Abstract
Purpose.
Sickle retinopathy (SR) is a major cause of vision loss in sickle cell disease (SCD). There are no strategies to prevent SR and treatments are extremely limited. The present study evaluated (1) the retinal pigment epithelial (RPE) cell as a hemoglobin producer and novel cellular target for fetal hemoglobin (HbF) induction, and (2) monomethylfumarate (MMF) as an HbF-inducing therapy and abrogator of oxidative stress and inflammation in SCD retina.
Methods.
Human globin gene expression was evaluated by RT–quantitative (q)PCR in the human RPE cell line ARPE-19 and in primary RPE cells isolated from Townes humanized SCD mice. γ-Globin promoter activity was monitored in KU812 stable dual luciferase reporter expressing cells treated with 0 to 1000 μM dimethylfumarate, MMF, or hydroxyurea (HU; positive control) by dual luciferase assay. Reverse transcriptase–qPCR, fluorescence-activated cell sorting (FACS), immunofluorescence, and Western blot techniques were used to evaluate γ-globin expression and HbF production in primary human erythroid progenitors, ARPE-19, and normal hemoglobin producing (HbAA) and homozygous βs mutation (HbSS) RPE that were treated similarly, and in MMF-injected (1000 μM) HbAA and HbSS retinas. Dihydroethidium labeling and nuclear factor (erythroid-derived 2)-like 2 (Nrf2), IL-1β, and VEGF expression were also analyzed.
Results.
Retinal pigment epithelial cells express globin genes and synthesize adult and fetal hemoglobin MMF stimulated γ-globin expression and HbF production in cultured RPE and erythroid cells, and in HbSS mouse retina where it also reduced oxidative stress and inflammation.
Conclusions.
The production of hemoglobin by RPE suggests the potential involvement of this cell type in the etiology of SR. Monomethylfumarate influences multiple parameters consistent with improved retinal health in SCD and may therefore be of therapeutic potential in SR treatment.
Keywords: fetal hemoglobin (HbF), sickle cell disease, sickle cell retinopathy, monomethylfumarate, retinal pigment epithelium (RPE)
Blindness in sickle cell disease is most debilitating and unfortunately common. Herein, we present for the first time, the retinal pigment epithelium as a novel target for fetal hemoglobin induction and therapeutic management of sickle cell retinopathy, and monomethylfumarate as a new and potent therapy for use in this purpose.
Introduction
Sickle cell disease (SCD) is a debilitating monogenic blood disorder caused by a β-globin gene mutation (βs) on chromosome 11, leading to the production of abnormal hemoglobin S (HbS) due to substitution of glutamic acid with valine in the sixth codon.1 The disease is characterized by a marked interpatient variability of painful episodes, organ damage, and survival. Complications are most severe in patients bearing a homozygous βs mutation (HbSS; sickle cell anemia). Most organs are affected by SCD. The eye is highly susceptible to damage, a factor thought to be congruent with the particularly small diameter of the vessels within the retina and therefore, the increased propensity for vaso-occlusive events.2,3 Indeed, sickle cell retinopathy (SR), a leading cause of vision loss and blindness in SCD, affects a high number of patients and often at a very young age. Of children with SCD, 20% to 95% are reported to develop SR, with a reported 10% blindness rate.4
There are no strategies to prevent or delay SR and options for treatment are extremely limited.
Current theory holds HbS production and its consequent polymerization within red blood cells (RBCs) under low oxygen conditions as the primary causative factor in SR and other SCD-related complications.1 Red cell sickling, vaso-occlusion and hemolysis are indisputably key components to the pathophysiology of SR; however, there is evidence for the critical involvement of other cell types and factors such as inflammation and oxidative stress in the pathogenesis of SR.5–7 To date however, little attention has been given to the identification and/or study of nonerythroid cellular targets and related molecular factors in SR.
Given that the mutation responsible for SCD occurs on the adult β-globin gene, SCD patients experience very few complications during the first 6 months of life when the expression of β-globin is low and that of fetal γ-globin is high. For this reason, therapies to reactivate the expression of γ-globin and recapitulate this early postnatal environment are highly attractive in terms of ameliorating clinical complications of SCD.8 Therapeutic reactivation of γ-globin and the consequent induction of fetal hemoglobin (HbF) production are efficacious in alleviating systemic complications of SCD, however little is known regarding the impact of HbF-inducing therapies in the retina. This may stem in part from the fact that advanced-stage SR often presents during adolescence and use of hydroxyurea (HU), the only Food and Drug Administration (FDA)-approved HbF-inducing drug, has not been consistently employed in children.9,10 An improved understanding of cellular and molecular mechanisms in SR and development of new therapies to prevent and treat this sight-debilitating disease are sorely needed. Our present investigation was designed to impact positively both of these needs as our primary goals were to (1) identify novel cellular targets in SR, and (2) develop a new potential therapy for SR treatment. Congruent with these goals, we evaluated specifically (1) the retinal pigment epithelial (RPE) cell as a producer of Hb and potential target for HbF induction in SR, and (2) monomethylfumarate (MMF) as a novel chemical HbF inducer in retinal and erythroid cells.
Fumaric acid esters (FAE) have demonstrated efficacy in reducing inflammation and oxidative stress in a number of tissue and cell types as evident from their use in diseases as diverse as psoriasis and multiple sclerosis.11–15 Fumaric acid esters are administered in the form of dimethylfumarate (DMF); however, MMF is the major bioactive component.16,17 Our prior work with MMF demonstrated the robust antioxidant effects of the compound in RPE, and confirmed its ability to activate anti-inflammatory GRP109A-mediated signaling in this cellular layer.14 Retinal pigment epithelial is a key regulator of immunity and inflammation in retina; it is an integral component of the outer blood–retinal barrier and performs a number of functions essential to the maintenance of normal retinal health and visual function.18
New among the many functions ascribed to RPE and central to our present investigation is Hb production.19 Hemoglobin synthesis is a property thought to be exclusive to RBCs as few nonerythroid cell types express the globin genes. The functional relevance of globin gene expression and Hb production by RPE is not well understood. In the present study, we evaluated this phenomenon in the context of its potential relevance to the development of retinal pathology in SCD, a disease in which defective Hb production is key. Along these lines, we confirm globin gene expression and Hb production in RPE, and demonstrate, for the first time, the induction of γ-globin gene expression and HbF synthesis by MMF in cultured human retinal cells and primary erythroid progenitors, and in the intact retina of a humanized mouse model of SCD. Consistent with MMF-induced γ-globin expression and HbF production in SCD retina, we demonstrate also a reduction of oxidative stress and inflammation in this tissue. The ability of RPE to produce Hb suggests the potential involvement of this cell type in the etiology of SR. Furthermore, our finding that MMF induces γ-globin expression and HbF production in erythroid and retinal cell types is highly supportive of the therapeutic potential of this compound in the treatment of systemic and retinal complications of SCD.
Methods
Tissue Culture and Drug Treatments
A dual-luciferase KU812 (leukemic) stable cell line was established previously in our laboratory and validated for screening potential γ-globin inducing agents.20,21 KU812 stable cells (1 × 106 cells/assay) were treated with varying concentrations (0–1000 μM) of DMF or MMF (Sigma-Aldrich Corp., St. Louis, MO, USA) for 48 hours. Cells cultured in the absence of drugs served as negative controls (UT, untreated), and cells treated with HU (100 μM; Sigma-Aldrich Corp.) as positive controls for γ-globin promoter activation. Following the treatment period, γ-globin expression was evaluated using the dual luciferase assay system in which firefly luciferase and renilla luciferase activity represent γ-globin and β-globin promoter activities, respectively.20 Trypan blue exclusion (0.4%) was used to monitor cell viability.
Key experimental findings observed in the KU812 stable cells were verified using human primary erythroid cells. Erythroid progenitors were generated in vitro from adult CD34+ stem cells (STEMCELL Technologies, Inc., Vancouver, British Columbia, Canada) using a two-stage culture system that achieves terminal erythroid differentiation. In brief, CD34+ stem cells (500,000) were grown in first medium consisting of Iscove Modified Dulbecco Media containing human AB serum, IL-3 (10 ng/mL), stem cell factor (10 ng/mL), and erythropoietin (2 IU/mL). All media and cell-culture supplements were obtained from Invitrogen/Life Technologies (Grand Island, NY, USA). On day 7, the erythroblasts were placed in Second medium with 2 IU/mL erythropoietin for the duration. On day 8, erythroid cells were treated with MMF (1000 μM), dimethylfumarate (200 μM), or HU (100 μM). At 48 hours post treatment, cells were harvested and total RNA and protein were prepared for RT–quantitative (q)PCR and Western blot analyses, respectively, or used for fluorescence-activated cell sorting (FACS) assay.
Identical treatments and experimentation were performed using the human RPE cell line ARPE-19 and primary RPE cell cultures established from the eyes of Hbaa (normal hemoglobin)- and HbSS- expressing Townes humanized knock-in SCD mice22 (described in greater detail below) per our established method of primary RPE cell isolation.23 Also in accordance with our previously described protocols,23,24 the ARPE-19 and primary RPE cells used in this study were cultured in Dulbecco's modified Eagle medium (DMEM)/F12 supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, and maintained at 37°C in a humidified chamber of 5% CO2. All treatments and associated experiments, except immunofluorescence microscopy, were performed using confluent RPE cell monolayers. Immunofluorescence analyses were performed using subconfluent cell cultures to enhance the resolution of HbF labeling within individual cells.
Animals and Intravitreal Injection
HbAA- and HbSS-expressing Townes humanized knock-in SCD mice22 (6-weeks old; n = 6; Jackson Laboratories, Bar Harbor, ME, USA) were used for intravitreal injection of MMF following our published protocol.14 In brief, animals were weighed and anesthetized using 1 μL/g body weight of a solution of ketamine (80 mg/mL) and xylazine (12 mg/mL). Then 5 μL of proparacaine solution (5% wt/vol) was administered topically to the eyes. Monomethylfumarate (1 μl; 10 mM solution prepared in 0.01 M PBS, pH 7.4) was then injected into the vitreous body of the right eye of each animal at the limbus; the left eye served as a contralateral control and received and equal volume of PBS. Taking into account a total estimated vitreous volume of 10 μL per mouse eye, the final concentration of MMF achieved in our experimental system was 1000 μM. Animals were killed 24 hours post injection via CO2 inhalation followed immediately by cervical dislocation, and eyes were harvested. Some eyes (n = 3 per treatment group) were flash frozen in liquid nitrogen and cryosectioned for use in immunofluorescence assays with FITC-conjugated anti–γ-globin antibody or for dihydroethidium labeling, while the remaining were dissected to isolate RPE and neural retinal tissues per our published method25 and total RNA prepared. All experiments involving animals adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Georgia Regents University (Augusta, GA, USA) institutional animal care and use committee.
Reverse Transcription–Quantitative Polymerase Chain Reaction
Globin gene expression was evaluated in primary human erythroid progenitors, ARPE-19 and primary HbAA- and HbSS-expressing humanized mouse RPE cells by RT-qPCR using primer pairs specific to the human α-, β-, and γ-globin genes.20,21 The expression of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), IL-1β, and VEGF-A or VEGF was also evaluated in RNA samples obtained from HbAA- and HbSS-expressing Townes humanized mouse eyes injected intravitreally with PBS (0.01 M, pH 7.4) or MMF (1000-μM final concentration). The sequences of the primer pairs specific to mouse Nrf2, IL-1β, and VEGF that were employed in this study have been published.14,24,26
FACS and Western Blot Analyses
Fluorescence-activated cell sorting was used to measure HbF protein relative to that of isotype control in primary human erythroid progenitors, ARPE-19, and HbAA- and HbSS-expressing primary humanized mouse RPE cells treated with or without DMF, MMF, or HU as detailed above. For FACS assays, 500,000 cells were collected after drug treatments, washed twice with PBS, fixed in 4% paraformaldehyde, and permeated with ice-cold acetone/methanol (4:1). Cells were then incubated with FITC-conjugated human anti–γ-globin antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) in phosphate buffered saline–Triton X-100 (PBT; 0.01 M PBS/0.1% BSA/0.1% Triton X-100) solution for 20 minutes to stain intracellular HbF. The labeled cells were then analyzed using a Becton Dickerson LSR-II flow cytometer (BD Bioscience, San Jose, CA, USA). Standard Western blotting techniques were used to confirm HbF protein expression in the various cells. For Western analyses, HbF protein was measured relative to that of β-actin using antihuman HbF antibody (1:1000; Bethyl Laboratories, Inc., Montgomery, TX, USA) and horseradish peroxidase-conjugated sheep IgG (1:1000; Santa Cruz Biotechnology).
Immunofluorescence Assays and Dihydroethidium Labeling
Fetal hemoglobin protein was localized in cultured retinal cells and in retinal cryosections prepared from the eyes of HbAA- and HbSS-expressing Townes humanized mice injected intravitreally with PBS or MMF using the FITC-conjugated antihuman γ-globin antibody (described above) and standard immunofluorescence methods. Double immunolabeling experiments were in retinal cryosections performed using the FITC-conjugated HbF antibody in conjunction with the following retinal cell-type specific markers: HFE (1:500; Alpha Diagnostics, San Antonio, TX, USA), NeuN (1:200; Millipore, Billerica, MA, USA), Vimentin (1:250; Millipore), Griffonia Simplicifolia isolectin-b4 (Vector Laboratories, Burlingame, CA, USA), coupled with Alexa Fluor 568 secondary antibody (1:1000; Invitrogen). Vascular endothelial growth factor protein expression was evaluated also in the retinal cryosections using a mouse monoclonal anti-VEGF antibody (1:100; AbCam, Cambridge, MA, USA) coupled with goat antimouse Alexa Fluor 488 secondary antibody (1:1500; Invitrogen). Superoxide production was measured in the retinal cryosections as an indicator of oxidative stress using dihydroethidium (DHE) also per our published method.24 The relative fluorescence intensity within the images obtained was determined via automated image analysis or ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
Data Analysis
Data are presented as mean ± SEM and are from at least five data points generated from at least three independent drug treatments. For in vivo studies, six animals were included per group and samples were run in duplicate. Paired Student's t-test was performed and differences were considered statistically significant at P less than 0.05.
Results
α-, β-, and γ-Globin Gene Expression in RPE
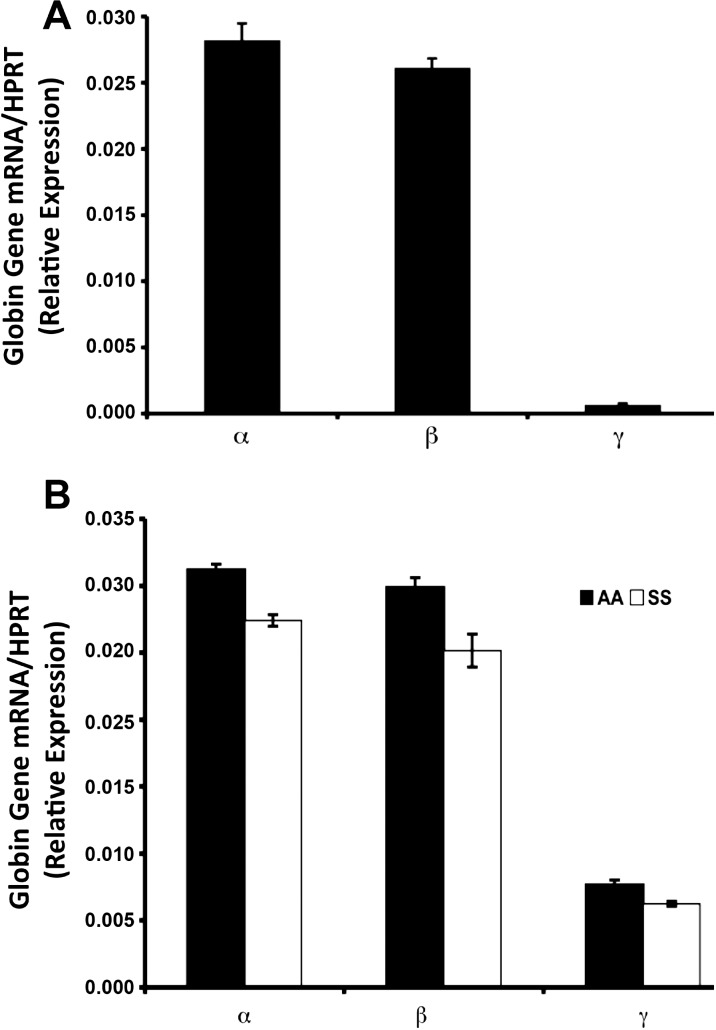
To synthesize hemoglobin, cells must express the α-, β-, and γ-globin genes during fetal and adult development. Adult hemoglobin A is a tetramer containing two α-globin subunits and two β-globin subunits (α2β2). Fetal hemoglobin, on the other hand, is composed of two α-globin and two γ-globin subunits. Evaluation of globin gene expression in ARPE-19 cells using standard RT-qPCR and primer pairs specific to human α-, β-, and γ- globin revealed the expression of mRNA transcripts specific to all three genes (Fig. 1A). Though a well-established and highly reputable model of cultured human RPE,27 the fact remains that ARPE-19 is a transformed retinal cell line, and therefore may have limited predictive power of RPE characteristics in vivo. As such, we sought to confirm findings obtained using ARPE-19 cells in a primary RPE cell culture system. Given our specific interest in SCD, we used the Townes humanized knock-in mouse, a fortuitous tool in that animals are engineered to express human rather than mouse globin genes and synthesize human Hb22; normal (HbAA) and sickle (HbSS) Hb producing animals are readily available. As observed in ARPE-19, primary RPE cultures established from HbAA- and HbSS-expressing animals were found to express human α-, β-, and γ-globin (Fig. 1B).
Figure 1.
Reverse transcription–qPCR analysis of endogenous human α-, β-, and γ-globin gene expression relative to that of hypoxanthine-guanine phosphoribosyltransferase 1 (HPRT; internal control) in (A) ARPE-19 cells and (B) HbAA (normal)- and HbSS (sickle)-primary RPE cells isolated from Townes humanized mouse retina.
Induction of γ-Globin Expression and HbF Production by DMF and MMF in Erythroid Cells
The above expression data confirm as reported previously by Tezel et al.,19 globin gene expression in RPE. Therapeutic elevation of γ-globin expression and HbF production is associated with improved outcomes in SCD,8 and there is evidence suggestive of a similar possible benefit in SR.28 Therefore, if the HbS produced locally within RPE cells is, in addition to that produced by erythroid cells, important in the pathophysiology of SR, then raising HbF levels in RPE cells would too be of benefit. However, HbF-inducing therapy has not been studied in RPE cells. Furthermore, the efficacy of FAE as HbF-inducing agents in erythroid or retinal cell types has not been tested.
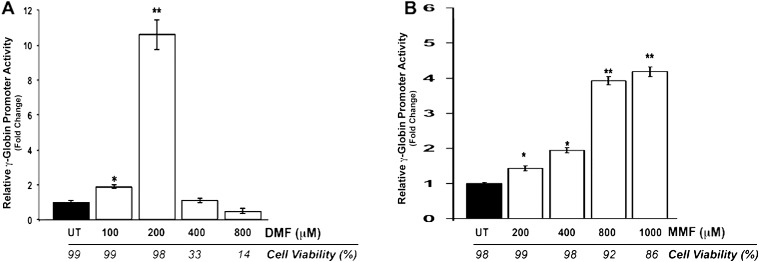
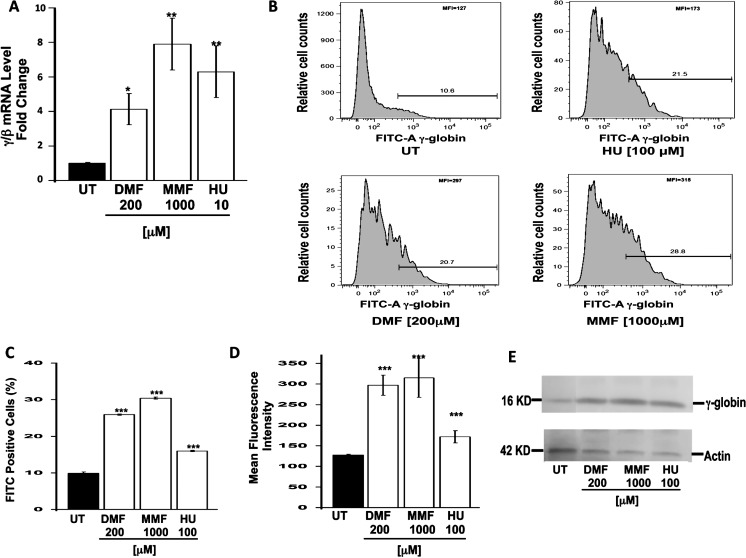
To address these issues, we started with erythroid cells, the principal cell type in the pathogenesis of SCD, as the methods for studying γ-globin expression and HbF induction in these cells are well established. We examined the potential of DMF and MMF as HbF-inducing agents. Dual luciferase assay of KU812 stable cells treated with DMF and MMF at concentrations ranging from 0 to 1000 μM revealed the robust induction of γ-globin promoter activity (Fig. 2). Maximal induction was obtained at 200 μM for DMF; increased cellular toxicity was associated with the use of DMF at higher concentrations as evidenced by the Trypan blue exclusion values of 33% to 14% for concentrations greater than 200 μM (Fig. 2A). Monomethylfumarate produced a dose-dependent induction of γ-globin promoter activity; maximal induction was observed at 1000 μM with minimal effects on the viability of the cells (Fig. 2B). To confirm these findings, additional experiments were performed using human primary erythroid progenitor cells (Fig. 3). Levels of γ- or β-globin mRNA were induced significantly by DMF and MMF (4- and 8-fold, respectively; Fig. 3A). Fluorescence-activated cell sorting analysis of HbF protein production in these cells demonstrated a significant increase in the number of HbF positive cells in DMF- and MMF-treated cells, respectively (Figs. 3B, 3C). The percent of HbF-positive cells in control, DMF- and MMF-treated cell cultures was 10, 28, and 32, respectively. Values in DMF- and MMF-treated cell cultures were significantly higher than in HU-treated (15%) cell cultures. Evaluation of the mean fluorescence intensity (MFI) in these cells revealed that not only was DMF and MMF treatment associated with an increase in the number of HbF positive cells but additionally that in the cells that were HbF positive in the DMF- and MMF-treated groups, the amount of HbF present was greater than that in the positive cells present in the untreated (control) or HU-treated groups (Fig. 3D). The robust increase in HbF protein induced by DMF and MMF was supported by Western blot analyses (Fig. 3E).
Figure 2.
Dual luciferase assay of γ-globin promoter activity in the human KU812 dual luciferase reporter stable cell line. KU812 stable cells established with the dual luciferase reporter construct were treated with varying concentrations (0–1000 μM) of DMF (A) or MMF (B) for 48 hours. Cells cultured in the absence of the drugs served as negative controls (UT) and those cultured with HU (100 μM) as positive controls. Dual luciferase reporter assay was used to evaluate γ- and β-globin promoter activity by monitoring firefly and renilla luciferase expression, respectively. Cell viability was monitored in parallel by trypan blue exclusion assay. *P < 0.05 and **P < 0.01 compared with untreated control.
Figure 3.
Induction of γ-globin gene expression and HbF production in human primary erythroid progenitors. Human adult CD34+ stem cells were grown in liquid culture to generate erythroid progenitors, which were subjected to DMF and MMF treatment at 200 and 1000 μM concentrations, respectively. Untreated cells served as negative controls, whereas cells cultured in the presence of 100 μM HU served as positive controls for γ-globin and HbF induction. (A) Total RNA was isolated and RT-qPCR analysis performed using primer pairs specific to the human β-globin, γ-globin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes. Globin gene expression was normalized to GAPDH before the γ/β mRNA ratio was calculated. Fluorescence-activated cell sorting analysis of HbF protein expression per cell was performed on treated and untreated cells using FITC-conjugated anti–γ-globin antibody. A representative FACS tracing is shown in (B), and in (C) the number of FITC-positive cells normalized to isotype controls is shown in graphical format. (D) Fetal hemoglobin protein values obtained via FACS were also analyzed as the mean concentration of HbF per cell measured by MFI. (E) Western blot analysis was used to evaluate HbF induction by the different agents. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with untreated control.
Induction of γ-Globin Expression and HbF Production in RPE Cells
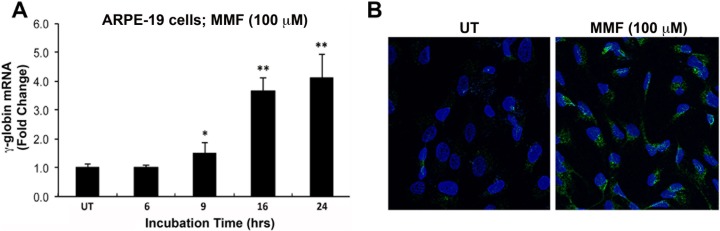
Studies described above demonstrate DMF- and MMF-mediated induction of γ-globin expression and HbF synthesis in human erythroid cells. To determine whether the same occurs in RPE, identical experiments were performed using ARPE-19 and primary humanized mouse RPE cells with the exception that in our studies of RPE, we focused solely on MMF. Following oral intake, DMF is not detectable in plasma as it is rapidly hydrolyzed and converted to MMF16,17; therefore, the latter is the active metabolite that mediates the effect in cells. This information coupled with the fact that we found acute and direct exposure of cells to high concentrations of DMF to be toxic led us to exclude DMF from studies conducted in RPE. As observed in KU812 and primary erythroid progenitor cells, treatment of ARPE-19 cells with a relatively low-dose of MMF (100 μM) produced a significant and sustained increase in γ-globin gene expression (Fig. 4A). Immunofluorescence analysis of HbF protein in these cells following the 24 hour treatment with MMF revealed a corresponding increase in HbF protein (Fig. 4B).
Figure 4.
Induction of γ-globin gene expression and HbF production in the human RPE cell line, ARPE-19. (A) Reverse transcriptase–qPCR analysis of γ-globin mRNA expression in control (UT) and MMF-treated cells (100 μM; 6–24 hours treatment). (B) Immunofluorescence analysis of HbF protein (green fluorescence) production in cells exposed to MMF (100 μM) for 24 hours. Cell nuclei were counterstained with DAPI (blue fluorescence). *P < 0.05 and **P < 0.01 compared with untreated control.
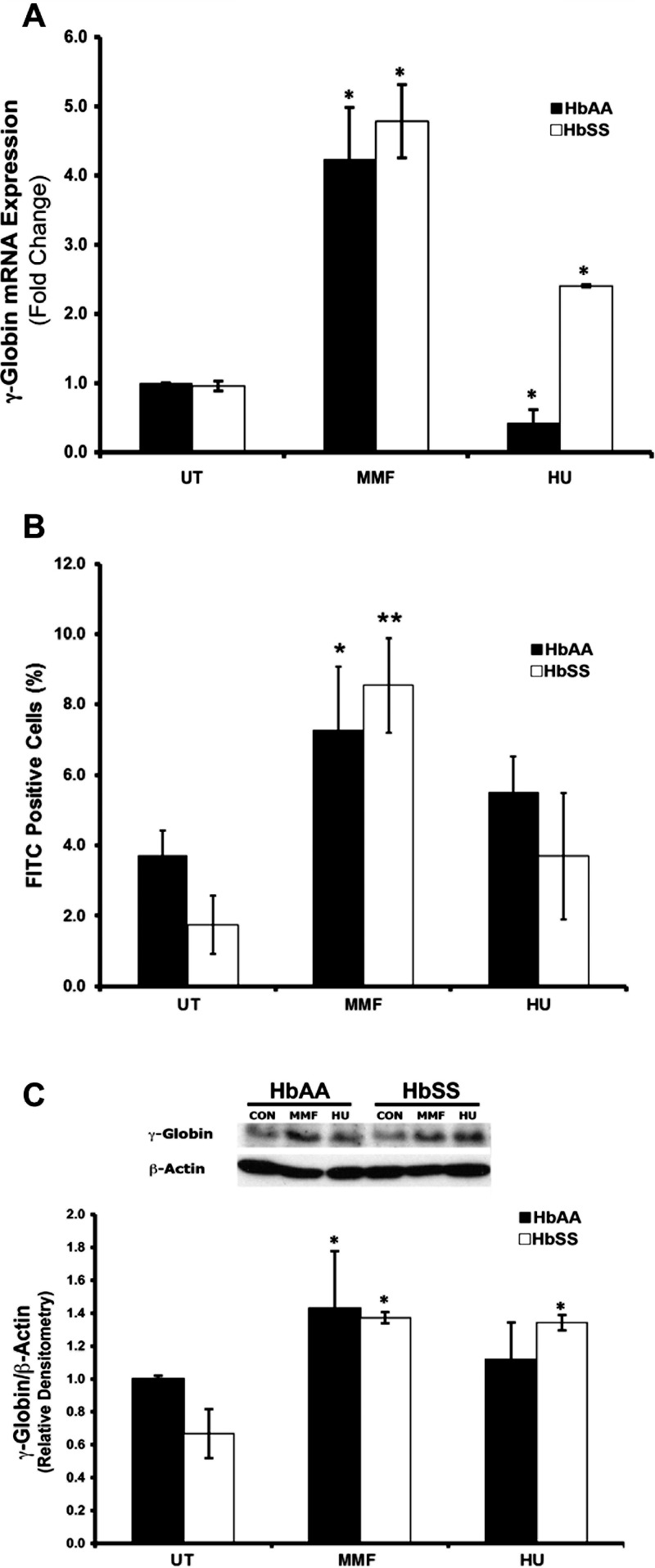
Findings in ARPE-19 cells were corroborated by studies performed using HbAA- and HbSS-expressing primary humanized mouse RPE cells (Fig. 5). Reverse transcriptase–qPCR analysis of MMF-treated cells revealed a significant (4- to 5-fold) increase of γ-globin expression in HbAA and HbSS RPE compared with untreated (UT) cells (Fig. 5A). Fluorescence-activated cell sorting and Western blot analyses confirmed the MMF-induced increase in γ-globin mRNA expression to be associated with a corresponding increase in HbF protein expression (Figs. 5B, 5C, respectively).
Figure 5.
Monomethylfumarate-mediated γ-globin gene induction and HbF production in primary RPE cells isolated from the eyes of HbAA (normal) and HbSS (SCD) Townes humanized knock-in mice. Primary RPE cells were isolated from HbAA and HbSS mouse eyes and subjected to no treatment (negative control; UT), MMF treatment (1000 μM) or HU treatment (positive control; 100 μM) for 24 hours. γ-Globin mRNA expression and HbF protein production were evaluated by (A) RT-qPCR and (B) FACS analysis. (C) Fetal hemoglobin protein expression was confirmed via Western blot coupled with densitometric analysis. *P < 0.05 and **P < 0.01 compared with corresponding AA or SS untreated control.
MMF Induces γ-Globin Expression and HbF Production in Retina In Vivo
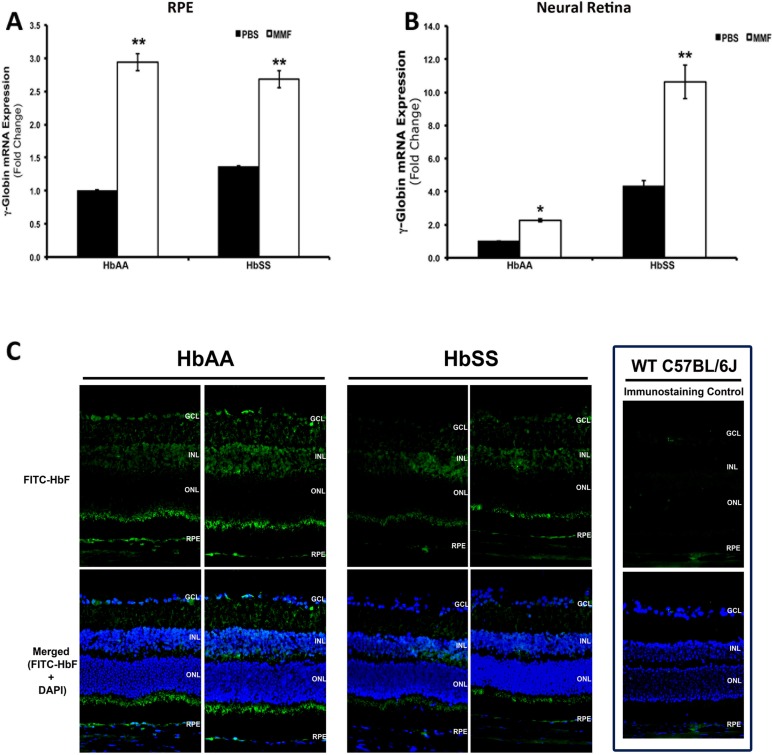
The studies detailed above confirm globin gene expression and consequent Hb production in RPE, and demonstrate the efficacy of MMF in the induction of γ-globin expression and associated HbF protein in this cell type and in erythroid cells. To determine whether these findings translate to the in vivo condition, we delivered MMF, at a final concentration of 1000 μM, a concentration similar to the dosages that were tested in our cell culture model systems, intravitreally to HbAA- and HbSS-expressing humanized mice. Monomethylfumarate treatment was associated with an approximately 3-fold induction of γ-globin expression in RPE tissues after intravitreal injection (Fig. 6A). Interestingly, the MMF-induced increase in γ-globin mRNA levels was not restricted to RPE but was present also in neural retina isolated from these same eyes (Fig. 6B). These data are supported by immunofluorescence analyses of HbF protein in retinal cryosections prepared from PBS- and MMF-injected eyes that demonstrate positive signal for antihuman FITC-HbF (green fluorescence) in the RPE and throughout the neural retina (Fig. 6C). The inclusion of retinal cryosections obtained from regular C57BL/6J wild type animals that express mouse and not human globin genes in the immunolabeling experiments confirmed the specificity of the antihuman FITC-HbF labeling that was detected.
Figure 6.
Monomethylfumarate-mediated induction of γ-globin and HbF protein in intact HbAA and HbSS humanized mouse retinas. Monomethylfumarate (1000 μM final concentration) or PBS (0.01 M, pH 7.4) was injected intravitreally into the eyes of live HbAA and HbSS mice (n = 6). Animals were killed at 24 hours post injection, eyes enucleated, and total RNA prepared from RPE and neural retina and used for RT-qPCR analysis of human γ-globin mRNA expression in (A) RPE and (B) neural retina. Additional eyes were used to prepare retinal cryosections (C). Immunofluorescence analysis of human HbF protein expression using FITC-conjugated HbF antibody (green fluorescence) was performed; DAPI nuclear stain (blue). *P < 0.05 and **P < 0.01 compared with respective PBS-injected HbAA or HbSS control.
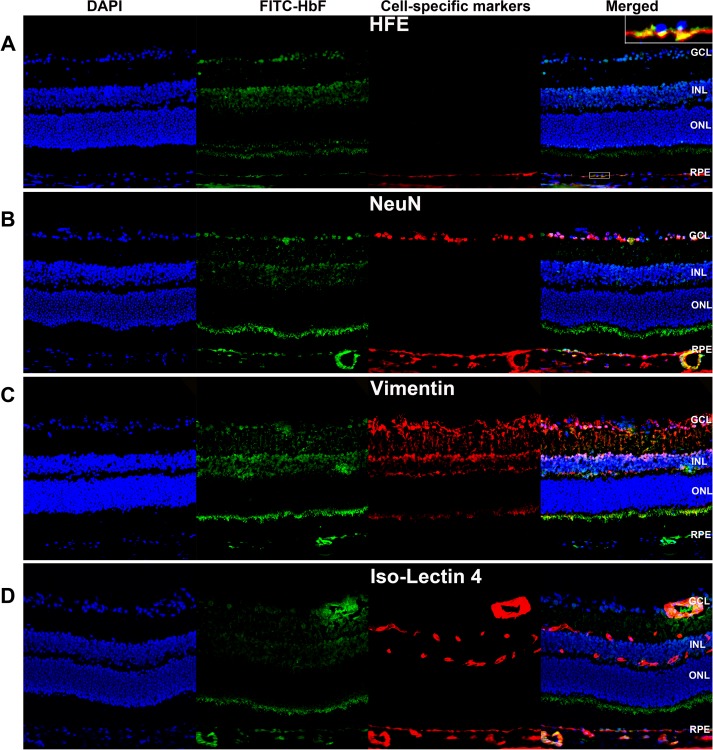
To determine definitively which retinal cell types express human HbF, additional PBS- and MMF-injected cryosections from HbAA- and HbSS-expressing animals were subjected to double-labeling immunofluorescence using the antihuman FITC-HbF antibody in conjunction with established retinal cell-type specific markers. Representative images obtained from MMF-injected HbSS-expressing retinal cryosections are shown in Figure 7. The hemochromatosis gene product HFE is an established marker of RPE basolateral membrane,25 therefore we used HFE to distinguish definitely the boundary between the RPE and the underlying choroid. A digitally magnified view of a region of the RPE cell layer, in which positive signals for FITC-HbF, HFE (red fluorescence), and DAPI (blue fluorescence) were merged (Fig. 7A, inset) demonstrates the presence of HbF protein expression within the RPE basolateral membrane, indicated by the yellow color produced upon the overlap of the positive signals for HFE and HbF, and in the cytoplasm of the RPE (green only signal above that of the red fluorescent signal in the merged image). Labeling of retinal cryosections with FITC-HbF and NeuN,29 a marker of retinal ganglion cells, or the Müller cell–specific marker vimentin30 demonstrated that the positive signal for HbF expression detected within the inner retina stems predominantly from HbF protein expression within Müller glia (Figs. 7B, 7C, respectively). As expected and indicated by double labeling immunofluorescence experiments using the endothelial cell–specific marker isolectin b4,31 HbF protein expression was detected also within some blood vessels in the choroid and in the inner retina (Fig. 7D).
Figure 7.
Monomethylfumarate induces HbF protein expression in RPE and Müller glial cells. Retinal cryosections prepared from HbSS-expressing mice that were injected intravitreally with MMF (1000 μM final concentration) and killed at 24 hours post injection were subjected to double-labeling immunofluorescence using FITC-conjugated HbF antibody (green fluorescence) in conjunction with the RPE basolateral membrane marker HFE (A), the ganglion cell marker NeuN (B), the Müller cell–specific marker Vimentin (C), or the endothelial cell-specific marker isolectin b4 (D). DAPI nuclear stain (blue).
MMF Reduces Oxidative Stress and Inflammation in SCD Retina
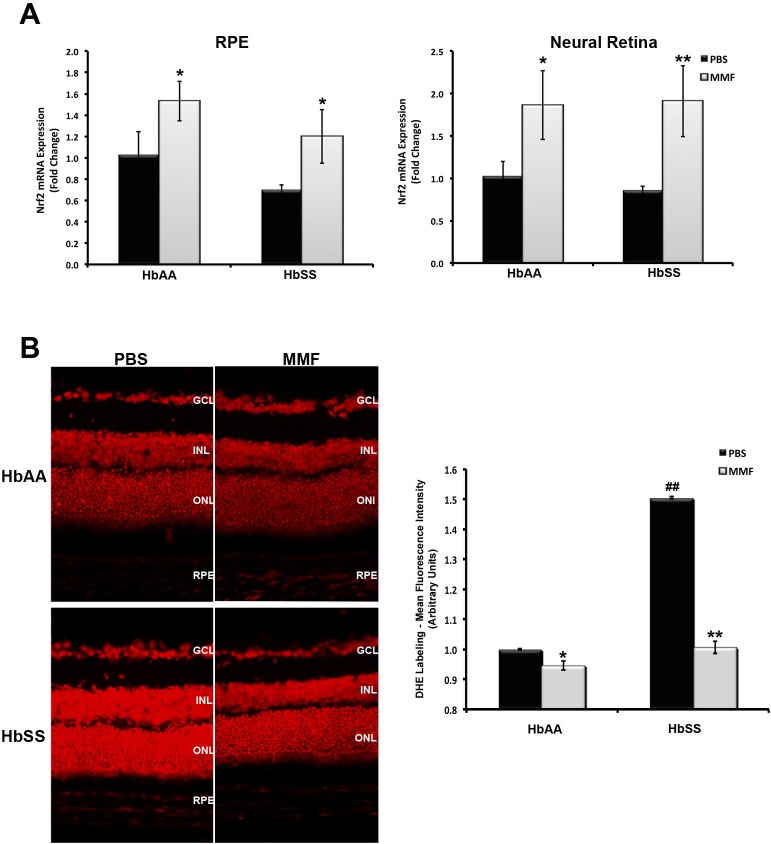
We have demonstrated that MMF induces γ-globin expression and HbF production in vitro and in vivo. However, we do not know the impact of this action on inflammation and oxidative stress, factors crucial to SR, in SCD retina. To address this, we evaluated Nrf2 expression in RNA samples obtained from PBS- and MMF-injected eyes by RT-qPCR (Fig. 8A). Basal levels of Nrf2 expression were reduced but not significantly lower in RPE and neural retinal tissues isolated from HbSS-expressing eyes than in similar tissues obtained from HbAA-expressing eyes. However, there was a significant and robust increase in Nrf2 expression in HbAA- and HbSS-expressing RPE and neural retina following MMF treatment. To confirm whether the MMF-mediated induction of Nrf2 was associated with increased antioxidant response pathway activation, retinal cryosections from PBS- and MMF-injected eyes were subjected to DHE labeling. The intensity of DHE staining was higher in retinal cryosections prepared from PBS-injected HbSS eyes than from PBS-treated HbAA eyes (Fig. 8B). Monomethylfumarate treatment abrogated the increase in superoxide production in HbSS retina as evidenced by the reduced intensity of DHE labeling in MMF-injected eyes.
Figure 8.
Monomethylfumarate reduces oxidative stress in HbSS retina. (A) Reverse transcriptase–qPCR analysis of Nrf2 expression was performed using total RNA prepared from the RPE and neural retina of MMF- and PBS (control)-injected HbAA and HbSS mouse eyes. (B) Dihydroethidium labeling (red fluorescence) followed by densitometric analysis was performed on retinal cryosections prepared from the same animals. *P < 0.05 and **P < 0.01 compared with respective PBS-injected HbAA or HbSS control; ##P < 0.01 compared with PBS-injected HbAA control.
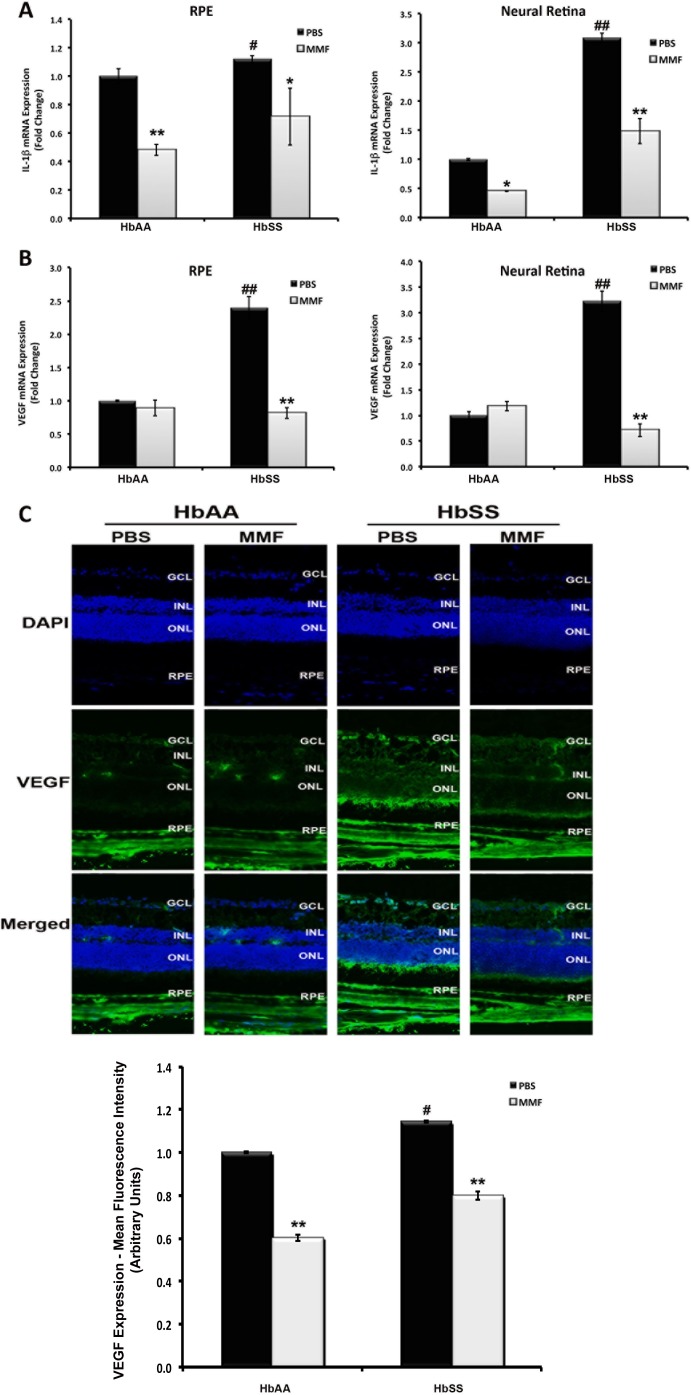
Interleukin-1β is a pro-inflammatory cytokine whose expression has been reported to increase in conditions of retinal ischemia and microvascular dysfunction/proliferative vascular disease,32 factors common to SR. The effects of IL-1β are purportedly mediated through the induction of the genes whose promoters are regulated via complex interactions with transcription factors such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), transcription factors that have been strongly implicated also in SCD.33 We next examined the expression of IL-1β as an indicator of inflammation in HbAA and HbSS retina. Reverse transcriptase–qPCR analyses revealed the upregulation of IL-1β expression in RPE and neural retinal tissues isolated from HbSS-expressing humanized mouse eyes (Fig. 9A). Interleukin-1β expression was significantly higher in HbSS RPE and neural retina than in HbAA RPE and neural retina. Treatment with MMF reduced significantly the expression of IL-1β both in HbAA- and HbSS-expressing RPE and neural retina suggesting a decrease in inflammation. To follow up this finding, we evaluated the expression of VEGF, another well-established marker of retinal inflammation and of pathologic angiogenesis,34,35 at the mRNA and protein levels (Figs. 9B, 9C). Vascular endothelial growth factor expression was significantly higher in RNA samples obtained from PBS-injected HbSS eyes compared with PBS-injected HbAA eyes (Fig. 8B). Monomethylfumarate treatment decreased VEGF expression in HbSS mouse RPE and neural retina to levels comparable to those in HbAA RPE and neural retina. Immunofluorescence and associated densitometric analyses of VEGF protein supported this finding (Fig. 9C).
Figure 9.
Monomethylfumarate reduces inflammation in HbSS retina. Reverse transcriptase–qPCR analysis of (A) IL-1β and (B) VEGF expression was performed using total RNA prepared from the RPE and neural retina of MMF- and PBS (control)-injected HbAA and HbSS humanized mouse eyes. (C) Vascular endothelial growth factor protein expression was evaluated in retinal cryosections prepared from these same animals using immunofluorescence means. *P < 0.05 and **P < 0.01 compared with respective PBS-injected HbAA or HbSS control. #P < 0.05 and ##P < 0.01 compared with PBS-injected HbAA control.
Discussion
Sickle cell disease is the most common genetic mutation among the US population and represents a major health cost in this country.2 Debilitating blindness occurs in 10% of sickle cell patients however there are no strategies to prevent SR, and options for treatment are limited. Present clinical management of SR includes diathermy, cryotherapy, and laser photocoagulation, strategies aimed at inducing the regression of neovascular lesions before they progress to bleeding and retinal detachment.6 While such strategies are effective in some patients, they are applicable relatively late in SR pathogenesis, effective only in proliferative or advanced-stage disease. This emphasizes strongly the definite and imperative need for development of new therapies to prevent or intervene “early” in SR. Toward this end, the identification of novel cellular and molecular targets is important.
We embarked upon the present studies with a purpose that was 3-fold: (1) to confirm globin gene expression and Hb production in RPE, (2) to determine whether MMF can induce γ-globin expression and HbF production in RPE cells, and (3) to investigate whether increased γ-globin expression and HbF production confer benefit to retina in terms of reduced inflammation and/or oxidative stress. Using ARPE-19 and primary RPE cells isolated from HbAA- and HbSS-expressing Townes humanized knock-in mice, we confirmed that RPE cells express the genes necessary to synthesize Hb. The ability of RPE to produce Hb suggests their potential involvement in the etiology of SR. Although not robust, there is clinical evidence of RPE and neuroretinal dysfunction early in SCD. Peachey et al.36,37 documented the existence of electrophysiological anomalies consistent with outer retinal cell dysfunction in the eyes of SCD patients with and without clinically detectable vascular pathology. Additionally, RPE disruptions such as black sunburst lesions in SCD are a common clinical finding.3,38 The induction of γ-globin expression and HbF protein production has been demonstrated extensively in peripheral erythrocytes and has proven benefit in terms of preventing and treating systemic complications of SCD. Given that in SCD, RPE cells should like RBCs, produce HbS, it is plausible that increasing HbF production in these cells in addition to RBCs might be of benefit in SR. The recent study by Estepp et al.27 demonstrating the incidence of SR to be significantly lower in patients with higher basal levels of HbF lends strong support to this notion, although the study did not examine in which cell types specifically the HbF was present. As such, we next sought to determine whether γ-globin expression and HbF expression could be induced in RPE. At present, HU is the only FDA-approved HbF-inducing drug available for use in SCD. Hydroxyurea has shown some efficacy in SCD but is associated with a number of major concerns including significant variability of patient responsiveness, bone marrow toxicity at high concentrations, and controversy over the optimal age to initiate therapy.9,10 Therefore, we evaluated not only the induction of γ-globin expression and HbF production in RPE but also, MMF as a feasible alternative to HU for HbF induction.
Fumaric acid esters are noted clinically for the robust antioxidant and antiinflammatory properties they elicit in a diverse spectrum of tissue and cell types.11–15 Others and we have shown these properties to stem directly from the activation of Nrf2 and downstream targets,14,15 an effect that according to the recent report by Macari et al.39 may be of benefit also in terms of inducing γ-globin and treating SCD. In the present study, approaches using established erythroid cell lines and γ-globin induction screening systems demonstrated γ-globin promoter activation by DMF and by MMF. Follow-up studies conducted using primary human erythroid progenitors confirmed the robust induction of γ-globin expression and HbF synthesis in the presence of both compounds. While DMF proved to be toxic at higher concentrations, MMF could be used at substantially high concentrations, stimulating γ-globin induction, and HbF protein production for sustained periods following the administration of a single dose.
Dimethylfumarate is the major component of the current United States FDA-approved FAE drug Tecfidera40; MMF is the major bioactive component and therefore the compound mediating the therapeutic effect in cells. Hence, we extrapolated the strategies used to study γ-globin induction in erythroid cells to RPE and moved forward focusing primarily on MMF. As found in erythroid cells, MMF proved to be quite effective in inducing γ-globin expression and HbF production in cultured ARPE-19 cells, HbAA-expressing primary humanized RPE cells, and HbSS-expressing primary RPE cells. Intravitreal delivery of MMF to the eyes of HbAA and HbSS humanized mice confirmed the in vivo efficacy of MMF in this regard. Reverse transcriptase–qPCR and immunofluorescence studies revealed the association of MMF treatment with γ-globin and HbF induction in the RPE and the neural retina of SCD mice. These findings suggest that MMF induces γ-globin and HbF production in RPE, RBCs, and as indicated by double immunolabeling experiments, other retinal cell types (i.e., Müller glia); hence, RPE may not be the only retinal cell type to produce Hb. Follow-up studies to investigate this possibility are well warranted.
The finding that γ-globin expression and HbF production can be induced in RPE and cell types within the neural retina is novel; however, understanding the therapeutic potential associated with such action is more important with respect to its possible use in the prevention and treatment of SR. Toward this end, we conducted RT-qPCR and immunofluorescence analyses of parameters relevant to oxidative stress and inflammation in samples obtained from MMF- and PBS-injected HbAA and HbSS humanized mouse eyes. Nuclear factor (erythroid-derived 2)-like 2 expression and DHE labeling were used to evaluate oxidative stress. Basal levels of oxidative stress were greater in tissues obtained from HbSS-expressing mouse retinas than in those obtained from HbAA-expressing mouse retinas. Monomethylfumarate treatment of these animals was associated with increased Nrf2 expression and decreased DHE staining, which supports a reduction of oxidative stress in HbSS retina. Similarly, the expression of IL-1β and VEGF, biomarkers of insufficient oxygen delivery to tissues and possible clinical indicators of tissue hypoxia and transfusion need,41 factors of particular relevance to SCD, was higher in HbSS RPE and neural retina, but reduced by MMF treatment, suggesting a coordinate reduction of inflammation as well.
Little is known regarding the importance of Hb production by RPE in healthy individuals or in SCD where abnormal HbS is produced. Likewise, additional data is required to determine the clinical impact of HbF-inducing therapies in the retina. Our present finding that MMF induces γ-globin expression and HbF production in retinal and erythroid cells is novel and supports the therapeutic potential of this compound in the treatment of retinal and systemic complications of SCD. Given the 10% incidence of vision loss and blindness among SCD patients,1,2 our study is of high clinical relevance. Furthermore, the fact that an FDA-approved formulation of our HbF-inducing drug of choice (DMF/MMF) has been demonstrated to be safe for prolonged use in humans with minimal toxicity heightens the potential for rapid extrapolation of our findings into clinical trials.
Acknowledgments
The authors thank Rajalakshmi Veeranan-Karmegan, Sudha Ananth, and Rachel Hutto for technical support.
Supported by a Pilot Project Award from the James and Jean Culver Vision Discovery Institute (Georgia Regents University, Augusta, GA, USA) and by National Institutes of Health (Bethesda, MD, USA) Grants EY022704 and HL069234.
Disclosure: W. Promsote, None; L. Makala, None; B. Li, None; S.B. Smith, None; N. Singh, None; V. Ganapathy, P; B.S. Pace, None; P.M. Martin, P
References
- 1. Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010; 376: 2018–2031 [DOI] [PubMed] [Google Scholar]
- 2. Elagouz M, Jyothi S, Gupta B, Sivaprasad S. Sickle cell disease and the eye: old and new concepts. Surv Ophthalmol. 2010; 55: 359–377 [DOI] [PubMed] [Google Scholar]
- 3. Ballas SK, Kesen MR, Goldberg MF, et al. Beyond the definitions of the phenotypic complications of sickle cell disease: an update on management. ScientificWorldJournal. 2012; 2012: 949535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenberg JB, Hutcheson KA. Pediatric sickle cell retinopathy: correlation with clinical factors. J AAPOS. 2011; 15: 49–53 [DOI] [PubMed] [Google Scholar]
- 5. Gee BE. Biologic complexity in sickle cell disease: implications for developing targeted therapeutics. ScientificWorldJournal. 2013; 2013: 6494146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kassim AA, DeBaun MR. Sickle cell disease, vasculopathy, and therapeutics. Ann Rev Med. 2013; 64: 451–466 [DOI] [PubMed] [Google Scholar]
- 7. Hebbel RP, Vercellotti GM, Pace BS, et al. The HDAC inhibitors trichostatin A and suberoylanilide hydroxamic acid exhibit multiple modalities of benefit for the vascular pathology of sickle transgenic mice. Blood. 2010; 115: 2483–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cools J. Using the hemoglobin switch for the treatment of sickle cell disease. Hematologica. 2012; 97: 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vichinsky EP, Lubin BH. A cautionary note regarding hydroxyurea in sickle cell disease. Blood. 1994; 83: 1124–1128 [PubMed] [Google Scholar]
- 10. Brawley OW, Cornelius LJ, Edwards LR, et al. NIH consensus development statement on hydroxyurea treatment for sickle cell disease. NIH Consens State Sci Statements. 2008; 25: 1–30 [PubMed] [Google Scholar]
- 11. Rostami YM, Mroweitz U. Fumaric acid esters. Clin Dermatol. 2008; 26: 522–526 [DOI] [PubMed] [Google Scholar]
- 12. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012; 360: 1098–1107 [DOI] [PubMed] [Google Scholar]
- 13. Bozard BR, Chothe PP, Tawfik A, et al. Regulation of proton-coupled folate transporter in retinal Müller cells by the antipsoriatic drug monomethylfumarate. Glia. 2012; 60: 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ananth S, Babu E, Veeranan-Karmegam R. Bozard Baldowski BR, Boettger T, Martin PM. Induction of the cystine/glutamate exchanger SLC7A11 in retinal pigment epithelial cells by the antipsoriatic drug monomethylfumarate. Invest Ophthalmol Vis Sci. 2013; 54: 1592–1602 [DOI] [PubMed] [Google Scholar]
- 15. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011; 134: 678–692 [DOI] [PubMed] [Google Scholar]
- 16. Kees F. Dimethylfumarate: a Janus-faced substance. Expert Opin Pharmacotherp. 2013; 14: 1559–1567 [DOI] [PubMed] [Google Scholar]
- 17. Litjens NA, Burggraaf J, van Strijen E, et al. Pharmacokinetics of oral fumarates in healthy subjects. Br J Clin Pharmacol. 2004; 58: 429–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010; 10: 802–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tezel TH, Geng L, Lato EB, et al. Synthesis and secretion of hemoglobin by retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2009; 50: 1911–1919 [DOI] [PubMed] [Google Scholar]
- 20. Zein S, Li W, Ramakrishnan V, et al. Identification of fetal hemoglobin-inducing agents using the human leukemia KU812 cell line. Exp Biol Med. 2010; 235: 1385–1394 [DOI] [PubMed] [Google Scholar]
- 21. Hebiguchi M, Hirokawa M, Guo YM, et al. Dynamics of human erythroblast enucleation. Int J Hematol. 2008; 88: 498–507 [DOI] [PubMed] [Google Scholar]
- 22. Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correlation of sickle cell disease by homologous recombination in embryonic stem cells. Blood. 2006; 108: 1183–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin PM, Ananth S, Cresci G, Roon P, Smith S, Ganapathy V. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol Vis. 2009; 15: 362–372 [PMC free article] [PubMed] [Google Scholar]
- 24. Promsote W, Veeranan-Karmegam R, Ananth S, et al. L-2-oxothiazolidine-4-carboxylic acid attenuates oxidative stress and inflammation in retinal pigment epithelium. Mol Vis. 2014; 20: 73–88 [PMC free article] [PubMed] [Google Scholar]
- 25. Martin PM, Gnana-Prakasam JP, Roon P, et al. Expression and polarized localization of the hemochromatosis gene product HFE in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2006; 47: 4238–4244 [DOI] [PubMed] [Google Scholar]
- 26. Gnana-Prakasam JP, Ananth S, Prasad PD, et al. Expression and iron-dependent regulation of succinate receptor GPR91 in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011; 52: 3751–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dun KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62: 55–69 [DOI] [PubMed] [Google Scholar]
- 28. Estepp HJ, Smeltzer MP, Wang WC, Hoehn ME, Hankins JS, Aygun B. Protection from sickle cell retinopathy is associated with elevated HbF levels and hydroxycarbamide use in children. Br J Hematology. 2013; 161: 402–405 [DOI] [PubMed] [Google Scholar]
- 29. Yang Y, Mao D, Chen X, et al. Decrease in retinal neuronal cells in streptozotocin-induced diabetic mice. Mol Vis. 2012; 18: 1411–1420 [PMC free article] [PubMed] [Google Scholar]
- 30. Umapathy NS, Dun Y, Martin PM, et al. Expression and function of system N glutamine transporters (SN1/SN2 or SNAT3/SNAT5) in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2008; 49: 5151–5160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tawfik A, Al-Shabrawey M, Roon P, et al. Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia. Invest Ophthalmol Vis Sci. 2013; 54: 939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kowluru RA, Odenbach S. Role of interleukin-β in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004; 88: 1343–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gonsalves C, Kalra VK. Endothelin-1-induced macrophage inflammatory protein-1beta expression in monocytic cells involves hypoxia-inducible factor-1alpha and AP-1 and is negatively regulated by microRNA-195. J Immunol. 2010; 185: 6253–6264 [DOI] [PubMed] [Google Scholar]
- 34. Kim SY, Mocanu C, Mcleod DS, et al. Expression of pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in sickle cell retina and choroid. Exp Eye Res. 2003; 77: 433–45 [DOI] [PubMed] [Google Scholar]
- 35. NaKao S, Arima M, Ishikawa K, et al. Intravitreal anti-VEGF therapy blocks inflammatory cell infiltration and re-entry into the circulation in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2012; 53: 4323–4328 [DOI] [PubMed] [Google Scholar]
- 36. Peachey NS, Charles HC, Lee CM, Fishman GA, Cunha-Vaz JG, Smith RT. Electroretinographic findings in sickle cell retinopathy. Arch Ophthalmol. 1987; 105: 934–938 [DOI] [PubMed] [Google Scholar]
- 37. Peachey NS, Gagliano DA, Jacobson MS, Derlacki DJ, Fishman GA, Cohen SB. Correlation of electroretinographic findings and peripheral retinal nonperfusion in patients with sickle cell retinopathy. Arch Ophthalmol. 1990; 108: 106–109 [DOI] [PubMed] [Google Scholar]
- 38. Fekrat S, Goldberg MF. Sickle cell retinopathy. In: Regillo CD, Brown GC, Flynn HW Jr, eds. Vitreoretinal Disease: The Essentials. New York: Thieme; 1999: 333–345 [Google Scholar]
- 39. Macari ER, Lowrey CH. Induction of human fetal Hb via the Nrf2 antioxidant response signaling pathway. Blood. 2011; 117: 5987–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Venci JV, Gandhi MA. Dimethyl fumarate (tecfidera): a new oral agent for multiple sclerosis. Ann Pharmacother. 2013; 47: 1697–1702 [DOI] [PubMed] [Google Scholar]
- 41. Tschirch E, Weber B, Koehne P, et al. Vascular endothelial growth factor as a marker for tissue hypoxia and transfusion need in anemic infants: a prospective clinical study. Pediatrics. 2009; 123: 784–790 [DOI] [PubMed] [Google Scholar]