between the U.S. and SEER areas. Secondly, liver cancer mortality data are based on the underlying causes of death on death certificates. When the cause of death is not fully documented, it can result in an undercount. Such errors are less likely with cancer incidence data, which are based on detailed abstractions of medical records. Thirdly, although HCC is the dominant histologic type of liver cancer, it is not the sole type, and rarer types do contribute to mortality rates. In addition, the liver is a common site of metastasis, thus some secondary liver cancers could be mistakenly over counted as primary liver cancer. Despite these potential sources of misclassification, incidence and mortality trends in SEER areas generally mirrored U.S. mortality trends.
As this is the first year in decades during which recent HCC incidence rates did not significantly increase, caution is warranted against over-interpretation. HCC incidence rates should be monitored over time to assess whether the direction of the trend turns downward, remains constant or increases. Strengths of the current study include the ability to examine HCC incidence in 28% and liver cancer mortality in 98% of the United States population by age, sex, and race/ethnicity. Limitations include the absence of data on etiological risk factors. Despite limitations, the data provide insight into changes in HCC incidence and liver cancer mortality rates across demographic strata of the United States population.
In summary, while overall liver cancer mortality rates increased during 2000–2010, recent HCC incidence rates did not significantly increase. Decreasing mortality rates among adult men aged 35–49 years and Asians/Pacific Islanders suggest that the peak of the epidemic may be near or have passed. Findings of geographically variable liver cancer mortality rates may help target affected areas.
Study Highlights.
1. What is current knowledge?
United States hepatocellular carcinoma (HCC) incidence and liver cancer mortality rates increased since the 1980s, with higher rates among men than women.
Rates are higher among Asians and Pacific Islanders than whites, with intermediate rates among Hispanics, blacks.
2. What is new here?
Incidence and mortality rates only increased among men who were black, Hispanic or white and 50+ years of age and among most women in these population subgroups. These increases were most pronounced among “baby-boomers”, 50–64 years of age.
During 2000–2010 age- and gender-specific incidence and mortality rates were stable or decreased among Asians/Pacific Islander age and gender subgroups.
Black and Hispanic baby-boomers now have higher incidence and mortality rates than Asian/Pacific Islander men of the same age.
Incidence and mortality rates decreased among 35–49 year old men in all four racial and ethnic groups.
Recent liver cancer mortality rates were higher in southern and coastal states than other areas.
Acknowledgments
Support: Division of Cancer Control and Population Sciences; Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health; Division of Cancer Prevention and Control, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention.
Abbreviations
- APC
annual percent change
- HCC
hepatocellular cancer
- HBV
Hepatitis B virus
- HCV
hepatitis C virus
- NAFLD
non-alcoholic fatty liver disease
- SEER
Surveillance, Epidemiology and End Results
- U.S
United States
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Publisher's Disclaimer: Disclaimer: Findings and conclusions are the authors and do not necessarily represent official positions of the Centers for Disease Control and Prevention or National Cancer Institute.
Author Contributions: Altekruse: Study concept and design, acquisition of data, analysis and interpretation of data; drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. Henley: study concept and design, acquisition of data, analysis and interpretation of data; drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, technical support. Cucinelli: Study design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis, technical and material support.
McGlynn: Study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, study supervision
References
- 1.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No.10. 2010 [cited 06-15-2013]; Available from: http://globocan.iarc.fr.
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–43. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizokami M, Tanaka Y. Molecular evolutionary analysis predicts the incidence of hepatocellular carcinoma in the United States and Japan. Cancer Chemother Pharmacol. 2004;54 (Suppl 1):S83–6. doi: 10.1007/s00280-004-0892-0. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub (1973–2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission. 2013 [cited 05-17-2013]; Available from: http://seer.cancer.gov.
- 7.International Classification of Diseases for Oncology. 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 8.National Vital Statistics, Centers for Disease Control and Prevention. Mortality Data. 2013 [cited 05-22-2013]; Available from: http://www.cdc.gov/nchs/deaths.htm.
- 9.International Statistical Classification of Diseases and Related Health Problems. Chapter II Neoplasms (C00-D48) (10) 2013 #x0005B;cited 05-25-2013]; Available from: http://apps.who.int/classifications/icd10/browse/2010/en.
- 10.Policy for Calculating Hispanic Mortality. National Cancer Institute Sureveillance, Epidemiology and End Results Program. 2013 [cited 05-05-2013]; Available from: http://seer.cancer.gov/seerstat/variables/mort/origin_recode_1990+/
- 11.Population projections. United States Census Bureau, United States Department of Commerce; 2013. [cited 05-07-2013]; Available from: http://www.census.gov/population/projections. [Google Scholar]
- 12.Standard Populations (Millions) for Age-Adjustment; Surveillance, Epidemiology and End Results. National Cancer Institute; 2013. [cited 05-06-2013]; Available from: http://seer.cancer.gov/stdpopulations/ [Google Scholar]
- 13.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Jenks G. The Data Model Concept in Statistical Mapping. International Yearbook of Cartography. 1967;7:186–190. [Google Scholar]
- 15.Cancer Trends Progress Report – 2011/2012 Update. National Cancer Institute; 2013. [cited 05-16-2013]; Available from: http://progressreport.cancer.gov/ [Google Scholar]
- 16.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hepatitis C FAQs for Health Professionals. Centers for Disease Control and Prevention; 2013. [cited 06-10-2013]; Available from: http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm. [Google Scholar]
- 18.Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol (Paris) 2010;58:273–7. doi: 10.1016/j.patbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 20.Hamed MA, Ali SA. Non-viral factors contributing to hepatocellular carcinoma. World J Hepatol. 2013;5:311–22. doi: 10.4254/wjh.v5.i6.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 22.Loomba R, Yang HI, Su J, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol. 2013;177:333–42. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1. 2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55:1047–55. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 25.Increasing prevalence of diagnosed diabetes--United States and Puerto Rico, 1995–2010. MMWR Morb Mortal Wkly Rep. 2012;61:918–21. [PubMed] [Google Scholar]
- 26.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 (Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia IB, Mack KA, Murphy W, et al. State-specific prevalence of selected chronic disease-related characteristics--Behavioral Risk Factor Surveillance System, 2001. MMWR Surveill Summ. 2003;52:1–80. [PubMed] [Google Scholar]
- 28.Persson EC, Quraishi SM, Welzel TM, et al. Risk of liver cancer among US male veterans with cirrhosis, 1969–1996. Br J Cancer. 2012;107:195–200. doi: 10.1038/bjc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed F, Perz JF, Kwong S, et al. National trends and disparities in the incidence of hepatocellular carcinoma, 1998–2003. Prev Chronic Dis. 2008;5:A74. [PMC free article] [PubMed] [Google Scholar]
- 30.Hepatocellular carcinoma - United States, 2001–2006. MMWR Morb Mortal Wkly Rep. 2010;59:517–20. [PubMed] [Google Scholar]
- 31.Fox AN, Jacobson IM. Recent successes and noteworthy future prospects in the treatment of chronic hepatitis C. Clin Infect Dis. 2012;55 (Suppl 1):S16–24. doi: 10.1093/cid/cis391. [DOI] [PubMed] [Google Scholar]
- 32.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 33.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong GL, Alter MJ, McQuillan GM, et al. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777–82. doi: 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda T, Saika K. Trends in liver cancer mortality rates in Japan, USA, UK, France and Korea based on the WHO mortality database. Jpn J Clin Oncol. 2012;42:360–1. doi: 10.1093/jjco/hys048. [DOI] [PubMed] [Google Scholar]
- 36.Saika K, Matsuda T. Time trends in liver cancer mortality (1980–2008) in Japan, the USA and Europe. Jpn J Clin Oncol. 2012;42:84. doi: 10.1093/jjco/hyr192. [DOI] [PubMed] [Google Scholar]